Sample Origin Effect on Chemical Reactivity of Tajogaite Volcanic Ashes for Ancient Mortar Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
3. Results and Discussion
3.1. Raw Volcanic Ash Characterization
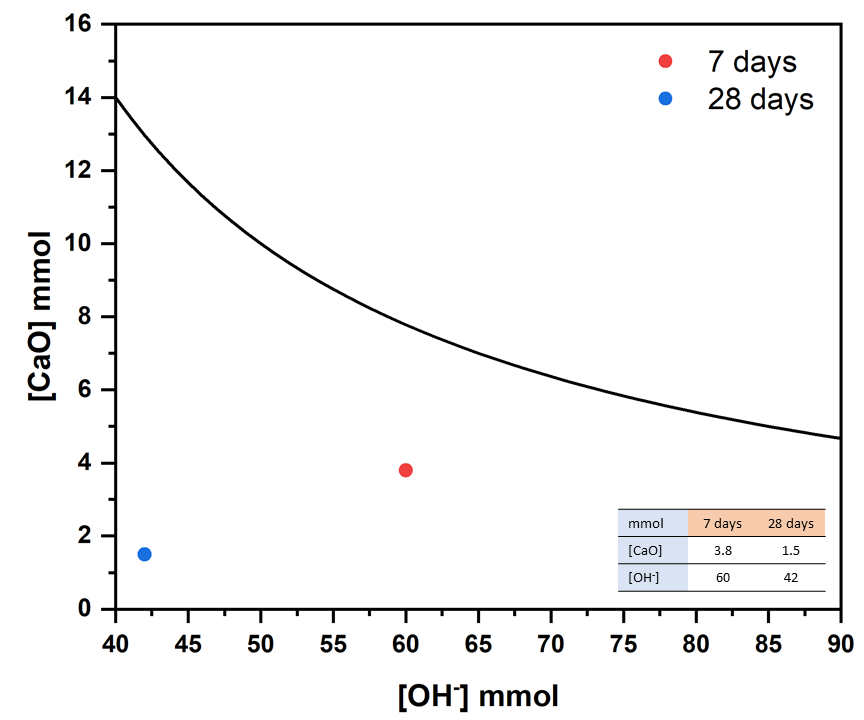
3.2. Pozzolanic Activity Test
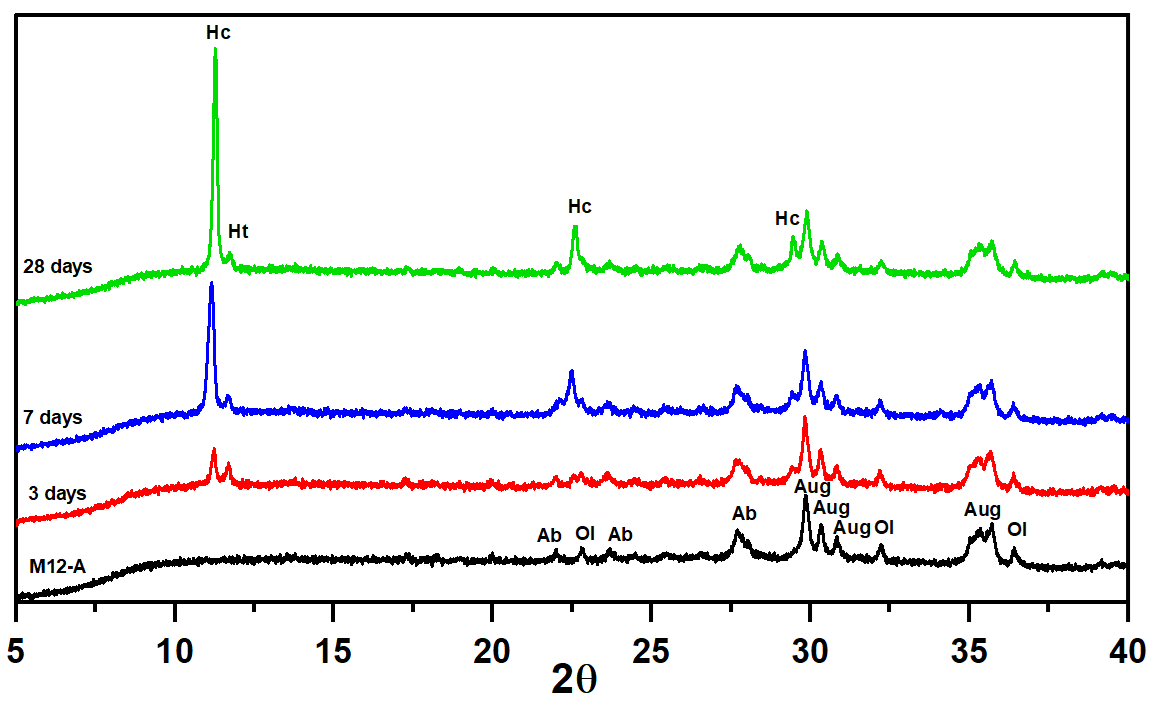
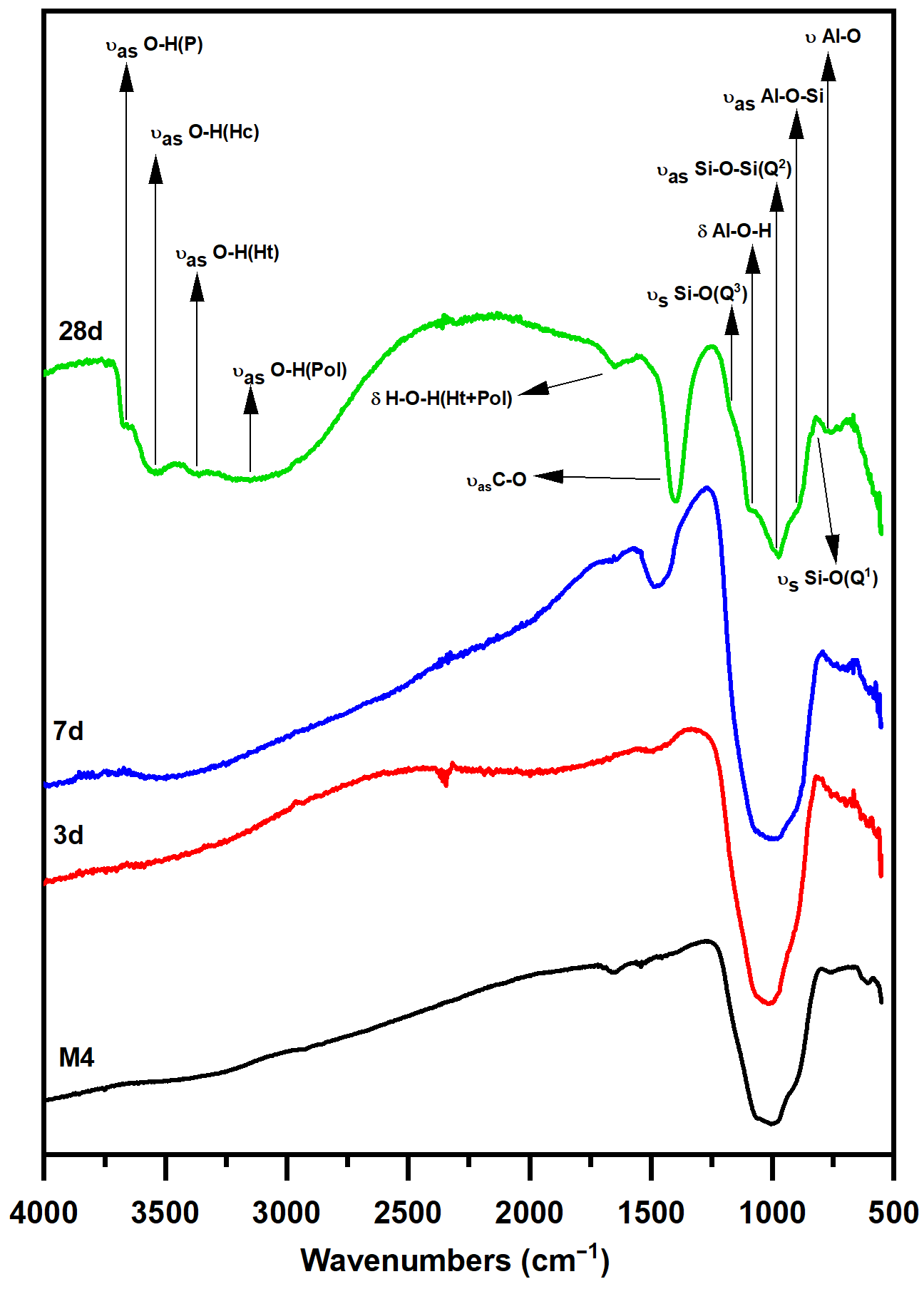
