A Novel Continuous Ultrasound-Assisted Leaching Process for Rare Earth Element Extraction: Environmental and Economic Assessment
Abstract
1. Introduction
2. Materials and Methods
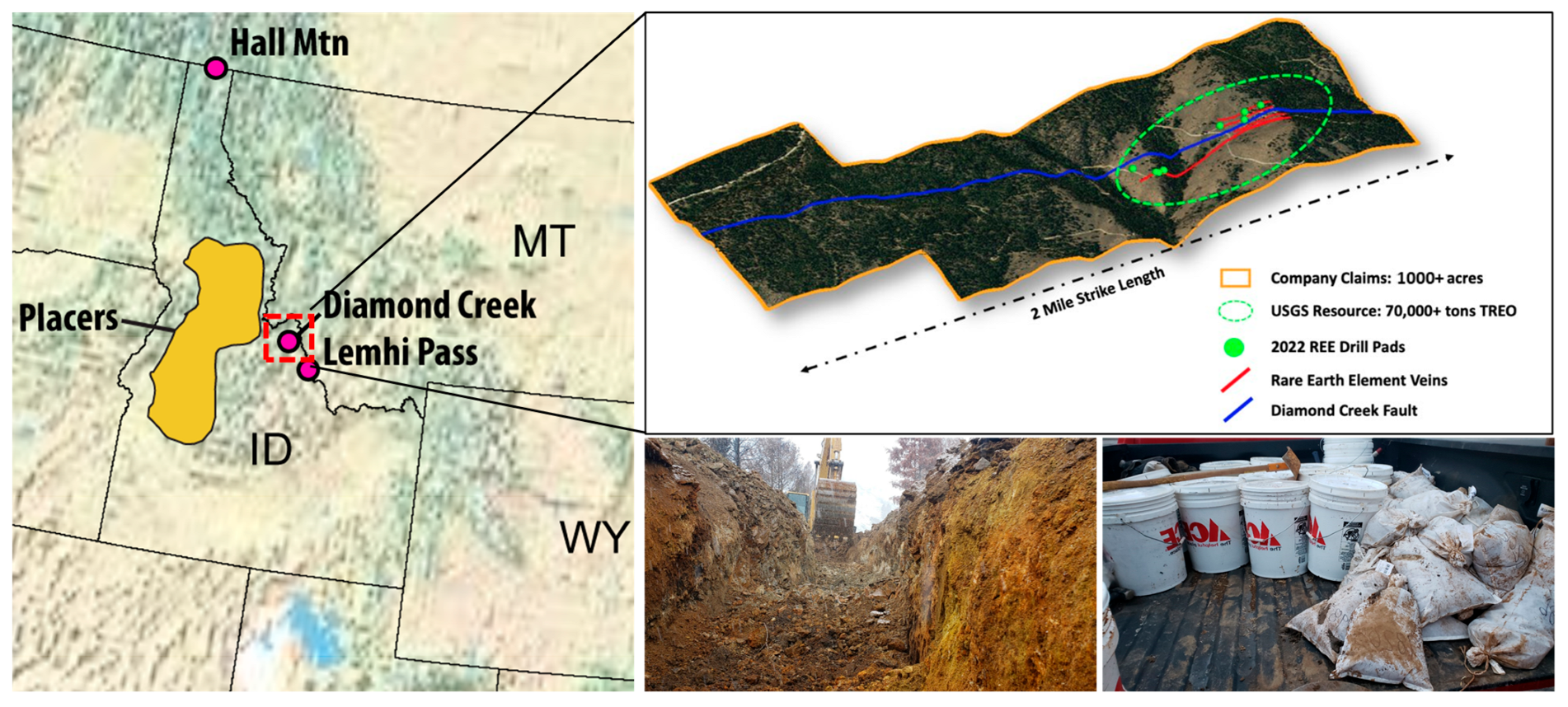
2.1. Sample Collection
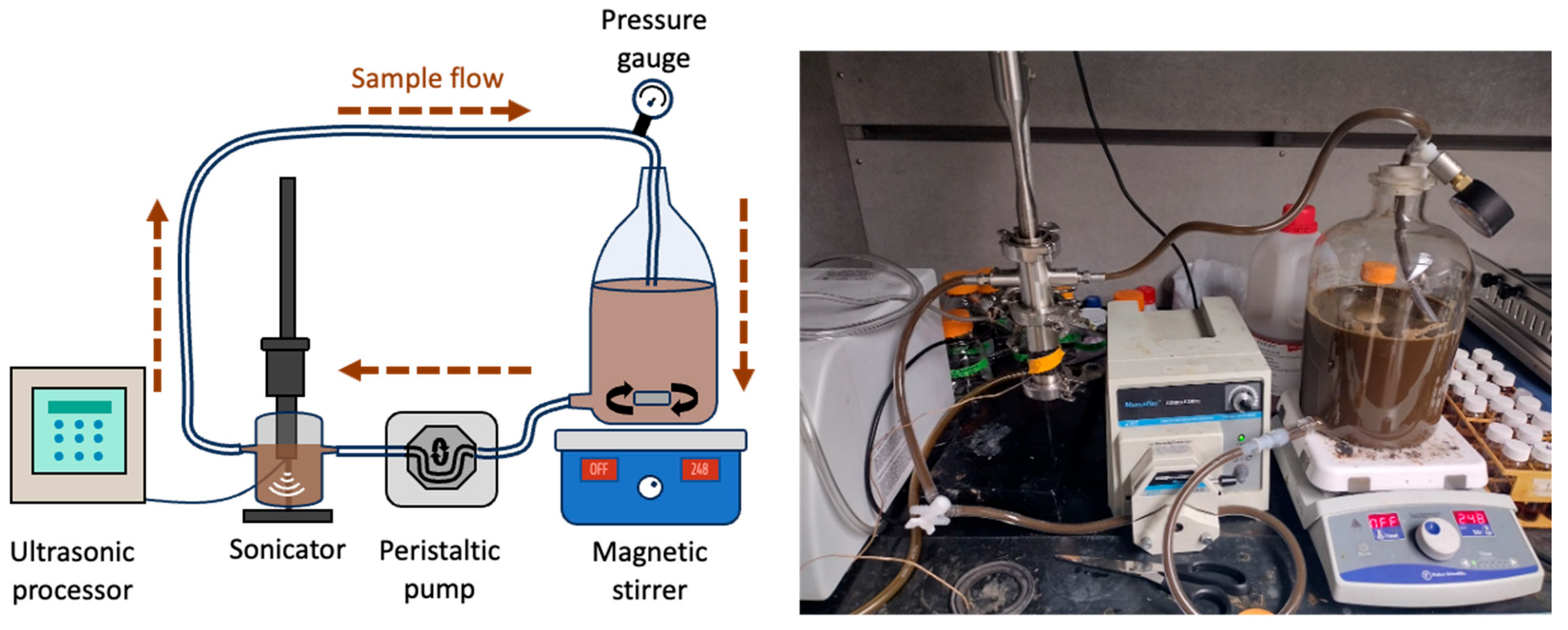
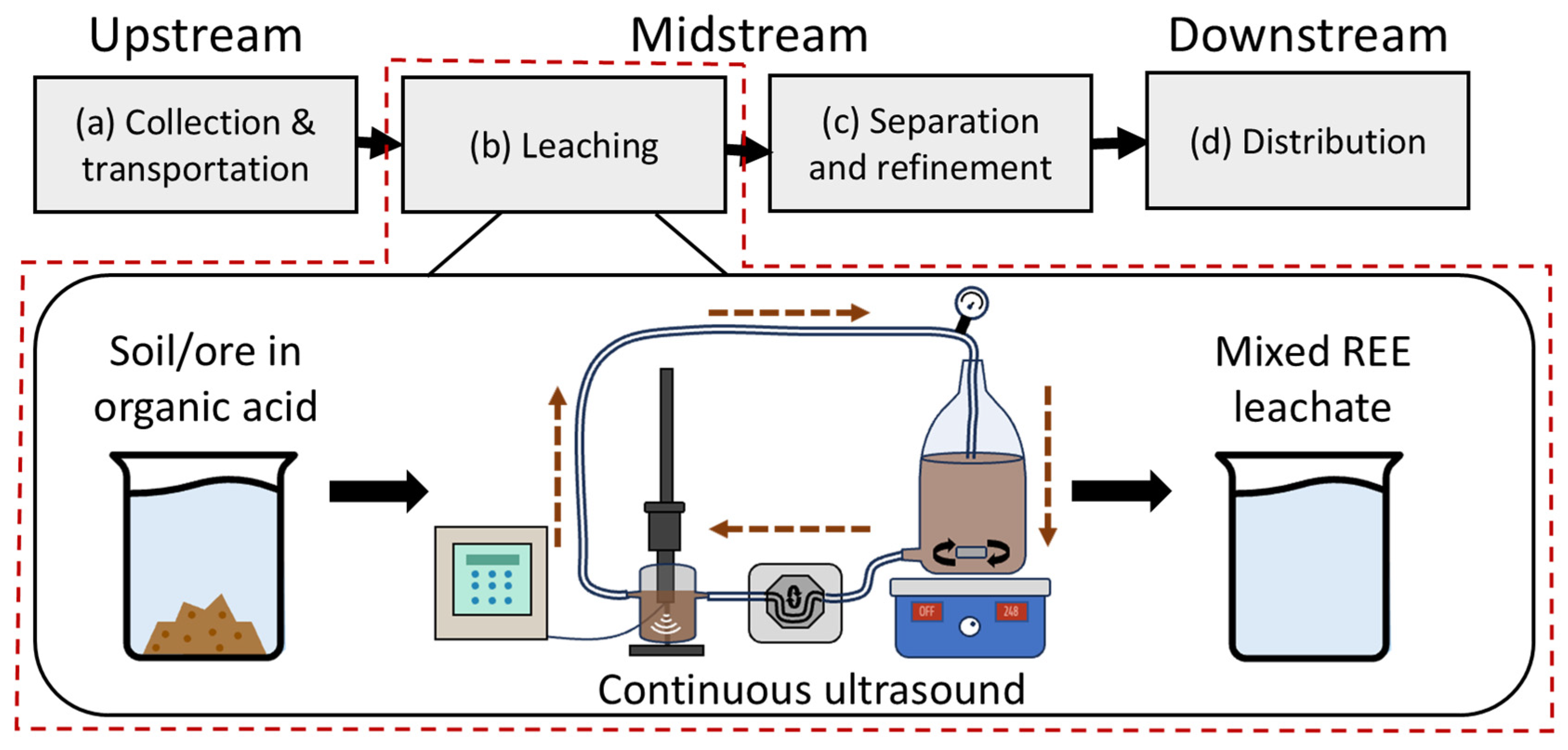
2.2. Continuous Ultrasound-Assisted Organic Acid Leaching
2.3. Analytical Methods
2.4. Environmental Impact Assessment
2.4.1. Goal and Scope
2.4.2. Life Cycle Inventory Analysis
2.4.3. Life Cycle Impact Assessment
2.4.4. Interpretation
2.5. Case Study
- 100,000 kg (100 metric tons) of soil containing a mixed REE concentration of 1.16% was processed.
- 142 kg of mixed REEs were extracted from 100,000 kg of soil using gluconic acid (12.2% yield).
- 202 kg of mixed REEs were extracted from 100,000 kg of soil using citric acid (17.4% yield).
- Energy data was measured in the laboratory-scale experiments and scaled linearly. Energy data of the key continuous ultrasound-assisted leaching steps were included in this study (i.e., ultrasound leaching and centrifugation of the resulting slurry). The energy used to power the facility was not considered.
- The capital, overhead, and variable costs are not considered in this study. A comprehensive evaluation of these costs can be found in Brown et al., 2024 [21].
- Emission factors from the Intergovernmental Panel on Climate Change for a 100-year time horizon (28 kg CO2 eq./kg CH4 and 265 kg CO2 eq./kg N2O) were used to calculate process emissions [26].
