Abstract
Chlorophylls and their derivatives have been extensively studied due to their unique and valuable properties, including their anti-mutagenic and anti-carcinogenic features. Nevertheless, high-purity-level chlorophylls extracted from natural sources are quite expensive because the methods used for their extraction have low selectivity and result in low yields. This study aimed to develop a “greener” and cost-effective technology for the extraction of chlorophylls from biomass using aqueous solutions of ionic liquids (ILs). Several aqueous solutions of ILs, with hydrotropic and surface-active effects were evaluated, demonstrating that aqueous solutions of surface-active ILs are enhanced solvents for the extraction of chlorophylls from spinach leaves. Operating conditions, such as the IL concentration and solid–liquid ratio, were optimized by a response surface methodology. Outstanding extraction yields (0.104 and 0.022 wt.% for chlorophyll a and b, respectively, obtained simultaneously) and selectivity (chlorophyll a/b ratio of 4.79) were obtained with aqueous solutions of hexadecylpyridinium chloride ([C16py]Cl) at moderate conditions of temperature and time. These extraction yields are similar to those obtained with pure ethanol. However, the chlorophyll a/b ratio achieved with the IL aqueous solution is higher than with pure ethanol (3.92), reinforcing the higher selectivity afforded by IL aqueous solutions as viable replacements to volatile organic compounds and allowing the obtainment of more pure compounds. Finally, the recovery and reuse of the solvent were evaluated by using a back-extraction step of chlorophylls using ethyl acetate. The results disclosed here bring new perspectives into the design of new approaches for the selective extraction of chlorophylls from biomass using aqueous solutions of surface-active ILs.
1. Introduction
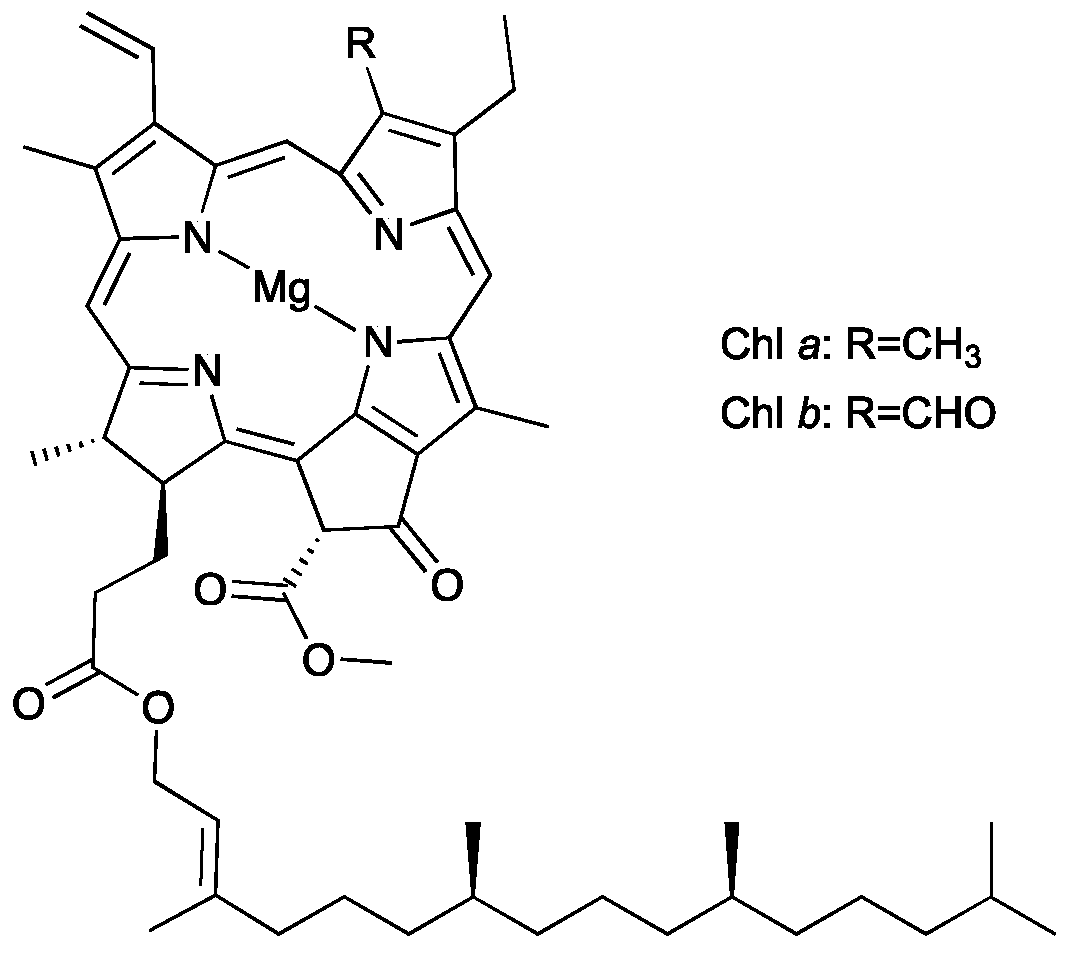
Chlorophylls are the main photoreceptors in photosynthesis and common are in nature; they are found in plants, algae and cyanobacteria [1]. Regarding plants, two chemical forms can be distinguished, namely chlorophyll a and b, usually present at a ratio of 3:1 [2]. Chlorophylls’ structure consists of a basic skeleton of porphyrin with a magnesium ion at the center of the aromatic plane. The difference between the two forms of chlorophyll consists in the presence of a methyl group at the C3 carbon for chlorophyll a, while in chlorophyll b a formyl group was found at the same position (Figure 1) [1,3,4].