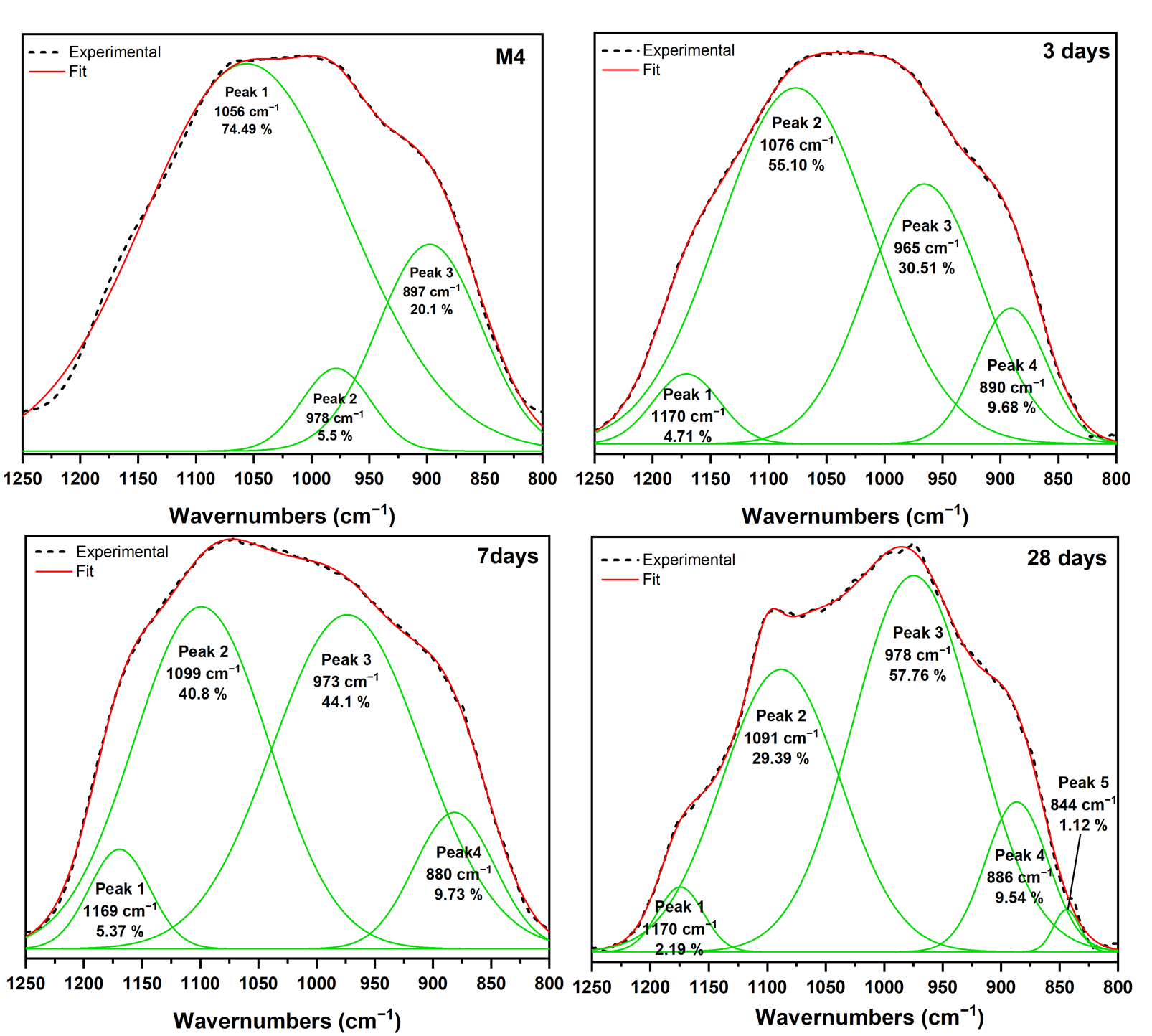
3.3. Pozzolanic Reaction Products Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casadevall, T.J. (Ed.) Volcanic Ash and Aviation Safety: Proceedings of the First International Symposium on Volcanic Ash and Aviation Safety; No. 2047; US Government Printing Office: Washington, DC, USA, 1994.
