Development of a Binder-Free Tetra-Metallic Oxide Electrocatalyst for Efficient Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Instrumentation
2.2. Method of Electrode Modification
3. Results and Discussion
3.1. Cyclic Voltammetry
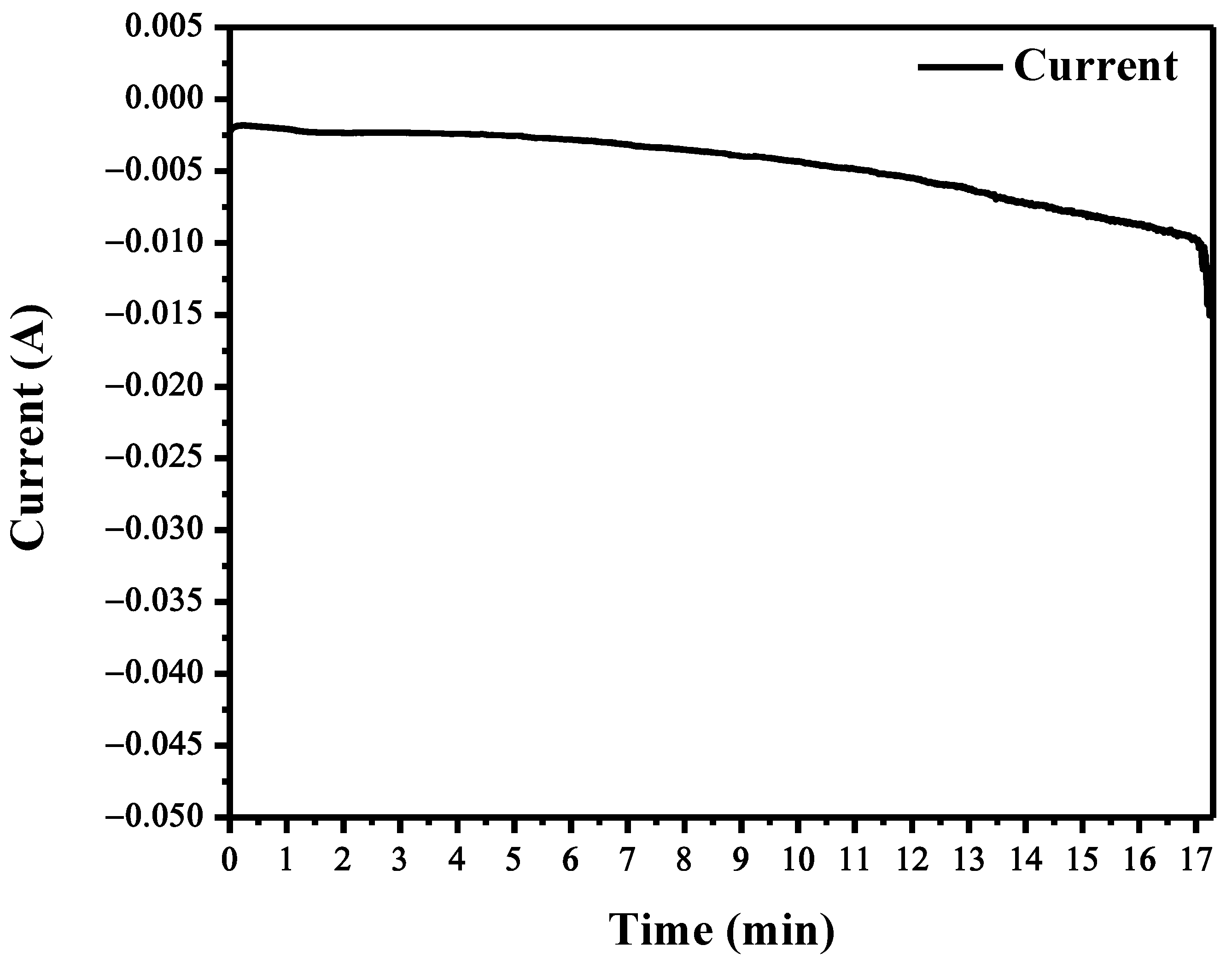
3.2. Electrodeposition through Chronoamperometry
3.3. Characterization of Modified FTO
3.3.1. XRD Analysis of FTO Modified with Tetra-Metallic Electro-Catalyst
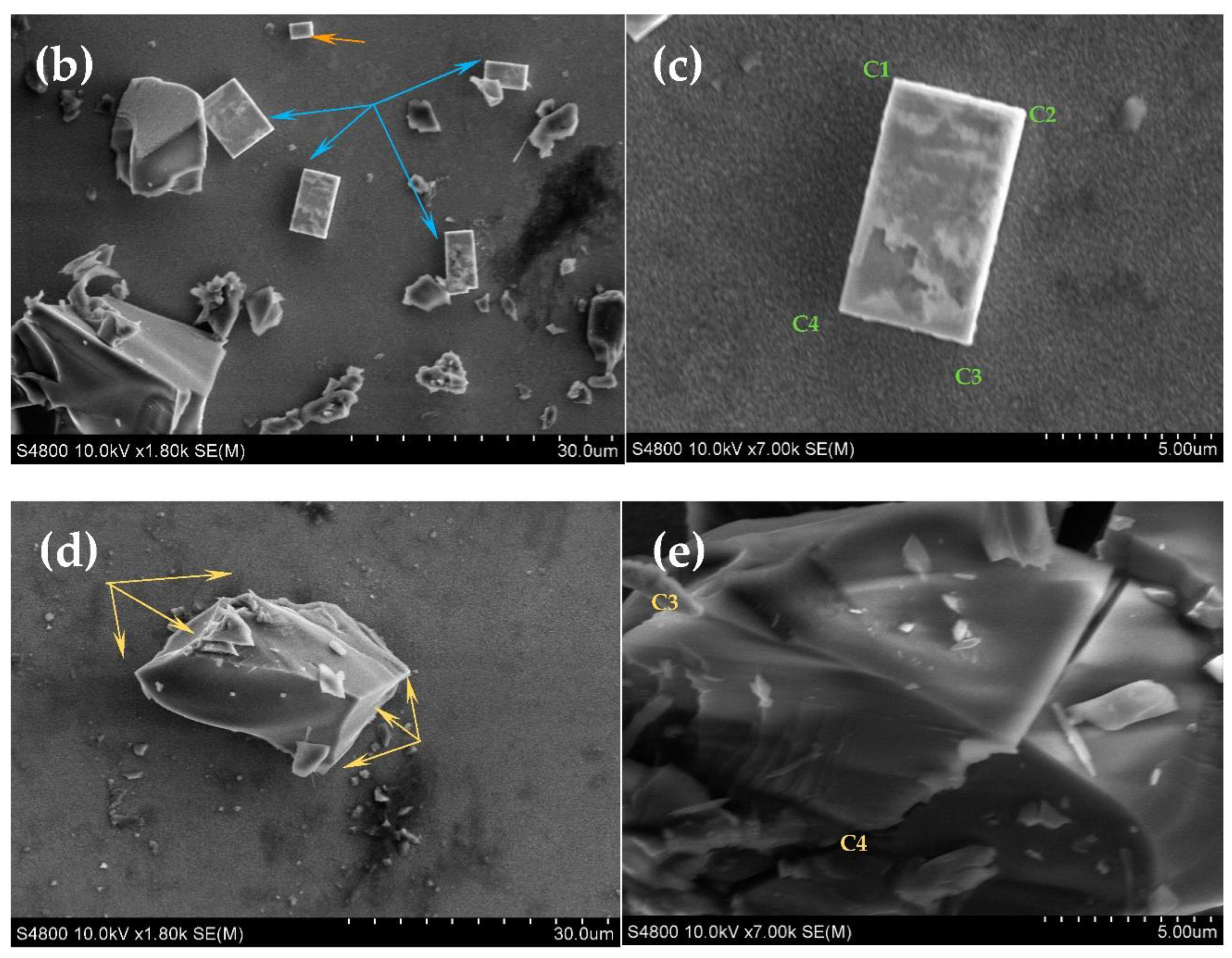
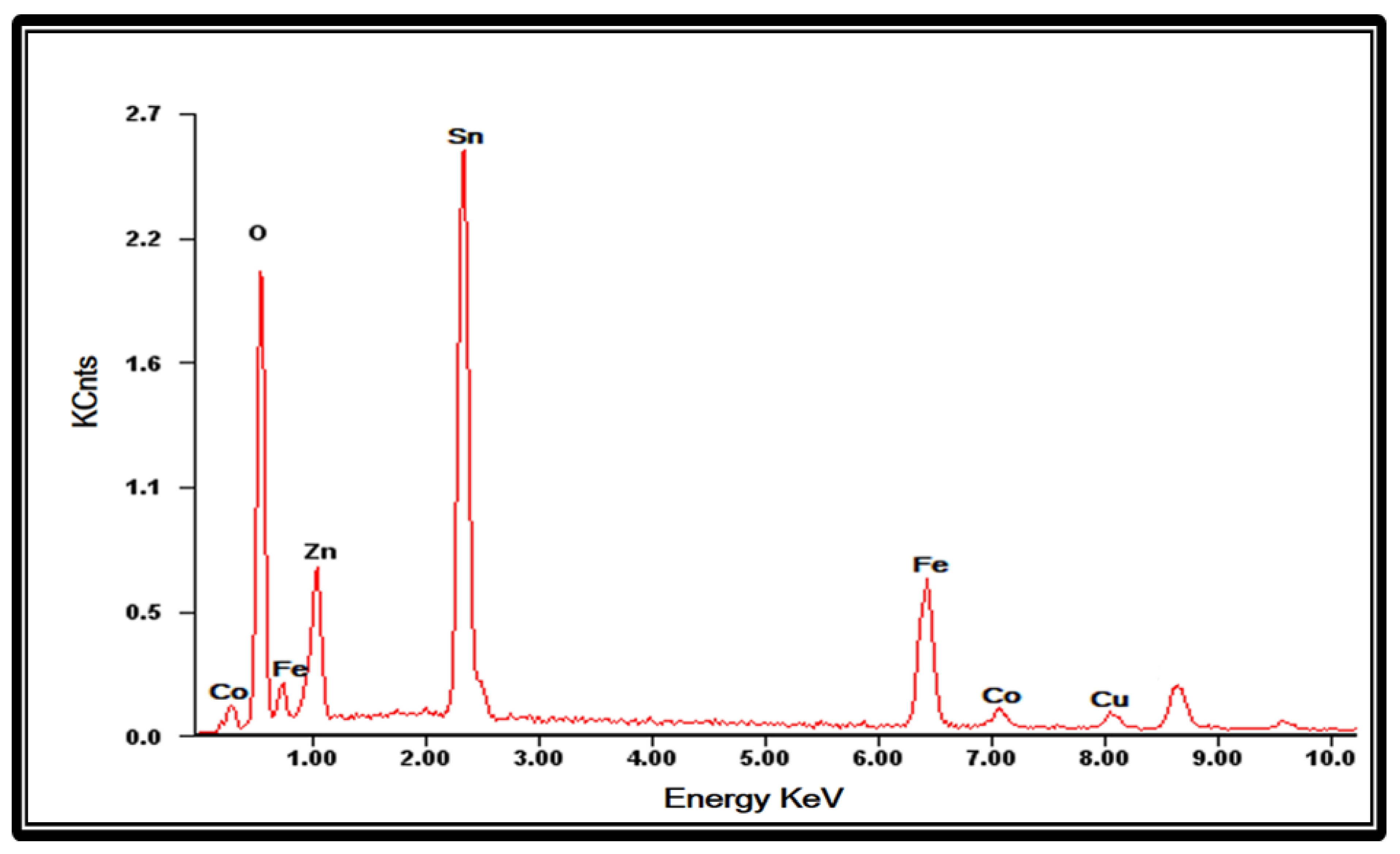
3.3.2. SEM and EDX Analysis of FTO Modified with Oxides of Tetra-Metallic Electrocatalyst
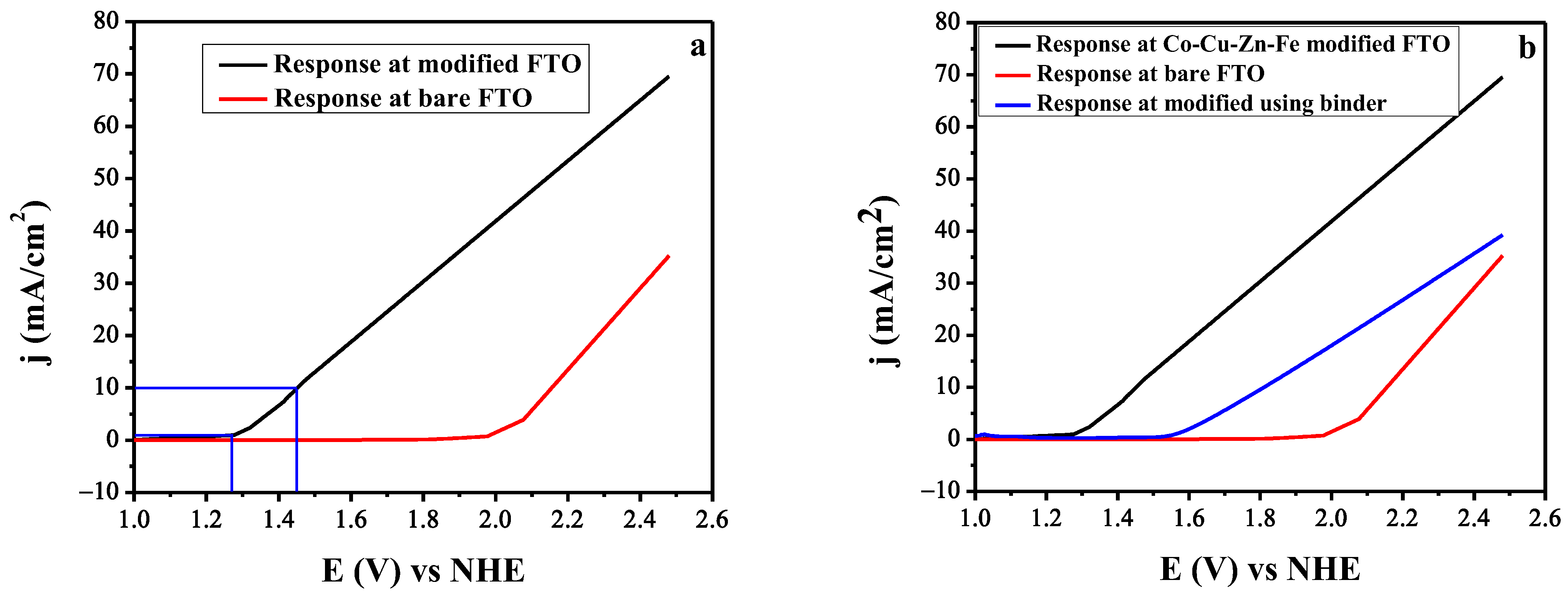
3.4. Linear Sweep Voltammetry for the OER Activity of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aldalbahi, A.; Mkawi, E.; Ibrahim, K.; Farrukh, M. Effect of sulfurization time on the properties of copper zinc tin sulfide thin films grown by electrochemical deposition. Sci. Rep. 2016, 6, 32431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Wijesinghe, M.; Perrin, K.; Ranchord, A.; Simmonds, M.; Weatherall, M.; Beasley, R. Routine use of oxygen in the treatment of myocardial infarction: Systematic review. Heart 2009, 95, 198–202. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merki, D.; Hu, X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 2011, 4, 3878–3888. [Google Scholar] [CrossRef] [Green Version]
- Vrubel, H.; Merki, D.; Hu, X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles. Energy Environ. Sci. 2012, 5, 6136–6144. [Google Scholar] [CrossRef] [Green Version]
- Hunter, B.M.; Gray, H.B.; Muller, A.M. Earth-abundant heterogeneous water oxidation catalysts. Chem. Rev. 2016, 116, 14120–14136. [Google Scholar] [CrossRef]
