Abstract
This review was devoted to outlining the use and potential increasing application of the Design of Experiment (DoE) approach to the rational and planned synthesis of inorganic nanomaterials, with a particular focus on polycrystalline nanostructures (metal and alloys, oxides, chalcogenides, halogenides, etc.) produced by sustainable wet chemistry routes based on a multi-parameter experimental landscape. After having contextualised the stringent need for a rational approach to inorganic materials’ synthesis, a concise theoretical background on DoE is provided, focusing on its statistical basis, shortly describing the different sub-methodologies, and outlining the pros and cons of each. In the second part of the review, a wider section is dedicated to the application of DoE to the rational synthesis of different kinds of chemical systems, with a specific focus on inorganic materials.
1. Introduction
Inorganic materials’ synthesis, also remarkably boosted by the requirement for robust, reproducible, and up-scalable approaches to materials’ development, supporting the energetic transition and renewable energies’ conversion and storage (e.g., catalysts, fuel cells, batteries, photovoltaics, etc.), is currently experiencing an actual renaissance aimed at matching a sustainable and green approach to synthesis with the demand for highly performing, stable, long-lasting, cost-effective and possibly multifunctional inorganic materials [,,,]. According to the paradigms of green chemistry [] and to the emerging issues of circular chemistry [,,,,,,,], currently adopted synthetic requirements [,] encompass (1) a low temperature of processing (<200 °C, to pursue energy consumption reduction), (2) a short processing time (again for time and energy consumption reduction), (3) routes ensuring high yield and high throughput, (4) the use of environmentally friendly solvents (e.g., water, polyols) and of earth-abundant precursors (to address materials’ criticality), in view of the application of these routes at an industrial scale, (5) the easy implementation of the synthetic procedure, (6) cost effectiveness, (7) reproducibility, and (8) up-scalability. This, in turn, implies the careful and informed optimisation of the whole experimental parameters’ landscape, entailing several factors such as (1) pH, (2) temperature and time of processing, (3) the chemical nature of the solvent (i.e., dielectric constant, polarity, viscosity, density), (4) the structure and composition of the precursors, (5) molar ratios among the reagents, (6) the nature and amount of additives (i.e., stabilizing ligands, surfactants, structure-directing agents, porogens, etc.), (7) ionic strength, and (8) pressure, which are in many cases also intertwined with and interdependent of each other (e.g., dielectric constant and temperature/pressure). To avoid time- and resources-consuming approaches, a methodological paradigm shift in approaching this complexity is urgently needed. The current research in chemical synthesis is still chiefly relying on traditional inductive approaches such as “trial and error” methodologies for the development of new drugs, molecules, materials, or chemical protocols: this approach derives from the researcher’s expertise, background, and knowledge. In many cases, the chemist approaches the research to optimise well-established and known recipes by continuously tuning and optimizing previous results and achievements. This safer approach, although simple and generally well accepted by literature, is, however, resource and time consuming and experimentally demanding, particularly when different parameters contribute in an interrelated fashion to affect the synthesis output, though their specific role is hardly identified. As a working example, the mentioned combined effect of temperature and pressure on dielectric constant, viscosity, and density of a suspension used for (even sub-critical) hydrothermal synthesis [,,] would firstly require a deep understanding of the relation between temperature and pressure on the mentioned chemical–physical properties of the dispersing medium and secondly of the influence these experimental parameters have on the dissolution/reprecipitation phenomena occurring during the synthesis and eventually on the outcomes of the synthesis itself, in terms of crystallinity and built crystalline phase, composition, and yield of the product(s) [,,,]. The main issue derives from the fact that, by manually changing one parameter at time, the obtained result does not represent the unconditional outcome but only a relative maximum in efficiency, causing a reduced yield for the products. An additional relevant point pushing towards the reduction of the experimental trials is the need to reduce the use of critical raw materials [,,,], and specifically metals [,], very often involved in the development of technologically relevant inorganic nanomaterials, such as catalysts and devices for solar energy conversion, e-mobility, wind energy production, etc. [,]. To reduce the experimental effort required for the synthesis optimisation, instead of a time- and resources-demanding systematic screening of all involved experimental parameters, a more rational approach is thus required. In this regard, a well-established and promising methodology, based on statistics, is the Design of Experiments (DoE) [,,,,] in which an impartial multi-variable analysis is carried out, thoroughly uncorrelated from user know-how, thus aseptic and focused only to boost the product yield in terms of sustainability (both environmental as well as economic) and efficiency, where the full experimental space can be truly explored. DoE is a well-known approach to optimise different processes and industrial manufacturing approaches, but its application to the specific field of inorganic materials chemistry is still relatively unexplored, at least at a scientific literature level, whereas its implementation at an industrial level is rapidly spreading.
In this review, we aimed at highlighting DoE main features by focusing on its potential application in the synthesis of inorganic materials, with a specific focus on wet chemistry and colloidal routes, which are the main methodological approaches of our research group at the University of Padova [,]. Related to this, we would like to explicitly point out that the choice of the examples reported concerning the DoE experimental methodology were in this case mainly related to wet inorganic chemistry, but the general considerations might apply also to solid-state and vapour-based inorganic synthesis methods. In particular, the DoE approach has been mostly implemented for optimizing the graphene synthesis [,] via chemical vapor deposition (CVD), although some examples concerning the growth of GaN rods [] and silicon [] through CVD could also be retrieved. DoE methodology has been also applied to support plasma-enhanced chemical vapor deposition (PECVD) synthesis (for example, of TiS2 [], SiNx [], and AlOx []), as well as to physical vapor deposition (PVD) for the synthesis of TiAlN [], ZrN, TiN [], and yttria-stabilised zirconia []. Concerning solid-state synthesis, very few examples could be found. DoE has been successfully applied to accomplish a more rational preparation of alumina-based nanocomposites [], ball-milling synthesis of electrolytes []. These few examples are neither comprehensive nor complete, and interested readers can refer to specialised literature on this. In addition, other approaches (for example, deep [] and machine [] learning, which will be mentioned later) have been also used to rationalise and optimise synthesis routes. The choice made in this review was made by the authors, also based on previous experiences, but does not represent a fully comprehensive view of the approaches to rationalise syntheses.
First, the theoretical framework underpinning DoE will be concisely described. In the second part of the review, some selected examples of the application of DoE to the synthesis of inorganic materials are provided and critically discussed. Eventually, perspective developments in this exciting field are highlighted.
2. Theoretical Background of DoE and Its Application to Inorganic Chemistry
The DoE can be applied in chemistry as a methodology to statistically optimise processes and reaction conditions based on different experimental parameters, possibly but not necessarily interdependent (e.g., temperature and dielectric constant of a solvent). The first conceptualisation of DoE was introduced by Fisher in 1935 [], who described a general problem of experiment design outlining which recurring factors’ combination would best describe the outcome of an experiment and how the response is influenced by the single factors. Since the outcome of a chemical reaction is typically the result of multiple reaction conditions (factors), it is therefore possible, by screening the ‘parameter space’ of a process, to optimise the desired outcome. Specifically, DoE is a powerful tool that aims (1) to optimise and minimise the numbers of experiments through a statistical approach, (2) to identify the effects of the investigated parameters on the outcomes, and (3) to predict the response of untested conditions within the range of the explored experimental domains. The advantage of applying DoE to inorganic synthesis also lies in the possibility of assessing the synergistic effect of several parameters on the final result of the synthetic pathways, which would not be possible with a one-variable-at-a-time (OVAT) approach []. Therefore, a Design of Experiment approach will enable a rational planning of the synthetic efforts by careful screening the experimental parameters that most influence the final features, taking into account also interaction thereof, to identify the most promising combinations among them to obtain the desired product. Within this framework, in the field of inorganic synthesis, the possibility to apply the DoE approach to figure out the relationship among the experimental parameters, the outcomes of the reaction, and the final functional properties of the inorganic nanomaterials is extremely attractive as well as being an approach that can lead to a comprehensive exploration of the whole experimental factors. Even if in literature several examples of a DoE approach so far concern the optimisation of pharmaceutical processes [,,], there are some studies related to the inorganic syntheses of different systems. A relevant disclaimer is that, being a statistical methodology, this approach is best applicable when large amounts of data can be retrieved. At the same time, it allows a more complete overview of the reaction conditions and it allows studying the synergistic and antagonistic interactions of the studied parameters, with an optimised (and limited) number of experiments, therefore reducing costs, time, and efforts. This approach can be used by researchers in two phases: (1) during an initial screening of a complex reaction with multiple parameters, thereby providing the understanding of the key factors impacting the outcome of a reaction, or (2) during the fine optimisation and tuning of a defined process, increasing yields or reducing costs.
