Exploring the Potential of Zeolites for Sustainable Environmental Applications
Abstract
1. Introduction
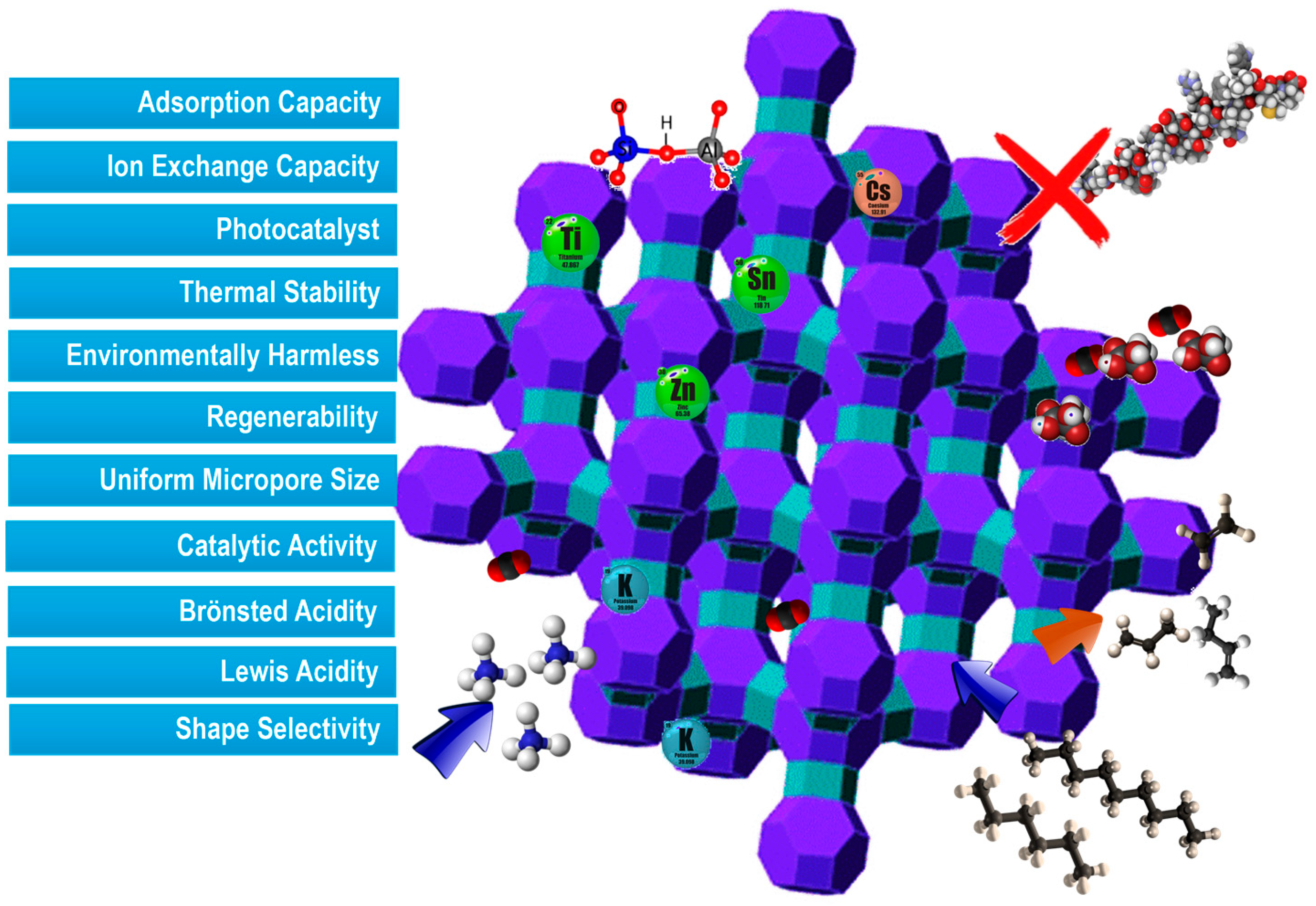
2. Zeolites for Sustainable Environmental Applications
2.1. Zeolites for Inorganic Contaminant Removal
2.2. Zeolites for the Removal of Organic Contaminants
2.3. Zeolites for CO2 Capture and Storage
2.4. Zeolites in Bioprocessing Applications
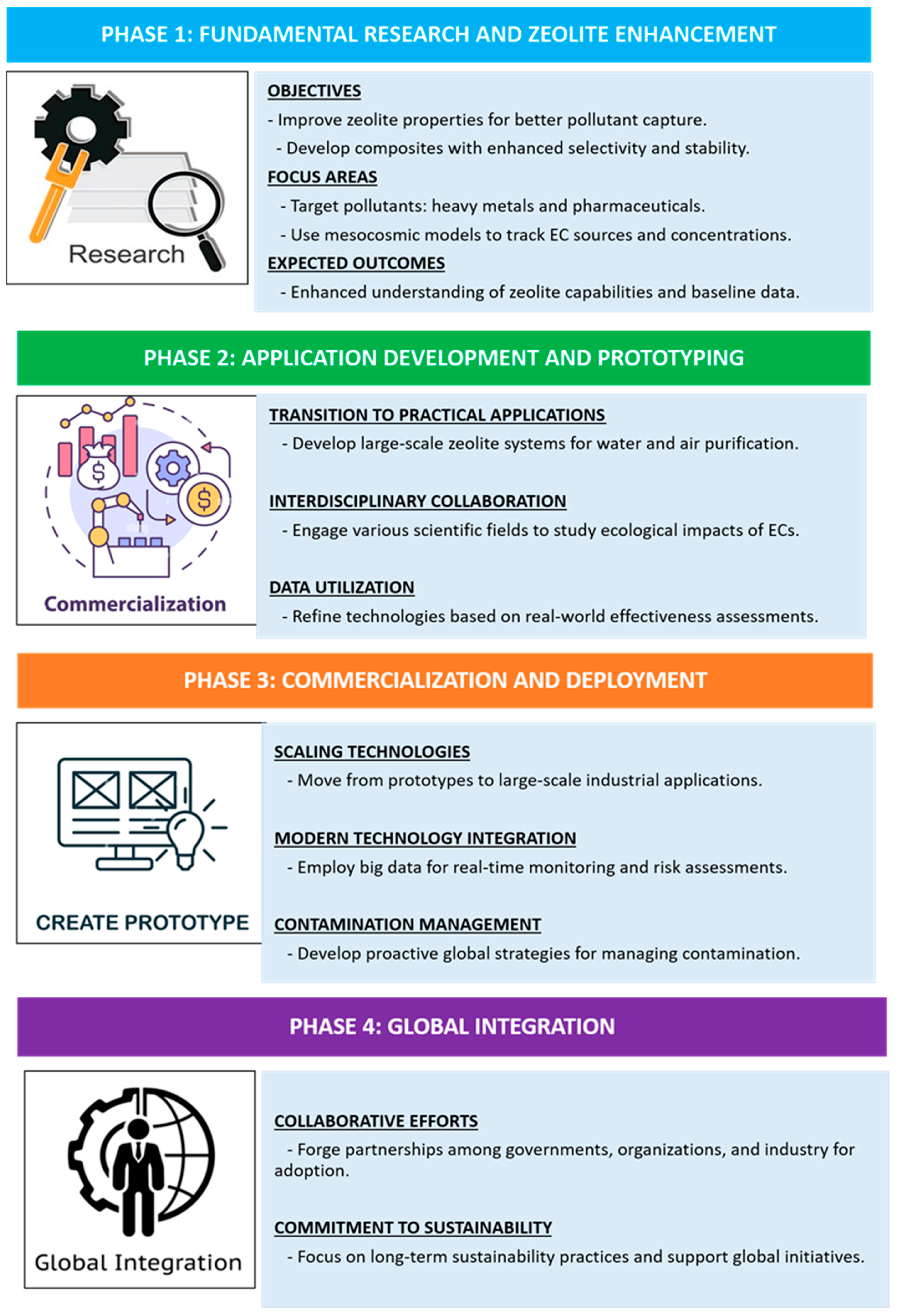
3. Toward the Development of a Sustainable Zeolite-Based Roadmap for Environmental Remediation
- Advancements in technology and material developments: this includes monitoring the progress of zeolite structures, surface functionalization, and composite materials to improve efficiency in applications such as adsorption, ion exchange, and catalysis.
- Market and industry alignment: it is essential to assess how zeolite-based materials meet the increasing global demand for sustainable environmental technologies, green chemistry, and renewable energy solutions.