- Stewart, C.; Horwell, C.; Plumlee, G.; Cronin, S.; Delmelle, P.; Baxter, P.; Oppenheimer, C. Protocol for analysis of volcanic ash samples for assessment of hazards from leachable elements. In IAVCEI Commissions Joint Report: International Volcanic Health Hazard Network and Cities and Volcanoes; International Volcanic Health Hazard Network, 2013. [Google Scholar]
- Suarez-Navarro, J.A.; Caño, A.; Puertas, F.; Alonso, M.M. Relationship between the chemical composition of cementitious materials and their radioactivity. In Proceedings of the 16th International Congress on the Chemistry of Cement, Bangkok, Thailand, 18–22 September 2023. [Google Scholar]
- Sánchez, E.; Vizcaino, G.; Mejía, F.; Cipriani, I. Análisis mineralógico y multielemental de la ceniza volcánica, producto de la erupción del Cotopaxi en 2015, por difracción de rayos X (XRD) y espectrometría de masas con plasma acoplado inductivamente (ICP-MS) y sus posibles aplicaciones e impactos. Infoanalítica 2018, 6, 9–23. [Google Scholar] [CrossRef]
- Kamseu, E.; Leonelli, C.; Perera, D.S.; Melo, U.C.; Lemougna, P.N. Investigation of the volcanic ash based cements as potential building materials. Interceram 2000, 58, 136–140. [Google Scholar]
- Tchakouté, H.K.; Elimbi, A.; Yanne, E.; Djangang, C.N. Utilisation of volcanic ashes for the production of cements cured at ambient temperature. Cem. Concr. Compos. 2013, 38, 75–813. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Melo, U.C.; Delplancke, M.P.; Rahier, H. Influence of the chemical and mineralogical composition on the reactivity of volcanic ashes during alkali activation. Ceram. Int. 2014, 40, 811–820. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Volcanic ashbased cement cements/concretes: The current state of the art and perspectives. Environ. Sci. Pollut. Res. 2017, 24, 4433–4446. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Wang, K.; Tang, Q.; Nzeukou, A.N.; Billong, N.; Melo, U.C.; Cui, X. Review on the use of volcanic ashes for engineering applications. Resour. Conserv. Recycl. 2018, 137, 177–190. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Mejía de Gutiérrez, R. Natural volcanic pozzolans as an available raw material for alkali-activated materials in the foreseeable future: A review. Constr. Build. Mater. 2018, 189, 109–118. [Google Scholar] [CrossRef]
- Rafat, S. Properties of concrete made with volcanic ash. Resour. Conserv. Recycl. 2012, 66, 40–44. [Google Scholar]
- Habert, G.; Choupay, N.; Montel, J.M.; Guillaume, D.; Escadeillas, G. Effects of the secondary minerals of the natural pozzolans on their pozzolanic activity. Cem. Concr. Res. 2008, 38, 963–975. [Google Scholar] [CrossRef]
- Celik, K.; Jackson, M.D.; Mancio, M.; Meral, C.; Emwas, A.H.; Mehta, P.K.; Monteiro, P.J.M. High-volume natural volcanic pozzolan and limestone powder as partial replacements for portland cement in self-compacting and sustainable concrete. Cem. Concr. Compos. 2014, 45, 136–147. [Google Scholar] [CrossRef]
- Hewlett, P. Lea’s Chemistry of Cement and Concrete; Elsevier Science: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Proske, T.; Hainer, S.; Rezvani, M.; Graubner, C.-A. Eco-friendly concretes with reduced water and cement contents—Mix design principles and laboratory tests. Cem. Concr. Res. 2013, 51, 38–46. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Purnell, P. Material nature versus structural nurture: The embodied carbon of fundamental structural elements. Environ. Sci. Technol. 2011, 46, 454–461. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs andcarbon emissions for geopolymer pastes in comparison to ordinary Portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete; Prentice Hall: Hoboken, NJ, USA, 2003. [Google Scholar]
- Hossain, K.M.A.; Lachemi, M. Performance of volcanic ash and pumice based blended cement concrete in mixed sulfate environment. Cem. Concr. Res. 2006, 36, 1123–1133. [Google Scholar] [CrossRef]
- Sabtan, A.A.; Shehata, W.M. Evaluation of engineering properties of scoria in central Harrat Rahat, Saudi Arabia. Bull. Eng. Geol. Environ. 2000, 59, 219–225. [Google Scholar] [CrossRef]
- Jackson, M.; Vola, G.; Všiansky’, D.; Oleson, J.; Scheetz, B.; Brandon, C.; Hohlfelder, R. Cement Microstructures and Durability in Ancient Roman Seawater Concretes. In Historic Mortars; Válek, J., Hughes, J.J., Groot, C.J.W.P., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 49–76. [Google Scholar]
- Mehta, P.K. Sustainable cements and concrete for the climate change era—A review. In Proceedings of the 2nd International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; pp. 1–10. [Google Scholar]
- Contrafatto, L. Recycled Etna volcanic ash for cement, mortar and concrete manufacturing. Constr. Build. Mater. 2017, 151, 704–713. [Google Scholar] [CrossRef]
- Ndjock, B.D.L.; Elimbi, A.; Cyr, M. Rational utilization of volcanic ashes based on factors affecting their alkaline activation. J. Non-Cryst. Solids 2017, 463, 31–39. [Google Scholar] [CrossRef]
- Massazza, F. Pozzolanic cements. Cem. Concr. Compos. 1993, 15, 185–214. [Google Scholar] [CrossRef]
- Shah, S.P.; Tregger, N. Improving fly ash cementitious materials for sustainable construction through nanotechnology. In Proceedings of the 2nd International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; pp. 11–21. [Google Scholar]
- Mertens, G.; Snellings, R.; Van Balen, K.; Bicer-Simsir, B.; Verlooy, P.; Elsen, J. Pozzolanic reactions of common natural zeolites with lime and parameters affecting their reactivity. Cem. Concr. Res. 2009, 39, 233–240. [Google Scholar] [CrossRef]
- Donatello, S.; Freeman-Pask, A.; Tyrer, M.; Cheeseman, C.R. Effect of milling and acid washing on the pozzolanic activity of incinerator sewage sludge ash. Cem. Concr. Compos. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Lin, K.L.; Chang, W.C.; Lin, D.F. Pozzolanic characteristics of pulverized incinerator bottom ash slag. Constr. Build. Mater. 2008, 22, 324–329. [Google Scholar] [CrossRef]
- Kakali, G.; Perraki, T.; Tsivilis, S.; Badogiannis, E. Thermal treatment of kaolin: The effect of mineralogy on the pozzolanic activity. Appl. Clay Sci. 2001, 20, 73–80. [Google Scholar] [CrossRef]
- Frías, M.; Sánchez de Rojas, M.I.; Rivera, J. Influence of calcining conditions on pozzolanic activity and reaction kinetics in paper sludge-calcium hydroxide mixes. In Proceedings of the 8th CANMET/ACI International Conference on Fly Ash, Silica Fume, Slag and Natural Pozzolans in Concrete, Las Vegas, NV, USA, 23–29 May 2004; pp. 879–886. [Google Scholar]
- Garcia, R.; Vigil de la Villa, R.; Vegas, I.; Frias, M.; Sanchez de Rojas, M.I. The pozzolanic properties of paper sludge waste. Constr. Build. Mater. 2008, 22, 1484–1490. [Google Scholar] [CrossRef]
- Frias, M.; Villar-Cocina, E.; Sanchez de Rojas, M.I.; Valencia-Morales, E. The effect that different pozzolanic activity methods has on the kinetic constants of the pozzolanic reaction in sugar cane straw-ash/lime systems: Application of a kinetic-diffusive model. Cem. Concr. Res. 2005, 35, 2137–2142. [Google Scholar] [CrossRef]
- Frias, M.; Villar-Cocina, E.; Valencia-Morales, E. Characterisation of sugar cane straw waste as pozzolanic material for construction: Calcining temperature and kinetic parameters. Waste Manag. 2007, 27, 533–538. [Google Scholar] [CrossRef]
- Frias, M.; Rodriguez, C. Effect of incorporating ferroalloy industry wastes as complementary cementing materials on the properties of blended cement matrices. Cem. Concr. Res. 2007, 30, 212–219. [Google Scholar] [CrossRef]
- EN 196-5:2011; Methods of Testing Cement—Part 5: Pozzolanicity Test for Pozzolanic Cement. Spanish Association for Standardization (UNE): Madrid, Spain, 2011.