- The emissions produced during continuous ultrasound-assisted leaching of 100,000 kg of soil were assumed to be 69,428,000 CO2 eq./kg CO2, 544 kg CO2 eq./kg N2O, and 2788 kg CO2 eq./kg CH4.
- The LCA study focuses on gaseous emissions and environmental impacts of the midstream ultrasound-assisted leaching process. The environmental impacts of upstream processes (collection and transportation) additional midstream processes (separation and refinement), and downstream processes (distribution) were not assessed in this study.
2.6. Techno-Economic Analysis
2.7. Sensitivity Analysis
3. Results
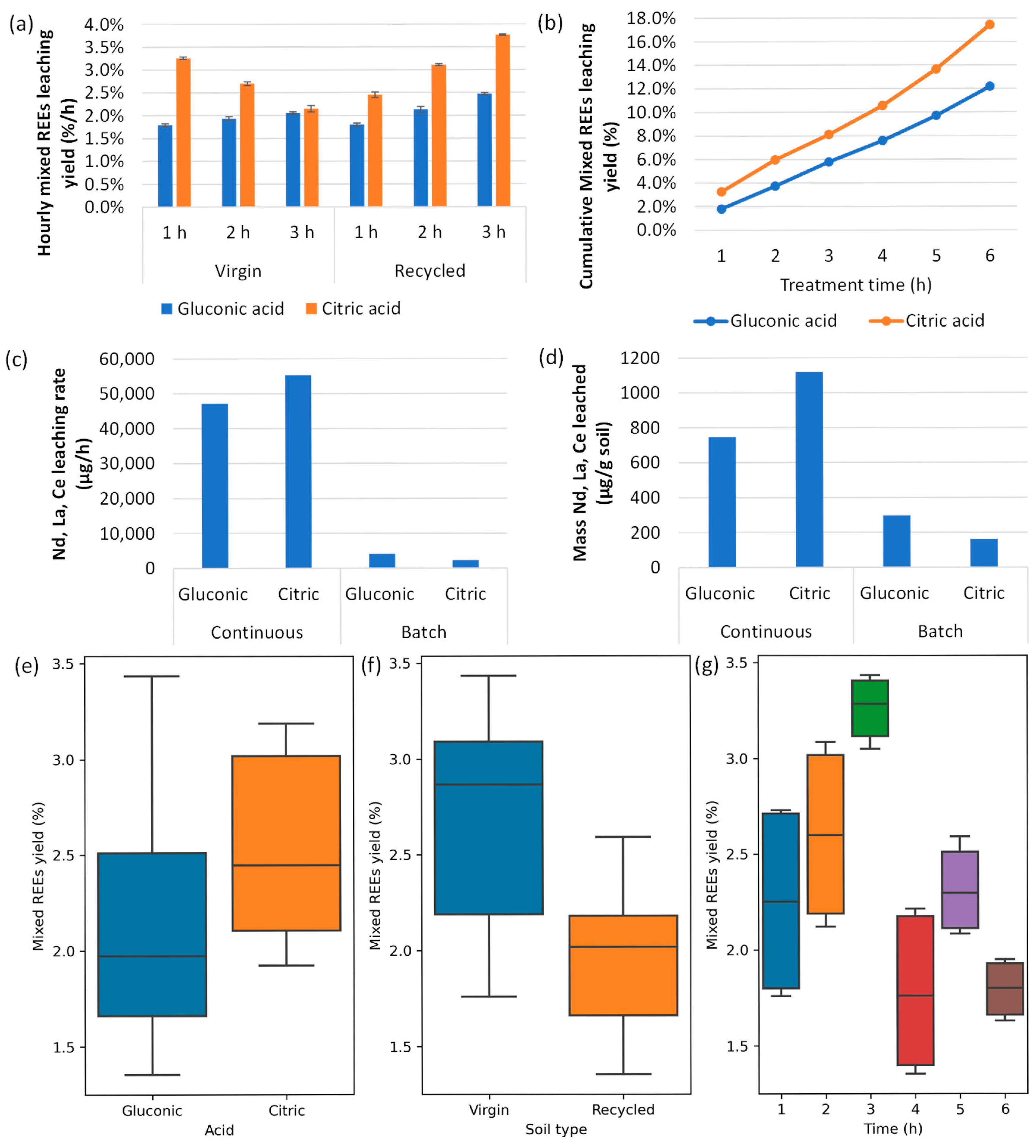
3.1. Laboratory Results
3.2. Data Analysis Results
3.3. Environmental Impact Assessment Results
3.4. Techno-Economic Analysis Results
3.5. Sensitivity Analysis Results
4. Discussion
- Exploration of ultrasound-assisted organic acid leaching with microbially produced biolixiviants (such as Gluconobacter oxydans and Aspergillus niger).
- Implementation of a mixed-organic acid leaching method.
- Improving organic acid leaching yield utilizing a combination of ultrasound and microwave-assisted methods.
- Further increasing the scale of ultrasound-assisted organic acid leaching to encourage industrial implementation.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Al | Aluminum |
| C | Mixed REE concentration of ICP-MS sample (μg/mL) |
| CAT | Annual total cost for the proposed process ($/yr) |
| Ce | Cerium |
| CE | Annual total cost of energy ($/yr) |
| CH4 | Methane |
| CO2 | Carbon dioxide |
