BiVO4-Based Systems Magnetron Sputtered with Silver Nanoparticles for the Artificial Photosynthesis Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Photocatalysts
2.2.2. Photocatalytic Reactions
3. Results and Discussion
3.1. Characterisation
3.2. Photocatalytic Activity Measurements
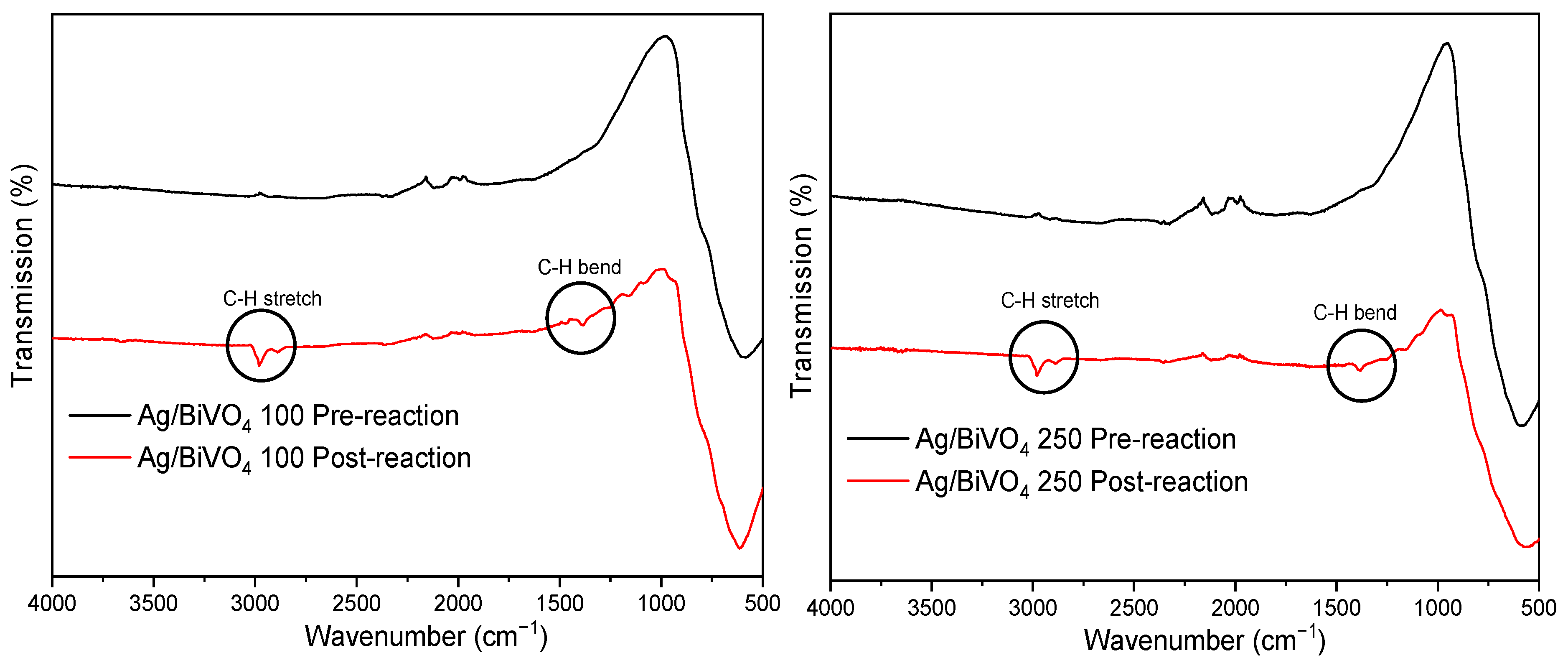
3.3. Post-Reaction Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, B.; Sun, L. Artificial photosynthesis: Opportunities and challenges of molecular catalysts. Chem. Soc. Rev. 2019, 48, 2216–2264. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, A.; Gornall, J.; Booth, B.; Dennis, E.; Falloon, P.; Kay, G.; McNeall, D.; McSweeney, C.; Betts, R. The importance of population, climate change and CO2 plant physiological forcing in determining future global water stress. Glob. Environ. Chang. 2013, 23, 1083–1097. [Google Scholar] [CrossRef]
- Kinney, P.L. Interactions of Climate Change, Air Pollution, and Human Health. Curr. Environ. Health Rep. 2018, 5, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Battin, T.J.; Luyssaert, S.; Kaplan, L.A.; Aufdenkampe, A.K.; Richter, A.; Tranvik, L.J. The boundless carbon cycle. Nat. Geosci. 2009, 2, 598–600. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Song, C. CO2 Conversion and Utilization: An Overview. In CO2 Conversion and Utilization; ACS: Washington, DC, USA, 2002. [Google Scholar]
- Kolobov, N.; Goesten, M.G.; Gascon, J. Metal–Organic Frameworks: Molecules or Semiconductors in Photocatalysis? Angew. Chem. 2021, 60, 26038–26052. [Google Scholar] [CrossRef]
- Yao, S.; He, J.; Gao, F.; Wang, H.; Lin, J.; Bai, Y.; Fang, J.; Zhu, F.; Huang, F.; Wang, M. Highly selective semiconductor photocatalysis for CO2 reduction. J. Mater. Chem. A Mater. Energy Sustain. 2023, 11, 12539–12558. [Google Scholar] [CrossRef]
