Designing a Photocatalyst: Relationship Between Surface Species and Specific Production of Desired ROS
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
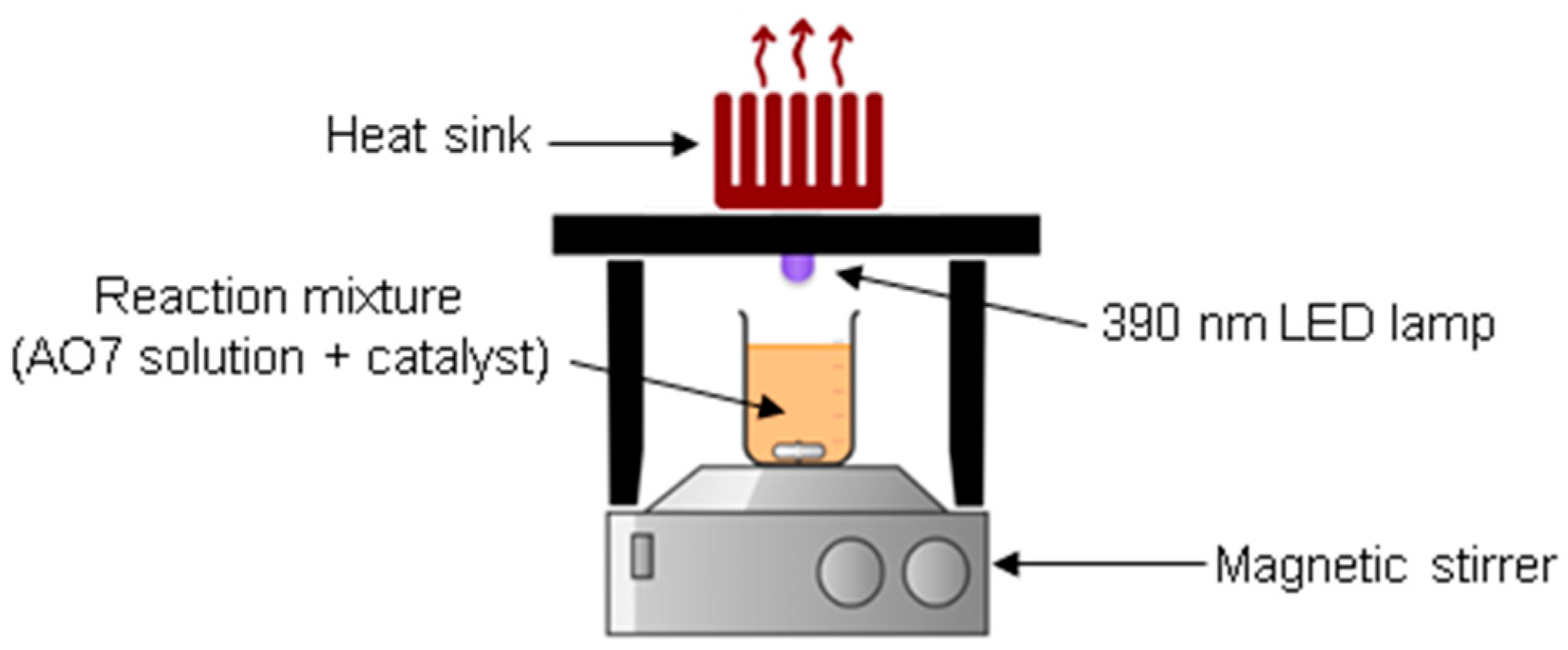
2.3. Catalytic Experiments
3. Results and Discussion
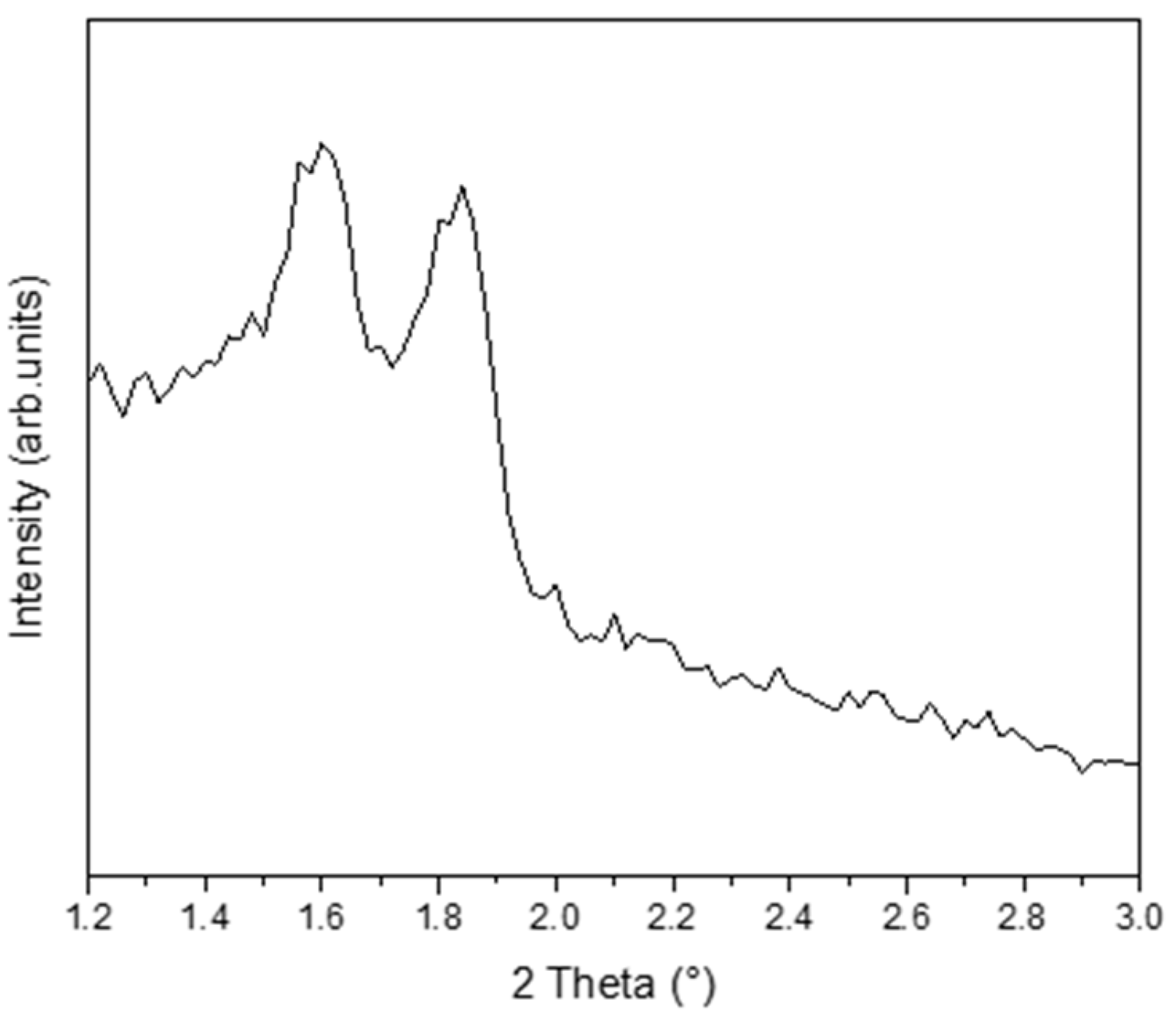
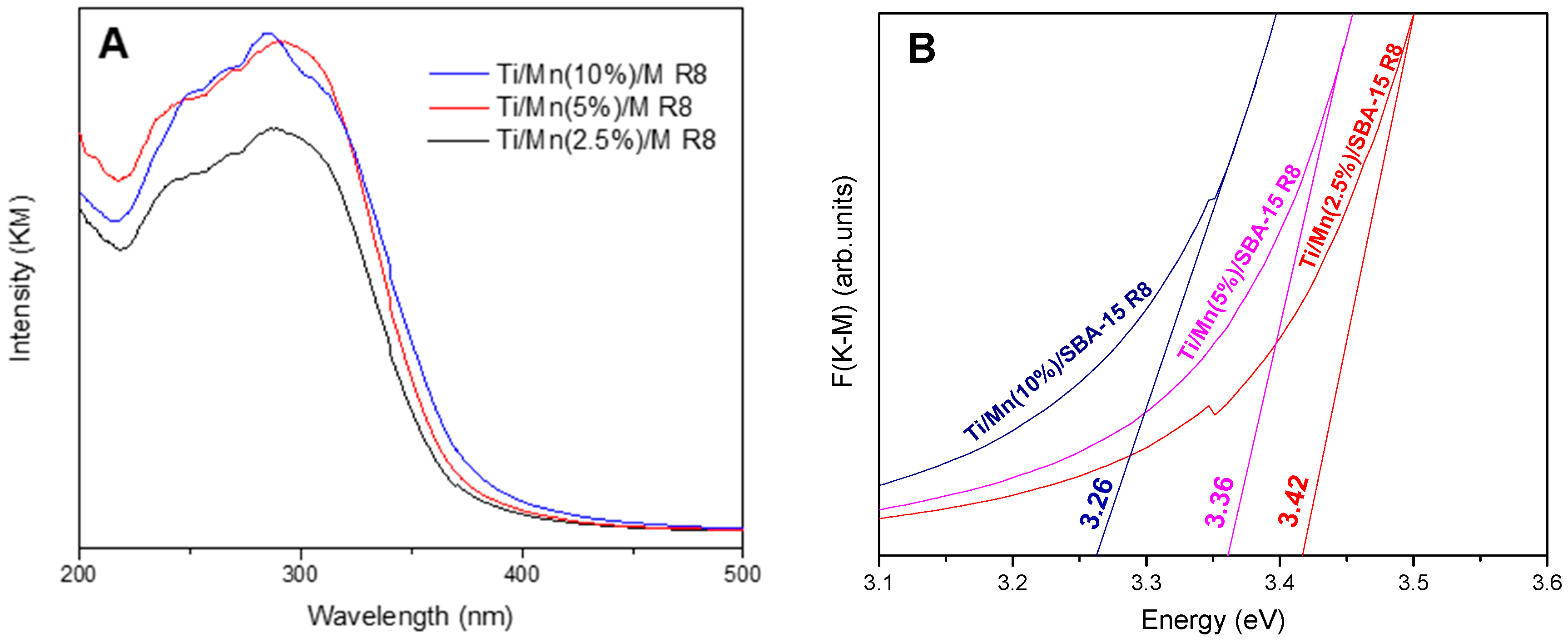
3.1. Characterization of Synthesized Materials
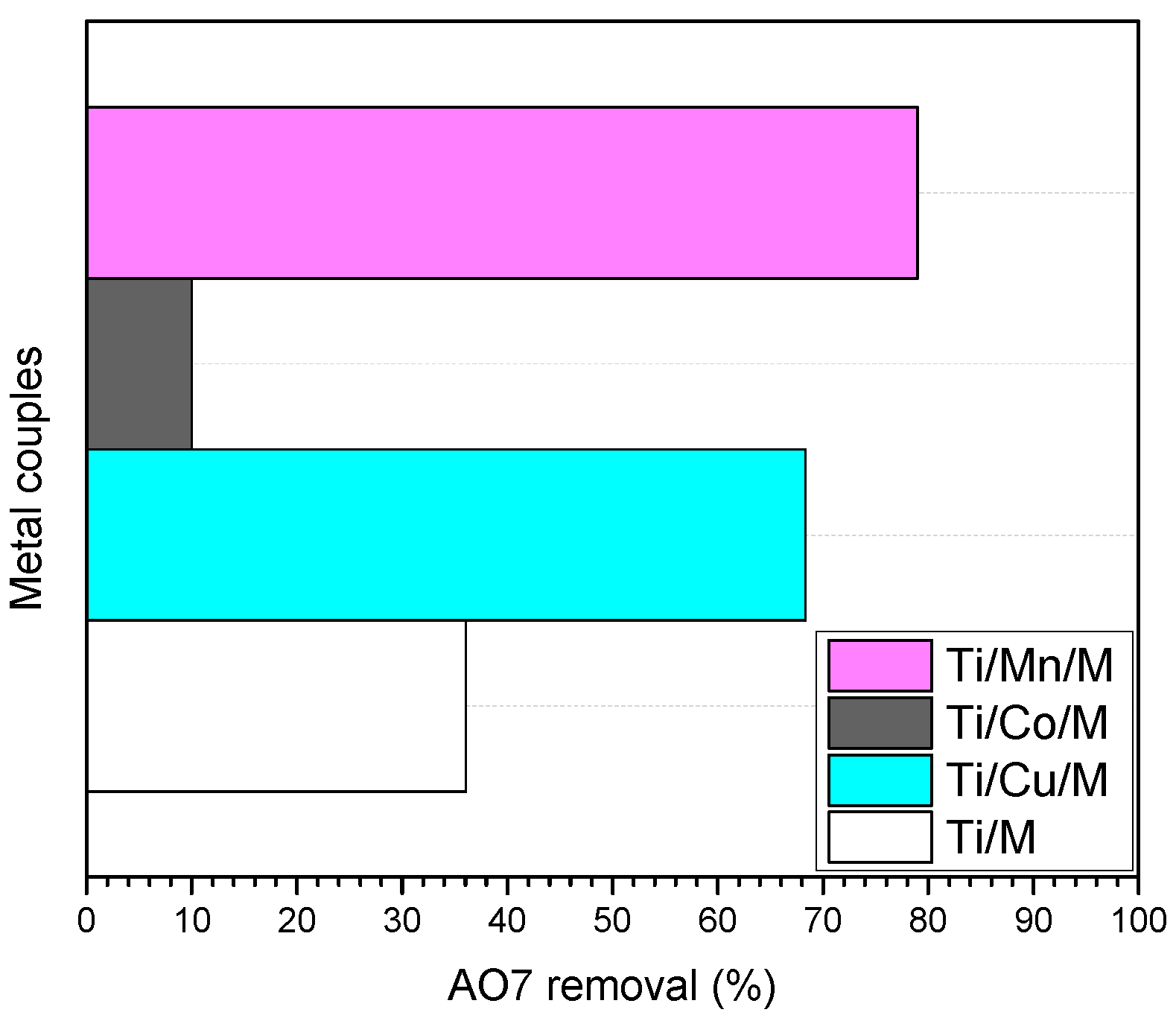
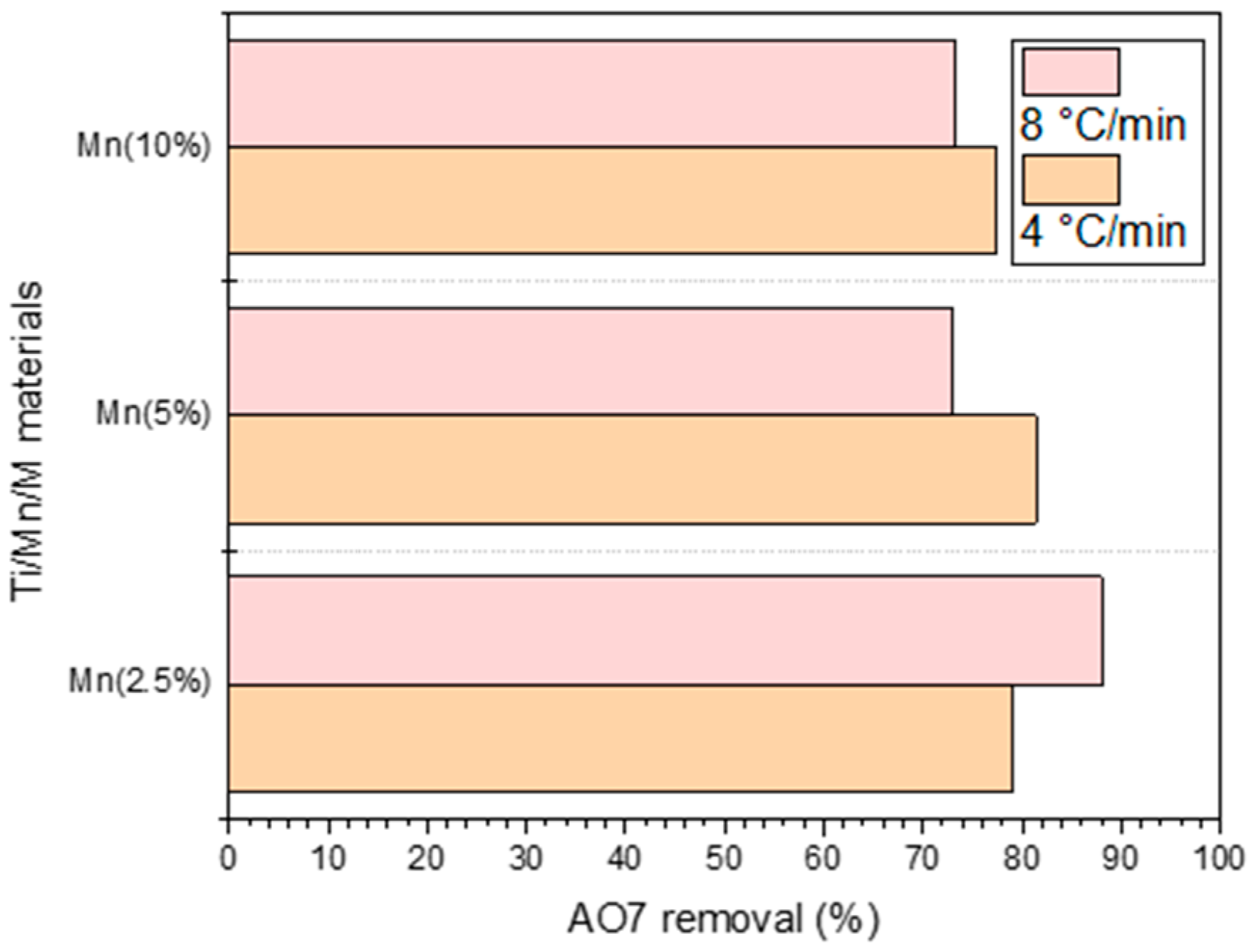
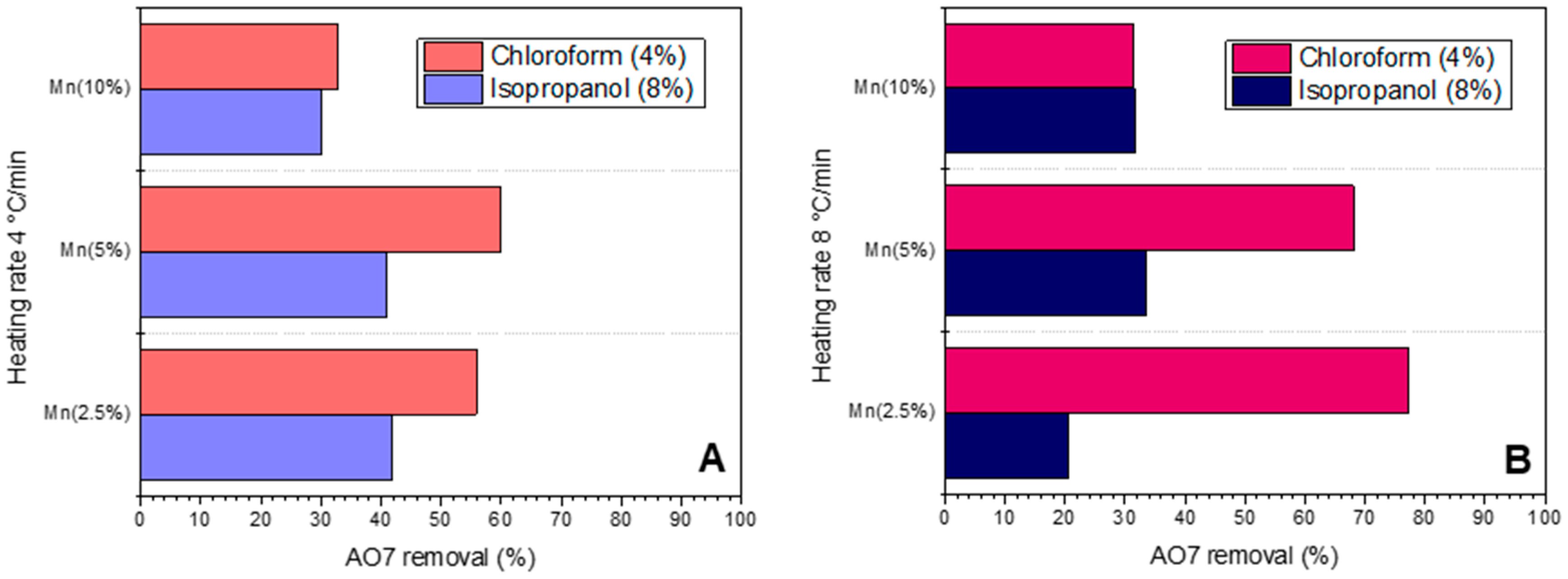
3.2. Photocatalytic Evaluation of Materials
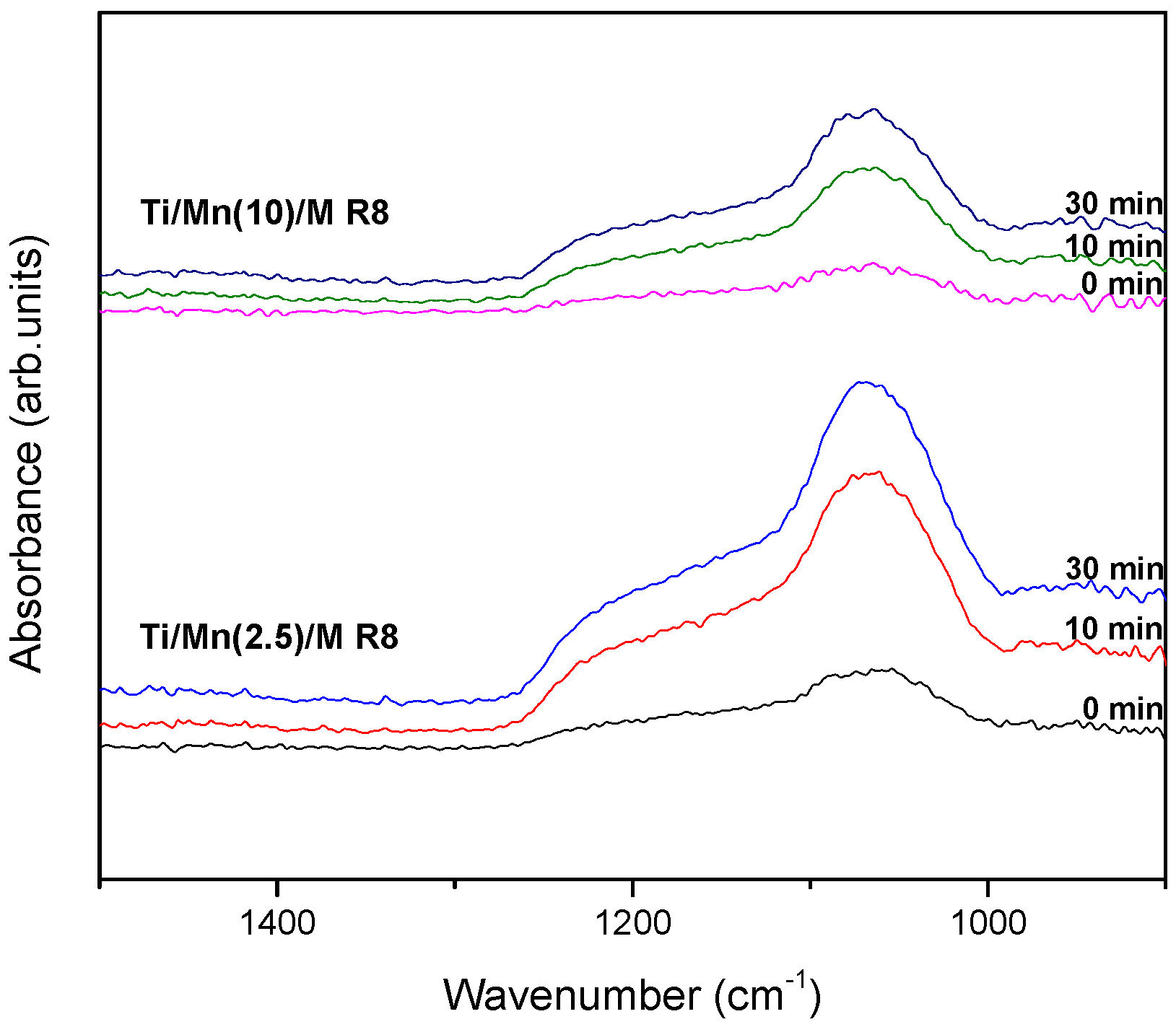
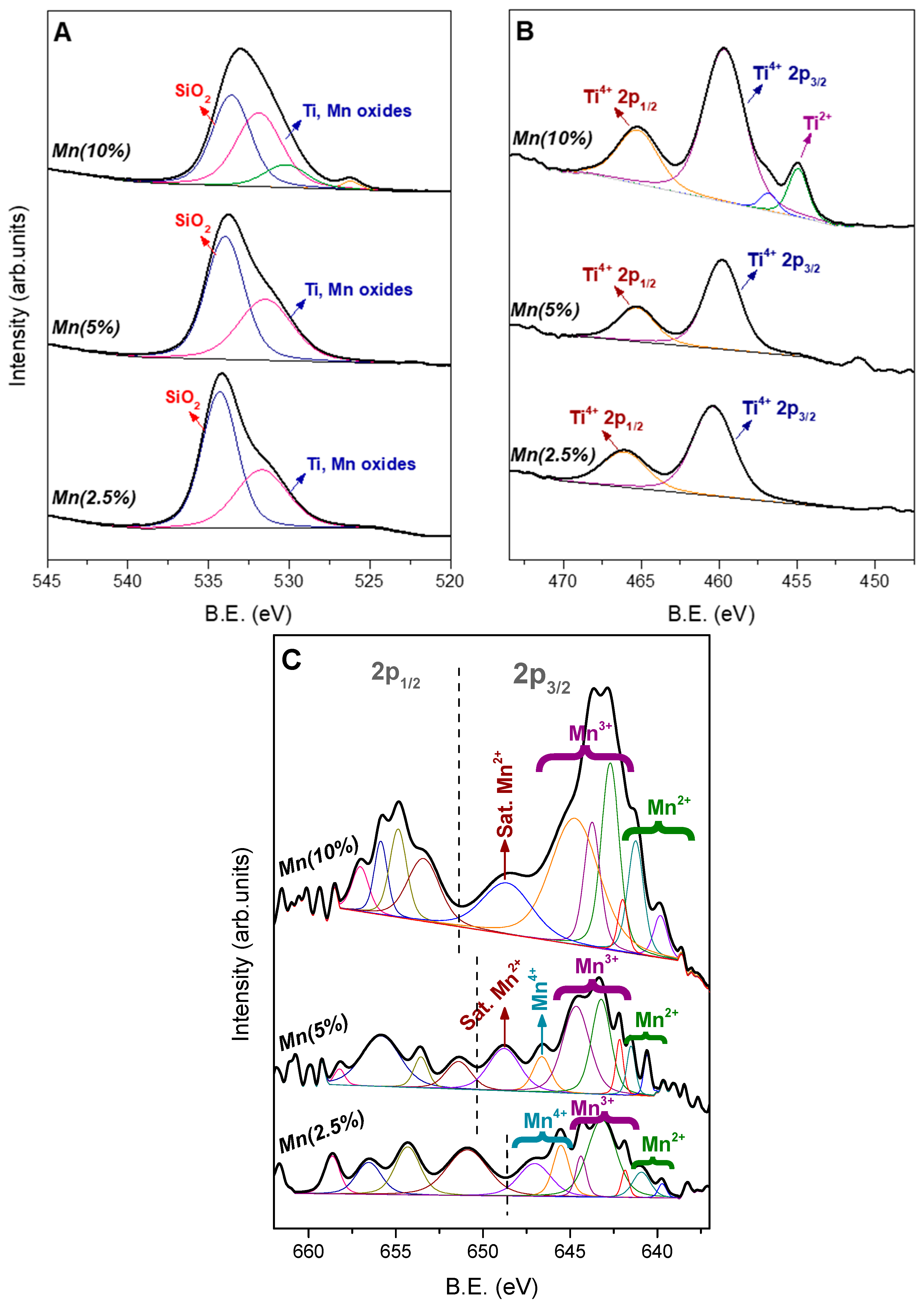
3.3. Characterization of Mn Species in the Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XPS | X-ray photoelectronic spectroscopy |
| UV–Vis | Ultraviolet and visible electromagnetic waves |
| DRS | Diffuse reflectance spectroscopy |
| AOP | Advanced oxidation processes |
| ROS | Reactive oxygen species |
| AO7 | Acid Orange 7 |
| TEOS | Tetraethoxysilane |
| XRD | X-ray diffraction |
| LED | Light-emitting diode |
| ATR-FTIR | Attenuated Total Reflectance–Fourier-Transform Infrared |
| BET | Brunauer–Emmett–Teller |
| PV | Pore volume |
| PD | Pore diameter |
| DR | Diffuse reflectance |
| KM | Kubelka–Munk |
| Eg | Band gap energy |
References
- Wang, J.; Wang, S. Reactive Species in Advanced Oxidation Processes: Formation, Identification and Reaction Mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, M.; Liu, J.; Guo, B. Recent Advances of SBA-15-Based Composites as the Heterogeneous Catalysts in Water Decontamination: A Mini-Review. J. Environ. Manag. 2020, 254, 109787. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced Oxidation Processes (AOPs) Based Wastewater Treatment—Unexpected Nitration Side Reactions—A Serious Environmental Issue: A Review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Rouster, P.; Pavlovic, M.; Szilagyi, I. Immobilization of Superoxide Dismutase on Polyelectrolyte-Functionalized Titania Nanosheets. ChemBioChem 2018, 19, 404–410. [Google Scholar] [CrossRef]
- Nazarewicz, R.R.; Bikineyeva, A.; Dikalov, S.I. Rapid and Specific Measurements of Superoxide Using Fluorescence Spectroscopy. J. Biomol. Screen. 2013, 18, 498–503. [Google Scholar] [CrossRef]
- Liu, R.H.; Fu, S.Y.; Zhan, H.Y.; Lucia, L.A. General Spectroscopic Protocol to Obtain the Concentration of the Superoxide Anion Radical. Ind. Eng. Chem. Res. 2009, 48, 9331–9334. [Google Scholar] [CrossRef]