| COA | Cost of purchasing organic acid ($/L) |
| CR | Annual total cost of reagents ($/yr) |
| CUP | Cost of utilities ($/kWh) |
| DoD | Department of defense |
| Dy | Dysprosium |
| E | Energy |
| EPA | U.S. environmental protection agency |
| Er | Erbium |
| ERCH4 | Emissions rate of CH4 (kg CO2 eq./kg CH4) |
| ERCO2 | Emissions rate of CO2 (kg CO2 eq./kg CO2) |
| ERN2O | Emissions rate of N2O (kg CO2 eq./kg N2O) |
| Fe | Iron |
| Gd | Gadolinium |
| H2SO4 | Sulfuric acid |
| Ho | Holmium |
| L | Continuous ultrasound-assisted leaching |
| La | Lanthanum |
| LCA | Life cycle assessment |
| LCIA | Life cycle impact assessment |
| LEF | GHG emissions factor for continuous ultrasound-assisted leaching (kg CO2 eq. per ton) |
| LEFCH4 | CH4 emission factor for continuous ultrasound-assisted leaching (kg CH4 per ton) |
| LEFCO2 | CO2 emission factor for continuous ultrasound-assisted leaching (kg CH4 per ton) |
| LEFN2O | N2O emission factor for continuous ultrasound-assisted leaching (kg N2O per ton) |
| LGWP | Continuous ultrasound-assisted leaching emissions (kg CO2 eq.) |
| M | Mass of REEs solubilized during the leaching reaction (μg) |
| MRL | Mass of REEs leached (metric ton) |
| NaOH | Sodium Hydroxide |
| Nd | Neodymium |
| NO2 | Nitrogen dioxide |
| PLS | Pregnant leach solution |
| Pr | Praseodymium |
| R | Reagents |
| REE | Rare earth element |
| Sc | Scandium |
| Sm | Samarium |
| Tb | Terbium |
| TEA | Techno-economic analysis |
| Ti | Titanium |
| TRL | Technology readiness level |
| U | Utilization |