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. Comparison of test methods to assess pozzolanic activity. Cem. Concr. Compos. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- Talero, R. Performance of metakaolin and Portland cements in ettringite formation as determined by ASTM C 452-68: Kinetic and morphological differences. Cem. Concr. Res. 2005, 35, 1269–1284. [Google Scholar] [CrossRef]
- Wild, S.; Gailius, A.; Hansen, H.; Pederson, L.; Szwabowski, J. Pozzolanic properties of a variety of european clay bricks. Build. Res. Inf. 1997, 25, 170–175. [Google Scholar] [CrossRef]
- Ulukaya, S.; Yüzer, N. Assessment of pozzolanicity of clay bricks fired at different temperatures for use in repair mortar. J. Mater. Civ. Eng. 2016, 28, 04016052. [Google Scholar] [CrossRef]
- Rahhal, V.; Talero, R. Influence of two different fly ashes on the hydration of Portland cements. J. Therm. Anal. Calorim. 2004, 78, 191–205. [Google Scholar] [CrossRef]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Sow, M.; Hot, J.; Tribout, C.; Cyr, M. Characterization of spreader stoker coal fly ashes (SSCFA) for their use in cement-based applications. Fuel 2015, 162, 224–233. [Google Scholar] [CrossRef]
- Dahlgren, R.; Shoji, S.; Nanzyo, M. Mineralogical characteristics of volcanic ash soils. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 1993; Volume 21, pp. 101–143. [Google Scholar]
- Shoji, S.; Kodayashi, S.; Yamada, I.; Masui, J.I. Chemical and mineralogical studies on volcanic ashes I. Chemical composition of volcanic ashes and their classification. Soil Sci. Plant Nutr. 1975, 21, 311–318. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Palkovic, S.D.; Bumajdad, A.; Soriano, C.; Büyüköztürk, O. Use of silica fume and natural volcanic ash as a replacement to Portland cement: Micro and pore structural investigation using NMR, XRD, FTIR and X-ray microtomography. Constr. Build. Mater. 2018, 158, 574–590. [Google Scholar] [CrossRef]
- Kouamo, H.T.; Elimbi, A.; Mbey, J.A.; Sabouang, C.N.; Njopwouo, D. The effect of adding alumina-oxide to metakaolin and volcanic ash on geopolymer products: A comparative study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Melo, U.C. Synthesis and thermal properties of inorganic polymers (geopolymers) for structural and refractory applications from volcanic ash. Ceram. Int. 2011, 37, 3011–3018. [Google Scholar] [CrossRef]
- Çetintaş, R.; Soyer-Uzun, S. Relations between structural characteristics and compressive strength in volcanic ash based one–part geopolymer systems. J. Build. Eng. 2018, 20, 130–136. [Google Scholar] [CrossRef]