- Mohan, H.; Vadivel, S.; Rajendran, S. Removal of harmful algae in natural water by semiconductor photocatalysis- A critical review. Chemosphere 2022, 302, 134827. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Wang, S.; Zhu, N.; Li, Z.; Zhao, L.; Wang, Y. Recent advances of semiconductor photocatalysis for water pollutant treatment: Mechanisms, materials and applications. Phys. Chem. Chem. Phys. 2023, 25, 25899–25924. [Google Scholar] [CrossRef]
- Wang, F.; Lu, Z.; Guo, H.; Zhang, G.; Li, Y.; Hu, Y.; Jiang, W.; Liu, G. Plasmonic Photocatalysis for CO2 Reduction: Advances, Understanding and Possibilities. Chem. Eur. J. 2023, 29, 202202716. [Google Scholar] [CrossRef]
- Kang, Q.; Ning, S.; Jiang, D.; Wang, Y.; Zhou, F. Semiconductor-based artificial photosynthesis for water-splitting and CO2 reduction. In Photosynthesis: From Plants to Nanomaterials; Academic Press: Cambridge, MA, USA, 2023; pp. 377–405. [Google Scholar]
- Coronado, J.M.; Fresno, F.; Hernández-Alonso, M.D.; Portela, R. The Role of Co-Catalysts: Interaction and Synergies with Semiconductors. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Green Energy and Technology; Springer: London, UK, 2013; Volume 71, pp. 195–216. [Google Scholar] [CrossRef]
- Wang, L.; Qi, G.; Liu, X. Ag/ɑ-Fe2O3 nanowire arrays enable effectively photoelectrocatalytic reduction of carbon dioxide to methanol. J. Power Sources 2021, 507, 230272. [Google Scholar] [CrossRef]
- Ueno, K.; Oshikiri, T.; Shi, X.; Zhong, Y.; Misawa, H. Plasmon-induced artificial photosynthesis. Interface Focus 2015, 5, 20140082. [Google Scholar] [CrossRef] [PubMed]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.-N.; Hwang, B.-J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Wang, F.; Jin, Z.; Xu, J. Cu2O nanoparticles decorated BiVO4 as an effective visible-light-driven p-n heterojunction photocatalyst for methylene blue degradation. Superlattices Microstruct. 2014, 74, 294–307. [Google Scholar] [CrossRef]
