Abstract
The plant-mediated synthesis of therapeutic metal nanoparticles is an intensively exploited field in the last decade. In particular, Salvia officinalis, considered one of these plants, was used in this work to synthesize silver particles. Here, we have used harmless substances to obtain silver particles and common characterization methods for quickly estimating sizes and shapes. Thus, UV–Visible spectroscopy helped us online-monitor and optimize the synthesis of silver particles and estimate the size of metallic particles in the stock solutions. The resulting eco-friendly synthesized silver particles were then separated and re-dispersed in water, to be analyzed by laser light scattering, transmission electron microscopy (TEM), and scanning electron microscopy (SEM) to prove their nanometric size and shape polydispersity. Furthermore, the role of citric acid in stabilizing colloidal solutions of silver nanoparticles was studied.
1. Introduction
Antimicrobial resistance is a major current issue for a wide range of researchers. This inconvenience appeared due to the excessive use of antimicrobials in daily life, especially in agriculture, zootechnics, and public health. They are currently struggling to eradicate pathogenic microorganisms like bacteria, fungi, viruses, parasites, and algae existing in environments using natural or synthetic chemicals. The irrational and sometimes abusive use of antimicrobial substances by less trained or profit-seeking people has periodically been counterbalanced by the adaptation and survival of the microorganisms even in the more hostile environments [1].
The used antimicrobial agents at this time with low molecular weight, like peracetic acid, hydrogen peroxide, sodium hypochlorite, chloramine, iodine, ethylene oxide, nitrogen, alcohol, phenols, aldehyde, chlorhexidine, and detergents, have a short duration of action and are considered environmentally toxic. Now, the research in the field is focused on new materials with enhanced antimicrobial properties, a prolonged time of action, and minimum toxicity toward the environment [2].
It is well known that the bacteria membrane is composed of phosphatidylethanolamine, phosphatidylglycerol, murein, cardiolipin, or lipopolysaccharides, while the fungus membrane is rich in sialic acid, glucan, chitin, or cellulose [3]. To fulfill their specific roles, the antimicrobials interact with the pathogens predominantly through electrostatic interactions. The van der Walls forces are the basis of the interactions between the charged chemical groups having an antimicrobial role and the oppositely charged chemical groups found on the surface structure of biological entities. Based on these specific secondary interactions, most antimicrobials can influence the growth and development of microorganisms. Related to their concentration, some substances record a bacteriostatic, bactericide, fungistatic, fungicide, virostatic, virucide, or sporicidal effect. The mechanism of action of these substances is controlled by the chemical structure of the attacked microorganism. After the attachment of substances to the cells, one of the following processes like cell membrane destruction, the inhibition of protein synthesis, or other metabolic pathways is very possible. Furthermore, the antimicrobial substance’s interference with deoxyribonucleic acid (ADN) or ribonucleic acid (ARN) synthesis could appear [4,5].
Chemically, the antimicrobials have been classified into a few large classes like quaternary ammonium groups bearing substances, molecules designed to mimic natural peptides, guanidine-type substances, halogenated molecules, and phosphonic and sulfonic derivatives [6,7]. In addition, most antimicrobial materials in recent years were designed based on inorganic particles like Ag, Au, Zn, Ce, and Fe or their oxides Ag2O, CuO, TiO2, ZnO, and MgO [8,9].
Due to their antimicrobial properties, the eco-friendly synthesized silver nanoparticles are considered a sustainable choice for designing food packaging and medical coatings. These silver particles with sizes in the nanometric or micrometric range are involved in designing antifungal, antibacterial, or anti-inflammatory materials. There are various methods for synthesizing therapeutic inorganic particles: physical, chemical, biological, or synergistic [10]. Usually, conventional synthesis methods of silver particles use expensive and hazardous substances [11]. In chemical synthesis, some authors used sodium salts of citric acid, sodium borohydride (NaBH4), or sodium hydroxide (NaOH) as reducing agents [12,13,14,15,16,17]. When using physical synthesis, other researchers refer to UV–Vis irradiation, thermal treatment, microwave irradiation, gamma irradiation, or ultrasonication [18,19,20,21,22,23]. Nowadays, it is imperative to find new ways to synthesize them by more economical, ecological, and safe methods [24]. In this regard, the research involving plant-mediated synthesis of therapeutic metallic particles has exploded in recent decades [25]. In particular, Salvia officinalis has started to be used as a source for reducing reagents [26,27,28,29,30,31,32].
Besides its use as a gastronomic spice or oil for cosmetic preparations, Salvia officinalis has a predestined name; the Latin “salvere” means to heal. The Greeks knew it as an efficient treatment for cognitive decline. Today, due to its antiseptic, astringent, and antioxidant properties, Salvia officinalis is used in various remedies for dysmenorrhea, diarrhea, tonsillitis, gingivitis, sore throat, asthma, digestive problems, depression, and protection against some types of cancer. Moreover, the infusions, decoctions, and extracts from this plant support bone health, lower blood sugar levels, improve the symptoms of menopause, and fight against skin aging. The nutritional value of this plant is due to the proteins, carbohydrates, minerals (Fe, Ca, Mg), and vitamins (K, B6) it contains. In the various structures of the plant, there are also chemical compounds with a reducing role toward the metal ions generated in a solution by the oxidation of an inorganic salt [33].
Considering the less toxic effects, but also the improvement of the biological properties of nanoparticles compared to silver ions, the number of studies investigating the biogenic synthesis of silver nanoparticles (AgNPs), using especially Salvia species, has increased. Siakavella I.K., together with their team, studied the influence of the origin and preparation conditions of some hydroglycolic extracts of cosmetic-quality Salvia officinalis on the properties of the synthesized silver nanoparticles [34]. Sreckovic N.Z. et al. used the roots and aerial parts of Salvia pratensis to prepare methanolic extracts and silver nanoparticles, respectively. They also tested the antimicrobial, antioxidant, hemolytic, and cytotoxic properties of AgNPs [35]. Besides its antimicrobial and antioxidant effects, the catalytic effect of AgNPs synthesized based on extracts of Salvia verticilata [36] and the antidiabetic activity of AgNPs synthesized using Salvia blepharophylla and Salvia greggii [37] were monitored as well. Laime-Oviedo L.A. et al. optimized the synthesis of AgNPs conjugated with the ethanolic fraction of a species of Salvia, namely Lepechinia meyenii (Walp.), using the response surface methodology and Plackett–Burman design [38]. Other works show the effect of green-synthesized silver nanoparticles, based on Salvia officinalis leaves, on the germinated plants of maize, compared with the silver ions [39].