- Parrino, F.; Livraghi, S.; Giamello, E.; Ceccato, R.; Palmisano, L. Role of Hydroxyl, Superoxide, and Nitrate Radicals on the Fate of Bromide Ions in Photocatalytic TiO2 Suspensions. ACS Catal. 2020, 10, 7922–7931. [Google Scholar] [CrossRef]
- Gülçin, I.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical Scavenging and Antioxidant Activity of Tannic Acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Mohammad, M.; Khan, A.Y.; Subhani, M.S.; Bibi, N.; Ahmad, S.; Saleemi, S. Kinetics and Electrochemical Studies on Superoxide. Res. Chem. Intermed. 2001, 27, 259–267. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Alnashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Bartosz, G. Use of Spectroscopic Probes for Detection of Reactive Oxygen Species. Clin. Chim. Acta 2006, 368, 53–76. [Google Scholar] [CrossRef]
- Burns, J.M.; Cooper, W.J.; Ferry, J.L.; King, D.W.; DiMento, B.P.; McNeill, K.; Miller, C.J.; Miller, W.L.; Peake, B.M.; Rusak, S.A.; et al. Methods for Reactive Oxygen Species (ROS) Detection in Aqueous Environments. Aquat. Sci. 2012, 74, 683–734. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, J.W.; Cha, Y.-N.; Kim, C. A Quantitative Nitroblue Tetrazolium Assay for Determining Intracellular Superoxide Anion Production in Phagocytic Cells. J. Immunoass. Immunochem. 2006, 27, 31–44. [Google Scholar] [CrossRef]
- Anastasescu, C.; Negrila, C.; Angelescu, D.G.; Atkinson, I.; Anastasescu, M.; Spataru, N.; Zaharescu, M.; Balint, I. Particularities of Photocatalysis and Formation of Reactive Oxygen Species on Insulators and Semiconductors: Cases of SiO2, TiO2 and Their Composite SiO2-TiO2. Catal. Sci. Technol. 2018, 8, 5657–5668. [Google Scholar] [CrossRef]
- Elías, V.R.; Ferrero, G.O.; Oliveira, R.G.; Eimer, G.A. Improved Stability in SBA-15 Mesoporous Materials as Catalysts for Photo-Degradation Processes. Microporous Mesoporous Mater. 2016, 236, 218–227. [Google Scholar] [CrossRef]
- Liou, T.H.; Liu, R.T.; Liao, Y.C.; Ku, C.E. Green and Sustainable Synthesis of Mesoporous Silica from Agricultural Biowaste and Functionalized with TiO2 Nanoparticles for Highly Photoactive Performance. Arab. J. Chem. 2024, 17, 105764. [Google Scholar] [CrossRef]
- Bai, H.; Li, X.; Hu, C.; Zhang, X.; Li, J.; Yan, Y.; Xi, G. Large-Scale, Three-Dimensional, Free-Standing, and Mesoporous Metal Oxide Networks for High-Performance Photocatalysis. Sci. Rep. 2013, 3, 2204. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.T.; Carrero, C.A.; Love, A.M.; Verel, R.; Hermans, I. Enhanced Two-Dimensional Dispersion of Group v Metal Oxides on Silica. ACS Catal. 2015, 5, 5787–5793. [Google Scholar] [CrossRef]