- Ding, K.; Chen, B.; Li, Y.; Zhang, Y.; Chen, Z. Comparative density functional theory study on the electronic and optical properties of BiMO4 (M = V, Nb, Ta). J. Mater. Chem. A Mater. Energy Sustain. 2014, 2, 8294–8303. [Google Scholar] [CrossRef]
- Loka, C.; Gelija, D.; Vattikuti, S.V.P.; Lee, K.-S. Silver-Boosted WO3/CuWO4 Heterojunction Thin Films for Enhanced Photoelectrochemical Water Splitting Efficiency. ACS Sustain. Chem. Eng. 2023, 11, 11978–11990. [Google Scholar] [CrossRef]
- Xu, X.; Du, M.; Chen, T.; Xiong, S.; Wu, T.; Zhao, D.; Fan, Z. New insights into Ag-doped BiVO4 microspheres as visible light photocatalysts. RSC Adv. 2016, 6, 98788–98796. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, B.X.; Wu, R.J.; Huang, C.L.; Chang, Y. Photoreduction of CO2 into CH4 Using Novel Composite of Triangular Silver Nanoplates on Graphene-BiVO4. Catalysts 2022, 12, 750. [Google Scholar] [CrossRef]
- Bakhtiarnia, S.; Sheibani, S.; Aubry, E.; Sun, H.; Briois, P.; Arab Pour Yazdi, M. One-step preparation of Ag-incorporated BiVO4 thin films: Plasmon-heterostructure effect in photocatalytic activity enhancement. Appl. Surf. Sci. 2022, 580, 152253. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.-S.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Wang, R. Preparation of Visible-Light-Driven Ag/BiVO4 Photocatalysts and Their Performance for Cr(VI) Reduction. ChemistrySelect 2022, 7, e202201348. [Google Scholar] [CrossRef]