- Anandhi, R.; Ravichandran, K.; Mohan, R. Conductivity enhancement of ZnO: F thin films by the deposition of SnO2: F over layers for optoelectronic applications. Mater. Sci. Eng. B 2013, 178, 65–70. [Google Scholar] [CrossRef]
- Carvalhal, R.F.; Sanches Freire, R.; Kubota, L.T. Polycrystalline Gold Electrodes: A Comparative Study of Pretreatment Procedures Used for Cleaning and Thiol Self-Assembly Monolayer Formation. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2005, 17, 1251–1259. [Google Scholar] [CrossRef]
- Ballesteros, J.; Gomez-Solis, C.; Torres-Martinez, L.; Juárez-Ramírez, I. Electrodeposition of Cu-Zn Intermetallic Compounds for its application as Electrocatalyst in the Hydrogen Evolution Reaction. Int. J. Electrochem. Sci. 2015, 10, 2892–2903. [Google Scholar]
- Akbar, M.; Shah, A.; Iftikhar, F.J.; Ali, G.; Han, H.; Rahman, G. In-situ formation of an efficient trimetallic (Cu-Zn-Ag) electrocatalyst for water oxidation. Int. J. Energy Res. 2021, 45, 2931–2944. [Google Scholar] [CrossRef]
- Liu, X.; Cui, S.; Qian, M.; Sun, Z.; Du, P. In situ generated highly active copper oxide catalysts for the oxygen evolution reaction at low overpotential in alkaline solutions. Chem. Commun. 2016, 52, 5546–5549. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Li, P.; Jin, Z.; Gao, T.; Wang, Y.; Guo, Y.; Xiao, D. Enhanced catalytic performance of ZnO-CoOx electrode generated from electrochemical corrosion of Co-Zn alloy for oxygen evolution reaction. Electrochim. Acta 2016, 222, 999–1006. [Google Scholar] [CrossRef]