- Competitive and regulatory landscape: understanding the alignment of zeolite technologies with existing and emerging environmental regulations, sustainability policies, and industrial standards
- R&D priorities and implementation challenges: identifying key innovation areas such as improving zeolite stability, scalability, and selectivity while addressing synthesis costs, resource availability, and large-scale applicability.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Gao, Z.R.; Lin, Q.F.; Liu, C.; Gao, F.; Lin, C.; Zhang, S.; Deng, H.; Mayoral, A.; Fan, W.; et al. A 3D extra-large-pore zeolite enabled by 1D-to-3D topotactic condensation of a chain silicate. Science 2023, 379, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Casci, J.L. Zeolite molecular sieves: Preparation and scale-up. Microporous Mesoporous Mater. 2005, 82, 217–226. [Google Scholar] [CrossRef]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Serrano, D.P.; Centi, G.; Diddams, P.A.; Čejka, J. Outlooks for zeolite catalysts in a low-carbon scenario. Catal. Today 2024, 426, 114365. [Google Scholar] [CrossRef]
- Van Donk, S.; Janssen, A.H.; Bitter, J.H.; de Jong, K.P. Generation, characterization, and impact of mesopores in zeolite catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef]
- Mancinelli, M.; Precisvalle, N.; Ardit, M.; Beltrami, G.; Gigli, L.; Catizzone, E.; Migliori, M.; Giordano, G.; Martucci, A. Thermal stability of templated ZSM-5 zeolites: An in-situ synchrotron X-ray powder diffraction study. Microporous Mesoporous Mater. 2023, 362, 112777. [Google Scholar] [CrossRef]
- Čejka, J.; Millini, R.; Opanasenko, M.; Serrano, D.P.; Roth, W.J. Advances and challenges in zeolite synthesis and catalysis. Catal. Today 2020, 345, 2–13. [Google Scholar] [CrossRef]
- Velty, A.; Corma, A. Advanced zeolite and ordered mesoporous silica-based catalysts for the conversion of CO2 to chemicals and fuels. Chem. Soc. Rev. 2023, 52, 1773–1946. [Google Scholar] [CrossRef]
- Ducamp, M.; Coudert, F.X. Systematic study of the thermal properties of zeolitic frameworks. J. Phys. Chem. C 2021, 125, 15647–15658. [Google Scholar] [CrossRef]
- Krivovichev, S. Topology of microporous structures. Rev. Mineral. Geochem. 2005, 57, 17–68. [Google Scholar] [CrossRef]
- Cruciani, G. Zeolites upon heating: Factors governing their thermal stability and structural changes. J. Phys. Chem. Solids 2006, 67, 1973–1994. [Google Scholar] [CrossRef]
- Bish, D.L.; Carey, J.W. Thermal behavior of natural zeolites. Rev. Mineral. Geochem. 2001, 45, 403–452. [Google Scholar] [CrossRef]
- Confalonieri, G.; Grand, J.; Arletti, R.; Barrier, N.; Mintova, S. CO2 adsorption in nanosized RHO zeolites with different chemical compositions and crystallite sizes. Microporous Mesoporous Mater. 2020, 306, 110394. [Google Scholar] [CrossRef]
- Rahmah, W.; Kadja, G.T.M.; Mahyuddin, M.H.; Saputro, A.G.; Dipojono, H.K.; Wenten, I.G. Small-pore zeolite and zeotype membranes for CO2 capture and sequestration—A review. J. Environ. Chem. Eng. 2022, 10, 108707. [Google Scholar] [CrossRef]
- Colella, C. Natural zeolites in environmentally friendly processes and applications. Stud. Surf. Sci. Catal. 1999, 125, 641–655. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A. Factors controlling the acidity of zeolites. Catal. Lett. 2015, 145, 162–172. [Google Scholar] [CrossRef]