| UE | Annual utilization rate of energy (kWh/yr) |
| UL | Annual ultrasound-assisted leaching utilization (metric tons/yr) |
| UOA | Annual organic acid utilization (L/metric ton) |
| V | Total reaction volume (mL) |
| Y | Yttrium |
| Yb | Ytterbium |
| Yi | Leaching yield (%) |
Appendix A
| Inputs | Quantity | Outputs | Quantity |
|---|---|---|---|
| Soil | 100,000 kg | - | - |
| Gluconic acid, 50% (w/w) | 2,232,000 kg | ||
| Citric acid, 100% anhydrous | 1,107,000 kg | - | - |
| Water (citric acid) | 1,107,000 kg | - | - |
| Electricity, medium voltage {US} | 2,584,000 kWh | - | - |
| - | - | Mixed REEs (gluconic acid) | 142 kg |
| - | - | Mixed REEs (citric acid) | 202 kg |
| - | - | CO2 emissions | 69,428,000 kg |
| - | - | N2O emissions | 544 kg |
| - | - | CH4 emissions | 2788 kg |
| - | - | Solid byproducts (gluconic acid) | 99,798 kg |
| Solid byproducts (citric acid) | 99,858 kg |
| Parameter | Definition | Value (Gluconic Acid) | Value (Citric Acid) | Unit |
|---|---|---|---|---|
| CAT | Annual total cost of the proposed process | 1,339,490 | 827,869 | $/yr |
| CR | Annual total cost of reagents | 1,055,250 | 588,629 | $/yr |
| CE | Annual total cost of energy | 284,240 | 284,240 | $/yr |
| COA | Cost of purchasing organic acids | 0.59 | 0.33 | $/L |
| UOA | Annual organic acid utilization | 1,800,000 | 1,800,000 | L/metric ton |
| UL | Annual ultrasound-assisted leaching utilization | 100 | 100 | Metric tons/yr |
| CUP | Cost of utilities | 0.11 | 0.11 | $/kWh |
| UE | Annual utilization rate of energy | 2,584,000 | 2,584,000 | kWh/yr |