- Kuang, M.; Wang, Q.; Ge, H.; Han, P.; Gu, Z.; Al-Enizi, A.M.; Zheng, G. CuCoOx/FeOOH Core–shell nanowires as an efficient bifunctional oxygen evolution and reduction catalyst. ACS Energy Lett. 2017, 2, 2498–2505. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Lu, Q.; Chen, B.; Chen, J.; Huang, Y.; Ma, Q.; Tan, C.; Yang, J.; Cao, X. Self-assembly of single-layer CoAl-layered double hydroxide nanosheets on 3D graphene network used as highly efficient electrocatalyst for oxygen evolution reaction. Adv. Mater. 2016, 28, 7640–7645. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chen, S.; Vasileff, A.; Qiao, S.Z. Anion and cation modulation in metal compounds for bifunctional overall water splitting. ACS Nano 2016, 10, 8738–8745. [Google Scholar] [CrossRef] [PubMed]
- Ishaque, M.; Shah, A.; Iftikhar, F.J.; Akbar, M. Development of transition metal based electrolyzer for efficient oxygen evolution reaction. J. Renew. Sustain. Energy 2020, 12, 024102. [Google Scholar] [CrossRef]
- Bahadur, A.; Hussain, W.; Iqbal, S.; Ullah, F.; Shoaib, M.; Liu, G.; Feng, K. A morphology controlled surface sulfurized CoMn2O4 microspike electrocatalyst for water splitting with excellent OER rate for binder-free electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 12255–12264. [Google Scholar] [CrossRef]
- Khalate, S.A.; Kadam, S.A.; Ma, Y.-R.; Kulkarni, S.B.; Parale, V.G.; Patil, U.M. Binder free cobalt iron phosphate thin films as efficient electrocatalysts for overall water splitting. J. Colloid Interface Sci. 2022, 613, 720–732. [Google Scholar] [CrossRef]
- Geiger, S.; Kasian, O.; Mingers, A.M.; Mayrhofer, K.J.; Cherevko, S. Stability limits of tin-based electrocatalyst supports. Sci. Rep. 2017, 7, 4595. [Google Scholar] [CrossRef] [PubMed]
- De Boer, F.; Van Santen, J.H.E.; Verwey, J.W. The electrostatic contribution to the lattice energy of some ordered spinels. J. Chem. Phys. 1950, 18, 1032–1034. [Google Scholar] [CrossRef]