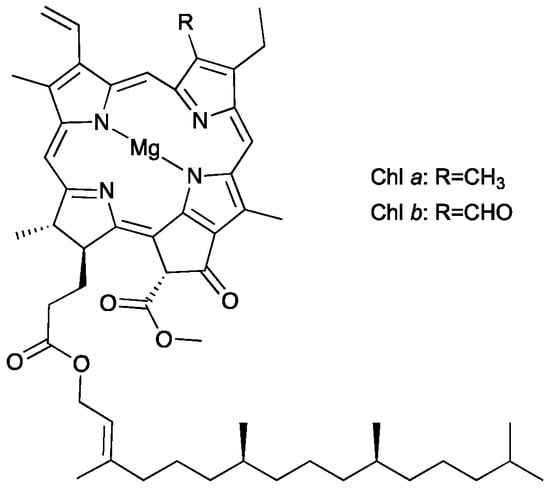
Figure 1.
Chemical structure of chlorophyll a and b.
In the last years, several studies have demonstrated the positive effects of natural pigments in human health [5]. Chlorophylls have been described as presenting anti-mutagenic and anti-carcinogenic properties due to their ability to accumulate in tumor tissues and to cause a photodynamic effect under laser radiation, producing reactive oxygen species and resulting in cancer cell death [6]. In addition, some data reported that chlorophylls were able to accelerate wound healing [1], help in cellular repair and increase hemoglobin levels in blood [7]. Recently, it was demonstrated that chlorophyll derivatives extracted from spinach are able to prevent oxidative DNA damage of human lymphocytes [8]. In addition to their recent medicinal applications, chlorophylls are extensively used as a natural colorant in food and cosmetic industries [1].
The extraction of chlorophylls, which are highly lipophilic compounds, from natural sources is usually carried out with acetone, dimethylsulfoxide, dioxane, ethanol and dimethylformamide [9]. In addition to the long processing times and high temperatures required, these solvents result in extracts with low selectivity and/or low yield [10]. The process can be even more complex and costly if isolated chlorophyll a or b are aimed.