2.1. Theoretical Background of DoE
In order to study the performance of a process/product and to try to systematically improve it, Design of Experiments (DoE) could be conducted. DoE deals with accurately planning, conducting, analyzing, and interpreting experiments so that valid and objective conclusions can be obtained about the process/product of interest.
In an experiment we deliberately manipulate some process/product variables, called factors, to investigate their effect on the desired output properties measured by response variables. It is worth noting that not all process/product variables are controllable by the experimenter. For this reason, controllable factors need to be distinguished from the uncontrollable ones (see Figure 1). For instance, in a synthesis based on the decomposition/reduction of tailor-made molecular precursor(s) to metal nanoparticles, controllable factors are, for instance, temperature and concentration of the precursor, whereas a not controllable factor is the oligomerisation degree and dynamics of the molecular precursor(s). An experiment is, therefore, a series of trials where the experimenter intentionally varies one or more controllable factors to observe changes in the output response and determine which factors affect the response variables.
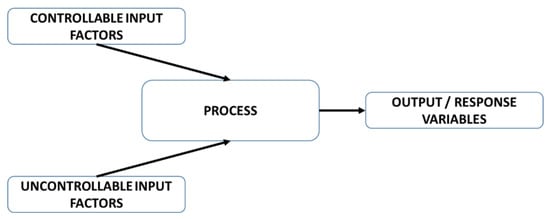
Figure 1.
Experiment description.
The experimenter needs to choose a few qualitative categories or numeric values, called levels, of the controllable factors to be tested in the experiment. Responses and factors are then measured or controlled on a randomised sample of experimental units. Each combination of factor levels tested on this sample represents an experimental condition, also called treatment or run. The entire set of runs is the design and its choice is a fundamental part in Design of Experiments.
The three basic principles [] of experimental designs are:
- randomisation: both the allocation of the experimental units to treatments and the order in which the individual runs are to be performed are randomly determined;
- blocking: a technique for dealing with known and controllable nuisance factors (i.e., factors having an impact on the response, but of no interest to the experimenter), blocking out their potential effect on the response; and
- replication: each factor combination is, generally, assigned to more than one experimental unit. Replicates are indeed multiple independent executions of the same experimental conditions, which are processed individually in the experiment and should be run in random order.
It should be noted that a replicate is not a repeated or duplicate measurement of the response variable, which, on the other hand, is meant to reflect the inherent variability in the measurement system.
Let us now focus on the case where we are interested in evaluating the impact of more than one controllable factor on a specific response. A valuable approach is to plan a factorial experiment in which factors vary simultaneously, instead of one at a time. A factorial design indeed allows us to investigate whether each factor has an effect on the response (main effect) but also whether interactions between/among factors exist, i.e., if the effect of one factor on the response is not the same for all the levels of the other factor(s).
When the number of factors is particularly high, the experiment becomes too expensive or time and resources consuming to be performed because the number of required runs to investigate all possible treatments rapidly increases. In these cases, the experimenter can decide not to run all possible treatments, focusing on a suitable subset of the runs, by planning a fractional factorial design [] instead of a full factorial design [] (see Figure 2).
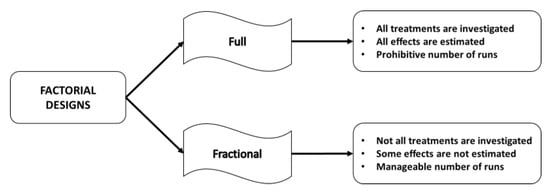
Figure 2.
Advantages and disadvantages of full and fractional designs.
It is worth noting that, in a full factorial design, all main effects and interactions can be estimated, but large numbers of factors and levels may result in a prohibitive number of runs. On the other hand, in a fractional factorial design the number of runs is reduced to a manageable size, minimizing time and cost, but some of the effects will be confounded (aliased).
Especially in the early stages of an experiment, a situation where many factors need to be investigated is quite common. Under such circumstances, experimenters commonly rely on screening experiments with factorial designs considering k factors, each at only two levels. As a working example, in a chemistry solution-based experiment, it can be chosen to vary pH and the temperature at two different levels, i.e., pH = 2 and pH = 5, and 100 °C and 150 °C, respectively. Screening experiments are widely used to identify the key factors affecting responses. After conducting them, the experimenter then proceeds with a general factorial design, increasing the number of levels to optimise factors’ levels according to process/product targets in response variables.
Full factorials and fractional factorials are only two examples of designs adopted in the context of response surface methodology (RSM) [,]. RSM is an experimental methodology based on a collection of statistical designs and procedures and has two main objectives:
- understanding the effect of factors on the response variable(s), possibly identifying also nonlinear trends, and
- identifying the optimal configuration of the levels of the factors, typically by a maximisation or a minimisation of the response variables.
The sequential steps of the RSM procedure are []:
- (1)
- Select factors (and their levels) and response variables. A screening experiment can be run to identify the most relevant factors.
- (2)
- Select an appropriate experimental design (usually an optimal design) and collect data.
- (3)
- Model the relationship between response variables and the factors.
- (4)
- Evaluate the quality of the fitted model.
- (5)
- Find optimal configurations of the factors’ levels.
One of the most common designs used in the response surface methodology is the Central Composite Design (CCD). CCDs allow us to estimate first- and second-order terms and model the response with curvature by adding center and star points to a factorial design [].
Another particularly relevant design in the chemical field is the mixture design []. It is used when the experiment involves a mixture of reagents and/or solvents and/or additives to form a solution/suspension/formulation. The goal is to determine if there is a combination of ingredients that improves the response variable according to pre-defined target values. In this case, a specific constraint needs to be considered differently from classical designs: The proportions (or molar fraction) all of the ingredients must add up to 100% (or 1, in the case of molar fraction, respectively). Then, in order to model the relationships between response variables and the factors, polynomial models are commonly adopted, but more complex techniques can be also used, such as machine learning methods [].
Sometimes the number of response variables is larger than 1. Several different regression models are, therefore, required, and in the final optimisation step the desirability approach [] is usually applied.
2.2. Selected Examples
The DoE methodology requires a specific and robust knowledge of the addressed topic. The reported examples focused on some of the most important sub-fields of modern inorganic chemistry that, although multidisciplinary, are at the interface between basic research and industrial research and development. For this reason, we will first illustrate the DoE application on a more simple topic such as solvent choice in a reaction and we will end the discussion by referring to outcomes obtained in the field of flow chemistry, competing with batch chemistry also in industrial research [].