- Verdolotti, L.; Iannace, S.; Lavorgna, M.; Lamanna, R. Geopolymerization reaction to consolidate incoherent pozzolanic soil. J. Mater. Sci. 2008, 43, 865–873. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A. Mineralogical and microstructural characterisation of alkali-activated fly ash/slag pastes. Cem. Concr. Compos. 2003, 25, 287–292. [Google Scholar] [CrossRef]
- Villa, C.; Pecina, E.T.; Torres, R.; Gomez, L. Geopolymer synthesis using alkaline activation of natural zeolite. Constr. Build. Mater. 2010, 24, 2084–2090. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deventer, J.S.J. Structural reorganisation of class F fly ash in alkaline silicate solution. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 49–66. [Google Scholar] [CrossRef]
- McMillan, P. Structural studies of silicate glasses and melts—Applications and limitations of Raman spectroscopy. Am. Mineral. 1984, 69, 622–644. [Google Scholar]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lukey, G.C. The characterisation of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Yamada, I.; Shoji, S. Relationships between particle size and mineral composition of volcanic ashes. Tohoku J. Agric. Res. 1975, 26, 7–10. [Google Scholar]
- Latif, D.O.; Rifa’i, A.; Suryolelono, K.B. Chemical characteristics of volcanic ash in Indonesia for soil stabilization: Morphology and mineral content. Geomate J. 2016, 11, 2606–2610. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Perera, D.S.; Cashion, J.D.; Blackford, G.M.; Zhang, Z.; Vance, E.R. Fe speciation in geoplymers with Si/Al molar ratio of 2. J. Eur. Ceram. Soc. 2007, 27, 2697–2703. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Jameson, G.N.L.; Rahier, H.; Chinje Melo, U.F. The role of iron in the formation of inorganic polymers (geopolymers) from volcanic ash: A 57Fe Mössbauer spectroscopy study. J. Mater. Sci. 2013, 48, 5280–5286. [Google Scholar] [CrossRef]
- Frías, M.; De Rojas, M.S.; Cabrera, J. The effect that the pozzolanic reaction of metakaolin has on the heat evolution in metakaolin-cement mortars. Cem. Concr. Res. 2000, 30, 209–216. [Google Scholar] [CrossRef]
- Agarwal, S.K. Pozzolanic activity of various siliceous materials. Cem. Concr. Res. 2006, 36, 1735–1739. [Google Scholar] [CrossRef]
- Qing, Y.E.; Zenan, Z.; Li, S.; Rongshen, C. A comparative study on the pozzolanic activity between nano-SiO2 and silica fume. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2006, 21, 153–157. [Google Scholar] [CrossRef]