The biosynthesis mechanism of silver nanoparticles has been proposed by the work of Balciunaitiene A. [32]. Here, successive phases are considered: The first is the reduction by plant metabolites and nucleation of the silver ions in the activation phase, followed by the second, consisting of the formation of larger particles from adjacent small silver particles in the growth phase. Next, the particle shape finishes in the termination phase, and the last one is the stabilization of resulting AgNPs by capping molecules from the plant extract. The size and shape of nanoparticles depend on the time, temperature, and pH as well as on reactant concentrations. These physical and chemical parameters are the main variables in the optimization experiments of the biosynthesis of nanoparticles [35,38].
The stabilizing or capping agents are essential in the preparation of nanoparticles because they prevent the clustering or aggregation of these, influencing the uncontrolled growth in the size of nanoparticles, and improving their colloidal stability. Thus, by controlling the size and morphology, the capping agents will enhance the biocompatibility of nanoparticles with different biomolecules and bioavailability in the target living cells and reduce their toxicity to the environment. It is known that the nanoparticles suitable for cellular uptake should have sizes below 100 nm and a polydispersity index less than 0.3 [40].
Researchers often need to corroborate information about particle size obtained from different methods; results show that sizes obtained from TEM are usually smaller than the values measured by DLS. It is considered that these mismatches derive from the physical principle of each method, the assumptions based on which the dimensions are calculated, or the sample preparation. TEM is a number-based technique that analyses the projected surface area resulting from the interaction of the electrons with a dry sample. DLS is an intensity-based technique that measures the hydrodynamic radius of particles dispersed in a solution, accepting a sphere shape equivalent of these. However, the data provided by DLS on mean size, standard deviation, size distribution, and polydispersity index are more plentiful than TEM data because the number of analyzed particles differs [41]. Also, the range of DLS measurements is limited to particles smaller than 10 μm because the larger ones are quite heavy to be involved in Brownian motion [32].
This study presents an eco-friendly approach to AgNPs synthesis and reveals the importance of handy characterization methods for quickly estimating their sizes and shapes. In addition, the possibility of citric acid playing the role of a capping agent for silver nanoparticles was evaluated.
2. Materials and Methods
2.1. Materials for Synthesis and Separation of Silver Nanoparticles
We used Salvia officinalis leaves as the vegetal material, silver nitrate from S.C. Chimopar Trading S.R.L. (Bucharest, Romania), citric acid from Sigma-Aldrich (Bucharest, Romania), ethanol from Merck (Darmstadt, Germany) as received, and Millipore (Burlington, MA, USA) purified water for preparing solutions.
To obtain the silver particles, the reaction times between silver nitrate and the vegetal extract were varied, and UV–Vis spectra were recorded. After 24 h reaction time, the resulting silver particles were separated by centrifugation at 10,000 rpm for 30 min. To remove the residues from the vegetal extract, the resulting nanoparticles were dispersed in water and were extra centrifuged. Next, after the purification step, the resulting AgNPs (sediment) were left in vials without a lid at room temperature for 24 h, then dried in an oven at 60 °C, and finally stored in a refrigerator. The dried and bulk AgNPs were used for SEM-EDX analysis, but for laser light scattering and TEM analysis, these were dispersed again in water.
2.2. UV–Vis Spectroscopy
The characterization of AgNPs included the UV–Vis spectroscopy for surface plasmon resonance (SPR) analysis in solution. The surface plasmons created at the separation limit of some metal particles under the action of an external electric field are quantized oscillations of surface charge. There are several resonance modes for surface plasmons on silver nanoparticles, the most important being the Mie mode equal to the unit. The Mie resonance mode of silver nanoparticles has an optically active dipole field configuration, and this dipole resonance occurs in the UV–Vis region [42].
The UV–Vis absorption spectra were recorded at different reaction times with a SPECORD 200 Analytik Jena spectrophotometer, equipped with 1 × 1 cm2 quartz cuvettes, by using the same solvent mixture as reference (1:1 volumetric ratio of water/ethanol). In the range of the 350 nm and 1000 nm wavelengths of the UV–Vis spectra, an absorption maximum characteristic of synthesized AgNPs was monitored around 430–450 nm. We chose to start recording UV–Vis spectra from 350 nm because the signals of the Ag+ ions from the pure AgNO3 solution (around 220 nm) and polyphenols (around 280 nm) from the vegetal extract were not of interest for our study [43].
2.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray (EDX) Spectroscopy
The sample’s particle morphology and elemental composition were analyzed with a Verios G4 UC Scanning electron microscope (Thermo Scientific, Brno, Czech Republic) equipped with an energy-dispersive X-ray spectroscopy analyzer (Octane Elect Super SDD detector, Pleasanton, CA, USA). For SEM investigation, the samples were fixed on aluminum stubs with double-adhesive carbon tape and coated with 6 nm platinum using a Leica EM ACE200 Sputter coater (Vienna, Austria) to provide electrical conductivity and to prevent charge buildup during exposure to the electron beam. The morphological study was carried out with an ETD detector (Everhart–Thornley detector) using an acceleration voltage of 30 kV and a beam current of 0.1 nA. For EDX analysis, the samples were analyzed using an acceleration voltage of 20 kV and a beam current of 6.4 nA.
2.4. Transmission Electron Microscopy (TEM)
TEM evaluated the size and morphology of AgNPs resulting from the green synthesis. The TEM equipment used for analysis is a microscope manufactured by the Hitachi Company (Minato-ku, Tokyo, Japan), HT7700 series, working at a maximum acceleration voltage of 120 kV. The special construction of the microscope can provide images in “high-contrast mode” or “high-resolution mode” without requiring additional adjustments, and the visual inspection of the sample is performed in daylight directly on the display. The samples were deposited from the liquid phase on Ted Pella 300 mesh copper grids covered with carbon film. They were subsequently subjected to drying under specific conditions corresponding to the analyzed material to avoid disturbing their morphology or appearance as much as possible.