| Organic Acid | Ore/Soil Type | Time (h) | Temperature (°C) |
|---|---|---|---|
| Gluconic acid | Virgin | 0 | 22.0 |
| Virgin | 1 | 35.0 | |
| Virgin | 2 | 40.3 | |
| Virgin | 3 | 45.9 | |
| Recycled | 1 | 36.9 | |
| Recycled | 2 | 41.2 | |
| Recycled | 3 | 46.8 | |
| Citric acid | Virgin | 0 | 22.0 |
| Virgin | 1 | 39.7 | |
| Virgin | 2 | 40.1 | |
| Virgin | 3 | 40.5 | |
| Recycled | 1 | 38.8 | |
| Recycled | 2 | 39.9 | |
| Recycled | 3 | 41.0 |
Appendix B
References
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Lopez, T. DOD Looks to Establish “Mine-to-Magnet” Supply Chain for Rare Earth Materials. 2024. Available online: https://www.defense.gov/News/News-Stories/Article/Article/3700059/dod-looks-to-establish-mine-to-magnet-supply-chain-for-rare-earth-materials/#:~:text=Since%202020%2C%20DOD%20has%20awarded,into%20metals%20and%20then%20magnets (accessed on 9 July 2024).
- McNulty, T.; Hazen, N.; Park, S. Processing the ores of rare-earth elements. MRS Bull. 2022, 47, 258–266. [Google Scholar] [CrossRef]
- Xue, H.; Lv, G.; Wang, L.; Zhang, T. Review of rare earth extraction and product preparation technologies and new thinking for clean utilization. Miner. Eng. 2024, 215, 108796. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Nayar, J. Not So Green Technology: The Complicated Legacy of Rare Earth Mining. Harvard International Review. 2021. Available online: https://hir.harvard.edu/not-so-green-technology-the-complicated-legacy-of-rare-earth-mining/ (accessed on 21 July 2024).