- Mohammed, S. Processing, effect and reactivity assessment of artificial pozzolans obtained from clays and clay wastes: A review. Constr. Build. Mater. 2017, 140, 10–19. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Elsen, J. Calorimetric evolution of the early pozzolanic reaction of natural zeolites. J. Therm. Anal. Calorim. 2010, 101, 97–105. [Google Scholar] [CrossRef]
- Rousselot, I.; Taviot-Guého, C.; Leroux, F.; Léone, P.; Palvadeau, P.; Besse, J.P. Insights on the structural chemistry of hydrocalumite and hydrotalcite-like materials: Investigation of the series Ca2M3+(OH)6Cl·2H2O (M3+: Al3+, Ga3+, Fe3+, and Sc3+) by X-ray powder diffraction. J. Solid State Chem. 2002, 167, 137–144. [Google Scholar] [CrossRef]
- Renaudin, G.; Francois, M.; Evrard, O. Order and disorder in the lamellar hydrated tetracalcium monocarboaluminate compound. Cem. Concr. Res. 1999, 29, 63–69. [Google Scholar] [CrossRef]
- Kuzel, H.J.; Baier, H. Hydration of calcium aluminate cements in the presence of calcium carbonate. Eur. J. Mineral. 1996, 8, 129–142. [Google Scholar] [CrossRef]
- Fernández, R.; Nebreda, B.; De la Villa, R.V.; García, R.; Frías, M. Mineralogical and chemical evolution of hydrated phases in the pozzolanic reaction of calcined paper sludge. Cem. Concr. Compos. 2010, 32, 775–782. [Google Scholar] [CrossRef]
- Lothenbach, B.; Matschei, T.; Möschner, G.; Glasser, F.P. Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cem. Concr. Res. 2008, 38, 1–18. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.M.; Martínez-Ramírez, S. Infrared spectroscopy in the analysis of building and construction materials. In Infrared Spectroscopy–Materials Science, Engineering and Technology; InTech: Bergharen, The Netherlands, 2012; p. 510. [Google Scholar]
- Fernon, V.; Vichot, A.; Colombet, P.; Van Damme, H.; Beguin, F. Synthesis and Structure of Calcium Aluminate Hydrates Intercalated by Aromatic Sulfonates. In Materials Science Forum; Trans Tech Publications Ltd.: Baech, Switzerland, 1994; Volume 152, pp. 335–338. [Google Scholar]
- Wiyantoko, B.; Kurniawati, P.; Purbaningtias, T.E.; Fatimah, I. Synthesis and characterization of hydrotalcite at different Mg/Al molar ratios. Procedia Chem. 2015, 17, 21–26. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in CASH gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Adamczyk, A.; Długoń, E. The FTIR studies of gels and thin films of Al2O3–TiO2 and Al2O3–TiO2–SiO2 systems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 89, 11–17. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near-, Mid-, and Far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Facio, D.S.; Luna, M.; Mosquera, M.J. Facile preparation of mesoporous silica monoliths by an inverse micelle mechanism. Microporous Mesoporous Mater. 2017, 247, 166–176. [Google Scholar] [CrossRef]