2.5. Light Scattering
Static and dynamic light scattering are currently used techniques to characterize the particles in the solution. The same phenomenon is involved in both methods, but the difference lies in the data collection and processing mode. The various experimental variants allow the recording of the scattered light at one or more scattering angles, and consequently using one or more detectors [44,45].
To obtain the most accurate results, it is strictly necessary to control some experimental conditions: calibration of the light scattering device with an appropriate solvent (e.g., toluene, decalin, or benzene) depending on the group of chemical substances analyzed. Another indication is the normalization of photodiode detectors with a solution of an isotropic material (ex. dextran, poly(styrene), or bovine serum albumin). The filtration of samples and solvents, degassing of working solutions, avoiding dust particles from the laboratory and work glassware, and the electrostatic counteraction of the aggregates in the solution are also imposed [46].
2.5.1. Static Light Scattering (SLS) at Multiple Angles θ
The laser light scattering involves recording under various scattering angles of the intensity of the light which comes from a laser and crosses a usually homogeneous solution. In current practice, the experimental device could include a HeNe laser, GaAs laser, or Ar3+ laser operating at the incident wavelength λ0 = 632.8 nm, 658 nm, or 514.5 nm, respectively. All dispersed molecules from solutions are considered entities able to produce the scattering of light [47].
The incident and scattered beams have the same wavelength in the classical light scattering phenomenon (elastic, Rayleigh, or static light scattering). Also, the fluctuations of the light intensities are averaged on a large time scale compared with the temporal scale of intensity fluctuations [44]. If the scattering entities have dimensions larger than the twentieth part of the value of the incident wavelength, the recommended equation used to describe the light scattering is the basic Zimm equation (Equations (1) and (2)). This equation, which connects the intensity of the scattered light and the properties of the molecules in a solution, was suggested by Bruno Hasbrouck Zimm in 1949, and represents the most widely used formalism for evaluating light scattering data [48].
The Zimm equation was applied here to extract the medium value of the radius of gyration (Rg) for particles existing in dilute solutions:
with
where K is the optical constant for the incident vertical polarized light, n is the refractive index of the medium, λ0 is the wavelength of the incident light, N is the Avogadro number, dn/dc is the refractive index increment, A2 is the second virial coefficient, Rθ is the Rayleigh ratio, θ is the scattering angle, MW is the weight-average molecular weight, and c represents the solution concentration [49].
(K × c)/Rθ = 1/MW + 2A2 × c + (16 π2 Rg/3λ0 MW) × sin2 (θ/2) + …
K = 2π2n2/λ04N × (dn/dc)2 × (1 + cos2θ)
The multiangle laser light scattering (MALLS) measurements were realized with a DAWN-DSP laser photometer (Wyatt Technology Corporation, Santa Barbara, CA, USA) consisting of a HeNe laser and a group of eighteen photodiodes arranged around the cell that contains the investigated solution. Generally, the detector photodiodes convert the light signals into electrical signals that will be processed with the Zimm, Debye, or Berry formalisms.
2.5.2. Dynamic Light Scattering (DLS) at θ = 173°
In dynamic light scattering experiments (known as quasi-elastic light scattering QELS or photon correlation spectroscopy PCS), the time dependence of the scattered light is provided by the diffusing centers in a solution and measured over a very narrow time range (from milliseconds to microseconds). The intensity fluctuations of the scattered light are related to the particle’s diffusion rate in solution, which performs a continuous Brownian motion. These data are usually presented in terms of diffusion coefficients and processed to obtain the size of the particles (radius or diameter). The linking between the particle size and diffusion results from Einstein’s theoretical model of spherical particles in Brownian motion, later becoming the Einstein–Stokes equation (Equation (3)):
where Rh is the Stokes radius derived from the hydrodynamic diameter of particle Dh, kB is Boltzmann constant, T represents the absolute temperature, η0 the viscosity of the solution, and D the translational diffusion coefficient [50].
Rh = kBT/6πη0D
The diffusion coefficients of the particles were measured with a Zetasizer Nano ZS instrument (Malvern Instruments, Malvern, UK) working in backscattering mode at θ = 173°. The resulting diffusion coefficients are converted into the hydrodynamic radius of particles having a shape/conformation equivalent to a sphere. If multiple species are present in the solution, a distribution of the diffusion coefficients is recorded resulting in a particle size distribution.
2.6. Colloidal Stability
In the case of colloidal dispersions, the zeta potential is the electrical potential recorded at the interface between particles and the surrounding fluid. This parameter is specific to a charged surface and predicts the long-term stability of a colloidal dispersion [51,52]. The zeta potential (value in mV) was recorded with the same Zetasizer Nano ZS instrument.
3. Results and Discussion
3.1. Synthesis of Silver Nanoparticles
Salvia officinalis leaves were collected in May from the N-E region of Romania, dried at room temperature, and then crushed with an electric grinder. A total of 50 mL of a mixture of ethanol–water (1:1 volume ratio) was added to 5 g of dried vegetal material. The preparation was left overnight and then boiled for 30 min to extract the main vegetable compounds. Next, the resulting solution was cooled, filtered through a 45 μm mesh, and stored in the refrigerator for future experimental steps. The plant extract thus obtained was dropped in a fresh 0.1 M solution of silver nitrate, prepared in the same mixture of solvents up to reaching a 1:1 volume ratio.
The literature has recorded the change of solution color as the first empirical sign of the silver particle formation due to the surface plasmon resonance (SPR) phenomenon [42,43]. In this work, mixing the vegetal extract (caramel brown color) with the colorless solution of silver salt precursor resulted in a greenish-gray color.