- Čejka, J.; Wichterlová, B. Acid-catalyzed synthesis of mono-and dialkyl benzenes over zeolites: Active sites, zeolite topology, and reaction mechanisms. Catal. Rev. 2002, 44, 375–421. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in adsorption processes: State of the art and future prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Jin, W.; Yang, Y.; Jin, J.; Xu, M.; Zhang, Z.; Dong, F.; Shao, M.; Wan, Y. Characterization of phosphate modified red mud-based composite materials and study on heavy metal adsorption. Environ. Sci. Pollut. Res. 2024, 31, 43687–43703. [Google Scholar] [CrossRef]
- Daer, S.; Kharraz, J.; Giwa, A.; Hasan, S.W. Recent applications of nanomaterials in water desalination: A critical review and future opportunities. Desalination 2015, 367, 37–48. [Google Scholar] [CrossRef]
- Swenson, P.; Tanchuk, B.; Bastida, E.; An, W.; Kuznicki, S.M. Water desalination and de-oiling with natural zeolite membranes—Potential application for purification of SAGD process water. Desalination 2012, 286, 442–446. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yu, J. Applications of zeolites in sustainable chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Jiménez-Reyes, M.; Almazán-Sánchez, P.T.; Solache-Ríos, M. Radioactive waste treatments by using zeolites. A short review. J. Environ. Radioact. 2021, 233, 106610. [Google Scholar] [CrossRef]
- Riley, B.J.; Chong, S.; Schmid, J.; Marcial, J.; Nienhuis; Bera, M.K.; Lee, S.; Canfield, N.L.; Kim, S.; Derewinski, M.A.; et al. Role of zeolite structural properties toward iodine capture: A head-to-head evaluation of framework type and chemical composition. ACS Appl. Mater. Interfaces 2022, 14, 18439–18452. [Google Scholar] [CrossRef]
- Pham, T.C.T.; Docao, S.; Hwang, I.C.; Song, M.K.; Moon, D.; Oleynikov, P.; Yoon, K.B. Capture of iodine and organic iodides using silica zeolites and the semiconductor behavior of iodine in a silica zeolite. Energy Environ. Sci. 2016, 9, 1050–1062. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, C.; Han, E.; Lee, H.; Cho, H.S.; Choi, M. Relationship between zeolite structure and capture capability for radioactive cesium and strontium. J. Hazard. Mater. 2021, 408, 124419. [Google Scholar] [CrossRef]
- Hijikata, T.; Koyama, T.; Aikyo, Y.; Shimura, S.; Kawanishi, M. Strontium adsorption characteristics of natural Zeolites for permeable reactive barrier in Fukushima Daiichi nuclear power station. J. Nucl. Sci. Technol. 2021, 58, 1079–1098. [Google Scholar] [CrossRef]
- Belviso, C. Special Issue “Sustainable Remediation Processes Based on Zeolites”. Processes 2021, 9, 2153. [Google Scholar] [CrossRef]
- de Gennaro, B.; Aprea, P.; Liguori, B.; Galzerano, B.; Peluso, A.; Caputo, D. Zeolite-rich composite materials for environmental remediation: Arsenic removal from water. Appl. Sci. 2020, 10, 6938. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, B.; Yin, H.; Meng, L.; Jin, W.; Wang, F.; Xu, J.; Al-Tabbaa, A. Application of zeolites in permeable reactive barriers (PRBs) for in-situ groundwater remediation: A critical review. Chemosphere 2022, 308, 136290. [Google Scholar] [CrossRef]
- Eberle, S.; Börnick, H.; Stolte, S. Granular natural zeolites: Cost-effective adsorbents for the removal of ammonium from drinking water. Water 2022, 14, 939. [Google Scholar] [CrossRef]
- Mancinelli, M.; Arfè, A.; Martucci, A.; Pasti, L.; Chenet, T.; Sarti, E.; Vergine, G.; Belviso, C. Evaluation for the removal efficiency of VOCs and heavy metals by zeolites-based materials in the wastewater: A case study in the Tito Scalo industrial area. Processes 2020, 8, 1519. [Google Scholar] [CrossRef]
- Wilfong, W.C.; Ji, T.; Duan, Y.; Shi, F.; Wang, Q.; Gray, M.L. Critical review of functionalized silica sorbent strategies for selective extraction of rare earth elements from acid mine drainage. J. Hazard. Mater. 2022, 424, 127625. [Google Scholar] [CrossRef]