Considering that chlorophylls are highly hydrophobic compounds with negligible solubility in water [11], the introduction of additives with co-solvency, hydrotropy or tensioactive effects to increase their solubility in water and improve extraction yield could be seen as a favorable approach. In this field, the use of aqueous solutions of ionic liquids (ILs) as alternative solvents can be considered. ILs are organic salts usually composed of poorly coordinated large organic cations and inorganic or organic anions. The first definition of ILs was proposed by Walden et al. [12] as salts with melting temperatures below the boiling point of water (100 °C) in order to differentiate these from typical salts. In the last years, the definition of ILs has been reviewed and updated by different authors [13,14]. Silva et al. [13], based on the phase-forming ability trend to create aqueous biphasic systems as a function of temperature, showed that ILs are organic salts capable of establishing a much wider range of interactions than inorganic salts, particularly dispersive-type interactions that are not possible in inorganic salts. More recently, Mariani et al. [14] proposed that “ILs are water-free organic salts with a melting temperature lower than decomposition temperature.” ILs have attracted high attention due to their outstanding properties, such as negligible vapor pressure, non-flammability, high thermal and chemical stabilities and enhanced solvation ability for organic, inorganic and organometallic compounds [15,16]. These characteristics are dependent on IL nature, meaning that the appropriate combination of their ions can be used to tune their properties [17]. However, the fact that they have negligible vapor pressure is not enough to claim that these compounds are truly “green,” although losses to the atmosphere are completely avoided when compared to traditional volatile organic solvents. In this line, toxicity and biodegradability of ILs must be known or investigated as well. Several studies have been carried out in recent years to assess the toxicity and biodegradability of ILs by varying the alkyl side chain length and number of alkyl groups at the cation and/or by combining various anions and cations [18]. Overall, the cation nature of ILs determines their toxicity, with their toxicity increasing with the increase in length of the alkyl side chain (increase in hydrophobicity) [19,20]. However, the opposite occurs with biodegradability and where the inclusion of ester groups increases their biodegradable nature. Commonly, the anion has a smaller impact in toxicity than the cation [21,22]. Moreover, the cytotoxicity of the ILs is another important topic to address when the final product is envisaged for human consumption. Although the molecular mechanisms behind ILs’ toxicity are still not completely understood, relevant studies have been developed in this line. Recent studies suggested that ILs’ cytotoxicity is directly correlated to cell membrane damage—increasing the alkyl chain length of one of the cation alkyl substituents results in higher toxicity [23]. Losada-Pérez et al. [23] investigated the interaction of amphiphilic 3-methylimidazolium-based ILs with a lipid bilayer as a model cell membrane to understand these ILs’ cytotoxicity at a molecular level. The authors concluded that the amphiphilic ILs essentially interact with lipid membranes such as ionic surfactants, suggesting that the cytotoxicity of amphiphilic ILs could be predicted from the assessment of environmental safety of commonly used ionic surfactants of similar alkyl chain structures and lengths. IL aqueous solutions may act as enhanced solvents in the solid–liquid extraction of high-value compounds from biomass [24,25,26,27,28], which can be achieved by their co-solvency, hydrotropic behavior or micelle formation [29], thus increasing the solubility of target products and extraction yield. In general, aqueous solutions of ILs display higher solubilization and extraction performances relative to small organic compounds when compared to the respective pure solvents, as well as a decreased viscosity [24,29].
Hydrotrope ILs at moderately high concentrations form aggregates with the target solute, which is usually a moderately hydrophobic low molecular weight compound, therefore enhancing its solubility in aqueous media [30,31,32]. The hydrotropic behavior of ILs was shown by determining the solubility of different biomolecules in aqueous solutions of ILs, where a significant increase in solubility (e.g., up to 84-fold for syringic acid) was observed [30]. Considering this solubility improvement, Faria et al. [30] showed that aqueous solutions of hydrotrope ILs are efficient solvents to extract syringic acid from Rocha pear peels, a residue from food industries. On the other hand, surface-active ILs have been reported as promising solvents for increasing the solubility of highly hydrophobic compounds in aqueous solutions. Bica and co-workers [33] reported the extraction of piperine from black pepper using surface-active ILs. Jin et al. [34] proposed a family of water–IL mixtures with amphiphilic anionic functional long-chain carboxylate ILs (LCC-IL) for the simultaneous dissolution of biomass and extraction of hydrophobic bioactive compounds. The authors studied the dissolution mechanism and demonstrated the formation of nanomicelles when tocopherol is dissolved in water/LCC-IL mixtures, meaning that the formation of IL aggregates achieved by the use of surface-active ILs allows the incorporation of hydrophobic bioactive compounds into the micelle core, thereby enhancing extraction yield. More recently, Freire and co-workers [35,36] showed the successful extraction of cynaropicrin and triterpenic acids from C. cardunculus L. leaves and apple peels, respectively, by using aqueous solutions of surface-active ILs. Ventura and co-workers [37] attempted the extraction of phycobiliproteins from red macroalgae Gracilaria sp. The structural features of the ILs were optimized, demonstrating that the more hydrophilic ILs better extract phicobiliproteins, while those of lower hydrophilicity are better extraction solvents for chlorophylls and carotenoids.
In the field of biomass, recent studies have demonstrated the ability of aqueous solutions of surface-active ILs in the extraction of chlorophylls from Spirulina [38] and wild-harvested algae [39]. More specifically, Martins et al. [38] showed that ammonium-based ILs and, in particular, tetradecyltrimethylammonium bromide ([N44414]Br) were able to increase the yield of extraction of chlorophyll a from Spirulina in 25% compared to the conventional methodology. On the other hand, Ventura and co-workers [39] demonstrated that the use of the tributyltetradecylphosphonium chloride ([P44414]Cl) allowed achieving a maximum extraction yield of chlorophyll from wild-harvested algae of 5.96 mg/g dry algae.