- Brown, R.M.; Mirkouei, A.; Reed, D.; Thompson, V. Current nature-based biological practices for rare earth elements extraction and recovery: Bioleaching and biosorption. Renew. Sustain. Energy Rev. 2023, 173, 113099. [Google Scholar] [CrossRef]
- Antonick, P.J.; Hu, Z.; Fujita, Y.; Reed, D.W.; Das, G.; Wu, L.; Shivaramaiah, R.; Kim, P.; Eslamimanesh, A.; Lencka, M.M.; et al. Bio-and mineral acid leaching of rare earth elements from synthetic phosphogypsum. J. Chem. Thermodyn. 2019, 132, 491–496. [Google Scholar] [CrossRef]
- Shi, S.; Pan, J.; Dong, B.; Zhou, W.; Zhou, C. Bioleaching of Rare Earth Elements: Perspectives from Mineral Characteristics and Microbial Species. Minerals 2023, 13, 1186. [Google Scholar] [CrossRef]
- Owusu-Fordjour, E.Y.; Yang, X. Bioleaching of rare earth elements challenges and opportunities: A critical review. J. Environ. Chem. Eng. 2023, 11, 110413. [Google Scholar] [CrossRef]
- Gergoric, M.; Ravaux, C.; Steenari, B.-M.; Espegren, F.; Retegan, T. Leaching and Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Organic Acids. Metals 2018, 8, 721. [Google Scholar] [CrossRef]
- Gasser, M.S.; Ismail, Z.H.; Abu Elgoud, E.M.; Abdel Hai, F.; Ali, O.I.; Aly, H.F. Process for lanthanides-Y leaching from phosphogypsum fertilizers using weak acids. J. Hazard. Mater. 2019, 378, 120762. [Google Scholar] [CrossRef] [PubMed]
- Lütke, S.F.; Oliveira, M.L.S.; Waechter, S.R.; Silva, L.F.O.; Cadaval, T.R.S.; Duarte, F.A.; Dotto, G.L. Leaching of rare earth elements from phosphogypsum. Chemosphere 2022, 301, 134661. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Rahmati, S.; Adavodi, R.; Birloaga, I.; Vegliò, F. Leaching of Rare Earth Elements from Permanent Magnet Swarf in Citric Acid: Effects of Acid Concentration on Extraction Kinetics. Metals 2023, 13, 1801. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Su, X.; Du, H.; Lu, Y.; Zhang, Q. Rare Earth Extraction from Phosphogypsum by Aspergillus niger Culture Broth. Molecules 2024, 29, 1266. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, S.; Wang, J.; Jiang, S.; Panchal, B.; Sun, Y. Bioleaching rare earth elements from coal fly ash by Aspergillus niger. Fuel 2023, 354, 129387. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.; Yu, X.; Kang, J.; Qiu, G.; Zhao, H.; Shen, L. Effective extraction of rare earth elements from ion-adsorption type rare earth ore by three bioleaching methods. Sep. Purif. Technol. 2024, 330, 125305. [Google Scholar] [CrossRef]
- Sakr, A.K.; Praneeth, S.; Dardona, M.; Kakaris Porter, D.; Tummala, C.M.; Roy, P.K.; Dittrich, T.M. Potential for eco-friendly recovery of rare earth elements from fly ash using carboxylic acids: A comparative study with mineral acids and environmental risk assessment for sustainable fly ash reuse. Chem. Eng. J. 2025, 503, 158355. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Pan, J.; Yang, F.; Long, X.; Yang, Y.; Zhou, C. Rare earth elements recovery and mechanisms from coal fly ash by column leaching using citric acid. Sep. Purif. Technol. 2025, 353, 128471. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.-P. A Review of the Application of Ultrasound in Bioleaching and Insights from Sonication in (Bio)Chemical Processes. Resources 2018, 7, 3. [Google Scholar] [CrossRef]
- Brown, R.M.; Struhs, E.; Mirkouei, A.; Raja, K.; Reed, D. Mixed rare earth metals production from surface soil in Idaho, USA: Techno-economic analysis and greenhouse gas emission assessment. Sci. Total Environ. 2024, 944, 173945. [Google Scholar] [CrossRef]
- Staatz, M.; Armbrustmacher, T.; Olson, J.; Brownfield, I.; Brock, M.; Lemons, J., Jr.; Coppa, L.V.; Clingan, B.V. Principal Thorium Resources in the United States; U.S. Geological Survey Circular: Reston, VA, USA, 1979. [Google Scholar]
- US Geological Survey. The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and a Global Perspective; US Geological Survey: Reston, VA, USA, 2010. [Google Scholar]
- Knudsen, L.; Steven, C.; Lewis, R.; Gillerman, V. Critical Mineral Atlas of Idaho; Idaho Geological Survey: Moscow, ID, USA, 2024. [Google Scholar]
- Jin, H.; Reed, D.W.; Thompson, V.S.; Fujita, Y.; Jiao, Y.; Crain-Zamora, M.; Fisher, J.; Scalzone, K.; Griffel, M.; Hartley, D.; et al. Sustainable Bioleaching of Rare Earth Elements from Industrial Waste Materials Using Agricultural Wastes. ACS Sustain. Chem. Eng. 2019, 7, 15311–15319. [Google Scholar] [CrossRef]
- Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; Nganga, J.; Prinn, R. Changes in Atmospheric Constituents and in Radiative Forcing. In Climate Change; Cambridge University Press: Cambridge, UK, 2007; Chapter 2. [Google Scholar]
- Thompson, V.S.; Gupta, M.; Jin, H.; Vahidi, E.; Yim, M.; Jindra, M.A.; Nguyen, V.; Fujita, Y.; Sutherland, J.W.; Jiao, Y.; et al. Techno-economic and Life Cycle Analysis for Bioleaching Rare-Earth Elements from Waste Materials. ACS Sustain. Chem. Eng. 2018, 6, 1602–1609. [Google Scholar] [CrossRef]
- Ma, Y.; Li, B.; Zhang, X.; Wang, C.; Chen, W. Production of Gluconic Acid and Its Derivatives by Microbial Fermentation: Process Improvement Based on Integrated Routes. Front. Bioeng. Biotechnol. 2022, 10, 864787. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Mattanovich, D.; Marx, H. Microbial production of organic acids for use in food. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 288–320. [Google Scholar] [CrossRef]
- ChemAnalyst. Gluconic Acid Price Trend and Forecast. 2025. Available online: https://www.chemanalyst.com/Pricing-data/gluconic-acid-1624 (accessed on 23 September 2025).