- Mijailović, N.R.; Nedić Vasiljević, B.; Ranković, M.; Milanović, V.; Uskoković-Marković, S. Environmental and pharmacokinetic aspects of zeolite/pharmaceuticals systems. Two facets of adsorption ability. Catalysts 2022, 12, 837. [Google Scholar] [CrossRef]
- Mangla, D.; Sharma, A.; Ikram, S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard. Mater. 2022, 425, 127946. [Google Scholar] [CrossRef]
- Song, J.Y.; Jhung, S.H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: Quantitative analyses of H-bonding in adsorption. J. Chem. Eng. 2017, 322, 366–374. [Google Scholar] [CrossRef]
- Sarti, E.; Chenet, T.; Stevanin, C.; Costa, V.; Cavazzini, A.; Catani, M.; Martucci, A.; Precisvalle, N.; Beltrami, G.; Pasti, L. High-silica zeolites as sorbent media for adsorption and pre-concentration of pharmaceuticals in aqueous solutions. Molecules 2020, 25, 3331. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Lu, S.; Zhao, B.; Wang, Z.; Xi, B.; Guo, W. Adsorption and biodegradation of sulfamethoxazole and ofloxacin on zeolite: Influence of particle diameter and redox potential. Chem. Eng. J. 2020, 384, 123346. [Google Scholar] [CrossRef]
- Senila, M.; Cadar, O. Modification of natural zeolites and their applications for heavy metal removal from polluted environments: Challenges, recent advances, and perspectives. Heliyon 2024, 10, e25303. [Google Scholar] [CrossRef]
- Chenet, T.; Mancinelli, M.; Sarti, E.; Costa, V.; D’Anna, C.; Martucci, A.; Pasti, L. Competitive Adsorption of 4-Hydroxybenzaldehyde and Toluene onto High Silica Zeolites. Environ. Process. 2024, 11, 49. [Google Scholar] [CrossRef]
- Rocha, L.C.C.; Zuquette, L.V. Evaluation of zeolite as a potential reactive medium in a permeable reactive barrier (PRB): Batch and column studies. Geosciences 2020, 10, 59. [Google Scholar] [CrossRef]
- de Sousa, D.N.R.; Insa, S.; Mozeto, A.A.; Petrovic, M.; Chaves, T.F.; Fadini, P.S. Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere 2018, 205, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Qian, C.; Ma, S.; Xiong, J. Sulfonamide antibiotics sorption by high silica ZSM-5: Effect of pH and humic monomers (vanillin and caffeic acid). Chemosphere 2020, 248, 126061. [Google Scholar] [CrossRef]
- Bal’zhinimaev, B.S.; Paukshtis, E.A.; Toktarev, A.V.; Kovalyov, E.V.; Yaranova, M.A.; Smirnov, A.E.; Stompel, S. Effect of water on toluene adsorption over high silica zeolites. Microporous Mesoporous Mater. 2019, 277, 70–77. [Google Scholar] [CrossRef]
- Mancinelli, M.; Ardit, M.; Chenet, T.; Pasti, L.; Martucci, A. Desorption of humic monomers from Y zeolite: A high-temperature X-ray diffraction and thermogravimetric study. Microporous Mesoporous Mater. 2024, 379, 113270. [Google Scholar] [CrossRef]
- Mancinelli, M.; Martucci, A.; Ahrens, L. Exploring the adsorption of short and long-chain per-and poly-fluoroalkyl substances (PFAS) to different zeolites using environmental samples. Environ. Sci. Water Res. Technol. 2023, 9, 2595–2604. [Google Scholar] [CrossRef]
- Ponge, C.A.; Sheehan, N.P.; Corbin, D.R.; Peltier, E.; Hutchison, J.M.; Shiflett, M.B. Zeolites for Sorption of PFAS from Water. Ind. Eng. Chem. Res. 2024, 63, 12102–12112. [Google Scholar] [CrossRef]
- Mancinelli, M.; Stevanin, C.; Ardit, M.; Chenet, T.; Pasti, L.; Martucci, A. PFAS as emerging pollutants in the environment: A challenge with FAU type and silver-FAU exchanged zeolites for their removal from water. J. Environ. Chem. Eng. 2022, 10, 108026. [Google Scholar] [CrossRef]
- Ponge, C.A.; Corbin, D.R.; Sabolay, C.M.; Shiflett, M.B. Designing zeolites for the removal of aqueous PFAS: A perspective. Ind. Chem. Mater. 2024, 2, 270–275. [Google Scholar] [CrossRef]