- Wahba, M.A.; Khaled, R.K.; Dawy, M. Tailored Bimetallic Zn/Ni and Zn/Ag MCM-41 Photocatalysts for Enhanced Visible-Light Photocatalytic Tetracycline Degradation. Sci. Rep. 2025, 15, 5725. [Google Scholar] [CrossRef]
- Wang, M.; Yang, L.; Guo, C.; Liu, X.; He, L.; Song, Y.; Zhang, Q.; Qu, X.; Zhang, H.; Zhang, Z.; et al. Bimetallic Fe/Ti-Based Metal–Organic Framework for Persulfate-Assisted Visible Light Photocatalytic Degradation of Orange II. ChemistrySelect 2018, 3, 3664–3674. [Google Scholar] [CrossRef]
- Khandekar, D.C.; Bhattacharyya, A.R.; Bandyopadhyaya, R. Role of Impregnated Nano-Photocatalyst (SnxTi(1−x)O2) inside Mesoporous Silica (SBA-15) for Degradation of Organic Pollutant (Rhodamine B) under UV Light. J. Environ. Chem. Eng. 2019, 7, 103433. [Google Scholar] [CrossRef]
- Shoneye, A.; Jiao, H.; Tang, J. Bimetallic FeOx-MOx Loaded TiO2 (M = Cu, Co) Nanocomposite Photocatalysts for Complete Mineralization of Herbicides. J. Phys. Chem. C 2023, 127, 1388–1396. [Google Scholar] [CrossRef]
- Bharati, B.; Mishra, N.C.; Sinha, A.S.K.; Rath, C. Unusual Structural Transformation and Photocatalytic Activity of Mn Doped TiO2 Nanoparticles under Sunlight. Mater. Res. Bull. 2020, 123, 110710. [Google Scholar] [CrossRef]
- Čižmar, T.; Lavrenčič Štangar, U.; Fanetti, M.; Arčon, I. Effects of Different Copper Loadings on the Photocatalytic Activity of TiO2-SiO2 Prepared at a Low Temperature for the Oxidation of Organic Pollutants in Water. ChemCatChem 2018, 10, 2982–2993. [Google Scholar] [CrossRef]
- Ullrich, A.; Rahman, M.M.; Azhar, A.; Kühn, M.; Albrecht, M. Synthesis of Iron Oxide Nanoparticles by Decomposition of Iron-Oleate: Influence of the Heating Rate on the Particle Size. J. Nanoparticle Res. 2022, 24, 183. [Google Scholar] [CrossRef]
- Ristig, S.; Cibura, N.; Strunk, J. Manganese Oxides in Heterogeneous (Photo)Catalysis: Possibilities and Challenges. Green 2015, 5, 23–41. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Mane, G.P.; Rane, V.; Tripathi, A.K.; Tyagi, A.K. Selective CO2 Photoreduction with Cu-Doped TiO2 Photocatalyst: Delineating the Crucial Role of Cu-Oxidation State and Oxygen Vacancies. J. Phys. Chem. C 2021, 125, 1793–1810. [Google Scholar] [CrossRef]
- Adamu, A.; Isaacs, M.; Boodhoo, K.; Abegão, F.R. Investigation of Cu/TiO2 Synthesis Methods and Conditions for CO2 Photocatalytic Reduction via Conversion of Bicarbonate/Carbonate to Formate. J. CO2 Util. 2023, 70, 102428. [Google Scholar] [CrossRef]