- Depla, A.; Lesthaeghe, D.; van Erp, T.S.; Aerts, A.; Houthoofd, K.; Fan, F.; Li, C.; Van Speybroeck, V.; Waroquier, M.; Kirschhock, C.E.A.; et al. 29Si NMR and UV− Raman investigation of initial oligomerization reaction pathways in acid-catalyzed silica sol− gel chemistry. J. Phys. Chem. C 2011, 115, 3562–3571. [Google Scholar] [CrossRef]
- Higl, J.; Hinder, D.; Rathgeber, C.; Ramming, B.; Lindén, M. Detailed in situ ATR-FTIR spectroscopy study of the early stages of CSH formation during hydration of monoclinic C3S. Cem. Concr. Res. 2021, 142, 106367. [Google Scholar] [CrossRef]
- Fernandez, L.; Alonso, C.; Hidalgo, A.; Andrade, C. The role of magnesium during the hydration of C3S and CSH formation. Scanning electron microscopy and mid-infrared studies. Adv. Cem. Res. 2005, 17, 9–21. [Google Scholar] [CrossRef]
- Barzgar, S.; Tarik, M.; Ludwig, C.; Lothenbach, B. The effect of equilibration time on Al uptake in CSH. Cem. Concr. Res. 2021, 144, 106438. [Google Scholar] [CrossRef]
- Djebaili, K.; Mekhalif, Z.; Boumaza, A.; Djelloul, A.X.P.S. XPS, FTIR, EDX, and XRD analysis of Al2O3 scales grown on PM2000 alloy. J. Spectrosc. 2015, 1, 868109. [Google Scholar]
- Liu, C.; Shih, K.; Gao, Y.; Li, F.; Wei, L. Dechlorinating transformation of propachlor through nucleophilic substitution by dithionite on the surface of alumina. J. Soils Sediments 2012, 12, 724–733. [Google Scholar] [CrossRef]
- Partyka, J.; Leśniak, M. Raman and infrared spectroscopy study on structure and microstructure of glass–ceramic materials from SiO2–Al2O3–Na2O–K2O–CaO system modified by variable molar ratio of SiO2/Al2O3. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 82–91. [Google Scholar] [CrossRef]
- Padmaja, P.; Anilkumar, G.M.; Mukundan, P.; Aruldhas, G.; Warrier, K.G.K. Characterisation of stoichiometric sol–gel mullite by fourier transform infrared spectroscopy. Int. J. Inorg. Mater. 2001, 3, 693–698. [Google Scholar] [CrossRef]




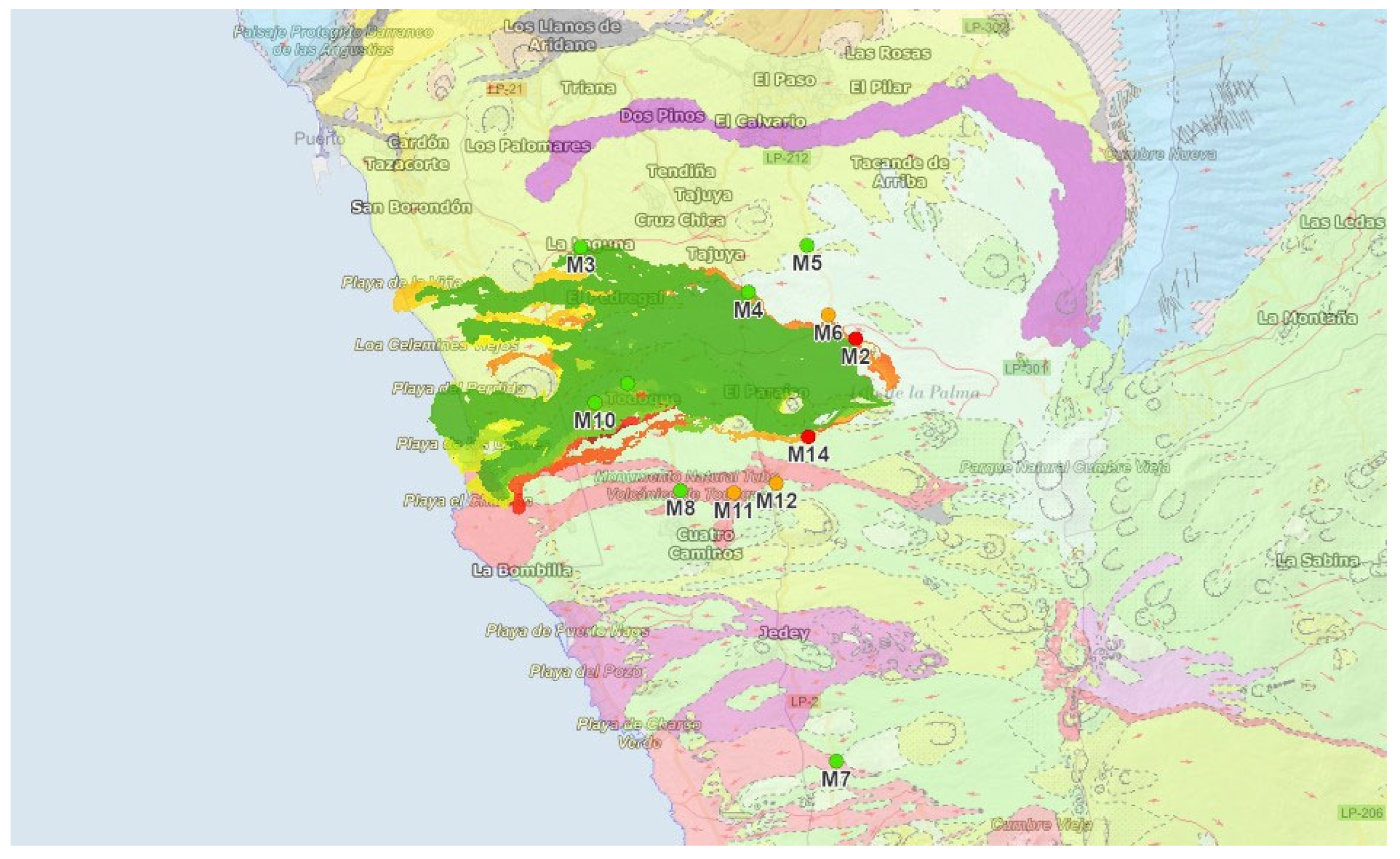
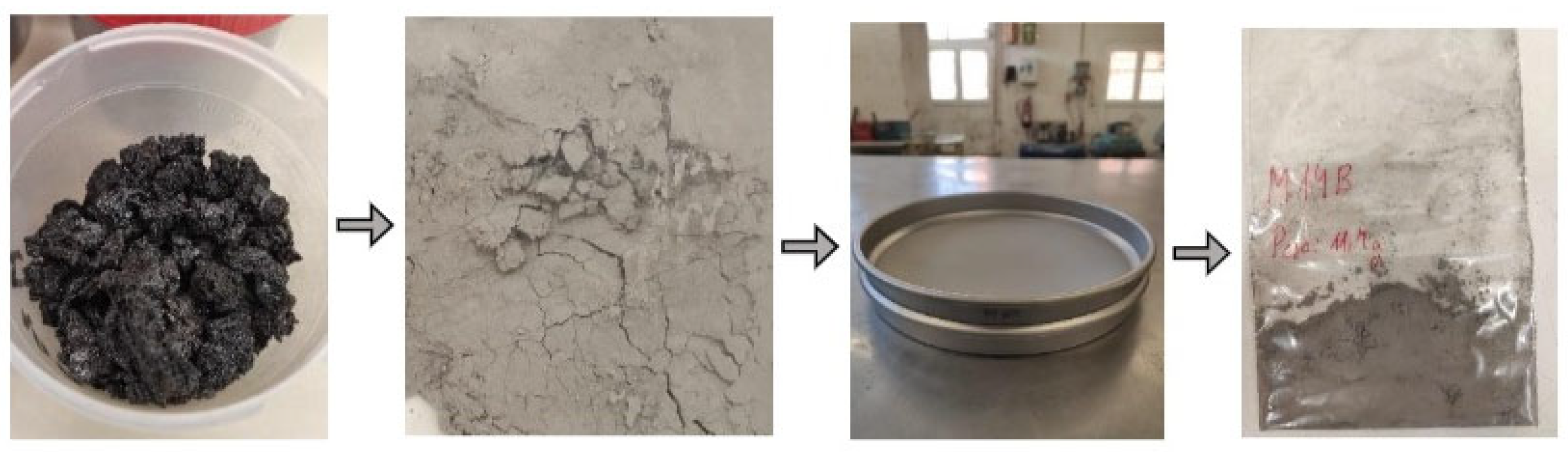

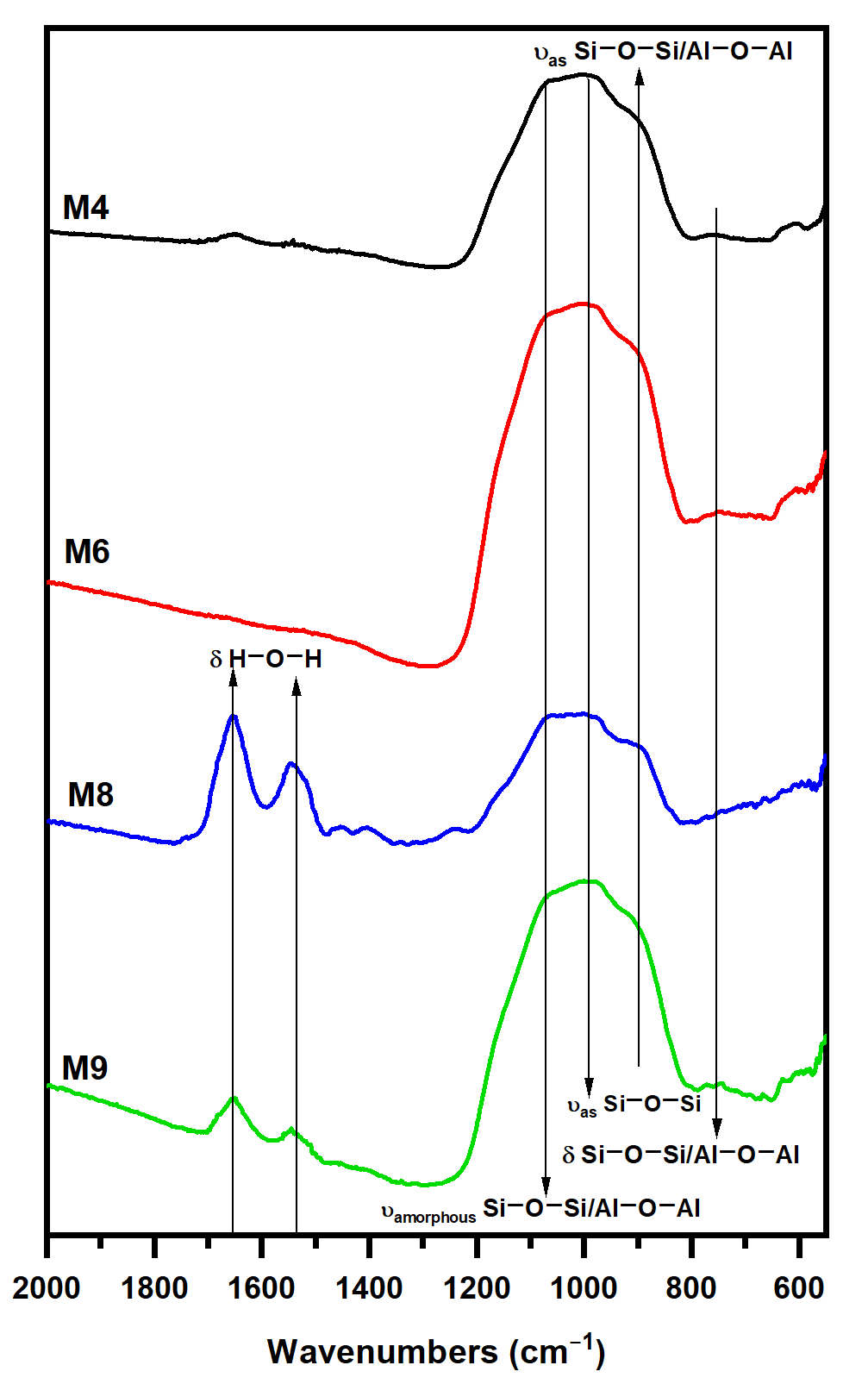

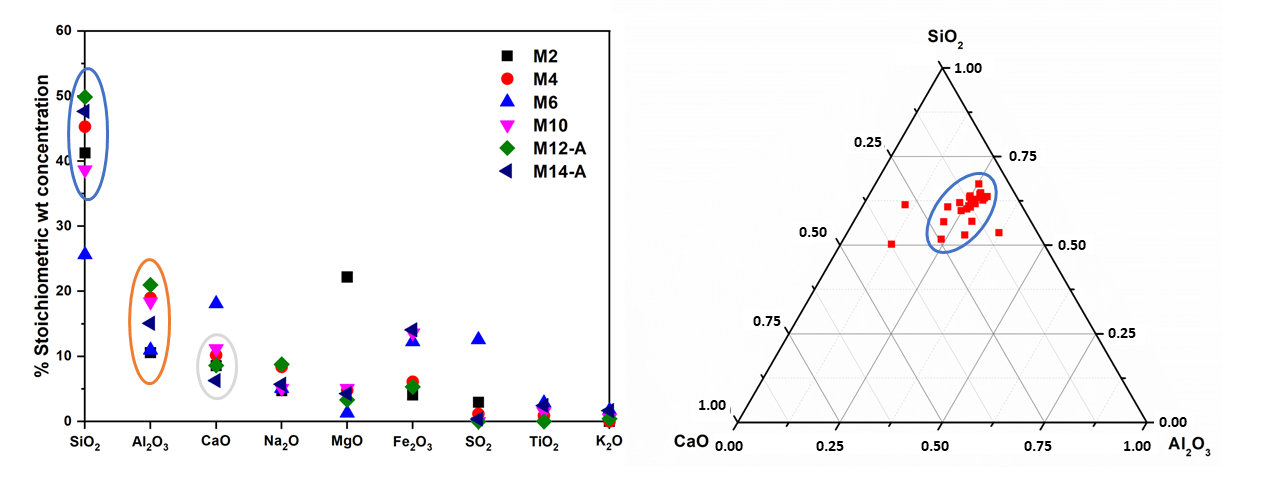

| Sample | Crater Orientation | Coordinates X | Coordinates Y | Sea Level (m) | Accumulation (cm) | |
|---|---|---|---|---|---|---|
| M2 | N | 28R 219405 | 3169339 | 803 | 60 | |
| M3 | NW | 28R 215674 | 3170678 | 302 | 0–1 | |
| M4 | NW | 28R 217951 | 3170010 | 522 | 0–1 | |
| M5 | N | 28R 218771 | 3170626 | 695 | 1–2 | |
| M6 | N | 28R 219039 | 3169670 | 738 | 12 | |
| M7 | S | 28R 219002 | 3163586 | 687 | 2 | |