Taking into account the valuable properties of chlorophylls as well as the advantages of ILs, we propose here the use of IL aqueous solutions as alternative solvents for the extraction of chlorophylls from spinach leaves by solid–liquid extraction at moderate temperature and time. To this end, an initial screening on various ILs with hydrotrope or surface-active behavior was conducted. After the identification of the most promising ILs, response surface methodologies were applied to optimize the operating conditions of the extractive process, namely the solid–liquid ratio and surface-active IL concentrations. As benchmark, the extraction of chlorophylls using ethanol was also studied. Finally, IL recovery and reuse have been attempted by the back-extraction of chlorophylls. To the best of our knowledge, no attempts have been previously reported in the literature on the use of aqueous solutions of surface-active ILs to improve selectivity in the extraction of chlorophylls or their a/b ratio from biomass.
2. Materials and Methods
2.1. Materials
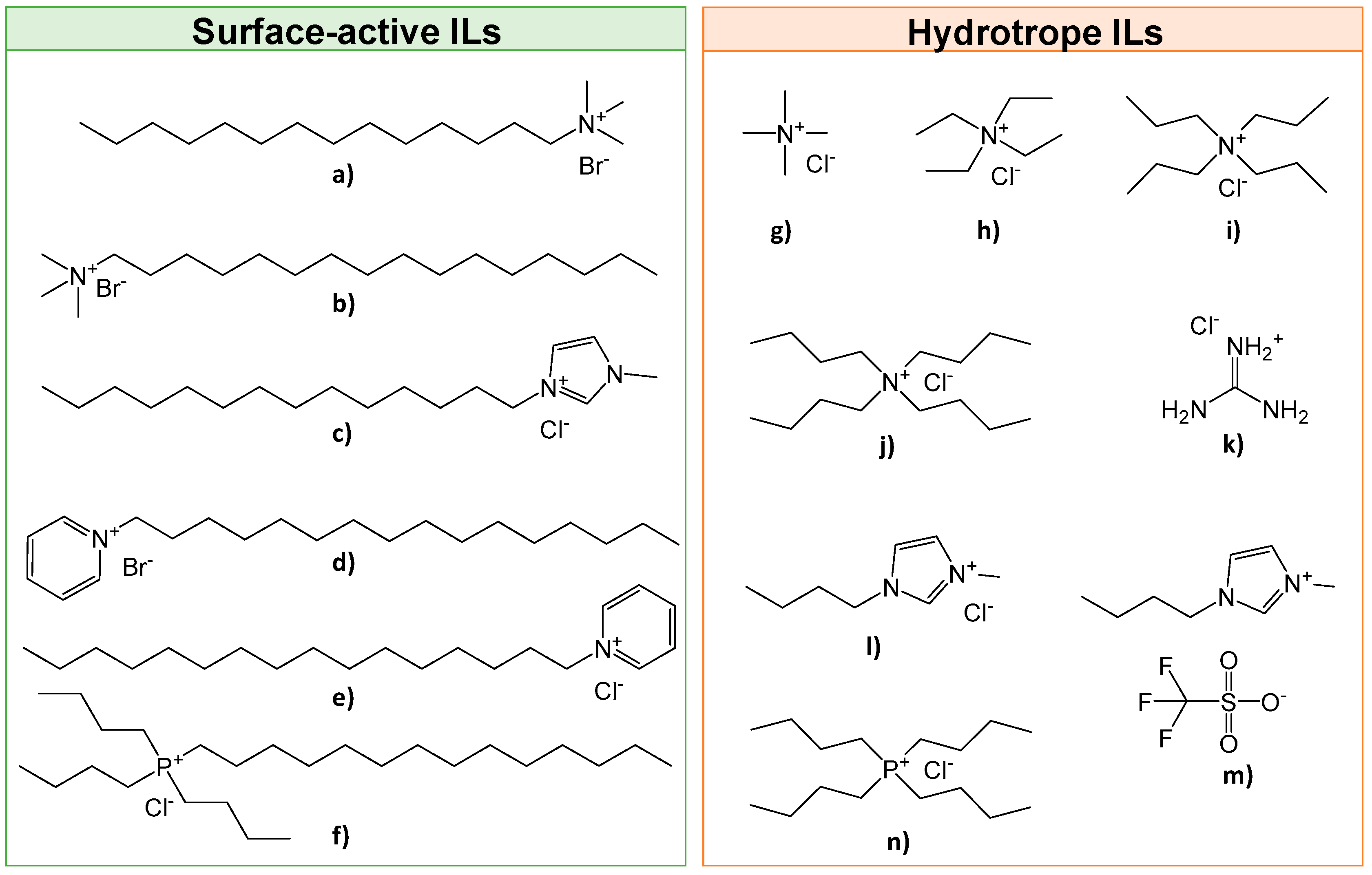
Spinach was purchased at a local market (Auchan Aveiro, distributed by VITACRESS, Aveiro, Portugal) and immediately frozen for storage. Before extraction, spinach (Spinacia oleracea) leaves were frozen with liquid nitrogen and ground by means of a coffee grinder until a green powder was obtained. Chlorophyll a standard (>95 wt.%) and chlorophyll b standard (>99 wt.%) were supplied by Sigma-Aldrich (Algés, Portugal). The ILs used, namely tetramethylammonium chloride, [N1111]Cl (97 wt.%); tetraethylammonium chloride, [N2222]Cl (98 wt.%); tetrapropylammonium chloride, [N3333]Cl (98 wt.%); tetrabutylammonium chloride, [N4444]Cl (97 wt.%); myristyltrimethylammonium bromide, [N11114]Br (99 wt.%); hexadecylpyridinium chloride, [C16py]Cl (99 wt.%); and cetylpyridium bromide, [C16py]Br (98 wt.%), were purchased from Sigma-Aldrich (Algés, Portugal). The ILs 1-methyl-3-tetradecylimidazolium chloride, [C14mim]Cl (98 wt.