- Mancinelli, M.; Martucci, A.; Salani, G.M.; Bianchini, G.; Gigli, L.; Plaisier, J.R.; Colombo, F. High-temperature behavior of Ag-exchanged Y zeolites used for PFAS sequestration from water. Phys. Chem. Chem. Phys. 2023, 25, 20066–20075. [Google Scholar] [CrossRef]
- Omo-Okoro, P.N.; Curtis, C.J.; Marco, A.M.; Melymuk, L.; Okonkwo, J.O. Removal of per- and polyfluoroalkyl substances from aqueous media using synthesized silver nanocomposite-activated carbons. J. Environ. Health Sci. Eng. 2021, 19, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Zeolite-based photocatalysts. Chem. Commun. 2004, 13, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Hamd, A.; Shaban, M.; Al-Senani, G.M.; Alshabanat, M.N.; Al-Ghamdi, A.; Dryaz, A.R.; Ahmed, S.A.; El-Sayed, R.; Soliman, N.K. Comprehensive evaluation of zeolite/marine alga nanocomposite in the removal of waste dye from industrial wastewater. Sci. Rep. 2023, 13, 8082. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, H.; Chen, Z.; Veksha, A.; Lisak, G.; You, C. Interaction between SO2 and NO in their adsorption and photocatalytic conversion on TiO2. Chemosphere 2020, 249, 126136. [Google Scholar] [CrossRef]
- Izadpanah, M.; Sarrafzadeh, M.H.; Rezaei-Dashtarzhandi, M.; Vojoudi, H. Aquatic center sewage reclamation and water reuse, using an integrated system combining adsorption, RO membrane system, and TiO2/Fe3O4 photocatalytic oxidation. J. Environ. Chem. Eng. 2021, 9, 104957. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Sourinejad, I.; Kazemian, H.; Rohani, S. Application of zeolites in aquaculture industry: A review. Rev. Aquac. 2018, 10, 75–95. [Google Scholar] [CrossRef]
- Zhao, Q.; Liao, C.; Chen, G.; Liu, R.; Wang, Z.; Xu, A.; Ji, S.; Shih, K.; Zou, L.; Duan, T. In situ confined synthesis of a copper-encapsulated silicalite-1 zeolite for highly efficient iodine capture. Inorg. Chem. 2022, 61, 20133–20143. [Google Scholar] [CrossRef]
- Mambetova, M.; Dossumov, K.; Baikhamurova, M.; Yergaziyeva, G. Sorbents Based on Natural Zeolites for Carbon Dioxide Capture and Removal of Heavy Metals from Wastewater: Current Progress and Future Opportunities. Processes 2024, 12, 2071. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Tufail, M.K.; Ifrahim, M.; Rashid, M.; Haq, I.U.; Asghar, R.; Uthappa, U.T.; Selvaraj, M.; Kurkuri, M. Chemistry of zeolites and zeolite based composite membranes as a cutting-edge candidate for removal of organic dyes & heavy metal ions: Progress and future directions. Sep. Purif. Technol. 2024, 354, 128739. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; Wang, J.; Zhou, J.; Huang, X.; Chen, L.; Bashir, S.; Liu, J.L. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem. Eng. J. 2020, 390, 124513. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H. Clean Laccase immobilized nanobiocatalysts (graphene oxide-zeolite nanocomposites): From production to detailed biocatalytic degradation of organic pollutant. Appl. Catal. B Environ. 2020, 268, 118443. [Google Scholar] [CrossRef]
- Hassan, F.; Mushtaq, R.; Saghar, S.; Younas, U.; Pervaiz, M.; Muteb Aljuwayid, A.; Habila, M.A.; Sillanpaa, M. Fabrication of graphene-oxide and zeolite loaded polyvinylidene fluoride reverse osmosis membrane for saltwater remediation. Chemosphere 2022, 307, 136012. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef]
- Fu, D.; Davis, M.E. Carbon dioxide capture with zeotype materials. Chem. Soc. Rev. 2022, 51, 9340–9370. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, S.; Yu, J. Metal sites in zeolites: Synthesis, characterization, and catalysis. Chem. Rev. 2022, 123, 6039–6106. [Google Scholar] [CrossRef]