Plant-mediated synthesis involves the reaction between phytochemicals (reduction agents), like terpenoids, flavonoids, anthocyanins, or polyphenols, and silver ions in its +1 oxidation state from AgNO3 (substrate), resulting in the metallic particles of silver Ag0. Phenolic acids, flavanols, and flavonoids are predominantly found in leaves, while the terpenes and terpenoids predominate in seeds, as the literature reports [24].
3.2. UV–Vis Spectroscopic Analysis
UV–Vis spectroscopy is known as a useful method for the optimization of nanoparticle synthesis. The vegetal filtrate was used as a reducing and stabilizer agent to obtain silver nanoparticles. Different fractions between the vegetal extract and AgNO3 were tested in the laboratory. Finally, an optimal ratio of 1:1 volume ratio was chosen to obtain silver particles in as large a number as possible.
The reducing reaction of the silver ions from the solution to Ag particles, catalyzed by phytochemicals, was monitored by UV–Vis analysis depending on the reaction time (Figure 1). Thus, an absorption maximum, specific to silver, was observed in the range 430–450 nm for the hydro-alcoholic mixture of plant decoct with AgNO3 solution. As the reaction time increased, the UV–Vis absorption peak increased, meaning that more AgNPs per unit were formed. Despite the increased yield of AgNPs, it is observed that a prolonged reaction time induces their agglomeration and instability [53]. As a result, an increase in intensity and a broadening of the absorption peak is sometimes accompanied by a red-shifting in the UV–Vis spectra [32].
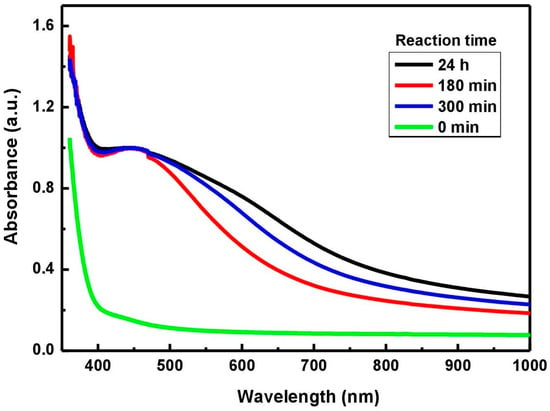
Figure 1.
Normalized UV–Vis spectra used to optimize the synthesis of Ag particles.
The silver particle sizes resulting from the mixture reacted for 24 h were estimated based on the full width at half maximum of the absorption band (FWHM) and Equation (4) [54]:
where ε0 = 4.9 is the frequency-independent part from the complex form of the silver particle’s dielectric constant, n = 1.345 is the refractive index of the medium, c = 3 × 108 is the light velocity (m/s), m = 9.1 × 10−31 is the electron mass (Kg), uF = 1.4 × 106 represents the electron velocity at Fermi energy (m/s); Nc = 5.86 × 1028 (for Ag) is the number of electrons per volume unit (electrons/m3), e = 1.6 × 10−19 is the electron charge (C), and w = FWHM.
R = [(ε0 + 2n2) × c × m × uF]/(2Nc × e2 × w)
According to the UV–Vis results (absorption maximum of 445 nm, absorbance of 0.856 at λmax/2, and an FWHM of 75 nm), the particle radius computed value (R) was 14.47 nm. Consequently, the estimated medium size of silver particles resulting in solution after 24 h of reaction was about 30 nm.
3.3. SEM and EDX Analysis
SEM analysis provides us with information about the structural morphology of nanoparticle surfaces, while EDX analysis was used to extract specific information about the element’s abundance in NPs. In our study, the SEM micrograph highlighted the silver nanoparticles embedded in a matrix of organic debris (Figure 2).

Figure 2.
SEM image of silver nanoparticles.
The elemental composition and its homogeneity distribution in the sample were proved by the EDX spectra recorded from three different areas (Figure 3).
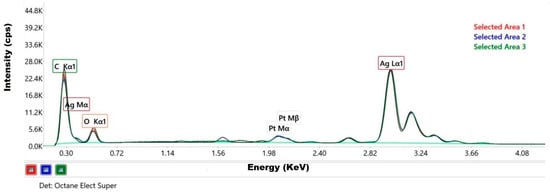
Figure 3.
EDX spectra of silver particle-containing sample (spectra overlay originating from 3 selected areas).
The qualitative analysis of these spectra revealed the presence of the following chemical elements in the sample: silver, carbon, and oxygen (Table 1).

Table 1.
Elemental composition of the silver particle-containing sample (data in triplicate).
The characteristic peaks of silver (L-shell transition energy with intense Ag(L) peak at 2.984 keV) confirmed that the nanoparticles were mainly composed of silver. The other distinct peaks recorded typically represented the absorption of carbon (K-shell transition energy with C(K) peak at 0.277 keV) and oxygen (K-shell transition energy with O(K) peak at 0.525 keV). These peaks are partially derived from the residual organic material of the plant extract used in the synthesis process of AgNPs and, to a lesser extent, from the ambient oxygen attached to the specimen or sample support [43,55]. This organic residue seems to have acted as a capping agent for the nanoparticles resulting in a green synthesis. Sreckovic N.Z. et al. worked with extracts from Salvia pratensis L. to obtain the synthesis of AgNPs and suggested that the primarily phenolic compounds were bound to the surface of the NPs [35]. We can also observe the presence of platinum (Pt(M) peak at 2.048 keV), which comes from the coverage of the SEM grid required for the examination.
3.4. TEM Analysis
The shape polydispersity is representative of nanoparticles originating from a plant-mediated synthesis. The AgNPs synthesized by Balciunaitiene A. et al. from Eucalyptus globulus and Salvia officinalis extracts were nearly spherical, and some had hexagonal or triangular shapes [32]. Also, Tanase C. et al. reported spherical and polygonal shapes for the resulting AgNPs using spruce bark extract [56]. Here, TEM images revealed small aggregates of silver nanoparticles embedded in organic matter. The inorganic particles have an average size of 50 nm and insufficiently defined morphologies like quasi-spheres, quasi-pyramids, or polygons (Figure 4).