- Liyanaarachchi, H.; Thambiliyagodage, C.; Liyanaarachchi, C.; Samarakoon, U. Efficient Photocatalysis of Cu Doped TiO2/g-C3N4 for the Photodegradation of Methylene Blue. Arab. J. Chem. 2023, 16, 104749. [Google Scholar] [CrossRef]
- Eldoma, M.A.; Alaswad, S.O.; Mahmoud, M.A.; Qudsieh, I.Y.; Hassan, M.; Bakather, O.Y.; Elawadi, G.A.; Abouatiaa, A.F.F.; Alomar, M.S.; Elhassan, M.S.; et al. Enhancing Photocatalytic Performance of Co-TiO2 and Mo-TiO2-Based Catalysts through Defect Engineering and Doping: A Study on the Degradation of Organic Pollutants under UV Light. J. Photochem. Photobiol. A Chem. 2024, 446, 115164. [Google Scholar] [CrossRef]
- Kunnamareddy, M.; Ganesan, S.; Hatamleh, A.A.; Alnafisi, B.K.; Rajendran, R.; Chinnasamy, R.; Arumugam, P.; Diravidamani, B.; Lo, H.M. Enhancement in the Visible Light Induced Photocatalytic and Antibacterial Properties of Titanium Dioxide Codoped with Cobalt and Sulfur. Environ. Res. 2023, 216, 114705. [Google Scholar] [CrossRef]
- Viale, F.E.; Winkler, E.L.; Lima, E.; Goya, G.F.; Benzaquén, T.B.; Elías, V.R.; Eimer, G.A.; Ferrero, G.O. Optimization of Photocatalytic Activity in Transition Metal-Modified Mesoporous Silicas: Fine-Tuning Properties to Elucidate Radical Reaction Pathways by EPR. J. Mater. Chem. C Mater. 2025, 13, 12440–12450. [Google Scholar] [CrossRef]
- Goto, H.; Hanada, Y.; Ohno, T.; Matsumura, M. Quantitative Analysis of Superoxide Ion and Hydrogen Peroxide Produced from Molecular Oxygen on Photoirradiated TiO2 Particles. J. Catal. 2004, 225, 223–229. [Google Scholar] [CrossRef]
- Šihor, M.; Reli, M.; Vaštyl, M.; Hrádková, K.; Matějová, L.; Kočí, K. Photocatalytic Oxidation of Methyl Tert-Butyl Ether in Presence of Various Phase Compositions of TiO2. Catalysts 2020, 10, 35. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Intelligent LED Solutions. T9090 1 PowerStar UV, ILH-XU01-Sxxx-SC211-WIR200 Datasheet; Intelligent LED Solutions: Thatcham, UK, 2017. [Google Scholar]
- Taiwan Semiconductor Lighting Corporation. T9090U-UNL1 High Power UV LED. T9090U-UNL1 Series Product Datasheet; Taiwan Semiconductor Lighting Corporation: Zhunan, Taiwan, 2017. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Janus, R.; Wądrzyk, M.; Lewandowski, M.; Natkański, P.; Łątka, P.; Kuśtrowski, P. Understanding Porous Structure of SBA-15 upon Pseudomorphic Transformation into MCM-41: Non-Direct Investigation by Carbon Replication. J. Ind. Eng. Chem. 2020, 92, 131–144. [Google Scholar] [CrossRef]