- Zhang, H.; bin Samsudin, I.; Jaenicke, S.; Chuah, G.K. Zeolites in catalysis: Sustainable synthesis and its impact on properties and applications. Catal. Sci. Technol. 2022, 12, 6024–6039. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Yu, J. Advances in catalytic applications of zeolite-supported metal catalysts. Adv. Mater. 2021, 33, 2104442. [Google Scholar] [CrossRef]
- Dusselier, M.; Van Wouwe, P.; Dewaele, A.; Jacobs, P.A.; Sels, B.F. Shape-selective zeolite catalysis for bioplastics production. Science 2015, 349, 78–80. [Google Scholar] [CrossRef]
- Faisal, A.; Holmlund, M.; Ginesy, M.; Holmgren, A.; Enman, J.; Hedlund, J.; Grahn, M. Recovery of L-arginine from model solutions and fermentation broth using zeolite-Y adsorbent. ACS Sustain. Chem. Eng. 2019, 7, 8900–8907. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Baloch, H.A.; Mazari, S.A.; Mubarak, N.M.; Sabzoi, N.; Aziz, S.; Soomro, S.A.; Abro, R.; Shah, S.F. A review of extractive fermentation via ion exchange adsorption resins opportunities, challenges, and prospects. Biomass Convers. Biorefin. 2021, 13, 3543–3554. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Feng, K.; Liu, J. Oriented fermentation of food waste towards high-value products: A review. Energies 2020, 13, 5638. [Google Scholar] [CrossRef]
- Munsch, S.; Hartmann, M.; Ernst, S. Adsorption and separation of amino acids from aqueous solutions on zeolites. Chem. Comm. 2021, 19, 1978–1979. [Google Scholar] [CrossRef] [PubMed]
- Polisi, M.; Fabbiani, M.; Vezzalini, G.; Di Renzo, F.; Pastero, L.; Quartieri, S.; Arletti, R. Amino acid encapsulation in zeolite MOR: Effect of spatial confinement. Phys. Chem. Chem. Phys. 2021, 23, 20541–20552. [Google Scholar] [CrossRef]
- Beltrami, G.; Martucci, A.; Pasti, L.; Chenet, T.; Ardit, M.; Gigli, L.; Cescon, M.; Suard, E. L-Lysine Amino Acid Adsorption on Zeolite L: A Combined Synchrotron, X-Ray and Neutron Diffraction Study. ChemistryOpen 2020, 9, 978–982. [Google Scholar] [CrossRef]
- Chen, T.; Wun, C.K.T.; Day, S.J.; Tang, C.C.; Lo, T.W.B. Enantiospecificity in achiral zeolites for asymmetric catalysis. Phys. Chem. Chem. Phys. 2020, 22, 18757–18764. [Google Scholar] [CrossRef]
- Chen, T.; Huang, B.; Day, S.; Tang, C.C.; Tsang, S.C.E.; Wong, K.; Lo, T.W.B. Differential Adsorption of L-and D-Lysine on Achiral MFI Zeolites as Determined by Synchrotron X-Ray Powder Diffraction and Thermogravimetric Analysis. Angew. Chem. Int. Ed. 2020, 59, 1093–1097. [Google Scholar] [CrossRef]
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical applications of zeolite-based materials: A review. Mater. Sci. Eng. C 2020, 116, 111225. [Google Scholar] [CrossRef]
- Elsherbeni, A.I.; Youssef, I.M.; Kamal, M.; Youssef, M.A.; El-Gendi, G.M.; El-Garhi, O.H.; Alfassam, H.E.; Rudayni, H.A.; Allam, A.A.; Moustafa, M.; et al. Impact of Adding Zeolite to broilers’ Diet and Litter on Growth, Blood Parameters, Immunity, and Ammonia Emission. Poult. Sci. 2024, 103, 103981. [Google Scholar] [CrossRef]
- Papaioannou, D.; Katsoulos, P.D.; Panousis, N.; Karatzias, H. The role of natural and synthetic zeolites as feed additives on the prevention and/or the treatment of certain farm animal diseases: A review. Microporous Mesoporous Mater. 2005, 84, 161–170. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Soldatkin, O.O.; Dzyadevych, S.V.; Soldatkin, A.P. Application of zeolites and zeolitic imidazolate frameworks in biosensor development. Biomater. Adv. 2022, 143, 213180. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef] [PubMed]