- Barakat, M.; Henaish, M.; Olofa, S.; Tawfik, A. Sintering behaviour of the spinel ferrite system Ni0.65Zn0.35Fe2−xCuxO4. J. Therm. Anal. Calorim. 1991, 37, 241–248. [Google Scholar] [CrossRef]
- Dehnicke, K. Darstellung und Eigenschaften von SnF2Cl2, SnF2(ONO2)2 und SnOF2. Chem. Ber. 1965, 98, 280–289. [Google Scholar] [CrossRef]
- Denes, G.; Pannetier, J.; Lucas, J.; Le Marouille, J. About SnF2 stannous fluoride. I. Crystallochemistry of α-SnF2. J. Solid State Chem. 1979, 30, 335–343. [Google Scholar] [CrossRef]
- Hanawalt, J.; Rinn, H.; Frevel, L. Chemical analysis by X-ray diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Pak, M.; Moshaii, A.; Siampour, H.; Abbasian, S.; Nikkhah, M. Cobalt-copper bimetallic nanostructures prepared by glancing angle deposition for non-enzymatic voltammetric determination of glucose. Microchim. Acta 2020, 187, 276. [Google Scholar] [CrossRef]
- Esmaeeli, A.; Ghaffarinejad, A.; Zahedi, A.; Vahidi, O. Copper oxide-polyaniline nanofiber modified fluorine doped tin oxide (FTO) electrode as non-enzymatic glucose sensor. Sens. Actuators B Chem. 2018, 266, 294–301. [Google Scholar] [CrossRef]
- Fondell, M.; Johansson, F.; Gorgoi, M.; von Fieandt, L.; Boman, M.; Lindblad, A. Phase control of iron oxides grown in nano-scale structures on FTO and Si (100): Hematite, maghemite and magnetite. Vacuum 2015, 117, 85–90. [Google Scholar] [CrossRef]
- Nickel, E. The new mineral cuprospinel (CuFe2O4) and other spinels from an oxidized ore dump at Baie Verte, Newfoundland. Canad. Mineral. 1973, 11, 1003–1007. [Google Scholar]
- Dana, J.D.; Dana, E.S.; Palache, C.; Frondel, C. System of Mineralogy: Silica Minerals; John Wiley & Sons: New York, NY, USA, 1962; Volume 3. [Google Scholar]
- Voncken, J.; Verkroost, T.W. Powder diffraction of cubic α-brass. Powder Diffr. 1997, 12, 228–229. [Google Scholar] [CrossRef]