%); 1-butyl-3-methylimidazolium triflate, [C4mim][CF3SO3] (99 wt.%); and 1-butyl-3-methylimidazolium chloride, [C4mim]Cl (99 wt.%), were purchased from Iolitec (Heilbronn, Germany). Guanidinium chloride, [Gdm]Cl (99 wt.%), was purchased from Merck (Algés, Portugal). IL hexadecyltrimethylammonium bromide, [N16111]Br (99 wt.%) was acquired from Fluka (Porto Salvo, Portugal). The ILs tributyltetradecylphosphonium chloride, [P44414]Cl (97 wt.%), and tetrabutylphosphonium chloride, [P4444]Cl (97 wt.%), were kindly supplied by Cytec Industries Inc. (Carnaxide, Portugal). The chemical structure of all ILs investigated is shown in Figure 2. ChemDraw 19.1© software was used for drawing all the chemical structures of the studied compounds. Before use, all ILs were dried, at least 24 h, under vacuum at moderate temperature (≈80 °C) to remove volatile impurities. The purity of each IL was further confirmed by 1H and 13C NMR spectra.
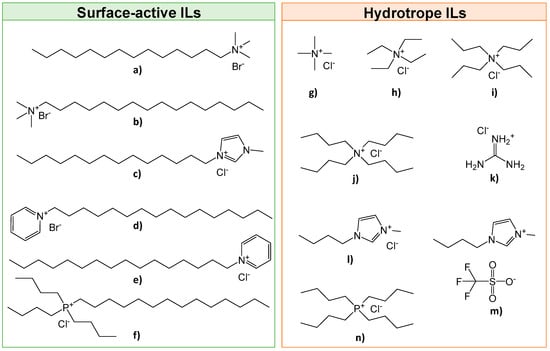
Figure 2.
Chemical structures of the ILs investigated: (a) [N11114]Br; (b) [N16111]Br; (c) [C14mim]Cl; (d) [C16py]Br; (e) [C16py]Cl; (f) [P44414]Cl; (g) [N1111]Cl; (h) [N2222]Cl; (i) [N3333]Cl; (j) [N4444]Cl; (k) [Gdm]Cl; (l) [C4mim]Cl; (m) [C4mim][CF3SO3]; and (n) [P4444]Cl.
2.2. Chlorophylls Extraction
Solid–liquid extractions were carried out in the dark in a Carousel 12 Plus Reaction Station from Radleys Tech (Germany) that is able to both stir and maintain temperature within ±1 °C. In all experiments, the temperature was kept constant at 25 °C and stirring at 600 rpm. At least three individual samples were prepared and quantified, allowing the determination of the average extraction yield and respective standard deviation. All aqueous solutions were prepared gravimetrically within ±10−4 g. Mixtures of spinach ground leaves and extraction solutions were prepared by weight taking into account the solid–liquid ratio. Several concentrations of IL in water, solid–liquid ratio and extraction time were optimized as described below.