- Villarroel Rocha, J.; Barrera, D.; Sapag, K. Improvement in the Pore Size Distribution for Ordered Mesoporous Materials with Cylindrical and Spherical Pores Using the Kelvin Equation. Top. Catal. 2011, 54, 121–134. [Google Scholar] [CrossRef]
- Trejda, M.; Drobnik, M.; Nurwita, A. Application of Microwave Radiation in the Grafting of Acidic Sites on SBA-15 Type Material. J. Porous Mater. 2021, 28, 1261–1267. [Google Scholar] [CrossRef]
- Nurwita, A.; Trejda, M. The Effect of Mesoporous Structure of the Support on the Oxidation of Dibenzothiophene. Int. J. Mol. Sci. 2023, 24, 16597. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Mikolei, J.J.; Richter, D.; Pardehkhorram, R.; Helbrecht, C.; Schabel, S.; Meckel, T.; Biesalski, M.; Ceolin, M.; Andrieu-Brunsen, A. Nanoscale Pores Introduced into Paper via Mesoporous Silica Coatings Using Sol-Gel Chemistry. Nanoscale 2023, 15, 9094–9105. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; García-Peñas, A.; Prashar, S.; Rodríguez-Diéguez, A.; Fischer-Fodor, E. Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation. Materials 2018, 11, 224. [Google Scholar] [CrossRef]
- Colmenares-Zerpa, J.; Chimentão, R.J.; Gispert-Guirado, F.; Peixoto, A.F.; Llorca, J. Preparation of SBA-15 and Zr-SBA-15 Materials by Direct-Synthesis and PH-Adjustment Methods. Mater. Lett. 2021, 301, 130326. [Google Scholar] [CrossRef]
- Gonçalves, N.P.F.; Paganini, M.C.; Armillotta, P.; Cerrato, E.; Calza, P. The Effect of Cobalt Doping on the Efficiency of Semiconductor Oxides in the Photocatalytic Water Remediation. J. Environ. Chem. Eng. 2019, 7, 103475. [Google Scholar] [CrossRef]
- Pradhan, D.; Falletta, E.; Dash, S.K. Enhanced and Rapid Photocatalytic Degradation of Toxic Dyes by Cobalt Oxide and Modified Cobalt Oxide under Solar Light Irradiation. Opt. Mater. 2023, 135, 113368. [Google Scholar] [CrossRef]
- Dong, G.; Hu, H.; Huang, X.; Zhang, Y.; Bi, Y. Rapid Activation of Co3O4 Cocatalysts with Oxygen Vacancies on TiO2 Photoanodes for Efficient Water Splitting. J. Mater. Chem. A Mater. 2018, 6, 21003–21009. [Google Scholar] [CrossRef]
- Elías, V.; Vaschetto, E.; Sapag, K.; Oliva, M.; Casuscelli, S.; Eimer, G. MCM-41-Based Materials for the Photo-Catalytic Degradation of Acid Orange 7. Catal. Today 2011, 172, 58–65. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The Absolute Energy Positions of Conduction and Valence Bands of selected Semiconducting Minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A.; Wawrzyńczak, A.; Nowak, I. Photocatalysis by Mixed Oxides Containing Niobium, Vanadium, Silica, or Tin. Catalysts 2025, 15, 118. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S. Molybdenum-Doped Anatase and Its Extraordinary Photocatalytic Activity in the Degradation of Orange II in the UV and Vis Regions. J. Phys. Chem. C 2010, 114, 19308–19317. [Google Scholar] [CrossRef]