2.2.1. Solvent Optimisation
When referring to solution processing and wet chemistry routes to inorganic materials, solvents and dispersing media play an essential role in the outcome of the synthesis, as well as in the stabilisation of the resulting product (e.g., nanoparticles). Chemico-physical properties such as dielectric constant and polarity, presence of protons and hydrogen bonding properties, viscosity, and density all dramatically affect solubilisation, diffusion, and precipitation/crystallisation of both precursors and end products. Identifying the most suitable dispersing medium might be useful to optimise the synthetic pathway, accomplish polymorph selection, and maximise purity and yield of the targeted material.
The DoE approach can be conveniently used for the choice of a particular solvent (or mixture thereof) in a new synthetic route [,]. First of all, it is convenient to write down a list of the desired properties that the solvent needs to possess, not limited to chemical constraints but also including socio-economic issues: (1) the ecological impact on environment and health, (2) cost, (3) availability on the market, (4) chemical specifications such as boiling point, vapor pressure, and density, and (5) conditions for possible recycling/reuse, disposal, and/or (if needed) substitution with greener/cheaper alternatives. It is now possible to realise a principal component analysis (PCA) map in which tabulated and calculated variables for each solvent are inserted and analysed. The reported map in Figure 3, for instance, shows a PCA solvent map in which hydrogen bonding properties and polarity of 136 solvents are correlated, thus allowing the analysis to rationally build a tri-dimensional “solvent space” (a cuboid) in which at each vertex a solvent class is located: This class derives from the results of the previous correlation and links several species that in principle are not connected to each other or do not belong to the same chemical family. This intriguing analysis allows the researcher to systematically change the solvent with a rationale: As in this case, hazardous dichloromethane can easily be replaced with 1,4-dioxane or dimethyl carbonate and, likewise, carcinogenic and highly toxic benzene can be replaced with xylene. It is straightforward to understand that the choice would fall on the cheaper, the less harmful, and the more convenient one [].
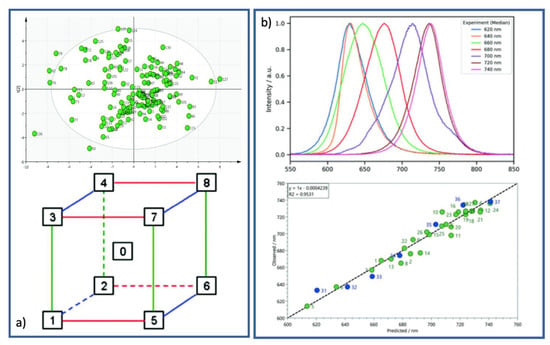
Figure 3.
(a) The PCA solvent map (top panel) and the cuboid representing the solvent space (bottom panel) (reproduced from Ref. [] with permission from the Royal Society of Chemistry). (b) The perovskite photoluminescence model validation experiment described in the text, where both S1-normalised PL emission (top panel) and observed vs. predicted wavelength PL, showing design experiments in green and validation experiments in blue (bottom panel), are presented (reproduced from Ref. [] with permission from the Royal Society of Chemistry).
Another recent example of critical solvent choice using DoE was used by a group from the University of Bath in collaboration with the Paul Marray Catalysis Consulting Ltd. firm in the UK []. In this work, the authors applied DoE for a rational understanding of the best conditions for designing halide perovskite nanocrystals with tunable emissive properties by the realisation of an empiric model taking into account a reactant-to-solvent weight ratio, ligand concentration, ligand-to-metal molar ratio, and non-polar solvent polarizability, thus obtaining withstanding adherence to experimental data (Figure 3b).
Solvent selection for optimizing the solubilisation process under the desired optimal reaction temperature is, therefore, a significant challenge in processability [,]. One perspective evolution of DoE would be the use of machine learning for the automatisation of process development: In a recent paper [], the authors, starting from a library of 459 solvents, divided them into 17 groups, each one characterised by a specific molecular descriptor, and obtained different recipes and better reaction conditions for the catalytic hydrogenation of a lactam.
The ultimate application of machine learning in chemistry design is the implementation of the Bayesian optimisation algorithm [] in the exploration of reaction space: Within this approach, even literature and the operator’s intuition that are already present in DoE boundary conditions are overcome, allowing us to balance the exploration of areas of uncertainty and the exploitation of available information. By doing so, Bayesian optimisation algorithms can be applied to different areas of research, enabling the selection of multiple experiments in parallel [].
2.2.2. Synthesis of Inorganic Nanomaterials and Nanoparticles
The interest in nanomaterials [] derives from the fact that, at this length scale, new properties are available and that these properties change drastically depending on nanomaterial size and/or shape. Nanoparticles are used in many chemical processes and often as catalysts with optimised dimensions to maximise efficiency and reduce volumes [,,].
As far as a Design of Experiment is concerned, it is worth considering its application in the design of inorganic functional nanomaterials. Nowadays, the possibility to finely optimise and control the size, morphology, and size distribution of inorganic nanoparticles is becoming increasingly relevant to confer tuneable and desired final functional properties to the nanomaterials. In this regard, a Design of Experiment approach applied to the inorganic synthesis of nanoparticles will enable assessing the relevant experimental parameters that most affect the outcomes of the reactions and, therefore, to determine which experimental conditions, e.g., temperature, concentration of precursor, pH, flow rate, etc., allow us to obtain the nanomaterials with the desired properties in terms of size, size distribution, etc. Going more into detail, Barglik-Chory et al. [] reported a DoE approach for the adjustment and tuning of the band gap energies of CdS nanoparticles, exploiting a colloidal synthesis with cysteine and glutathione as biostabilisers. Indeed, it is well known that the size of the nanoparticles affects their band gap energies, and, therefore, it is of significant importance to understand the role played by the various experimental parameters on the final size of the nanoparticles. This can be evaluated, as an output response of the synthesis, either directly by determining the Feret diameter obtained by direct imaging (i.e., TEM or SEM images) or indirectly by analyzing UV-Vis absorption spectra.
The discussed study relates to the analysis of the influence of three different experimental parameters, i.e., the pH value and the relative amount of the stabiliser (cysteine or glutathione) and of the sulfur source (1,1,1,3,3,3-hexamethyl-disi-lathiane) on the band gap energies of CdS nanoparticles by measuring, as measurable output, the UV-Vis absorption spectra of samples synthesised with different reaction parameters. As a result, as an example of the cysteine-stabilised nanoparticles, it was possible to observe that the position of the band gap was significantly influenced by the three considered factors of pH value, the amount of the stabiliser, and the concentration of the sulfur source. In addition, it was found that the effect of pH on both cysteine and the sulfur source also affects the final band gap of the nanoparticles. These synergistic parameter interactions, which provide thus an exciting tool to tune synthesis outcomes, can be identified only by the simultaneous variation of parameter values as provided by DoE.
A further example of the application of DoE in the inorganic synthesis can be found in the work of Sadat-Shojai et al. []. The study focused on the optimisation of the hydrothermal synthesis of hydroxyapatite (HAp) nanoparticles, which is a widely exploited biomaterial due to its biocompatibility, bioactivity, and favorable osteointegration []. In particular, the synthesis of particles with optimised size, morphology, crystallinity, and stoichiometry is becoming a focus of interest in the biomedical fields. Within this framework, and exploiting a DoE approach, this paper reported the controlled growth of HAp nanoparticles with tuneable size, morphology, crystallinity, and stoichiometry by adjusting the experimental parameters. Reactant concentration, pH, temperature, time of the hydrothermal treatment, and presence of urea in the reaction medium were considered as the process variables that may influence the characteristics of HAp nanoparticles. As a conclusion, the paper presented a general methodology to predict the suitable conditions for the hydrothermal synthesis of shape-controlled HAp nanoparticles by performing a systematic evaluation of the most influencing variables in the synthesis of HAp by the hydrothermal method and of their effects on the final HAp properties.