- Sánchez, O.A.; Rodríguez, J.L.; Barrera-Andrade, J.M.; Borja-Urby, R.; Valenzuela, M.A. High performance of Ag/BiVO4 photocatalyst for 2,4-Dichlorophenoxyacetic acid degradation under visible light. Appl. Catal. A Gen. 2020, 600, 117625. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Vattikuti, S.V.P.; Manne, R.; Manjula, G.; Munirathnam, K.; Mallapur, S.; Marraiki, N.; Mohammed, A.; Reddy, L.V.; Rajesh, M.; et al. Sono-chemical synthesis of silver quantum dots immobilized on exfoliated graphitic carbon nitride nanostructures using ginseng extract for photocatalytic hydrogen evolution, dye degradation, and antimicrobial studies. Nanomaterials 2021, 11, 2918. [Google Scholar] [CrossRef] [PubMed]
- Naughton, E.; Sullivan, J.A. Influence of the presence of RuO2 on the reactivity of Fe2O3 in the artificial photosynthesis reaction. Sustain. Chem. Environ. 2024, 8, 100167. [Google Scholar] [CrossRef]
- Morais, E.; O’Modhrain, C.; Thampi, K.R.; Sullivan, J.A. RuO2/TiO2 photocatalysts prepared via a hydrothermal route: Influence of the presence of TiO2 on the reactivity of RuO2 in the artificial photosynthesis reaction. J. Catal. 2021, 401, 288–296. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, X.; Wei, C.; Chen, L. Ag-Bi/BiVO4 chain-like hollow microstructures with enhanced photocatalytic activity for CO2 conversion. App. Catal. A Gen. 2020, 594, 117459. [Google Scholar] [CrossRef]
- Xie, Y.-c.; Chen, J.H.; Lin, W.-Y.; Wu, R.-J.; Fegade, U.; Patil, N.; Ansar, S.; Pandey, S. Triangular silver nanoplates-BiVO4 composite for the photocatalytic CO2 reduction under irradiating LED light source. Opt. Mater. 2023, 143, 114141. [Google Scholar] [CrossRef]
- Huang, N.; Li, X.; Xing, T.; Sun, F.; Yang, M.; Yang, J.; Zhao, Y.; Wang, H. Ag Nanoparticle-Decorated BiVO4 Electrodeposited Film as Photoanode for Enhancing the Performance of a Photocatalytic H2O2 Fuel Cell. Energy Fuels 2024, 38, 19029–19037. [Google Scholar] [CrossRef]