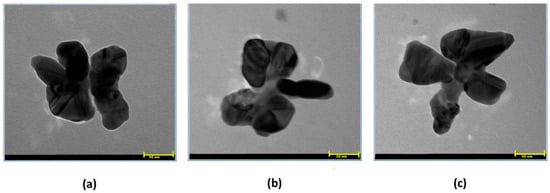
Figure 4.
(a–c) TEM micrographs of silver nanoparticles (scale of 50 nm).
In the case of our samples, the individual AgNPs covered by traces of organic material began to self-organize in the most favorable energetic form, predominantly in flower-type structures that seem partially overlapped.
3.5. SLS Analysis
The literature records various methods of representing laser light scattering experimental data based on the Zimm, Debye, Berry, Guinier, or Kratky formalisms (Equations (5)–(9), respectively):
K × c/Rθ = f (sin2 (θ/2) + a × c)
Rθ/K × c = f (sin2 (θ/2) + a × c)
(K × c/Rθ)0.5 = f (sin2 (θ/2) + a × c)
Rθ = f (sin2 (θ/2) + a × c)
sin2 (θ/2) Rθ = f (sin (θ/2) + a × c)
If the Zimm method fails, meaning that the plot does not have an appropriate form that allows the extraction of the interest quantities, it is recommended to use other formalisms in combination with the exclusion of data related to large θ scattering angles [49].
Because, in our experiments, a series of uncertainties regarding the value of the parameters appeared, we consider that the Debye plot is appropriate for the optimal data analysis of the light scattering signals. The values of Rg for the samples represented mediated values specific to all single or self-associated particles located in the path of the laser beam. Thus, for silver nanoparticles re-dispersed in water, the experimental value of the radius of gyration was 39 nm.
3.6. DLS Analysis
The Z-average particle diameter and size distribution (polydispersity index, PDI) of the obtained silver particles were measured by DLS. The size was measured three times with six runs of 4 min for each (1 min for equilibrium and 3 min for measurement) at 25 °C. The refractive indices, RI, for the dispersant medium (water) and the dispersant material (silver) were set to 0.133 and 0.135, respectively. Thus, a mean value of about 64 nm for the hydrodynamic radius was recorded for silver nanoparticles.
The size distribution of the particles was quasi-trimodal according to Figure 5a. The largest peak was assigned to the individual silver nanoparticles existing in large numbers in the solution, while the “tail” observed in the signal was due to the aggregates formed in small numbers between the silver nanoparticles. In addition, the polydispersity index of 0.43 obtained in this study suggested that the silver nanoparticles were quite monodispersed [57].
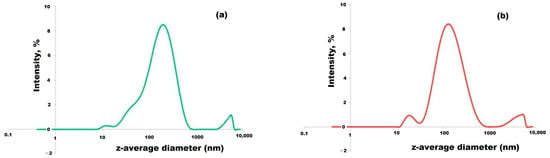
Figure 5.
Size distributions of silver particles before (a) and after (b) addition of sodium citrate.
In the literature, the semi-empirical significance of the aspect ratio (Rg/Rh) was the following: the value of 1.6 was consistent with an extended rod-like shape in solution; the value of 1.3 was characteristic of a random coil structure, while the value of 0.77 was specific to monodisperse compact spheres like globular proteins [58]. Based on the experimental values of Rg (39 nm) and Rh (64 nm), the estimated form factor for our plant-mediated synthesized silver nanoparticles was 0.6. Thus, we can suppose that the subunit value is characteristic of the monodisperse spherical-type particles. In reality, most particles are not perfectly spherical; consequently, their apparent hydrodynamic size could significantly differ from their true physical size. In their attempt to estimate the 3D volumes of the irregular, non-spherical “50 nm” silver nanoparticles using the electron tomography technique, Little C. A. et al. found that AgNPs can have volumes over 45% less than that of the “theoretical” circumscribed sphere [59].
3.7. Effect of Citric Acid on Silver Particles Obtained by Biogenic Synthesis
Citric acid is an important natural compound and exists as an industrial chemical (E331), in which the following three forms of the sodium salts of citric acid are reunited: monosodium citrate, disodium citrate, and trisodium citrate. After the plant-mediated synthesis of the silver nanoparticles, the impact of citric acid added to the colloidal solution of the silver nanoparticles was studied. In this regard, aqueous solutions with the same concentration as the nanoparticle solutions, and mixed by stirring in equal volumetric ratio, were prepared.
The capping or stabilizing agents prevent the over-growth of nanoparticles and inhibit their aggregation in the colloidal system. Thus, later, they are uptaken by the living cells, integrated into their metabolism, and efficiently removed from the environment. The capping agents should be non-toxic, biocompatible, biodegradable, and biosoluble and show a synergistic action with the nanoparticles to enhance their specific biological role. Over time, the researchers have tested the stability of nanoparticle colloidal solutions in the presence of varying capping agents: cetyltrimethylammonium bromide, sodium dodecyl sulfate, poly(vinylpyrrolidone), poly(ethylene glycol), poly(vinyl alcohol), chitosan, alginate, starch, sodium carboxy methyl cellulose, bovine serum albumin, ethylenediaminetetraacetic acid, decanethiol, thioglycerol, and plant extracts [60,61,62,63,64]. The system made up of nanoparticles covalently linked with the capping ligands can be considered a stabilized nanocomposite due to steric hindrances [61].
Trisodium citrate dihydrate was used by dos Santos Corrêa A. et al. as a reducing agent of gold chloride solution in the chemical synthesis of gold nanoparticles [65]. Dehydrated trisodium citrate was used by Oprica L. et al. as a reducing agent of silver ions from AgNO3 and a capping agent for the resulting AgNPs [63]. In our case, too, it seems that citric acid acted as a capping agent and attached to the surface of the silver particles re-dispersed in the colloidal solution. Also, this fact emerges from the narrowing of the size distribution of the particles analyzed by dynamic light scattering. In addition, a trimodal distribution of particle size was recorded in our sample. It was observed that there was a clearer definition of the three peaks in Figure 5b after the addition of citrate. Analyzing each range of particle sizes, the citrate managed to reduce the polydispersity of the particles. However, there is a small probability of citrate playing the role of a reducing agent because there was no more AgNO3 (precursor salt) but only silver metallic particles in a neutral state.