In a further paper, the synthesis of iron oxide nanoparticles to be used as anode materials in Li-ion batteries via coprecipitation was studied by exploiting a factorial design of experiment methodology to investigate the influence of pH, medium temperature, Fe3+/Fe2+ ratio, and reaction time on the crystallite size, taken as an output factor []. X-ray diffraction evidenced as the crystallite size decreased with increased pH and the Fe3+/Fe2+ molar ratio, therefore enabling the rapid optimisation of a robust and reproducible protocol for the preparation of monodispersed maghemite nanoparticles.
Analogously, a DoE approach was implemented to prepare pure and chitosan-coated Fe3O4 nanoparticles [] for the extraction and green removal of chromium (VI) from water samples. The optimised experimental parameters’ space encompassed pH, contact time, adsorbent dosage, and agitation speed, assessed and evaluated by DoE method and investigated by response surface methodology (RSM).
The same kind of magnetic nanoparticles deposited on a potato peel (PP) was used for remediation from Pb2+. The L16 (4(boolean AND)4) method of Taguchi DOE was used for the optimisation of the adsorption condition, showing that at pH 6 with 10 min of contact time and a dose of 15 g/L can give more than 90% removal efficiency of Pb2+. Contour maps, Taguchi response analysis, and analysis of variance (ANOVA) suggested that pH has a dominant contribution in the removal of Pb2+ [].
DoE can also be used to enhance synthesis reproducibility. A method for the synthesis of silver nanoparticles (AgNP) using gallic acid as reductant was optimised using DoE strategies based on response surface methodologies. Fractional factorial design was used in the screening stage. The obtained AgNP presented improved repetitivity and reproducibility of photophysical properties between batches compared to the synthesis method reported in literature [].
Nanosized titanium oxide (TiO2) powders prepared by conventional and microwave hydrothermal methods by forced hydrolysis of TiOCl2 were followed by DoE and different characterisation methods. The effects of mixing time, HPC, and TiO2 concentration and their mutual interactions on shear stress were evaluated with a Design of Experiment (DoE) approach [].
In a further study, a one-step custom Design of Experiment was employed for the simultaneous screening and optimisation of raw yield (Y-1) and percent yield (Y-2) with heating temperature T (190–220 °C) and reaction time t (1.0–2.5 h) as the predictors in a conventional solvothermal magnetite nanoparticles’ (NPs) synthesis [].
A further application of DoE to rapid and high-throughput screening of inorganic nanomaterials relates to the development of new heterogeneous catalysts in industrial research. The field of catalysts’ design and upscale production is characterised by an extremely large parameter space, implying that an increase in throughput in the preparation and testing of candidate substances is mandatory in order to identify a new and effective catalyst in a reasonable timeframe. In a paper by Duff et al. [], the authors developed a workflow in which 10,000 substances per day were synthesised and their activity tested in a heterogeneously catalysed gas phase reaction (alkene epoxidation). The synthesis was based on a careful dosing of precursor solutions onto a single substrate, using one ink-jet printer technology and subsequent thermal treatment. For the activity testing, the product stream of each candidate was carried out through a detection layer, where the target product was converted into a fluorescent substance, then detected and assessed by locally resolved fluorescence spectroscopy. Since even an effective throughput of 10,000 substances per day does not allow mapping the whole parameter space in a practicable amount of time, a combination of evolutionary optimisation and data mining was explored.
2.2.3. Precious Metal NP Optimisation for Exhaust Gas After-Treatment
An everyday example of the application of nanomaterials can be taken from the heterogeneous reactions happening in the exhaust after-treatment systems of fuel vehicles, where precious metal nanoparticles are used to abate harmful gases resulting from the fuel combustion inside the engine. Being scarce, expensive, and recognised as critical raw materials by the European Commission [], these metals are already used in the form of nanoparticles (NPs) to maximise the surface available for the reactions, while at the same time reducing their amount. Nevertheless, smaller does not necessarily mean better, and for certain applications stability towards sintering/coalescence and durability contribute to the overall performance throughout time. Within this context, a DoE was used by our group, within a collaboration with Umicore AG&Co. KG, to optimise the reaction conditions of a hydrothermal colloidal synthesis of Pt NPs as a possible approach to tune the resulting particle size [].
Indeed, relevant physical properties of water vary under hydrothermal treatments, such as viscosity, ionic product, and dielectric constant, that change inside the autoclaves used in the synthesis upon temperature (and consequent autogenous pressure) increase [,,]. From prior art and researcher experience, the resulting reagent solubilisation and product crystallisation were known to be functions of the temperature, pH, and molar ratio of the reagents (precious metal complexes and polymeric stabiliser). Nevertheless, with the reagents, set of factors, and reaction set up having never been applied before, it was important to investigate the interplay among the different experimental parameters involved and to single out the most relevant ones in ruling the synthesis output in terms of targeted size.
The reduction of the number of experiments to be performed was particularly important in this case because of the high criticality of the precious metals involved (Pd, Pt) [,,].
In case of a larger number of factors, the experimental cost required tends to be high as all possible combinations of factors must be taken into account. Usually, fractional factorial designs are preferred in this case to reduce the size of the design matrix. Various applications of model-based experimental designs can be found in the review by Franceschini et al. []. When the number of parameter combination leads to an affordable number of experiments, the so-called full-factorial method can be used, where the additive effects on a response for each of the input factors, as well as the interaction between them, are determined. Before setting the experiment and the DoE matrix, a few experiments were performed to understand the feasibility of the applied reaction conditions in order to choose upper and lower values in the DoE matrix that would not alter the stability of the colloidal suspensions. The range of temperatures was known to span between 120 °C (temperature needed to provide enough energy to the system to reduce the Pt metal salt) but lower than about 200 °C, which is the deformation temperature of the Teflon liner (polytetrafluoroethylene (PTFE)) inside the autoclave. The pH values below 8 showed the formation of precipitates (aquo-hydroxo complexes of the chosen Pt precursor) whereas the upper value of 10 was given by the dilution of the basic Pt solution. Finally, the chosen ranges for the atomic ratio between the Pt and the polymeric stabiliser (polyvinylpyrrolidone (PVP)) were selected in order to obtain stable suspensions.
Once the applicable parameter space for the hydrothermal syntheses was defined, to screen the impact of the three selected parameters (temperature, pH, and molar ratio between precious metal complexes and polymeric stabiliser), each with two possible values (low or high) in a full-factorial experiment design mode, eight (=23) experiments were conducted. By measuring the Feret diameter of at least 300 particles for each experiment (which can be considered as internal replicates of each set of analyzed experimental condition), the average NPs’ diameter was used as the outcome. A model was constructed, based on an Analysis of Variance (ANOVA) approach, that was able to fit for the selected DoE design the reaction outcomes [].
As outlined in Figure 4 (Pareto Chart), only one of the factors resulted as statistically relevant in determining a size variation of the resulting NPs, whereas the effect of the other parameters was negligible. Despite the reduced number of experiments, the chosen DoE approach allowed the identification of the main parameter (the pH, terms B in the Pareto Chart in Figure 4) responsible for the particle growth under the tested experimental conditions.
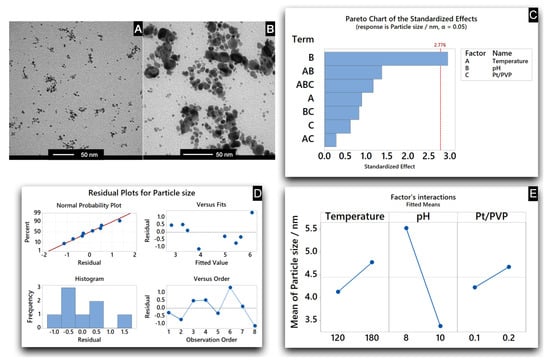
Figure 4.