- Bai, S.; Li, Q.; Han, N.; Zhang, K.; Tang, P.; Feng, Y.; Luo, R.; Li, D.; Chen, A. Synthesis of novel BiVO4/Cu2O heterojunctions for improving BiVO4 towards NO2 sensing properties. J. Colloid Interface Sci. 2020, 567, 37–44. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J.; Li, Y. Enhanced visible light photocatalytic activity of novel polymeric g-C3N4 loaded with Ag nanoparticles. Appl. Catal. A Gen. 2011, 409–410, 215–222. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, H.Y.; Ma, C.C.; Mao, F.; Xue, B. Ag/g-C3N4 photocatalysts: Microwave-assisted synthesis and enhanced visible-light photocatalytic activity. Catal. Commun. 2016, 79, 45–48. [Google Scholar] [CrossRef]
- Wan, J.; Liu, E.; Fan, J.; Hu, X.; Sun, L.; Tang, C.; Yin, Y.; Li, H.; Hu, Y. In-situ synthesis of plasmonic Ag/Ag3PO4 tetrahedron with exposed {111} facets for high visible-light photocatalytic activity and stability. Ceram. Int. 2015, 41, 6933–6940. [Google Scholar] [CrossRef]
- Booshehri, A.Y.; Chun-Kiat Goh, S.; Hong, J.; Jiang, R.; Xu, R. Effect of depositing silver nanoparticles on BiVO4 in enhancing visible light photocatalytic inactivation of bacteria in water. J. Mater. Chem. A Mater. Energy Sustain. 2014, 2, 6209–6217. [Google Scholar] [CrossRef]
- Huang, K.; Lv, Y.; Zhang, W.; Sun, S.; Yang, B.; Chi, F.; Ran, S.; Liu, X. One-step synthesis of Ag3PO4/Ag photocatalyst with visible-light photocatalytic activity. Mater. Res. 2015, 18, 939–945. [Google Scholar] [CrossRef]
- Singh, S.; Bharti, A.; Meena, V.K. Green synthesis of multi-shaped silver nanoparticles: Optical, morphological and antibacterial properties. J. Mater. Sci,. Mater. Electron. 2015, 26, 3638–3648. [Google Scholar] [CrossRef]