- ChemAnalyst. Citric Acid Price Trend and Forecast. 2025. Available online: https://www.chemanalyst.com/Pricing-data/citric-acid-1438 (accessed on 23 September 2025).
- Energy Sage. Cost of Electricity in Idaho. 2023. Available online: https://www.energysage.com/local-data/electricity-cost/id (accessed on 28 January 2025).
- Aston, J.E.; Thompson, V.S.; Fujita, Y.; Reed, D.W. Metabolic flux modeling of Gluconobacter oxydans enables improved production of bioleaching organic acids. Process Biochem. 2022, 122, 350–356. [Google Scholar] [CrossRef]
- Reed, D.W.; Fujita, Y.; Daubaras, D.L.; Jiao, Y.; Thompson, V.S. Bioleaching of rare earth elements from waste phosphors and cracking catalysts. Hydrometallurgy 2016, 166, 34–40. [Google Scholar] [CrossRef]
- Boness, H.V.M.; De Sá, H.C.; Dos Santos, E.K.P.; Canuto, G.A.B. Sample Preparation in Microbial Metabolomics: Advances and Challenges. In Microbial Natural Products Chemistry; Pacheco Fill, T., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; Volume 1439, pp. 149–183. [Google Scholar] [CrossRef]
- Shuai, Z.; Zhu, Y.; Gao, P.; Han, Y. Rare earth elements resources and beneficiation: A review. Miner. Eng. 2024, 218, 109011. [Google Scholar] [CrossRef]
- Ray, A.R.; Tripathy, B.C.; Mishra, S. Leaching of rare earth elements from phosphogypsum via citric acid medium: Optimization through central composite design and kinetics studies. Environ. Sci. Pollut. Res. 2025, 32, 4076–4089. [Google Scholar] [CrossRef]
- Gillerman, V.S. Rare Earth Elements and Other Critical Metals in Idaho. GeoNote 2011, 44, 4. [Google Scholar]
- Mukaba, J.-L.; Eze, C.P.; Pereao, O.; Petrik, L.F. Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques. Minerals 2021, 11, 1051. [Google Scholar] [CrossRef]
- Konrad-Schmolke, M.; Halama, R.; Wirth, R.; Thomen, A.; Klitscher, N.; Morales, L.; Schreiber, A.; Wilke, F.D.H. Mineral dissolution and reprecipitation mediated by an amorphous phase. Nat. Commun. 2018, 9, 1637. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Panda, S.K.; Mandal, D.; Parhi, P.K. Ultrasound and Microwave assisted leaching of neodymium from waste magnet using organic solvent. Hydrometallurgy 2019, 185, 61–70. [Google Scholar] [CrossRef]
- Widjaja, L.N.; Lie, J.; Soetaredjo, F.E.; Liu, J.-C. Microwave-assisted extraction of rare earth elements from phosphogypsum—Effect of hydrogen peroxide addition. Chem. Eng. Process Process Intensif. 2024, 200, 109800. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, L.; Yang, K. Application of the Microwave and Ultrasonic Combined Technique in the Extraction of Refractory Complex Zinc Ore. Metals 2023, 13, 356. [Google Scholar] [CrossRef]
- Chandaliya, V.K.; Biswas, P.P.; Dash, P.S.; Sharma, D.K. Producing low-ash coal by microwave and ultrasonication pretreatment followed by solvent extraction of coal. Fuel 2018, 212, 422–430. [Google Scholar] [CrossRef]
| Organic Acid | Acid Concentration | REE Source | Pulp Density | Percent REEs Leached | Reference |
|---|---|---|---|---|---|
| Citric | 1 M | Nd magnet waste | 3% | 100% | [11] |
| Citric | 1 M | Phosphogypsum | 20% | 83% | [12] |
| Citric | 3 M | Phosphogypsum | 5% | 62% | [13] |
| Citric | 1.45 M | REE magnet swarf | 10% | 90% | [14] |