DoE for the investigation of the main reaction parameter responsible for the nanoparticle size increase under hydrothermal synthesis conditions. Minitab was used for the data analysis and model generation. (A) TEM micrograph of Pt NPs obtained with the optimised reaction parameters. (B) TEM micrograph of Pt NPs obtained at the beginning of the study. (C) The Pareto Chart is reported, ranking the significance of the different effect as analysed by Minitab. (D) The Residual plot is also reported, providing an overview of the generated model to fit the experimental results and presenting the homoscedasticity of the residuals vs different variables. (E) The Factor’s interaction plot is reported, providing an overview of the impact of the different factors on the overall mean particle size. (Portions of information contained in this publication/book are printed with permission of Minitab, LLC. All such material remains the exclusive property and copyright of Minitab, LLC. All rights reserved. Minitab, LLC (2021), www.minitab.com) (accessed on 29 December 2021).
Instead, temperature and molar ratio between precious metal complexes and polymeric stabiliser were evidenced to be less relevant; therefore, their contribution reported in the Pareto Chart of Figure 4 resulted as below the threshold of statistically relevant parameters. Likewise, also the mutual interactions pH–temperature, temperature–molar ratio, and pH–molar ratio did not remarkably affect the reaction output.
As general comments for the performed DoE, the goodness of the generated model to fit the outcomes was confirmed by the linearity of the regression as reported in the residual plots in Figure 4. The model assumptions, such as linearity of relations and normal distribution of residuals, were adequate even if the low number of experiments could not provide strong evidence for the homoscedasticity (i.e., when all the aleatory variables have a similar variance) and the independency of errors. Overall, the standard variance for the values, the random distribution against the sequence of experiments, and the normal distribution of the residuals resulted in a good regression analysis. At the end of the study, the Pt NPs obtained with the optimised reaction parameters (Figure 4, TEM B) resulted as bigger than those obtained at the beginning of the study (Figure 4, TEM A).
2.2.4. Application to Flow Chemistry
Microfluidic and, generally speaking, flow chemistry are branches of research that are projected for industrial evolution because of the reduction in the experimental volumes and, accordingly, of the used reactants [,]. The opportune designing of starting experimental conditions is notably well accepted by entrepreneurs who can improve the quality and the quantity of goods to be sold. Several examples have already been reviewed and presented in literature [].
Its application to the field of inorganic chemistry synthesis is largely less impactful than that to the field of organic chemistry and drug design, but relevant contributions are emerging in the state of the art [,,,,,,]. Indeed, microfluidic approaches offer a promising route for the synthesis of high-quality and highly monodisperse inorganic nanoparticles. In microfluidic reactors, fluids flow in a parallel fashion, providing mixing of the reactants by diffusion of the molecules across the interface between the fluids []. The limited dimension of the reactor and the large ratio of surface to volume ensures thermal homogeneity across the reactor as well as a tight control on the mass and heat transfer. Therefore, it can be assumed that the reaction mixture is homogeneous with respect to the concentration and temperature []. In addition, microfluidic approaches, suitably optimised, allow the temporal separation of the two steps of nucleation and growth of nanoparticles, since the nucleation initiates with the efficient rapid mixing of the reactants in the microreactor and subsequently the growth of particles occurs, allowing the generation of nanoparticles with highly precise sizes and shapes [,].
In relation to microfluidics, and within a colloidal regime framework, DoE approaches are also useful for smartly controlling the nanoscale interactions of colloidal building blocks in order to obtain high-throughput functional materials. In particular, supraparticles (i.e., complex spherical structures obtained by self-assembling of evaporated emulsion droplets in flow reactors) yield is strongly affected by different physico-chemical properties (size, dispersity, porosity, and so on) that need rationalisation. In a recent paper [], the authors showed the possibility of up-scaling the continuous flow production of these structures in a defined range size (from 0.1 to 10 micron) by carefully tuning the involved experimental parameters.
A further contribution [], concerning a design of experiment approach for flow synthesis, was applied to the synthesis of citrate functionalised calcium phosphate CaP NPs aided by sonication using a continuous flow wet chemical precipitation, and the effect of some of the most relevant process factors (i.e., reactant flow rate, sonication amplitude, and maturation time) on the physico-chemical properties of the NPs were evaluated. From the statistical data analysis, the authors evidenced that CaP NP dimensions are influenced by the reactor flow rate, while the crystalline domain dimensions and product purity are influenced by the maturation process.
Even metal-organic frameworks (MOFs) are promising candidates for DoE application: Indeed, the daily productivity (defined as kg of MOF m−3d−1) by microfluidics can be boosted by several orders of magnitude by proper optimisation of synthesis parameters such as residence time, linker concentration, metal/linker volumetric ratio, and solvent choice [].
Another interesting example is the effort to optimise the parameters in a reactor flow during the synthesis of biocatalysts exploiting 3D printed scaffolds []: In such a way, the system automatically chooses the best result of the DoE analysis and runs the experiments by remote controlling the equipment, saving time and avoiding human intervention.
Other works concerning the application of DoE for flow chemistry reactions, not necessarily related to inorganic chemistry, are present in the literature: one work reports on a liquid-phase dehydrogenation, which is strongly influenced by temperature and liquid flee flow []. A further study in which the amount of carcinogenic reagents for performing a reaction of hydrogenation is strongly reduced [] was reported. A simultaneous self-optimisation strategy combining IR and mass spectrometry [] or exotic nanomole-scale reaction screening optimisation for pharmaceutical purposes [] were reported. Finally, continuous flow synthesis of an anticancer molecule in which an interesting synergic double application of DoE and high-throughput experimentation methodologies was presented [].
3. Conclusions and Perspectives
The increasing contribution of inorganic materials chemistry for strategically relevant technologies such as CO2 reduction and valorisation, heterogeneous catalysis, energy conversion and storage, and electric mobility urgently requires the implementation of rapid, up-scalable, and cost-effective synthetic approaches to highly performing functional inorganic materials. This in turn relies on the systematic screening of several (and in many cases interrelated) experimental parameters and, with the experimental parameters’ landscape dramatically influencing size, size distribution, shape, and morphology, a rational and rapid exploration of these parameters becomes a ruling factor in determining a possible industrial implementation of a given synthesis route. In this framework, DoE can rapidly become a relevant methodological tool supporting inorganic materials’ design and optimisation, also complying with the growing requirement of reducing the water and carbon footprint of the preparation approaches. Nevertheless, it should be pointed out that, particularly in complex synthetic systems and in the presence of tightly interrelated and interdependent parameters, even robust chemical intuition and knowledge are not always sufficient to support a reliable rationalisation. Since not every reaction is suited to be simplified in such a manner, whether a high-throughput screening approach is effective or not has to be considered on a case-by-case basis. In some cases, high throughput becomes only possible at the cost of abstraction and simplification with a concomitant reduction in knowledge gain per individual experiment. Finally, from the multidisciplinary point of view, it is worth noting that robust chemical knowledge and chemical intuition might not be sufficient to rationally address modern inorganic materials’ synthesis, and support from statisticians is required to implement reliable and fruitful DoE.
Author Contributions
All authors to conceptualization, writing and reviewing tasks. All authors have read and agreed to the published version of the manuscript.