- Fang, W.; Lin, Y.; Xv, R.; Shang, X.; Fu, L. Band-gap and interface engineering by Ni doping and CoPi deposition of BiVO4 photoanode to boost photoelectrochemical water splitting. Electrochim. Acta 2023, 437, 141511. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, Y.; Ma, F.; Zhang, Z.; Wei, X.; Zhu, G. Structural stability, band structure and optical properties of different BiVO4 phases under pressure. J. Mater. Sci. 2016, 51, 6662–6673. [Google Scholar] [CrossRef]
- Zhong, K.; Gao, H.; Feng, J.; Zhang, Y.; Lai, K. Facile synthesis of Z-scheme Se/BiVO4 heterojunction with enhanced visible-light-driven photocatalytic performance. J. Mater. Sci. 2019, 54, 10632–10643. [Google Scholar] [CrossRef]
- Kelly, A.; Knowles, K.M. Crystallography and Crystal Defects, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lv, F.Z.; Zhao, W.; Zhong, Y.; Hu, C.H.; Zhou, H.Y.; Chen, R. Synthesis and Photocatalytic Activity of Ag-Doped BiVO4. Adv. Mater. Res. 2013, 734–737, 2204–2209. [Google Scholar] [CrossRef]
- Xu, B.; Zada, A.; Wang, G.; Qu, Y. Boosting the visible-light photoactivities of BiVO4 nanoplates by Eu doping and coupling CeOx nanoparticles for CO2 reduction and organic oxidation. Sustain. Energy Fuels 2019, 3, 3363–3369. [Google Scholar] [CrossRef]
- Barberio, M.; Barone, P.; Imbrogno, A.; Xu, F. CO2 adsorption on silver nanoparticle/carbon nanotube nanocomposites: A study of adsorption characteristics. Phys. Status Solidi (B) Basic Res. 2015, 252, 1955–1959. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, H.; Qian, J.; Su, H.; Lee, K.-J.; Cheng, T.; Xiao, H.; Yano, J.; Goddard, W.A.; Crumlin, E.J.; et al. Dramatic differences in carbon dioxide adsorption and initial steps of reduction between silver and copper. Nat. Commun. 2019, 10, 1875. [Google Scholar] [CrossRef] [PubMed]