- González-Rodríguez, J.; Fernández, L.; Bava, Y.B.; Buceta, D.; Vázquez-Vázquez, C.; López-Quintela, M.A.; Feijoo, G.; Moreira, M.T. Enhanced Photocatalytic Activity of Semiconductor Nanocomposites Doped with Ag Nanoclusters under UV and Visible Light. Catalysts 2020, 10, 31. [Google Scholar] [CrossRef]
- Wongburapachart, C.; Pornaroontham, P.; Kim, K.; Rangsunvigit, P. Photocatalytic Degradation of Acid Orange 7 by NiO-TiO2/TiO2 Bilayer Film Photo-Chargeable Catalysts. Coatings 2023, 13, 141. [Google Scholar] [CrossRef]
- Nunes, M.J.; Lopes, A.; Pacheco, M.J.; Ciríaco, L. Visible-Light-Driven AO7 Photocatalytic Degradation and Toxicity Removal at Bi-Doped SrTiO3. Materials 2022, 15, 2465. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, G.; Zhang, J. High-Efficiency Degradation of Orange II by Co78Si8B14/g-C3N4 Composite Catalyst in a Visible-Light-Assisted Peroxymonosulfate Activation System. Materials 2025, 18, 1733. [Google Scholar] [CrossRef]
- Biswas, B.D.; Datta, J.; Purkayastha, M.D.; Das, D.; Ray, P.P.; Dutta, A.; Majumder, T.P. Electrical and Photocatalytic Properties of Composites of Manganese and Titanium Oxides. Surf. Interfaces 2020, 20, 100606. [Google Scholar] [CrossRef]
- Hafeez, M.; Afyaz, S.; Khalid, A.; Ahmad, P.; Khandaker, M.U.; Sahibzada, M.U.K.; Ahmad, I.; Khan, J.; Alhumaydhi, F.A.; Emran, T.B.; et al. Synthesis of Cobalt and Sulphur Doped Titanium Dioxide Photocatalysts for Environmental Applications. J. King Saud. Univ. Sci. 2022, 34, 102028. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Didwal, P.N.; Mulani, S.R.; Patil, M.S.; Devan, R.S. Photocatalytic Activity of MnTiO3 Perovskite Nanodiscs for the Removal of Organic Pollutants. Heliyon 2021, 7, e07297. [Google Scholar] [CrossRef]
- Osgouei, M.S.; Khatamian, M.; Kakili, H. Improved Visible-Light Photocatalytic Activity of Mn3O4-Based Nanocomposites in Removal of Methyl Orange. Mater. Chem. Phys. 2020, 239, 122108. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Zhang, J.; Gui, Y.; Liu, L. Enhanced Photocatalytic Performance of Mn-Doped CeO2 Nanoribbons for VOCs Degradation: Investigating Charge Rearrangement and Local Electric Field Effects. J. Environ. Chem. Eng. 2023, 11, 111445. [Google Scholar] [CrossRef]