Funding
This specific paper received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The University of Padova is gratefully acknowledged for administrative and logistic support to the research activity. S.G. additionally thanks DFG for a Mercator Fellow position at KIT funded by the DFG within the SFB 1441—Project-ID 426888090 and DAAD (Germany) for a visiting professorship at JLU. F.L. and S.G. acknowledge the Interdepartmental Centre Giorgio Levi Cases for Energy Economics and Technology of the University of Padova for the funding of the project AMON-RA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gatti, T.; Lamberti, F.; Mazzaro, R.; Kriegel, I.; Schlettwein, D.; Enrichi, F.; Lago, N.; Di Maria, E.; Meneghesso, G.; Vomiero, A.; et al. Opportunities from Doping of Non-Critical Metal Oxides in Last Generation Light-Conversion Devices. Adv. Energy Mater. 2021, 11, 2101041. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials for Strategic Technologies and Sectors in the EU—A Foresight Study. 2020. Available online: https://data.europa.eu/doi/10.2873/865242 (accessed on 20 December 2021).
- Kloo, L. Inorganic chemistry for renewable energy conversion and storage. Dalton Trans. 2014, 43, 14924–14925. [Google Scholar] [CrossRef] [PubMed]
- Salviulo, G.; Lavagnolo, M.C.; Dabalà, M.; Bernardo, E.; Polimeno, A.; Sambi, M.; Bonollo, F.; Gross, S. Enabling Circular Economy: The Overlooked Role of Inorganic Materials Chemistry. Chem.—Eur. J. 2021, 27, 6676–6695. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press Inc.: Oxford, UK, 2000. [Google Scholar]
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular chemistry to enable a circular economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Clark, J.H.; Zuin, V.G. Rethinking chemistry for a circular economy. Science 2020, 367, 369–370. [Google Scholar] [CrossRef]
- Clark, J.H. Green biorefinery technologies based on waste biomass. Green Chem. 2019, 21, 1168–1170. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Herrero-Davila, L.; Sherwood, J. Circular economy design considerations for research and process development in the chemical sciences. Green Chem. 2016, 18, 3914–3934. [Google Scholar] [CrossRef] [Green Version]
- Linder, M. Ripe for disruption: Reimagining the role of green chemistry in a circular economy. Green Chem. Lett. Rev. 2017, 10, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Loste, N.; Roldán, E.; Giner, B. Is Green Chemistry a Feasible Tool for the Implementation of a Circular Economy? Environ. Sci. Pollut. Res. 2020, 27, 6215–6227. [Google Scholar] [CrossRef]
- Thomas, J.M.; Raja, R. Designing Catalysts for Clean Technology, Green Chemistry, and Sustainable Development. Annu. Rev. Mater. Sci. 2005, 35, 315–350. [Google Scholar] [CrossRef]
- Everts, S. GREEN CHEMISTRY Environmentally friendly synthesis of niacin generates less inorganic waste. Chem. Eng. News 2008, 86, 15. [Google Scholar] [CrossRef]
- Bretos, I.; Diodati, S.; Jiménez, R.; Tajoli, F.; Ricote, J.; Bragaggia, G.; Franca, M.; Calzada, M.L.; Gross, S. Low-Temperature Solution Crystallization of Nanostructured Oxides and Thin Films. Chem.—Eur. J. 2020, 26, 9157–9179. [Google Scholar] [CrossRef]
- Diodati, S.; Dolcet, P.; Casarin, M.; Gross, S. Pursuing the Crystallization of Mono- and Polymetallic Nanosized Crystalline Inorganic Compounds by Low-Temperature Wet-Chemistry and Colloidal Routes. Chem. Rev. 2015, 115, 11449–11502. [Google Scholar] [CrossRef] [PubMed]
- Einarsrud, M.-A.; Grande, T. 1D oxide nanostructures from chemical solutions. Chem. Soc. Rev. 2013, 43, 2187–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabenau, A. The Role of Hydrothermal Synthesis in Preparative Chemistry. Angew. Chem. Int. Ed. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Byrappa, K.Y.M. Handbook of Hydrothermal Technology; Elsevier: New York, NY, USA, 2013. [Google Scholar]
- Walton, R.I. Subcritical solvothermal synthesis of condensed inorganic materials. Chem. Soc. Rev. 2002, 31, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Modeshia, D.R.; Walton, R.I. Solvothermal synthesis of perovskites and pyrochlores: Crystallisation of functional oxides under mild conditions. Chem. Soc. Rev. 2010, 39, 4303–4325. [Google Scholar] [CrossRef]
- European Commission. Report on Critical Raw Materials and the Circular Economy. 2018. Available online: https://data.europa.eu/doi/10.2873/331561 (accessed on 20 December 2021).
- European Commission. List of Critical Raw Materials for the EU (COM/2017/0490 Final). 2017. Available online: https://op.europa.eu/en/publication-detail/-/publication/d34eb321-985d-11e7-b92d-01aa75ed71a1/language-en/format-PDF/source-252345577 (accessed on 20 December 2021).
- Hagelüken, C. Bedeutung des EU Kreislaufwirtschaftspakets für das Metallrecycling. Chem. Ing. Tech. 2017, 89, 17–28. [Google Scholar] [CrossRef]
- Espinoza, L.T.; Schrijvers, D.; Chen, W.-Q.; Dewulf, J.; Eggert, R.; Goddin, J.; Habib, K.; Hagelüken, C.; Hurd, A.J.; Kleijn, R.; et al. Greater circularity leads to lower criticality, and other links between criticality and the circular economy. Resour. Conserv. Recycl. 2020, 159, 104718. [Google Scholar] [CrossRef]
- Benedetti, B.; Caponigro, V.; Ardini, F. Experimental Design Step by Step: A Practical Guide for Beginners. Crit. Rev. Anal. Chem. 2020, 1–14. [Google Scholar] [CrossRef]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Leardi, R. D-Optimal Designs. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 1–11. [Google Scholar]
- Fitzpatrick, D.; Ley, S. Engineering chemistry for the future of chemical synthesis. Tetrahedron 2018, 74, 3087–3100. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.A. The Design of Experiments. Nature 1936, 137, 252–254. [Google Scholar] [CrossRef]
- Papon, R.; Pierlot, C.; Sharma, S.; Shinde, S.M.; Kalita, G.; Tanemura, M. Optimization of CVD parameters for graphene synthesis through design of experiments. Phys. Status Solidi B 2016, 254, 1600629. [Google Scholar] [CrossRef]
- San, F.G.B.; Dursun, S.; Yazici, M.S. Optimization of the PEMFC operating parameters for cathode in the presence of PtCo/CVD graphene using factorial design. Int. J. Energy Res. 2019, 43, 4506–4519. [Google Scholar] [CrossRef]
- Arízaga, G.G.C.; Herrera, G.S.; Fischer, A.; López, O.E.C. Influence of reaction conditions on the growth of GaN rods in an ammono-CVD reactor. J. Cryst. Growth 2011, 319, 19–24. [Google Scholar] [CrossRef]
- Ramadan, Z.; Im, I.-T.; Park, C.W. Process Optimization and Modeling of the Silicon Growth in Trichlorosilane-Hydrogen Gas Mixture in a Planetary CVD Reactor. IEEE Trans. Semicond. Manuf. 2021, 34, 1–8. [Google Scholar] [CrossRef]
- Hößler, D.; Ernst, M. Optimization of a TiSi2 formation based on PECVD Ti using DoE methodology. Solid-State Electron. 2019, 158, 51–58. [Google Scholar] [CrossRef]