- Czanderna, A.W. The interaction of carbon dioxide and ethane with silver. J. Colloid Interface Sci. 1966, 22, 482–490. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Z.; Wang, D.; Guo, T.; Hu, Y. In-situ growth of p-type Ag2O on n-type Bi2O2S with intimate interfacial contact for NIR light-driven photocatalytic CO2 reduction. Appl. Surf. Sci. 2022, 601, 154185. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yu, H.; Yu, J.; Liu, S. Ag2O as a New Visible-Light Photocatalyst: Self-Stability and High Photocatalytic Activity. Chem. Eur. J. 2011, 17, 7777–7780. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Gao, M.; Shen, J.; Pu, X.; Zhang, Z.; Lin, H.; Wang, X. BiVO4/Bi4Ti3O12 heterojunction enabling efficient photocatalytic reduction of CO2 with H2O to CH3OH and CO. Appl. Catal. B Environ. 2020, 270, 118876. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Hiragond, C.; Ali, S.; Sorcar, S.; In, S.-I. Hierarchical Nanostructured Photocatalysts for CO2 Photoreduction. Catalysts 2019, 9, 370. [Google Scholar] [CrossRef]
- Wu, H.; Kong, X.Y.; Wen, X.; Chai, S.P.; Lovell, E.C.; Tang, J.; Ng, Y.H. Metal–Organic Framework Decorated Cuprous Oxide Nanowires for Long-lived Charges Applied in Selective Photocatalytic CO2 Reduction to CH4. Angew. Chem. (Int. Ed.) 2021, 60, 8455–8459. [Google Scholar] [CrossRef]
- Yang, R.; Zhu, R.; Fan, Y.; Hu, L.; Chen, Q. In situ synthesis of C-doped BiVO4 with natural leaf as a template under different calcination temperatures. RSC Adv. 2019, 9, 14004–14010. [Google Scholar] [CrossRef]
- Regmi, C.; Dhakal, D.; Lee, S.W. Visible-light-induced Ag/BiVO4 semiconductor with enhanced photocatalytic and antibacterial performance. Nanotechnology 2018, 29, 064001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, X.; Qian, Y.; Yang, L.; Liao, H. Nanocrystalline Silver Particles: Synthesis, Agglomeration, and Sputtering Induced by Electron Beam. J. Colloid Interface Sci. 1999, 209, 347–349. [Google Scholar] [CrossRef] [PubMed]

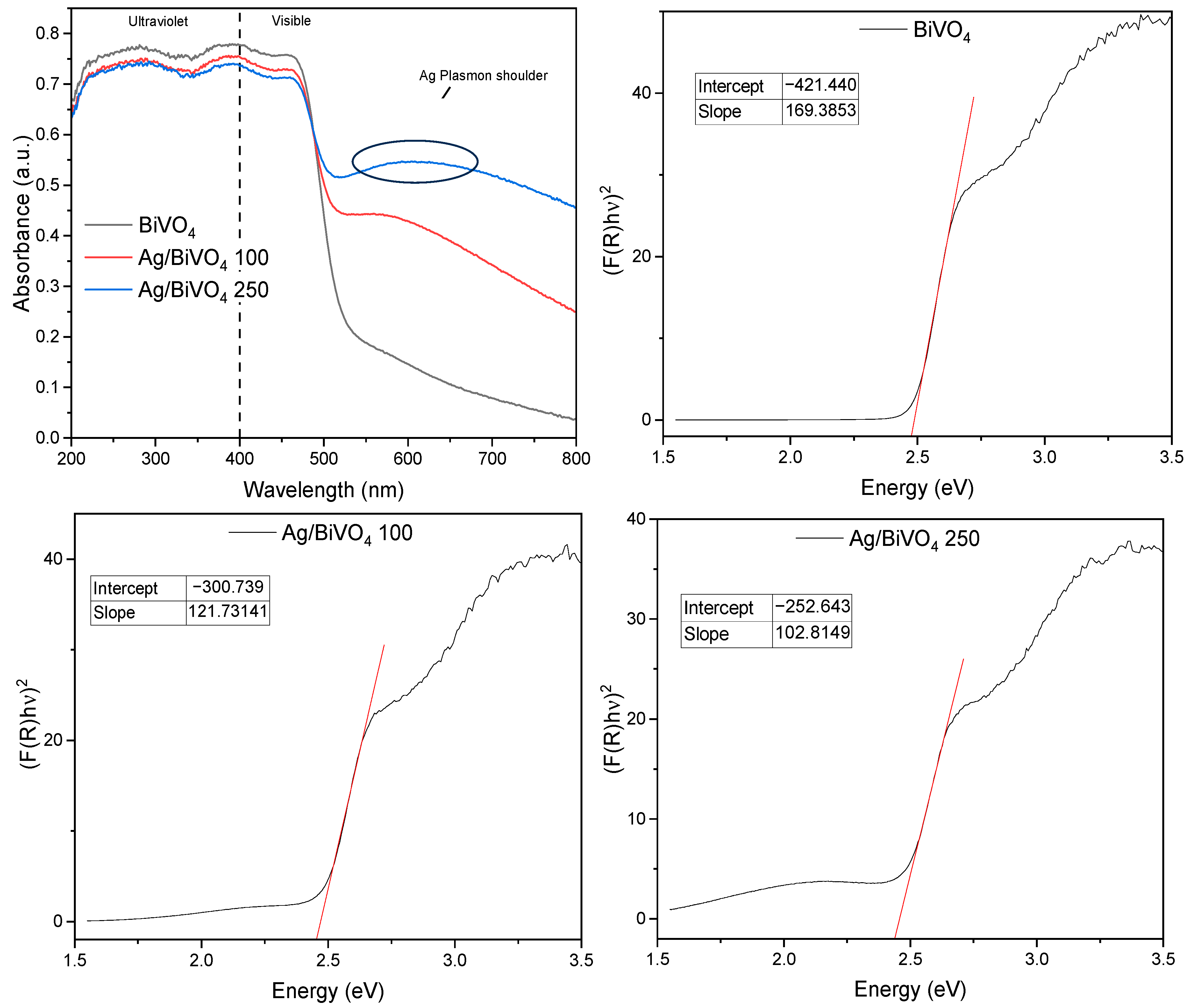
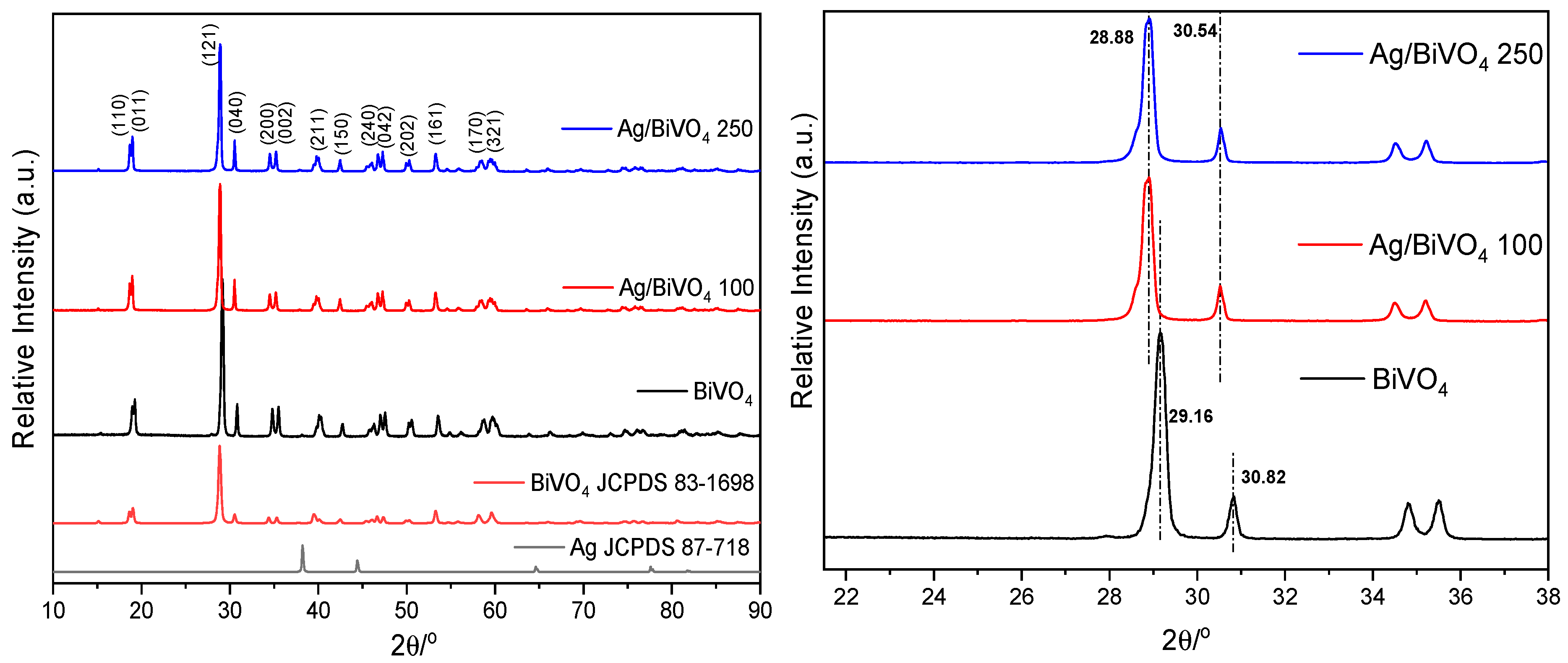