| M8 | SW | 28R 216959 | 3167330 | 458 | 3 | |
| M9 | W | 28R 216268 | 3168801 | 344 | 3 | |
| M10 | W | 28R 215816 | 3168552 | 286 | 1–1.5 | |
| M11 | SW | 28R 217687 | 3167280 | 542 | 10 | |
| M12 | a | SW | 28R 218273 | 3167395 | 611 | 7 |
| b | SW | 28R 218273 | 3167395 | 611 | 10 | |
| c | SW | 28R 218273 | 3167395 | 611 | 9 | |
| M14 | a | SW | 28R 218725 | 3168017 | 661 | 120 |
| b | SW | 28R 218725 | 3168017 | 661 | 60 | |
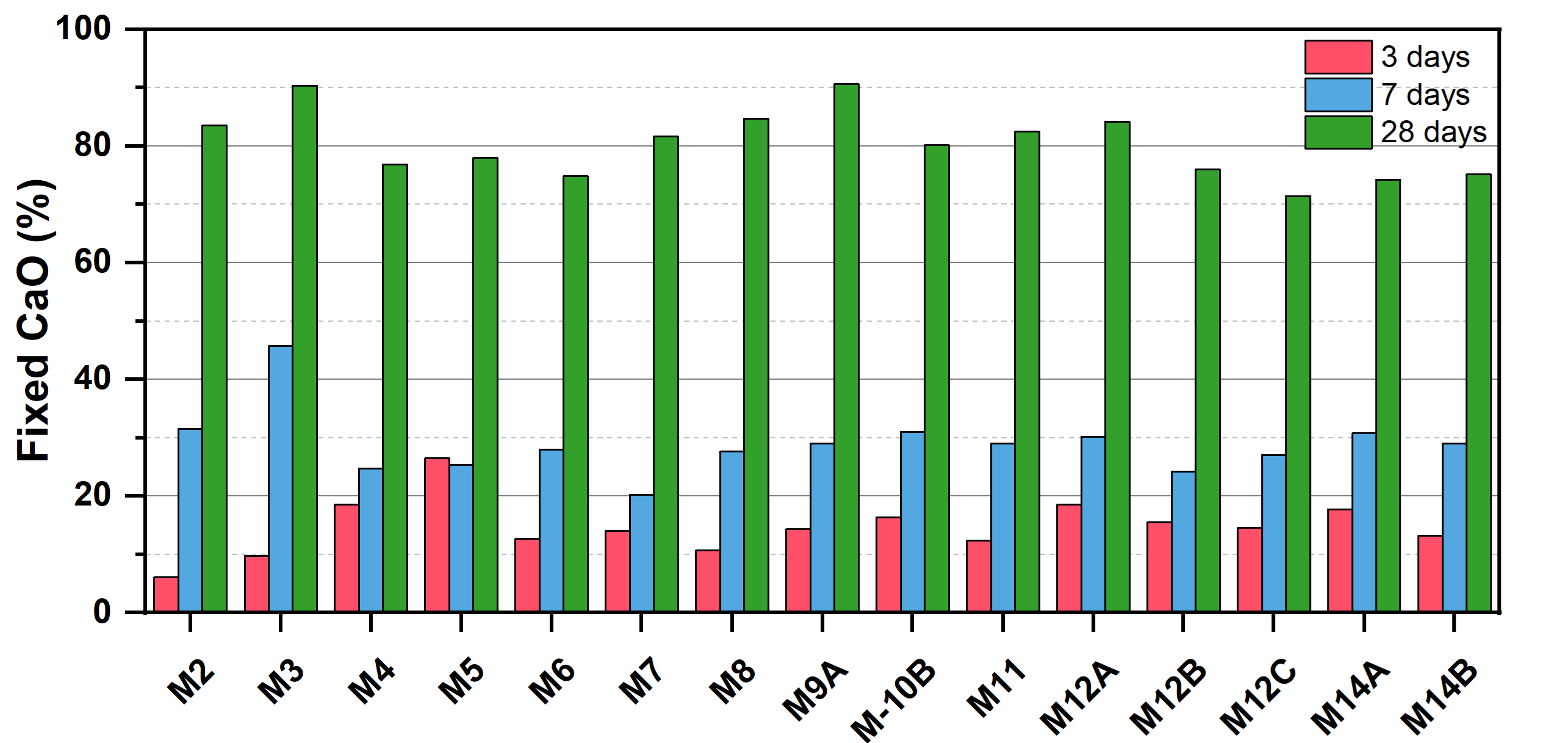
| [CaO]–3 Days | [CaO]–7 Days | [CaO]–28 Days | ||||
|---|---|---|---|---|---|---|
| Sample | mM/L | % vol. Fixed | mM/L | % vol. Fixed | mM/L | % vol. Fixed |
| M2 | 1.08 | 6.11 | 5.58 | 31.56 | 14.77 | 83.51 |
| M3 | 1.73 | 9.79 | 8.08 | 45.70 | 15.98 | 90.38 |
| M4 | 3.28 | 18.55 | 4.38 | 24.77 | 13.58 | 76.81 |
| M5 | 4.68 | 26.47 | 4.48 | 25.34 | 13.78 | 77.94 |
| M6 | 2.23 | 12.61 | 4.93 | 27.88 | 13.23 | 74.83 |
| M7 | 2.48 | 14.03 | 3.58 | 20.25 | 14.43 | 81.62 |
| M8 | 1.88 | 10.63 | 4.88 | 27.60 | 14.98 | 84.73 |
| M9 | 2.53 | 14.31 | 5.13 | 29.02 | 16.03 | 90.67 |
| M10 | 2.88 | 16.29 | 5.48 | 31.00 | 14.18 | 80.20 |
| M11 | 2.18 | 12.33 | 5.13 | 29.02 | 14.58 | 82.47 |
| M12-A | 3.28 | 18.55 | 5.33 | 30.15 | 14.88 | 84.16 |
| M12-B | 2.73 | 15.44 | 4.28 | 24.21 | 13.43 | 75.96 |
| M12-C | 2.58 | 14.59 | 4.78 | 27.04 | 12.63 | 71.44 |
| M14-A | 3.13 | 17.70 | 5.43 | 30.71 | 13.13 | 74.26 |
| M14-B | 2.33 | 13.18 | 5.13 | 29.02 | 13.28 | 75.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basar, I.; Torrens-Martín, D.; Fernández-Carrasco, L.; Caiza, C.; Martínez-Bofill, J.; Hürlimann, M. Sample Origin Effect on Chemical Reactivity of Tajogaite Volcanic Ashes for Ancient Mortar Repair. Sustain. Chem. 2025, 6, 18. https://doi.org/10.3390/suschem6030018
Basar I, Torrens-Martín D, Fernández-Carrasco L, Caiza C, Martínez-Bofill J, Hürlimann M. Sample Origin Effect on Chemical Reactivity of Tajogaite Volcanic Ashes for Ancient Mortar Repair. Sustainable Chemistry. 2025; 6(3):18. https://doi.org/10.3390/suschem6030018
Chicago/Turabian StyleBasar, Imren, David Torrens-Martín, Lucía Fernández-Carrasco, Cristhian Caiza, Joan Martínez-Bofill, and Marcel Hürlimann. 2025. "Sample Origin Effect on Chemical Reactivity of Tajogaite Volcanic Ashes for Ancient Mortar Repair" Sustainable Chemistry 6, no. 3: 18. https://doi.org/10.3390/suschem6030018
APA StyleBasar, I., Torrens-Martín, D., Fernández-Carrasco, L., Caiza, C., Martínez-Bofill, J., & Hürlimann, M. (2025). Sample Origin Effect on Chemical Reactivity of Tajogaite Volcanic Ashes for Ancient Mortar Repair. Sustainable Chemistry, 6(3), 18. https://doi.org/10.3390/suschem6030018