- Bucio, T.D.; Khokhar, A.Z.; Lacava, C.; Stankovic, S.; Mashanovich, G.Z.; Petropoulos, P.; Gardes, F.Y. Material and optical properties of low-temperature NH3 -free PECVD SiNx layers for photonic applications. J. Phys. D Appl. Phys. 2017, 50, 025106. [Google Scholar] [CrossRef]
- Kim, K.; Borojevic, N.; Winderbaum, S.; Duttagupta, S.; Zhang, X.; Park, J.; Hameiri, Z. Investigation of industrial PECVD AlOx films with very low surface recombination. Sol. Energy 2019, 186, 94–105. [Google Scholar] [CrossRef]
- Yu, D.; Wang, C.; Cheng, X.; Zhang, F. Optimization of hybrid PVD process of TiAlN coatings by Taguchi method. Appl. Surf. Sci. 2008, 255, 1865–1869. [Google Scholar] [CrossRef]
- Chou, W.-J.; Sun, C.-H.; Yu, G.-P.; Huang, J.-H. Optimization of the deposition process of ZrN and TiN thin films on Si(100) using design of experiment method. Mater. Chem. Phys. 2003, 82, 228–236. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, Z.; Zhong, X.; Shao, F.; Zhao, H.; Zhuang, Y.; Sheng, J.; Ni, J.; Tao, S. Deposition Behavior of PS-PVD Yttria Partially Stabilized Zirconia Coatings. J. Therm. Spray Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Araújo, A.J.; Alves, H.P.; Andrade, R.M.; Campos, L.F.; Macedo, D.A.; Pinho, A.L.; Nascimento, R.M.; Paskocimas, C.A. Designing experiments for the optimization of solid-state synthesis and characterization of alumina-based composites. Ceram. Int. 2019, 45, 8525–8532. [Google Scholar] [CrossRef]
- Chable, J.; Martin, A.; Bourdin, A.; Body, M.; Legein, C.; Jouanneaux, A.; Crosnier-Lopez, M.-P.; Galven, C.; Dieudonné, B.; Leblanc, M.; et al. Fluoride solid electrolytes: From microcrystalline to nanostructured tysonite-type La0.95Ba0.05F2.95. J. Alloy. Compd. 2017, 692, 980–988. [Google Scholar] [CrossRef]
- Ryan, K.; Lengyel, J.; Shatruk, M. Crystal Structure Prediction via Deep Learning. J. Am. Chem. Soc. 2018, 140, 10158–10168. [Google Scholar] [CrossRef]
- Gzyl, A.S.; Oliynyk, A.O.; Adutwum, L.A.; Mar, A. Solving the Coloring Problem in Half-Heusler Structures: Machine-Learning Predictions and Experimental Validation. Inorg. Chem. 2019, 58, 9280–9289. [Google Scholar] [CrossRef]
- Draheim, C.; De Crécy, F.; Hansen, S.; Collnot, E.-M.; Lehr, C.-M. A Design of Experiment Study of Nanoprecipitation and Nano Spray Drying as Processes to Prepare PLGA Nano- and Microparticles with Defined Sizes and Size Distributions. Pharm. Res. 2015, 32, 2609–2624. [Google Scholar] [CrossRef]
- Parikh, K.J.; Sawant, K.K. Comparative Study for Optimization of Pharmaceutical Self-Emulsifying Pre-concentrate by Design of Experiment and Artificial Neural Network. AAPS PharmSciTech 2018, 19, 3311–3321. [Google Scholar] [CrossRef]
- Turk, C.T.S.; Oz, U.C.; Serim, T.M.; Hascicek, C. Formulation and Optimization of Nonionic Surfactants Emulsified Nimesulide-Loaded PLGA-Based Nanoparticles by Design of Experiments. AAPS PharmSciTech 2013, 15, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Box, J.F.; Fisher, R.A. Design of Experiments. Am. Stat. 1980, 34, 1–7. [Google Scholar]
- Antony, J. Fractional Factorial Designs. In Design of Experiments for Engineers and Scientists, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 87–112. [Google Scholar]
- Antony, J. Full Factorial Designs. In Design of Experiments for Engineers and Scientists, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 63–85. ISBN 9780080994178. [Google Scholar]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B 1951, 13, 1–45. [Google Scholar] [CrossRef]
- Arboretti, R.; Ceccato, R.; Pegoraro, L.; Salmaso, L. Design of Experiments and machine learning for product innovation: A systematic literature review. Qual. Reliab. Eng. Int. 2021, 38, 1131–1156. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Eriksson, L.; Johansson, E.; Wikström, C. Mixture design-Design generation, PLS analysis, and model usage. Chemom. Intell. Lab. Syst. 1998, 43, 1–24. [Google Scholar] [CrossRef]
- Holtze, C.; Boehling, R. Batch or flow chemistry?—A current industrial opinion on process selection. Curr. Opin. Chem. Eng. 2022, 36, 100798. [Google Scholar] [CrossRef]
- Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef]
- Zhang, C.; Amar, Y.; Cao, L.; Lapkin, A.A. Solvent Selection for Mitsunobu Reaction Driven by an Active Learning Surrogate Model. Org. Process Res. Dev. 2020, 24, 2864–2873. [Google Scholar] [CrossRef]
- Murray, P.M.; Bellany, F.; Benhamou, L.; Bučar, D.-K.; Tabor, A.B.; Sheppard, T.D. The application of design of experiments (DoE) reaction optimisation and solvent selection in the development of new synthetic chemistry. Org. Biomol. Chem. 2015, 14, 2373–2384. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.W.; Forfar, L.; Liang, X.; Cameron, P.J. Using design of experiment to obtain a systematic understanding of the effect of synthesis parameters on the properties of perovskite nanocrystals. React. Chem. Eng. 2021, 6, 709–719. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Amar, Y.; Schweidtmann, A.M.; Deutsch, P.; Cao, L.; Lapkin, A. Machine learning and molecular descriptors enable rational solvent selection in asymmetric catalysis. Chem. Sci. 2019, 10, 6697–6706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, B.J.; Stevens, J.; Li, J.; Parasram, M.; Damani, F.; Alvarado, J.I.M.; Janey, J.M.; Adams, R.P.; Doyle, A.G. Bayesian reaction optimization as a tool for chemical synthesis. Nature 2021, 590, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Roussel, R.; Gonzalez-Aguilera, J.P.; Kim, Y.-K.; Wisniewski, E.; Liu, W.; Piot, P.; Power, J.; Hanuka, A.; Edelen, A. Turn-key constrained parameter space exploration for particle accelerators using Bayesian active learning. Nat. Commun. 2021, 12, 5612. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, H.; Roebben, G.; Mech, A.; Gibson, N.; Kestens, V.; Linsinger, T.P.J.; Riego Sintes, J. An Overview of Concepts and Terms Used in the European Commission’s Definition of Nanomaterial, EUR 29647 EN; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, G. (Ed.) Nanoparticles; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barglik-Chory, C.; Remenyi, C.; Strohm, H.; Müller, G. Adjustment of the Band Gap Energies of Biostabilized CdS Nanoparticles by Application of Statistical Design of Experiments. J. Phys. Chem. B 2004, 108, 7637–7640. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Atai, M.; Nodehi, A. Design of experiments (DOE) for the optimization of hydrothermal synthesis of hydroxyapatite nanoparticles. J. Braz. Chem. Soc. 2011, 22, 571–582. [Google Scholar] [CrossRef]