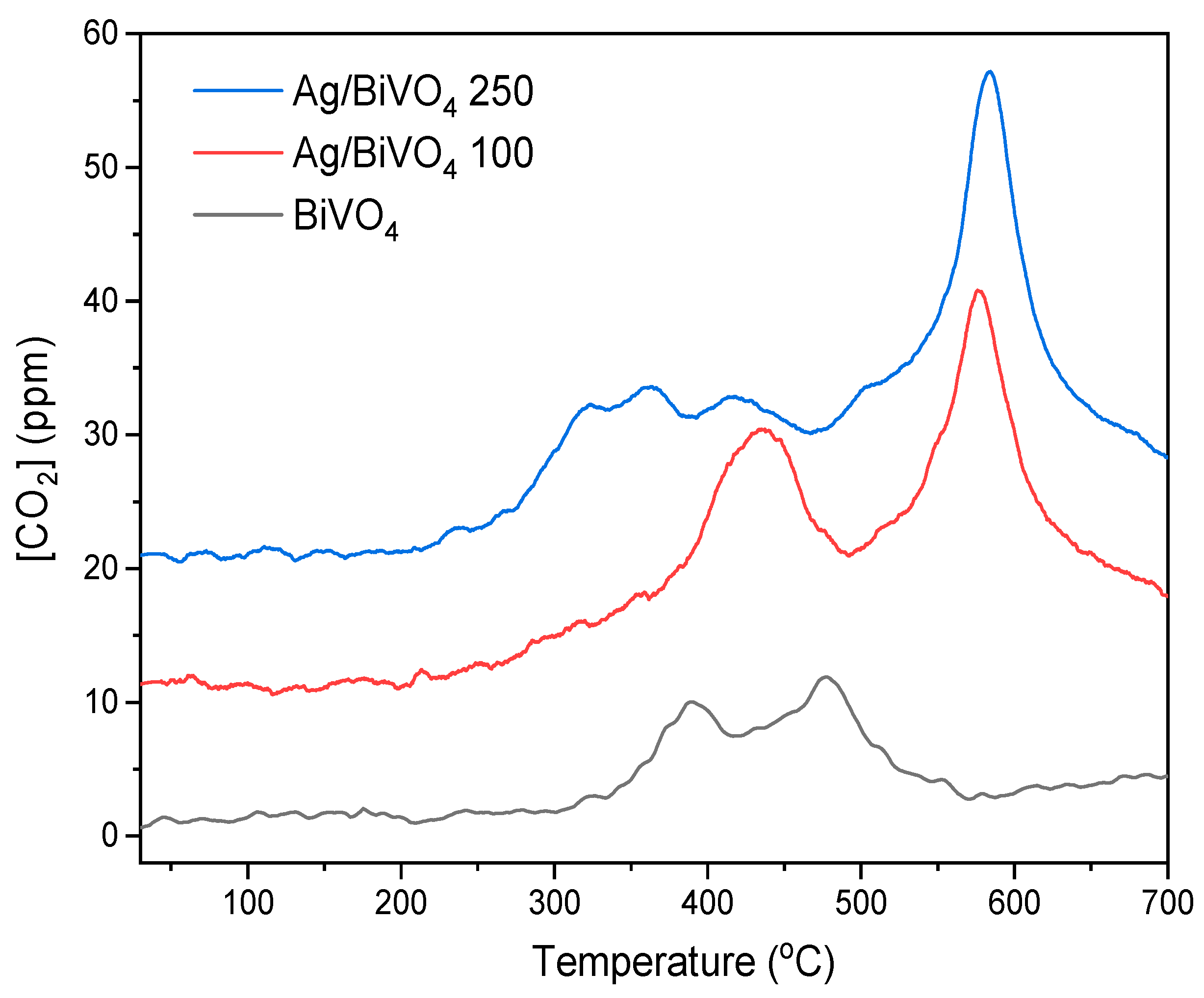
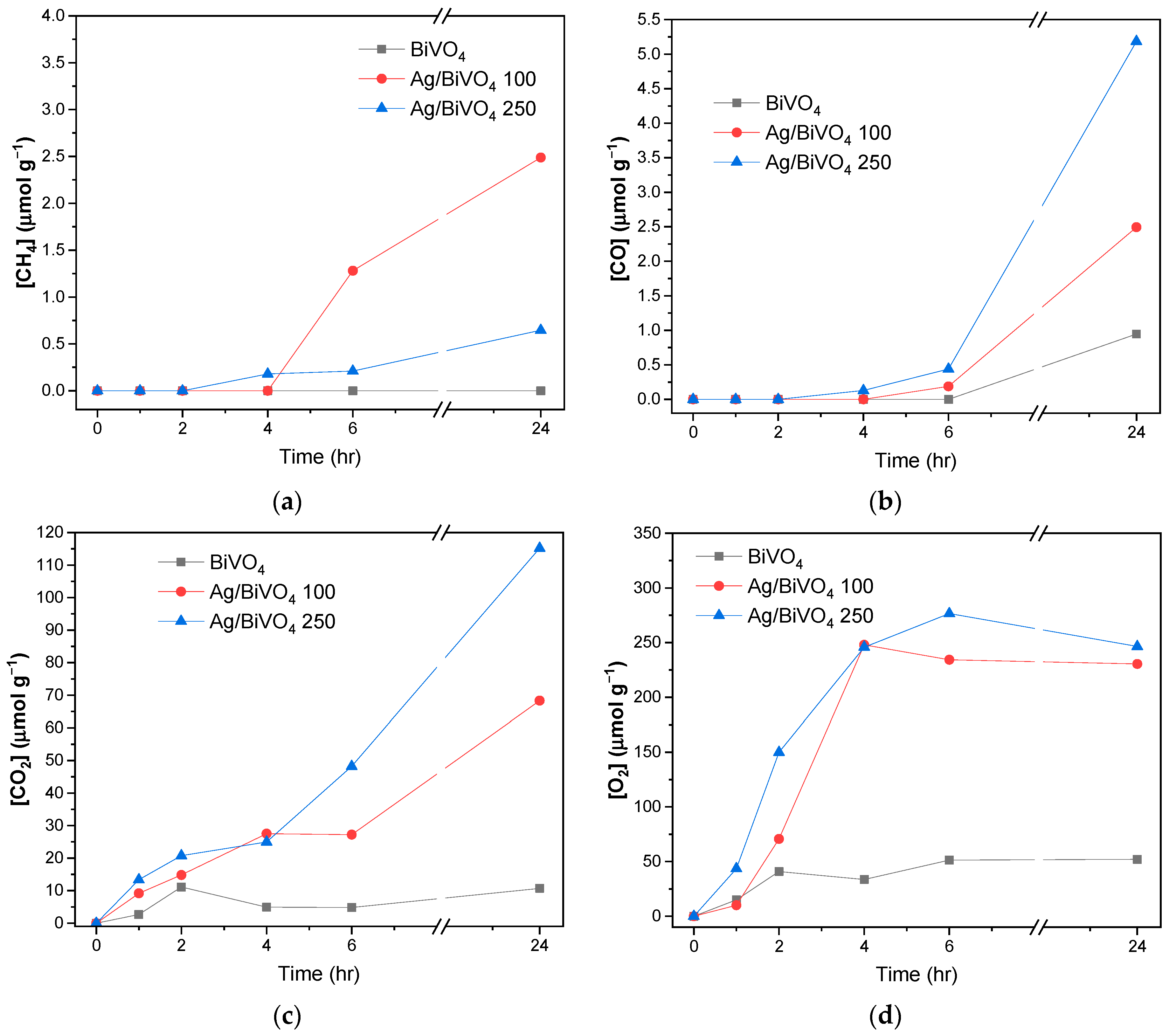

| Sample | Wt. % Ag | Colour |
|---|---|---|
| BiVO4 | 0 | Yellow |
| Ag/BiVO4 100 | 0.26 | Khaki |
| Ag/BiVO4 250 | 0.73 | Dark Green |
| Sample | Band Gap (eV) |
|---|---|
| BiVO4 | 2.49 |
| Ag/BiVO4 100 | 2.47 |
| Ag/BiVO4 250 | 2.46 |
| Sample | [CO2] (μmol/g) | [Ag] (moles/20 mg) |
|---|---|---|
| BiVO4 | 10 | 0 |
| Ag/BiVO4 100 | 32 | 4.8 × 10−7 |
| Ag/BiVO4 250 | 34 | 1.4 × 10−6 |
| Sample | Surface Area (m2/g) |
|---|---|
| BiVO4 | 1.46 |
| Ag/BiVO4 100 | 1.45 |
| Ag/BiVO4 250 | 1.45 |
| Sample | CO/μmol g−1 | CH4/μmol g−1 |
|---|---|---|
| BiVO4 | 0.95 | 0 |
| Ag/BiVO4 100 | 2.49 | 2.49 |
| Ag/BiVO4 250 | 5.19 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naughton, E.; Kohlrausch, E.C.; Alves Fernandes, J.; Sullivan, J.A. BiVO4-Based Systems Magnetron Sputtered with Silver Nanoparticles for the Artificial Photosynthesis Reaction. Sustain. Chem. 2025, 6, 4. https://doi.org/10.3390/suschem6010004
Naughton E, Kohlrausch EC, Alves Fernandes J, Sullivan JA. BiVO4-Based Systems Magnetron Sputtered with Silver Nanoparticles for the Artificial Photosynthesis Reaction. Sustainable Chemistry. 2025; 6(1):4. https://doi.org/10.3390/suschem6010004
Chicago/Turabian StyleNaughton, Eva, Emerson C. Kohlrausch, Jesum Alves Fernandes, and James A. Sullivan. 2025. "BiVO4-Based Systems Magnetron Sputtered with Silver Nanoparticles for the Artificial Photosynthesis Reaction" Sustainable Chemistry 6, no. 1: 4. https://doi.org/10.3390/suschem6010004
APA StyleNaughton, E., Kohlrausch, E. C., Alves Fernandes, J., & Sullivan, J. A. (2025). BiVO4-Based Systems Magnetron Sputtered with Silver Nanoparticles for the Artificial Photosynthesis Reaction. Sustainable Chemistry, 6(1), 4. https://doi.org/10.3390/suschem6010004