Unlike other methods that require standard molecules and rely on questionable assumptions, DLS offers direct and accurate measurements of the hydrodynamic size. Along with the zeta potential measurements, DLS is a valuable method for comparing the stability of different formulations because the particle size and surface charge depend on their micro-environment. In our study, through variation of the particle characteristics parameters, it is possible to monitor a good substance stabilization of nanoparticles effect in solution. In the literature, the low PDI values (below 0.7) were correlated with a narrow and uniform distribution of the particle sizes [66], and zeta potential values in the range of ±10÷20 mV predicted the relative stability of the colloidal system [67].
Our findings reveal that after the citric acid addition to the silver nanoparticle dispersion, parameters like the size, polydispersity index, and zeta potential were slightly modified, as Table 2 shows. In agreement with other results, the PDI was about 0.4 and the zeta potential was about −19 mV, meaning that the citric acid has a small influence on the colloidal stability of these nanoparticles. It seems that the citric acid capped the silver particles and acted just as a dispersant for them.

Table 2.
Solution properties of synthesized silver particles before and after addition of citric acid (CI) (mean values and standard deviations resulting from measurements in triplicate).
4. Conclusions
From this work, firstly, we conclude that the vegetal extract, water, and ethanol could be the main eco-friendly materials recommended for obtaining therapeutic silver particles. Along with this, UV–Vis spectroscopy represented a useful method for the size optimization of the synthesized particles. After a 24 h reaction time, silver particles with a mean size of 30 nm in solution were obtained. Furthermore, we confirmed the nanometric range of eco-friendly synthesized silver particles by using static and dynamic laser light scattering methods and TEM images. The EDX spectroscopy proved the presence of Ag in the analyzed samples. Citric acid added to the colloidal suspensions of the AgNPs plays the role of a capping agent but not a stabilizing agent of these particles.
Author Contributions
Conceptualization, A.G.G.; methodology, A.G.G. and V.C.G.; validation, A.G.G. and V.C.G.; formal analysis, A.G.G. and V.C.G.; investigation, A.G.G. and V.C.G.; resources, A.G.G. and V.C.G.; data curation, A.G.G. and V.C.G.; writing—original draft preparation, A.G.G.; writing—review and editing, A.G.G. and V.C.G.; visualization, A.G.G. and V.C.G.; supervision, A.G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in article, and further inquiries are available on request from the corresponding authors.
Acknowledgments
The authors appreciate the technical-scientific support of Irina Popescu, Irina Mihaela Pelin, Florica Doroftei, and Liviu Sacarescu.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lode, H.M. Clinical impact of antibiotic-resistant Gram-positive pathogens. Clin. Microbiol. Infect. 2009, 15, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Bonilla, A.; Fernandes-Garcia, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Hungo, W.B.; Longworth, A.R. Some aspects of the mode of action of chlorhexidine. J. Pharm. Pharmacol. 1964, 16, 655–662. [Google Scholar]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef] [PubMed]
- Kurtjak, M.; Aničić, N.; Vukomanovicć, M. Inorganic nanoparticles: Innovative tools for antimicrobial agents. In Antibacterial Agents; Kumavath, R.N., Ed.; IntechOpen Ltd.: London, UK, 2017; pp. 39–60. [Google Scholar]
- Zhang, X.F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534–1568. [Google Scholar] [CrossRef]
- Vega-Baudrit, J.; Gamboa, S.M.; Rojas, E.R.; Martínez, V.V. Synthesis and characterization of silver nanoparticles and their application as an antibacterial agent. Int. J. Biosens. Bioelectron. 2019, 5, 166–173. [Google Scholar] [CrossRef]
- Gils, P.S.; Ray, D.; Sahoo, P.K. Designing of silver nanoparticles in gum Arabic based semi-ipn hydrogel. Int. J. Biol. Macromol. 2010, 46, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Sabio, L.; Sosa, A.; Delgado-López, J.M.; Dominguez-Vera, J.M. Two-sided antibacterial cellulose combining probiotics and silver nanoparticles. Molecules 2021, 26, 2848. [Google Scholar] [CrossRef] [PubMed]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Synthesis and catalytic properties of silver nanoparticles supported on porous cellulose acetate sheets and wet-spun fibers. Carbohydr. Polym. 2017, 157, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Sivudu, K.S.; Mohan, Y.M.; Sreedhar, B.; Raju, K.M. Controlled silver nanoparticles synthesis in semi-hydrogel networks of poly(acrylamide) and carbohydrates: A rational methodology for antibacterial application. Carbohydr. Polym. 2009, 75, 463–471. [Google Scholar] [CrossRef]
- Attarad, A.; Ihsan, U.H.; Javeed, A.; Muhammad, S.; Naveed, A.; Muhammad, Z. Synthesis of Ag-NPs impregnated cellulose composite material: Its possible role in wound healing and photocatalysis. IET Nanobiotechnol. 2017, 11, 477–484. [Google Scholar]
- Al Rugaie, O.; Abdellatif, A.A.H.; El-Mokhtar, M.A.; Sabet, M.A.; Abdelfattah, A.; Alsharidah, M.; Aldubaib, M.; Barakat, H.; Abudoleh, S.M.; Al-Regaiey, K.A.; et al. Retardation of bacterial biofilm formation by coating urinary catheters with metal nanoparticle-stabilized polymers. Microorganisms 2022, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.J.B.; Marques, P.A.A.P.; Neto, C.P.; Tridando, T.; Daina, S.; Sadocco, P. Antibacterial activity of nanocomposites of silver and bacterial or vegetable cellulosic fibers. Acta Biomater. 2009, 5, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.W.; Kim, I.S.; Ni, Q.Q. A comparative study on synthesis of AgNPs on cellulose nanofibers by thermal treatment and DMF for antibacterial activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1179–1195. [Google Scholar] [CrossRef] [PubMed]