- Pu’Ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.; Idris, M.; Lee, T. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Golmohammad, M.; Mirhabibi, A.; Golestanifard, F.; Kelder, E.M. Optimizing Synthesis of Maghemite Nanoparticles as an Anode for Li-Ion Batteries by Exploiting Design of Experiment. J. Electron. Mater. 2015, 45, 426–434. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Sahebi, H.; Zandavar, H.; Mirsadeghi, S. Fabrication of Fe3O4 nanoparticles coated by extracted shrimp peels chitosan as sustainable adsorbents for removal of chromium contaminates from wastewater: The design of experiment. Compos. Part B Eng. 2019, 175, 107130. [Google Scholar] [CrossRef]
- Jalees, M.I. Synthesis and application of magnetized nanoparticles to remove lead from drinking water: Taguchi design of experiment. J. Water Sanit. Hyg. Dev. 2020, 10, 56–65. [Google Scholar] [CrossRef]
- Núñez, R.N.; Veglia, A.V.; Pacioni, N.L. Improving reproducibility between batches of silver nanoparticles using an experimental design approach. Microchem. J. 2018, 141, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Baldassari, S.; Corradi, A.B.; Bondioli, F.; Ferrari, A.; Romagnoli, M.; Villa, C. DOE analyses on aqueous suspensions of TiO2 nanoparticles. J. Eur. Ceram. Soc. 2008, 28, 2665–2671. [Google Scholar] [CrossRef]
- Sani, S.; Adnan, R.; Iqbal, M.A.M. One-step statistical design of experiment for the screening and optimization of magnetite nanoparticles yields from solvothermal synthesis. Microporous Mesoporous Mater. 2020, 312, 110775. [Google Scholar] [CrossRef]
- Duff, D.G.; Ohrenberg, A.; Voelkening, S.; Boll, M. A Screening Workflow for Synthesis and Testing of 10,000 Heterogeneous Catalysts per Day– Lessons Learned. Macromol. Rapid Commun. 2003, 25, 169–177. [Google Scholar] [CrossRef]
- Spolaore, F. Size- and Shape-Controlled Syntheses of Metal and Alloy Nano-Particles by Sustainable and Green Colloidal and Wet Chemistry Routes for Automotive Applications. Ph.D. Thesis, University of Padova, Padua, Italy, 2020. [Google Scholar]
- Franceschini, G.; Macchietto, S. Model-based design of experiments for parameter precision: State of the art. Chem. Eng. Sci. 2008, 63, 4846–4872. [Google Scholar] [CrossRef]
- Khan, R.M. Problem Solving and Data Analysis Using Minitab; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- DeMello, J.; DeMello, A. Microscale Reactors: Nanoscale Products. Lab Chip. 2004, 4, 11–15. [Google Scholar]
- Elvira, K.S.; Solvas, X.C.; Wootton, R.C.R.; de Mello, A.J. The Past, Present and Potential for Microfluidic Reactor Technology in Chemical Synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Taylor, C.J.; Baker, A.; Chapman, M.R.; Reynolds, W.R.; Jolley, K.E.; Clemens, G.; Smith, G.E.; Blacker, A.J.; Chamberlain, T.W.; Christie, S.D.R.; et al. Flow chemistry for process optimisation using design of experiments. J. Flow Chem. 2021, 11, 75–86. [Google Scholar] [CrossRef]
- Tajoli, F.; Dengo, N.; Mognato, M.; Dolcet, P.; Lucchini, G.; Faresin, A.; Grunwaldt, J.-D.; Huang, X.; Badocco, D.; Maggini, M.; et al. Microfluidic Crystallization of Surfactant-Free Doped Zinc Sulfide Nanoparticles for Optical Bioimaging Applications. ACS Appl. Mater. Interfaces 2020, 12, 44074–44087. [Google Scholar] [CrossRef] [PubMed]
- Dengo, N.; Faresin, A.; Carofiglio, T.; Maggini, M.; Wu, L.; Hofmann, J.P.; Hensen, E.J.M.; Dolcet, P.; Gross, S. Ligand-free ZnS nanoparticles: As easy and green as it gets. Chem. Commun. 2020, 56, 8707–8710. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hassan, A.; Sandre, O.; Cabuil, V. Microfluidics in Inorganic Chemistry. Angew. Chem. Int. Ed. 2010, 49, 6268–6286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.M.; Mathies, A.R.A.; Alivisatos, A.P. Size-Controlled Growth of CdSe Nanocrystals in Microfluidic Reactors. Nano Lett. 2003, 3, 199–201. [Google Scholar] [CrossRef]
- Nightingale, A.M.; de Mello, J.C. Microscale synthesis of quantum dots. J. Mater. Chem. 2010, 20, 8454–8463. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Bannock, J.H.; Krishnadasan, S.H.; O’Mahony, F.T.F.; Haque, S.A.; Sloan, J.; Drury, C.; McIntyre, R.; Demello, J.C. Large-scale synthesis of nanocrystals in a multichannel droplet reactor. J. Mater. Chem. A 2013, 1, 4067–4076. [Google Scholar] [CrossRef] [Green Version]
- Edel, J.B.; Fortt, R.; Demello, J.C.; Demello, A.J. Microfluidic routes to the controlled production of nanoparticlesElectronic supplementary information ESI available: Image of the central portion of the micromixer chip. See http://www.rsc.org/suppdata/cc/b2/b202998g/. Chem. Commun. 2002, 1136–1137. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Plunkett, A.; Eldridge, C.; Schneider, G.A.; Domènech, B. Controlling the Large-Scale Fabrication of Supraparticles. J. Phys. Chem. B 2020, 124, 11263–11272. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Dotti, A.; Adamiano, A.; Fabbi, C.; Quarta, E.; Colombo, P.; Catalucci, D.; De Luca, C.; Iafisco, M. Calcium Phosphate Nanoparticle Precipitation by a Continuous Flow Process: A Design of Experiment Approach. Crystals 2020, 10, 953. [Google Scholar] [CrossRef]
- Bagi, S.D.; Yuan, S.; Rojas-Buzo, S.; Shao-Horn, Y.; Román-Leshkov, Y. A continuous flow chemistry approach for the ultrafast and low-cost synthesis of MOF-808. Green Chem. 2021. [Google Scholar] [CrossRef]
- Valotta, A.; Maier, M.C.; Soritz, S.; Pauritsch, M.; Koenig, M.; Brouczek, D.; Schwentenwein, M.; Gruber-Woelfler, H. 3D printed ceramics as solid supports for enzyme immobilization: An automated DoE approach for applications in continuous flow. J. Flow Chem. 2021, 11, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Tan, J.; Davidson, M.G.; Bull, S.; Hutchby, M.; Mattia, D.; Plucinski, P. Continuous-flow liquid-phase dehydrogenation of 1,4-cyclohexanedione in a structured multichannel reactor. React. Chem. Eng. 2018, 4, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Örkényi, R.; Beke, G.; Riethmüller, E.; Szakács, Z.; Kóti, J.; Faigl, F.; Éles, J.; Greiner, I. Environmentally Friendly Synthesis of Indoline Derivatives using Flow-Chemistry Techniques. Eur. J. Org. Chem. 2017, 2017, 6525–6532. [Google Scholar] [CrossRef] [Green Version]
- Fath, V.; Lau, P.; Greve, C.; Weller, P.; Kockmann, N.; Röder, T. Simultaneous self-optimisation of yield and purity through successive combination of inline FT-IR spectroscopy and online mass spectrometry in flow reactions. J. Flow Chem. 2021, 11, 285–302. [Google Scholar] [CrossRef]
- Perera, D.; Tucker, J.W.; Brahmbhatt, S.; Helal, C.J.; Chong, A.; Farrell, W.; Richardson, P.; Sach, N.W. A platform for automated nanomole-scale reaction screening and micromole-scale synthesis in flow. Science 2018, 359, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Biyani, S.A.; Qi, Q.; Wu, J.; Moriuchi, Y.; Larocque, E.A.; Sintim, H.O.; Thompson, D.H. Use of High-Throughput Tools for Telescoped Continuous Flow Synthesis of an Alkynylnaphthyridine Anticancer Agent, HSN608. Org. Process Res. Dev. 2020, 24, 2240–2251. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).