After the extraction step, the aqueous solutions were separated from biomass by centrifugation (at 4000 rpm for 30 min using a centrifuge 5804, Eppendorf AG, Nussloch, Germany). The quantification of chlorophylls in each solution was carried out by UV-Vis spectroscopy by using a multi-mode microplate reader Synergy HT, BioTek Instruments (Lexington, MA, USA). Calibration curves were prepared using the commercial standards of chlorophylls a and b in IL aqueous solutions. OriginPro 8.0 was used for the spectral deconvolution of peaks at 649 and 665 nm that correspond to the maximum absorption wavelengths of chlorophylls b and a, respectively. Absorbance was recorded in duplicate for each sample. The amount of chlorophylls a and b in spinach leaves was calculated according to the weight of chlorophylls (a or b) present in the extract divided by the weight of dried biomass used. Selectivity was calculated as the ratio between the content of chlorophyll a and the content of chlorophyll b in each sample.
2.3. Surface Response Methodology
Surface response methodology was applied to simultaneously analyze various operating conditions and allow the identification of the most significant parameters that enhance the chlorophylls’ extraction yield. In a 2k surface response methodology, there are k factors that contribute to a different response, and the data are treated according to a second-order polynomial equation as follows:
where is the response variable and , , and are the adjusted coefficients for the intercept, linear, quadratic and interaction terms, respectively, and and are independent variables. This model allows drawing surface response curves, and by using analysis, the optimal conditions can be determined. Based on the results obtained in the initial screening with several IL, [C16py]Cl IL was selected to perform a 22 factorial planning, with the aim of optimizing the extractive process of chlorophylls from spinach leaves, i.e., the concentration of IL and solid–liquid ratio. A 22 factorial planning has been defined by the central point (zero level), the factorial points (1 and −1, level one) and the axial points (level α)—see Table S1 in the Supplementary Materials. The independent variables coded levels used in factorial planning are presented in Table S2 in Supplementary Materials. The axial points are encoded at a distance α from the central point, as described by Equation (2).