- Metsger, R.; Willman, A.; Van Ness, C. Field Guide to the Friedensville Mine of the New Jersey Zinc Company. In Conjunction with the Northeast Section Meeting of the Geological Society of America at Allentown, Pennsylvania, March; New Jersey Zinc Co.: Sussex, NJ, USA, 1973. [Google Scholar]
- Kopeli, N.I.S. Electrodeposition and Characterization Of Cu-Zn Alloy Films Obtained from a Sulfate Bath. Mater. Tehnol. 2014, 48, 221–226. [Google Scholar]
- Liu, X.; Cui, S.; Sun, Z.; Ren, Y.; Zhang, X.; Du, P. Self-supported copper oxide electrocatalyst for water oxidation at low overpotential and confirmation of its robustness by Cu K-edge X-ray absorption spectroscopy. J. Phys. Chem. C 2016, 120, 831–840. [Google Scholar] [CrossRef]
- Song, Y.; Ichimura, M. H2O2 treatment of electrochemically deposited Cu2O thin films for enhancing optical absorption. Int. J. Photoenergy 2013, 2013, 738063. [Google Scholar] [CrossRef] [Green Version]
- Blanco, M.; Barragan, J.; Barelli, N.; Noce, R.; Fugivara, C.S.; Fernández, J.; Benedetti, A.V. On the electrochemical behavior of Cu–16% Zn–6.5% Al alloy containing the β′-phase (martensite) in borate buffer. Electrochim. Acta 2013, 107, 238–247. [Google Scholar] [CrossRef]
- Hacıibrahimoğlu, M.Y.; Bedir, M.; Yavuz, A. Structural and Corrosion Study of Uncoated and Zn-Cu Coated Magnesium-Based Alloy. Metals 2016, 6, 322. [Google Scholar] [CrossRef] [Green Version]
- Haque, F.; Rahman, M.; Ahmed, E.; Bakshi, P.; Shaikh, A. A Cyclic Voltammetric Study of the Redox Reaction of Cu (II) in Presence of Ascorbic Acid in Different pH Media. Dhaka Univ. J. Sci. 2013, 61, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Khelladi, M.; Mentar, L.; Boubatra, M.; Azizi, A. Study of nucleation and growth process of electrochemically synthesized ZnO nanostructures. Mater. Lett. 2012, 67, 331–333. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Han, C.; Chen, Y.; Wei, C.; Li, B.; Kang, F. Enhancement on cycle performance of Zn anodes by activated carbon modification for neutral rechargeable zinc ion batteries. J. Electrochem. Soc. 2015, 162, A1439–A1444. [Google Scholar] [CrossRef]
- Mentar, L. A study of the electrodeposition of Co–Cu alloys thin films on FTO substrate. Ionics 2012, 18, 223–229. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Wei, L.; Karahan, H.E.; Zhai, S.; Liu, H.; Chen, X.; Zhou, Z.; Lei, Y.; Liu, Z.; Chen, Y. Amorphous bimetallic oxide–graphene hybrids as bifunctional oxygen electrocatalysts for rechargeable Zn–air batteries. Adv. Mater. 2017, 29, 1701410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Gu, F.; Li, Y.; Sun, W.; Ye, S.; Rao, H.; Liu, Z.; Bian, Z.; Huang, C. Mixed-organic-cation tin iodide for lead-free perovskite solar cells with an efficiency of 8.12%. Adv. Sci. 2017, 4, 1700204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Han, Y.; Ji, W.; Qiao, S.; Zhou, X.; Liu, R.; Han, X.; Huang, H.; Liu, Y.; Kang, Z. A high-performance reduced graphene oxide/ZnCo layered double hydroxide electrocatalyst for efficient water oxidation. Dalton Trans. 2014, 43, 15119–15125. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Qian, L.; Tian, Y.; Li, Y.; Sun, X.; Duan, X. Ternary NiFeMn layered double hydroxides as highly-efficient oxygen evolution catalysts. Chem. Commun. 2016, 52, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Kou, T.; Gao, H.; Peng, Z.; Zhang, Z. Eutectic-derived mesoporous Ni-Fe-O nanowire network catalyzing oxygen evolution and overall water splitting. Adv. Energy Mater. 