- Kakuma, Y.; Nosaka, A.Y.; Nosaka, Y. Difference in TiO2 Photocatalytic Mechanism between Rutile and Anatase Studied by the Detection of Active Oxygen and Surface Species in Water. Phys. Chem. Chem. Phys. 2015, 17, 18691–18698. [Google Scholar] [CrossRef]
- Namiki, A.; Tanimoto, K.; Nakamura, T.; Ohtake, N.; Suzaki, T. XPS Study on the Early Stages of Oxidation of Si(100) by Atomic Oxygen. Surf. Sci. 1989, 222, 530–554. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Puangpetch, T.; Ramakul, P.; Serivalsatit, K.; Ponchio, C.; Hunsom, M. Ultra-Fast Green Synthesis of a Defective TiO2 Photocatalyst towards Hydrogen Production. RSC Adv. 2024, 14, 24213–24225. [Google Scholar] [CrossRef]
- Fetisov, A.V.; Kozhina, G.A.; Estemirova, S.K.; Fetisov, V.B.; Gulyaeva, R.I. XPS Study of the Chemical Stability of DyBa2Cu3O6+δ Superconductor. Phys. C Supercond. Its Appl. 2015, 508, 62–68. [Google Scholar] [CrossRef]
- Paengjun, N.; Vibulyaseak, K.; Ogawa, M. Heterostructural Transformation of Mesoporous Silica–Titania Hybrids. Sci. Rep. 2021, 11, 3210. [Google Scholar] [CrossRef]
- Ianhez-Pereira, C.; Onofre, Y.J.; Magon, C.J.; Rodrigues, A.d.G.; de Godoy, M.P.F. The Interplay between Mn Valence and the Optical Response of ZnMnO Thin Films. Appl. Phys. A Mater. Sci. Process 2020, 126, 337. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS Determination of Mn Oxidation States in Mn (Hydr)Oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Banerjee, D. Interpretation of XPS Mn(2p) Spectra of Mn Oxyhydroxides and Constraints on the Mechanism of MnO 2 Precipitation. Am. Mineral. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, J.; Han, R.; Wang, Z.; Christie, P.; Zhang, S. Sustained Production of Superoxide Radicals by Manganese Oxides under Ambient Dark Conditions. Water Res. 2021, 196, 117034. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Y.; Zhou, C.; Yu, T. ROS Scavenging Manganese-Loaded Mesoporous Silica Nanozymes for Catalytic Anti-Inflammatory Therapy. Adv. Powder Technol. 2023, 34, 103886. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, S.; Dai, Y.; Liu, C.C.; Zhang, H. Effect of MnO2 Phase Structure on the Oxidative Reactivity toward Bisphenol A Degradation. Environ. Sci. Technol. 2018, 52, 11309–11318. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Xu, X.; Wan, B.; Wang, Q.; Borkiewicz, O.J.; Li, Y.; Chen, H.; Lu, A.; Tang, Y. Photocatalytic Oxidation of Dissolved Mn(II) on Natural Iron Oxide Minerals. Geochim. Cosmochim. Acta 2021, 312, 343–356. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Yang, T.; Mao, S.; Zhao, H. Peroxymonosulfate Activation for Preferential Generation of Hydroxyl Radical with Atomic Mn Anchored TiO2 in Photoelectrochemical Process. Environ. Funct. Mater. 2023, 2, 13–24. [Google Scholar] [CrossRef]