- Chook, S.W.; Chia, C.H.; Zakaria, S.; Neoh, H.M.; Jamal, R. Effective immobilization of silver nanoparticles on regenerated cellulose-chitosan composite membrane and its antibacterial activity. New J. Chem. 2017, 41, 5061–5065. [Google Scholar] [CrossRef]
- Barrera, N.; Guerrero, L.; Debut, A.; Santa-Cruz, P. Printable nanocomposites of polymers and silver nanoparticles for antibacterial devices produced by DoD technology. PLoS ONE 2018, 13, e0200918. [Google Scholar] [CrossRef]
- Van Phu, D.; Quoc, L.A.; Duy, N.N.; Lan, N.T.K.; Du, B.D.; Luan, L.Q.; Hien, N.Q. Study on antibacterial activity of silver nanoparticles synthesized by gamma irradiation method using different stabilizers. Nanoscale Res. Lett. 2014, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Francesko, A.; Ivanova, K.; Hoyo, J.; Pérez-Rafael, S.; Petkova, P.; Fernandes, M.M.; Heinze, T.; Mendoza, E.; Tzanov, T. Bottom-up layer-by-layer assembling of antibacterial freestanding nanobiocomposite films. Biomacromolecules 2018, 19, 3628–3636. [Google Scholar] [CrossRef] [PubMed]
- Davarnejad, R.; Azizi, A.; Asadi, S.; Mohammadi, M. Green synthesis of copper nanoparticles using Centaurea cyanus plant extract: A cationic dye adsorption application. Iran. J. Chem. Chem. Eng. 2022, 41, 1–14. [Google Scholar]
- Mashwani, Z.R.; Khan, M.A.; Khan, T.; Nadhman, A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Baharara, J.; Namvar, F.; Mousavi, M.; Ramezani, T.; Mohamad, R. Anti-angiogenesis effect of biogenic silver nanoparticles synthesized using Salvia officinalis on chick chorioalantoic membrane (CAM). Molecules 2014, 19, 13498–13508. [Google Scholar] [CrossRef] [PubMed]
- Albeladi, S.S.R.; Malik, M.A.; Al-thabaiti, S.A. Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards Congo Red dye Degradation. J. Mater. Res. Technol. 2020, 9, 10031–10044. [Google Scholar] [CrossRef]
- Sharifi, F.; Sharififar, F.; Soltanian, S.; Doostmohammadi, M.; Mohamadi, N. Synthesis of silver nanoparticles using Salvia officinalis extract: Structural characterization, cytotoxicity, antileishmanial and antimicrobial activity. Nanomed. Res. J. 2020, 5, 339–346. [Google Scholar]
- Metwally, D.M.; Alajmi, R.A.; El-Khadragy, M.F.; Al-Quraishy, S. Silver nanoparticles biosynthesized with Salvia officinalis leaf exerts protective effect on hepatic tissue injury induced by Plasmodium chabaudi. Front. Vet. Sci. 2021, 7, 620665. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Hoppe, H.; Okoh, A.I. Plant-based synthesis of silver nanoparticles using aqueous leaf extract of Salvia officinalis: Characterization and its antiplasmoidal activity. J. Clust. Sci. 2021, 32, 101–109. [Google Scholar] [CrossRef]
- Saud, M.A.; Saud, N.A.; Hamad, M.A.; Farhan Gar, L. Role of Salvia officinalis silver nanoparticles in attenuation renal damage in rabbits exposed to methotrexate. Arch. Razi Inst. 2022, 77, 151–162. [Google Scholar]
- Balciunaitiene, A.; Liaudanskas, M.; Puzeryt, V.; Viskelis, J.; Janulis, V.; Viskelis, P.; Griskonis, E.; Jankauskait, V. Eucalyptus globulus and Salvia officinalis extracts mediated green synthesis of silver nanoparticles and their application as an antioxidant and antimicrobial agent. Plants 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Siakavella, I.K.; Lamari, F.; Papoulis, D.; Orkoula, M.; Gkolfi, P.; Lykouras, M.; Avgoustakis, K.; Hatziantoniou, S. Effect of plant extracts on the characteristics of silver nanoparticles for topical application. Pharmaceutics 2020, 12, 1244. [Google Scholar] [CrossRef] [PubMed]
- Sreckovic, N.Z.; Nedic, Z.P.; Monti, D.M.; D’Elia, L.; Dimitrijevic, S.B.; Mihailovic, N.R.; Katanic Stankovic, J.S.; Mihailovic, V.B. Biosynthesis of silver nanoparticles using Salvia pratensis L. aerial part and root extracts: Bioactivity, biocompatibility, and catalytic potential. Molecules 2023, 28, 1387. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic, V.; Sreckovic, N.; Nedic, Z.P.; Dimitrijevic, S.; Matic, M.; Obradovic, A.; Selakovic, D.; Rosic, G.; Katanic Stankovic, J.S. Green synthesis of silver nanoparticles using Salvia verticillata and Filipendula ulmaria extracts: Optimization of synthesis, biological activities, and catalytic properties. Molecules 2023, 28, 808. [Google Scholar] [CrossRef] [PubMed]
- Geremew, A.; Gonzalles, J., III; Peace, E.; Woldesenbet, S.; Reeves, S.; Brooks, N., Jr.; Carson, L. Green synthesis of novel silver nanoparticles using Salvia blepharophylla and Salvia greggii: Antioxidant and antidiabetic potential and effect on foodborne bacterial pathogens. Int. J. Mol. Sci. 2024, 25, 904. [Google Scholar] [CrossRef] [PubMed]
- Laime-Oviedo, L.A.; Soncco-Ccahui, A.A.; Peralta-Alarcon, G.; Arenas-Chávez, C.A.; Pineda-Tapia, J.L.; Díaz-Rosado, J.C.; Alvarez-Risco, A.; Del-Aguila-Arcentales, S.; Davies, N.M.; Yáñez, J.A.; et al. Optimization of synthesis of silver nanoparticles conjugated with Lepechinia meyenii (Salvia) using Plackett-Burman design and response surface methodology—Preliminary antibacterial activity. Processes 2022, 10, 1727. [Google Scholar] [CrossRef]
- Sehnal, K.; Hosnedlova, B.; Docekalova, M.; Stankova, M.; Uhlirova, D.; Tothova, Z.; Kepinska, M.; Milnerowicz, H.; Fernandez, C.; Ruttkay-Nedecky, B.; et al. An assessment of the effect of green synthesized silver nanoparticles using sage leaves (Salvia officinalis L.) on germinated plants of maize (Zea mays L.). Nanomaterials 2019, 9, 1550. [Google Scholar] [CrossRef]