2018, 8, 1701347. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Li, F.; Fang, Y.; Tian, M.; An, X.; Fu, Y.; Jin, J.; Ma, J. Precious-metal-free Co–Fe–Ox coupled nitrogen-enriched porous carbon nanosheets derived from Schiff-base porous polymers as superior electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 6505–6512. [Google Scholar] [CrossRef]
- Li, X.; Ye, N.; Liu, H.; Li, C.; Huang, Y.; Zhu, X.; Feng, H.; Lin, J.; Huang, L.; Wu, J.; et al. Bifunctional iron doped CuS catalysts towards highly efficient overall water electrolysis in the alkaline electrolyte. Int. J. Hydrogen Energy 2022, 47, 16719–16728. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, G.; Yu, T.; Chen, J.; Shen, F.; Luo, L.; Zou, Y.; Yin, S. Carbon encapsulated FeWO4-Ni3S2 nanosheets as a highly active catalyst for overall water splitting at large current density. Chem. Eng. J. 2022, 434, 134669. [Google Scholar] [CrossRef]
- Fatemeh Yaghoubidoust, F.; Wicaksono, D.H.B.; Chandren, S.; Nur, H. Effect of graphene oxide on the structural and electrochemical behavior of polypyrrole deposited on cotton fabric. J. Molec. Struct. 2014, 1075, 486–493. [Google Scholar] [CrossRef]
- Nimal, R.; Yahya, R.; Shah, A.; Khan, M.A.; Zia, M.A.; Shah, I. Development of Electrolyzer Using NiCo(OH)2 Layered Double Hydroxide Catalyst for Efficient Water Oxidation Reaction. Nanomaterials 2022, 12, 1819. [Google Scholar] [CrossRef] [PubMed]

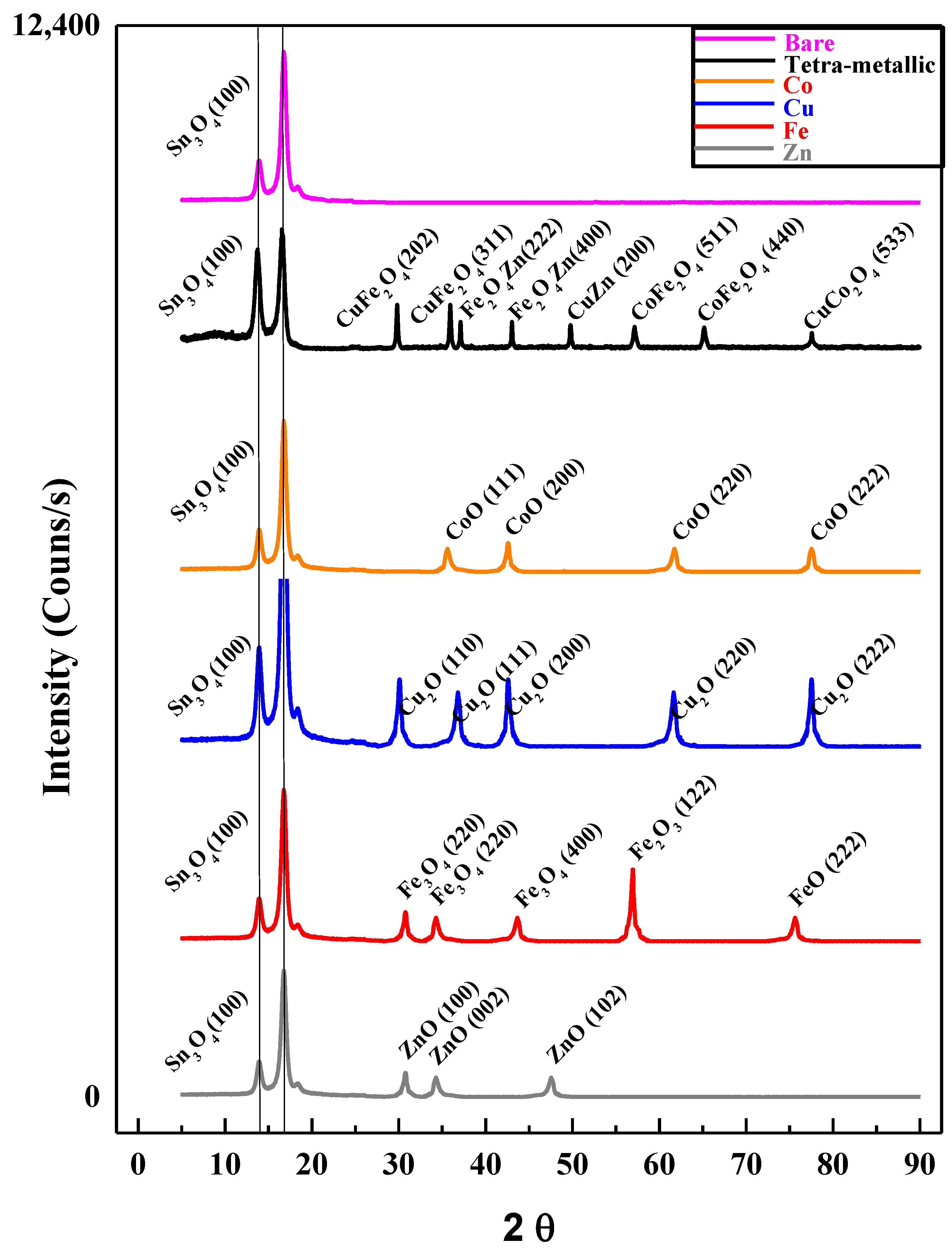

| Alloy Name | Chemical Formula | Peak Position (2θ) | Crystal Type | Space Groups | hkl |
|---|---|---|---|---|---|
| Cuprospinel | CuFe2O4 | 29.81 | Tetragonal | F | 202 |
| 35.93 | 311 | ||||
| Zinc Iron Oxide | ZnFe2O4 | 37.11 | Cubic | Fd3m | 222 |