For comparison purposes, a 22 factorial planning was performed with ethanol (Supplementary Materials, provided in Table S3). The obtained results were statistically analyzed with a confidence level of 95%. Student’s t-test was used to check the statistical significance of adjusted data. The adequacy of the model was determined by evaluating the lack of fit, the regression coefficient (R2) and the F-value obtained from the analysis of variance (ANOVA) that was generated. Statsoft Statistica 10.0© software was used for all statistical analyses and for representing response surfaces.
2.4. Chlorophylls Recovery and Solvent Reuse
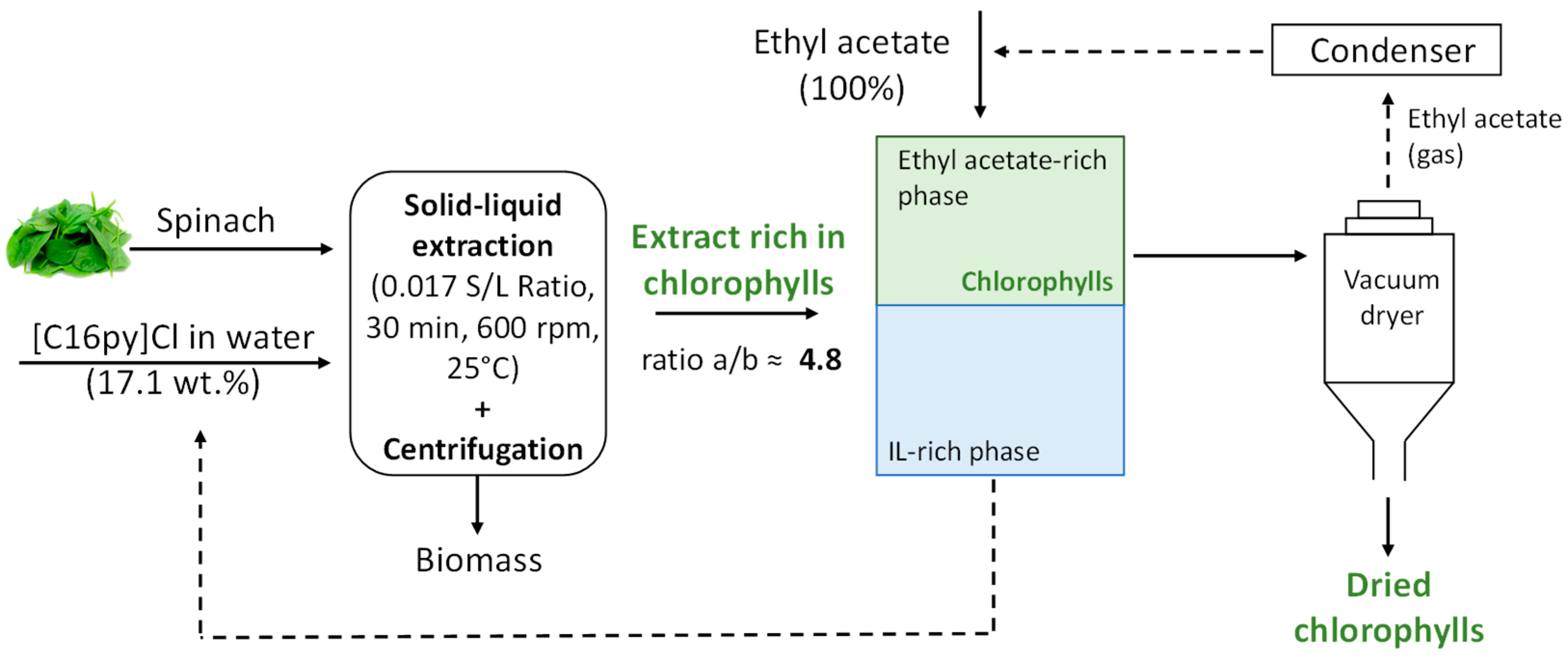
With the goal of developing a more sustainable extraction process, the reuse of the IL aqueous solution as solvent after chlorophyll recovery was addressed. The recovery of chlorophylls from the IL aqueous solution, after solid−liquid extraction, was carried out by liquid–liquid extraction. To this end, ethyl acetate was added to the IL aqueous solutions containing the extract obtained under the optimum operating conditions ([C16py]Cl at 17.1 wt.%, solid–liquid ratio of 0.017, 30 min at 25 °C) in a ratio of 1:1 (v:v). After equilibration, organic and aqueous phases were separated. Chlorophylls were back-extracted to the ethyl acetate (top) phase. After phase separation, the recovered fractions were analyzed by UV-Vis spectroscopy to evaluate the recovery of chlorophylls a and b, as described before. The IL aqueous solution was reused three times with a new sample of grounded spinach, and the respective extraction performance was addressed.
3. Results and Discussion
3.1. Screening of the IL Chemical Structure to Efficiently Extract Chlorophylls from Spinach Leaves
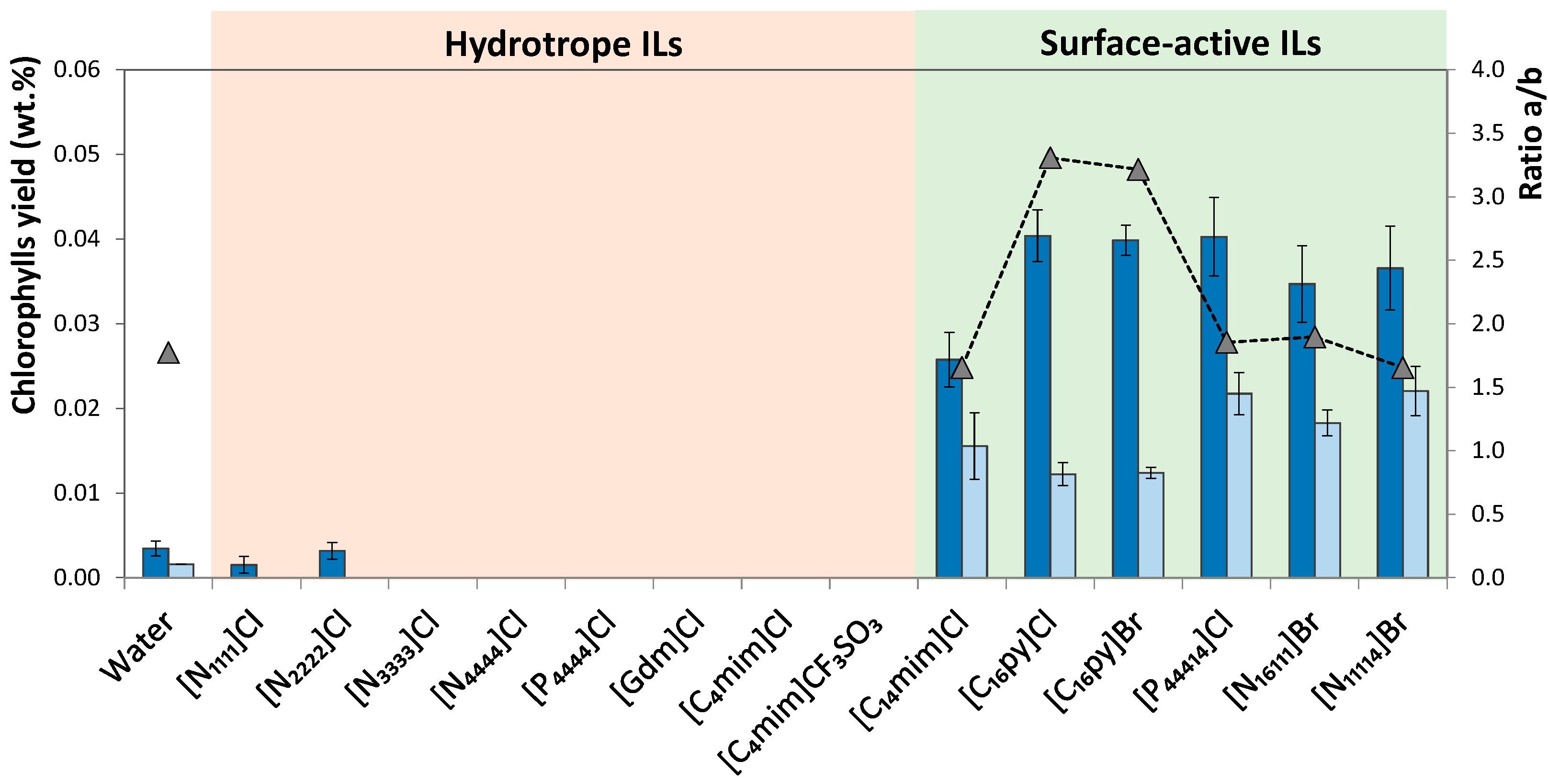