- Bayat, M.; Zargar, M.; Chudinova, E.; Astarkhanova, T.; Pakina, E. In vitro evaluation of antibacterial and antifungal activity of biogenic silver and copper nanoparticles: The first report of applying biogenic nanoparticles against Pilidium concavum and Pestalotia sp. fungi. Molecules 2021, 26, 5402. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Eccles, J.W.L.; Banger, U.; Bromfield, M.; Christian, P.; Harvey, A.J.; Thomas, P. UV-Vis plasmon studies of metal nanoparticles. J. Phys. Conf. Ser. 2010, 241, 012090. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 2013, 8, 318. [Google Scholar] [CrossRef]
- Russo, P.S.; Streletzky, K.A.; Huberty, W.; Zhang, X.; Edwin, N. Characterization of polymers by static light scattering. In Molecular Characterization of Polymers. A Fundamental Guide; Malik, M.I., Mays, J., Shah, M.R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 499–532. [Google Scholar]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Maconi, G.; Kassamakov, I.; Penttila, A.; Gritsevich, M.; Hæggström, E.; Muinonen, K. Experimental light scattering by small particles: System design and calibration. In Proceedings of the Optical Measurement Systems for Industrial Inspection X, Munich, Germany, 26 June 2017; Volume 10329, p. 103292S. [Google Scholar]
- Brar, S.K.; Verma, M. Measurement of nanoparticles by light-scattering techniques. Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Scattering Methods: Basic Principles and Application to Polymer and Colloidal Solutions, Part I, (Lang, P.; Summer Term, 2004). Available online: https://www.yumpu.com/en/document/read/33367130/basic-principles-and-application-to-polymer-and-colloidal-solutions- (accessed on 28 March 2023).
- Podzimek, S. Light Scattering, Size Exclusion Chromatography and Asymmetric Flow Field Flow Fractionation. Powerful Tools for Characterization of Polymers, Proteins and Nanoparticles; John Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of light scattering techniques to nanoparticle characterization and development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- ISO 13099-2; Colloidal Systems—Methods for Zeta Potential Determination. International Organization for Standardization (ISO): Geneva, Switzerland, 2012. Available online: https://cdn.standards.iteh.ai/samples/52832/93e1dae3987f437499cc05c56778471e/ISO-13099-2-2012.pdf (accessed on 9 March 2024).
- Zeta Potential—An Introduction in 30 Minutes, Technical Note from Malvern. Available online: https://www.research.colostate.edu/wp-content/uploads/2018/11/ZetaPotential-Introduction-in-30min-Malvern.pdf (accessed on 8 March 2024).
- Susanthy, D.; Santosa, S.J.; Kunarti, E.S. The synthesis and stability study of silver nanoparticles prepared by using p-aminobenzoic acid as reducing and stabilizing agent. Indones. J. Chem. 2018, 18, 421–427. [Google Scholar] [CrossRef]
- Madhu, G.; Kumar, A.S.; Nair, S.K. Sunlight-induced honey-mediated green synthesis of silver nanoparticles. AIP Conf. Proc. 2019, 2162, 020101. [Google Scholar]
- De Leersnyder, I.; Rijckaert, H.; De Gelder, L.; Van Driessche, I.; Vermeir, P. High variability in silver particle characteristics, silver concentrations, and production batches of commercially available products indicates the need for a more rigorous approach. Nanomaterials 2020, 10, 1394. [Google Scholar] [CrossRef]
- Tanase, C.; Berta, L.; Coman, N.A.; Rosca, I.; Man, A.; Toma, F.; Mocan, A.; Nicolescu, A.; Jakab-Farkas, L.; Biró, D.; et al. Antibacterial and antioxidant potential of silver nanoparticles biosynthesized using the spruce bark extract. Nanomaterials 2019, 9, 1541. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterization and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Burchard, W. Light scattering from polysaccharides as soft materials. In Soft matter Characterization; Borsali, R., Pecora, R., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 463–603. [Google Scholar]
- Little, C.A.; Batchelor-McAuley, C.; Young, N.P.; Compton, R.G. Shape and size of non-spherical silver nanoparticles: Implications for calculating nanoparticle number concentrations. Nanoscale 2018, 10, 15943–15947. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front. Nanotechnol. 2022, 3, 801620. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Pamanji, S.R.; Jeon, H.; Ahn, C.W. Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Oprica, L.; Andries, M.; Sacarescu, L.; Popescu, L.; Pricop, D.; Creanga, D.; Balasoiu, M. Citrate-silver nanoparticles and their impact on some environmental beneficial fungi. Saudi J. Biol. Sci. 2020, 27, 3365–3375. [Google Scholar] [CrossRef]
- Patel, K.; Bharatiy, B.; Mukherjee, T.; Soni, T.; Shukla, A.; Suhagi, B.N. Role of stabilizing agents in the formation of stable silver nanoparticles in aqueous solution: Characterization and stability study. J. Dispers. Sci. Technol. 2017, 38, 626–631. [Google Scholar] [CrossRef]
- Dos Santos Corrêa, A.; Contreras, L.A.; Keijok, W.J.; Barcelos, D.H.F.; Pereira, A.C.H.; Kitagawa, R.R.; Scherer, R.; de Oliveira Gomes, D.C.; da Silva, A.R.; Endringer, D.C.; et al. Virola oleifera-capped gold nanoparticles showing radical-scavenging activity and low cytotoxicity. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Nasiriboroumand, M.; Montazer, M.; Barani, H. Preparation and characterization of biocompatible silver nanoparticles using pomegranate peel extract. J. Photochem. Photobiol. B Biol. 2018, 179, 98–104. [Google Scholar] [CrossRef]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).