| 43.03 | 400 | ||||
| Copper Zinc Oxide | CuZnO2 | 49.77 | Cubic | Pm3m | 200 |
| Cobalt Iron Oxide | CoFe2O4 | 57.15 | Cubic | Fd3m | 511 |
| 65.17 | 440 | ||||
| Copper Cobalt Oxide | CuCo2O4 | 77.57 | Cubic | Fd3m | 533 |
| Element | Wt.% | At.% |
|---|---|---|
| Sn | 41.07 | 51.12 |
| O | 33.72 | 32.74 |
| Cu | 4.50 | 1.54 |
| Zn | 6.04 | 2.83 |
| Fe | 10.61 | 9.74 |
| Co | 4.06 | 2.03 |
| Catalyst | η (mV) at 10 mA/cm2 | Refs |
|---|---|---|
| Fe0.5Co0.5Ox | 257 | [44] |
| Mn3O4@CoxMn3-xO4 Core shell NPs | 246 | [45] |
| ZnCo LDH/reduced GO | 430 | [46] |
| CoFe2O4/C | 240 | |
| Ternary NiFeMn LDH | 310 | [47] |
| Ni2Fe1-O NW | 244 | [48] |
| Co3Fe7Ox/N-pC-450 Nanosphere | 328 | [49] |
| ZnO-CoOx Nanosphere | 276 | [14] |
| Iron doped CuS nanocrystal CuFe0.6S1.6 | 302 | [50] |
| 3D Carbon encapsulated FeWO4-Ni3S2 nanosheet | 200 | [51] |
| Co-Cu-Fe-Zn oxides | 216 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asad, M.; Shah, A.; Iftikhar, F.J.; Nimal, R.; Nisar, J.; Zia, M.A. Development of a Binder-Free Tetra-Metallic Oxide Electrocatalyst for Efficient Oxygen Evolution Reaction. Sustain. Chem. 2022, 3, 286-299. https://doi.org/10.3390/suschem3030018
Asad M, Shah A, Iftikhar FJ, Nimal R, Nisar J, Zia MA. Development of a Binder-Free Tetra-Metallic Oxide Electrocatalyst for Efficient Oxygen Evolution Reaction. Sustainable Chemistry. 2022; 3(3):286-299. https://doi.org/10.3390/suschem3030018
Chicago/Turabian StyleAsad, Muhammad, Afzal Shah, Faiza Jan Iftikhar, Rafia Nimal, Jan Nisar, and Muhammad Abid Zia. 2022. "Development of a Binder-Free Tetra-Metallic Oxide Electrocatalyst for Efficient Oxygen Evolution Reaction" Sustainable Chemistry 3, no. 3: 286-299. https://doi.org/10.3390/suschem3030018