- He, Z.W.; Zou, Z.S.; Ren, Y.X.; Tang, C.C.; Zhou, A.J.; Liu, W.; Wang, L.; Li, Z.; Wang, A. Roles of zero-valent iron in anaerobic digestion: Mechanisms, advances and perspectives. Sci. Total Environ. 2022, 852, 158420. [Google Scholar] [CrossRef]
- Jin, H.Y.; He, Z.W.; Ren, Y.X.; Tang, C.C.; Zhou, A.J.; Liu, W.; Liang, B.; Li, Z.H.; Wang, A. Current advances and challenges for direct interspecies electron transfer in anaerobic digestion of waste activated sludge. Chem. Eng. J. 2022, 450, 137973. [Google Scholar] [CrossRef]
- Montalvo, S.; Huiliñir, C.; Borja, R.; Sánchez, E.; Herrmann, C.J.B.T. Application of zeolites for biological treatment processes of solid wastes and wastewaters—A review. Bioresour. Technol. 2020, 301, 122808. [Google Scholar] [CrossRef]
- Zhao, C.; Sharma, A.; Ma, Q.; Zhu, Y.; Li, D.; Liu, Z.; Yang, Y. A developed hy-brid fixed-bed bioreactor with Fe-modified zeolite to enhance and sustain biohydrogen production. Sci. Total Env. 2021, 758, 143658. [Google Scholar] [CrossRef]
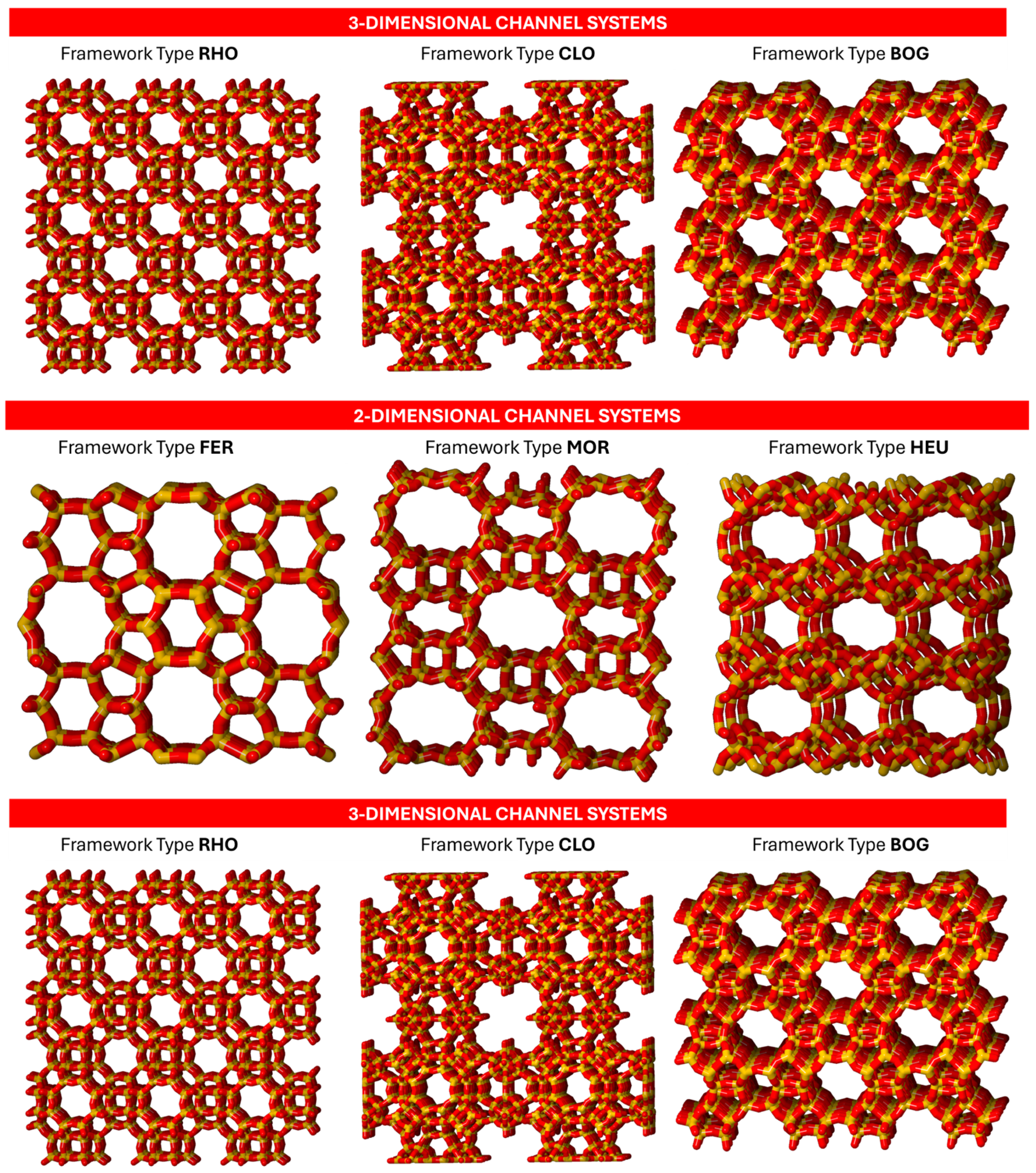

| Extra-large pores | ||
| CLO | Cloverite | <100> 20 4.0 × 13.2 *** | <100> 8 3.8 × 3.8 *** |
| VFI | VPI-5 | [001] 18 12.7 × 12.7 * |
| AET | AlPO-8 | [001] 14 7.9 × 8.7 * |
| CFI | CIT-5 | [010] 14 7.2 × 7.5 * |
| DON | UTD-1F | [010] 14 8.1 × 8.2 * |
| OSO | OSB-1 | [001] 14 5.4 × 7.3 * Ʇ [001] 8 2.8 × 3.3 ** |
| Large pores | ||
| AFI | AlPO-5 | [001] 12 7.3 × 7.3 * |
| *BEA | Beta | <100> 12 6.6 × 6.7 ** [001] 12 5.6 × 5.6 * |
| BOG | Boggsite | [100] 12 7.0 × 7.0 * [010] 10 5.5 × 5.8 * |
| CAN | Cancrinite | [001] 12 5.9 × 5.9 * |
| FAU | Faujasite | <111> 12 7.4 × 7.4 *** |
| GME | Gmelinite | [001] 12 7.0 × 7.0 * Ʇ [001] 8 3.6 × 3.9 ** |
| ISV | ITQ-7 | <100> 12 6.1 × 6.5 ** [001] 12 5.9 × 6.6 * |
| LTL | Linde Type L | [001] 12 7.1 × 7.1 * |
| MAZ | Mazzite | [001] 12 7.4 × 7.4 * | [001] 8 3.1 × 3.1 *** |
| MOR | Mordenite | [001] 12 6.5 × 7.0 * {[010] 8 3.4 × 4.8 * [001] 8 2.6 × 5.7} ** |
| MTW | ZSM-12 | [010] 12 5.6 × 6.0 * |
| OFF | Offretite | [001] 12 6.7 × 6.8 * Ʇ [001] 8 3.6 × 4.9 ** |
| Medium pores | ||
| DAC | Dachiardite | [010] 10 3.4 × 5.3 * [001] 8 3.7 × 4.8 * |
| EPI | Epistilbite | [100] 10 3.4 × 5.6 * [001] 8 3.7 × 4.5 * |
| FER | Ferrierite | [001] 10 4.2 × 5.4 * [010] 8 3.5 × 4.8 * |
| HEU | Heulandite | {[001] 10 3.1 × 7.5 * + 8 3.6 × 4.6 *} × [100] 8 2.8 × 4.7 * |
| MFI | ZSM-5 | {[100] 10 5.1 × 5.5 [010] 10 5.3 × 5.6} *** |
| STI | Stilbite | [100] 10 4.7 × 5.0 * [001] 8 2.7 × 5.6 * |
| TER | Terranovaite | [100] 10 5.0 × 5.0 * [001] 10 4.1 × 7.0 * |
| NAT | Natrolite | <100> 8 2.6 × 3.9 ** [001] 9 2.5 × 4.1 * |
| Small pores | ||
| ABW | Li-A | [001] 8 3.4 × 3.8 * |
| BRE | Bikitaite | [010] 8 2.8 × 3.7 * |
| CHA | Chabazite | Ʇ [001] 8 3.8 × 3.8 *** |
| LTA | Linde Type A | <100> 8 4.1 × 4.1 *** |
| YUG | Yugawaralite | [100] 8 2.8 × 3.6 * [001] 8 3.1 × 5.0 * |
| VNI | VPI-9 | {<110> 8 3.1 × 4.0 [001] 8 3.5 × 3.6} *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancinelli, M.; Martucci, A. Exploring the Potential of Zeolites for Sustainable Environmental Applications. Sustain. Chem. 2025, 6, 9. https://doi.org/10.3390/suschem6010009
Mancinelli M, Martucci A. Exploring the Potential of Zeolites for Sustainable Environmental Applications. Sustainable Chemistry. 2025; 6(1):9. https://doi.org/10.3390/suschem6010009
Chicago/Turabian StyleMancinelli, Maura, and Annalisa Martucci. 2025. "Exploring the Potential of Zeolites for Sustainable Environmental Applications" Sustainable Chemistry 6, no. 1: 9. https://doi.org/10.3390/suschem6010009
APA StyleMancinelli, M., & Martucci, A. (2025). Exploring the Potential of Zeolites for Sustainable Environmental Applications. Sustainable Chemistry, 6(1), 9. https://doi.org/10.3390/suschem6010009