| Oxalic | 45 mM | Coal fly ash | 5% | 31% | [16] |
| Citric | 5 M | Phosphogypsum | 67% | 63% | [15] |
| Gluconic | 100 mM | Ion adsorption clay | 67% | 100% | [17] |
| Pyruvic | 4 M | Coal fly ash | 14% | 74% | [18] |
| Citric | 1 M | Coal fly ash | 0.25% | 27% | [19] |
| Impact Categories | Unit | Gluconic Acid | Citric Acid |
|---|---|---|---|
| Fossil fuel depletion | MJ surplus | 9.10 × 104 | 9.03 × 104 |
| Eutrophication | kg N eq. | 4.59 ×102 | 4.56 ×102 |
| Global warming | kg CO2 eq. | 7.70 × 105 | 7.70 × 105 |
| Acidification | kg SO2 eq. | 4.91 × 102 | 4.87 × 102 |
| Ozone depletion | kg CFC-11 eq. | 8.07 × 10−3 | 8.02 × 10−3 |
| Non-carcinogenics | CTUh | 1.90 × 10−2 | 1.93 × 10−2 |
| Smog | kg O3 eq. | 3.38 × 103 | 3.36 × 103 |
| Ecotoxicity | CTUe | 6.10 × 105 | 6.06 × 105 |
| Carcinogenics | CTUh | 4.46 × 10−3 | 4.43 × 10−3 |
| Respiratory effects | kg PM2.5 eq. | 5.22 × 101 | 5.18 × 101 |
| Item | Description | Quantity/kg REEs Leached | Unit Price | Cost/kg REEs Leached | Reference |
|---|---|---|---|---|---|
| Gluconic acid | - | 12,719 L | $0.59/L | $7457 | [30] |
| Citric acid | - | 8917 L | $0.33/L | $2916 | [31] |
| Electricity, medium voltage {US}|market group for|APOS, U | Gluconic | 18,259 kWh | $0.11/kWh | $2008 | [32] |
| Citric | 12,802 kWh | $0.11/kWh | $1408 | [32] |
| Organic Acid | Source | Energy Usage (kWh/kg REEs Leached) | Reagent Cost ($/kg REEs Leached) |
|---|---|---|---|
| Gluconic | Commercial | 18,259 | 7457 |
| Citric | Commercial | 12,802 | 2916 |
| Gluconic | Microbial | 18,419 | 54 |
| Citric | Microbial | 12,915 | 38 |
| Reference | kg CO2 eq./ton Material Processed | Mass REEs Leached (kg/ton Material) | kg CO2 eq./kg REEs Leached | REE Source | Leaching Method |
|---|---|---|---|---|---|
| [27] | 1.00 × 102 | 26.10 | 3.84 × 100 | FCC catalysts | Biolixiviant |
| [25] | 5.80 × 101 | 3.24 | 1.79 × 101 | FCC catalysts | Biolixiviant |
| [21] | 1.90 × 105 | 11.18 | 1.70 × 104 | REE-rich soil | Gluconic acid |
| This study | 7.70 × 105 | 1.42 | 5.42 × 105 | REE-rich soil | Gluconic acid |
| This study | 7.70 × 105 | 2.02 | 3.81 × 105 | REE-rich soil | Citric acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.M.; Struhs, E.; Mirkouei, A.; Reed, D. A Novel Continuous Ultrasound-Assisted Leaching Process for Rare Earth Element Extraction: Environmental and Economic Assessment. Sustain. Chem. 2025, 6, 33. https://doi.org/10.3390/suschem6040033
Brown RM, Struhs E, Mirkouei A, Reed D. A Novel Continuous Ultrasound-Assisted Leaching Process for Rare Earth Element Extraction: Environmental and Economic Assessment. Sustainable Chemistry. 2025; 6(4):33. https://doi.org/10.3390/suschem6040033
Chicago/Turabian StyleBrown, Rebecca M., Ethan Struhs, Amin Mirkouei, and David Reed. 2025. "A Novel Continuous Ultrasound-Assisted Leaching Process for Rare Earth Element Extraction: Environmental and Economic Assessment" Sustainable Chemistry 6, no. 4: 33. https://doi.org/10.3390/suschem6040033
APA StyleBrown, R. M., Struhs, E., Mirkouei, A., & Reed, D. (2025). A Novel Continuous Ultrasound-Assisted Leaching Process for Rare Earth Element Extraction: Environmental and Economic Assessment. Sustainable Chemistry, 6(4), 33. https://doi.org/10.3390/suschem6040033