- Nevárez-Martínez, M.C.; Kobylanski, M.P.; Mazierski, P.; Wółkiewicz, J.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.J.; Zaleska-Medynska, A. Self-Organized TiO2-MnO2 Nanotube Arrays for Efficient Photocatalytic Degradation of Toluene. Molecules 2017, 22, 564. [Google Scholar] [CrossRef]
- Cheeseman, S.; Christofferson, A.J.; Kariuki, R.; Cozzolino, D.; Daeneke, T.; Crawford, R.J.; Truong, V.K.; Chapman, J.; Elbourne, A. Antimicrobial Metal Nanomaterials: From Passive to Stimuli-Activated Applications. Adv. Sci. 2020, 7, 1902913. [Google Scholar] [CrossRef]
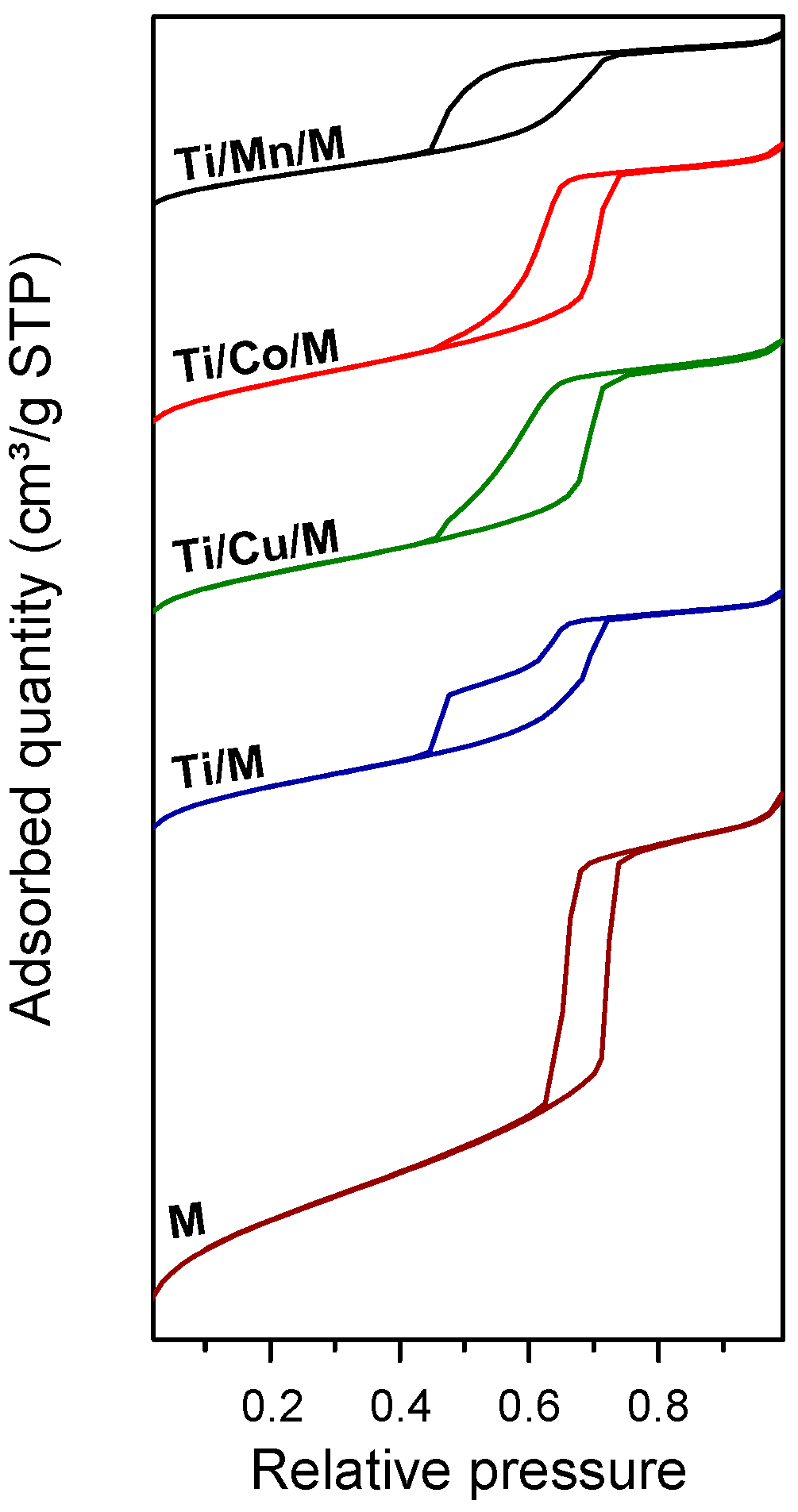

| Material | SBET (m2g−1) a | PD (nm) b | PV (cm3g−1) b | Eg (eV) c |
|---|---|---|---|---|
| M | 816 | 5.8 | 1.03 | --- |
| Ti/M | 485 | 4.6 | 0.52 | 3.51 |
| Ti/Cu/M | 399 | 5.5 | 0.55 | 3.44 |
| Ti/Co/M | 403 | 5.5 | 0.56 | 1.49 |
| Ti/Mn/M | 289 | 4.9 | 0.35 | 3.27 |
| Material | Mn2+ (%) | Mn3+ (%) | Mn4+ (%) | Total (%) |
|---|---|---|---|---|
| Ti/Mn(2.5%)/M R8 | 10.67 | 53.72 | 35.61 | 100.00 |
| Ti/Mn(5%)/M R8 | 26.58 | 64.50 | 8.92 | 100.00 |
| Ti/Mn(10%)/M R8 | 29.02 | 70.98 | 0.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viale, F.E.; Elías, V.R.; Benzaquén, T.B.; Goya, G.F.; Eimer, G.A.; Ferrero, G.O. Designing a Photocatalyst: Relationship Between Surface Species and Specific Production of Desired ROS. Sustain. Chem. 2025, 6, 31. https://doi.org/10.3390/suschem6040031
Viale FE, Elías VR, Benzaquén TB, Goya GF, Eimer GA, Ferrero GO. Designing a Photocatalyst: Relationship Between Surface Species and Specific Production of Desired ROS. Sustainable Chemistry. 2025; 6(4):31. https://doi.org/10.3390/suschem6040031
Chicago/Turabian StyleViale, Fabrizio E., Verónica R. Elías, Tamara B. Benzaquén, Gerardo F. Goya, Griselda A. Eimer, and Gabriel O. Ferrero. 2025. "Designing a Photocatalyst: Relationship Between Surface Species and Specific Production of Desired ROS" Sustainable Chemistry 6, no. 4: 31. https://doi.org/10.3390/suschem6040031
APA StyleViale, F. E., Elías, V. R., Benzaquén, T. B., Goya, G. F., Eimer, G. A., & Ferrero, G. O. (2025). Designing a Photocatalyst: Relationship Between Surface Species and Specific Production of Desired ROS. Sustainable Chemistry, 6(4), 31. https://doi.org/10.3390/suschem6040031