A first screening on aqueous solutions of ILs with hydrotrope or surface-active behavior was carried out in order to evaluate the most promising IL for the extraction of chlorophylls. The same operating conditions were kept in all experiments, namely the IL concentration at 10 wt.%, a spinach-solvent weight fraction ratio of 1:50 (solid–liquid ratio (R) = 0.02) and an extraction time of 30 min at 25 °C. The effect of different ILs on the extraction yield of chlorophylls (ratio between the weight of each extracted chlorophyll and the weight of dried biomass) and selectivity towards chlorophyll a (given by the chlorophylls a/b ratio) is presented in Figure 3, providing as well the same parameters for pure water as solvent
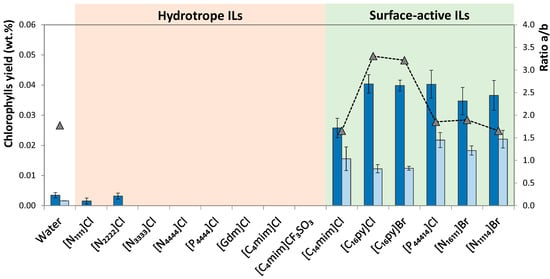
Figure 3.
Yield of extracted chlorophyll a (■) and chlorophyll b (■) from spinach leaves using IL aqueous solutions (IL at 10wt.%; R = 0.02; 30 min; 25 °C; and 600 rpm) and ratio of chlorophyll a/b (▲).
Although the amount of extracted chlorophylls depends on IL chemical structure (anion and cation), in general, surface-active ILs perform better in the extraction of chlorophylls than ILs with hydrotropic characteristics. More specifically, aqueous solutions of most ILs with hydrotrope behavior ([N1111]Cl, [N2222]Cl, [N3333]Cl, [N4444]Cl, [Gdm]Cl, [C4mim]Cl, [C4mim][CF3SO3] and [P4444]Cl) present poor extraction performance, with extractions similar or even worse than pure water (Figure 2). In fact, most of these IL aqueous solutions result in a negligible extraction yield (below the quantification limit). Therefore, hydrotropy is not a relevant main mechanism on promoting chlorophylls’ solubility in aqueous media and further extraction from biomass. On the other hand, extraction efficiency increases significantly with surface-active ILs, i.e., with ILs composed of long alkyl side chains that are able to form micelles in aqueous solutions [40]. The surface-active ILs here studied were [C14mim]Cl, [C16py]Cl, [C16py]Br, [P44414]Cl, [N16111]Br and [N14111]Br. Taking into consideration the critical micellar concentration (CMC) values for the surfactant ILs under study (cf. Table S4), it is possible to guarantee that the concentrations of all ILs are well above their CMC, thus allowing the formation of micelles and the incorporation of chlorophylls, which are hydrophobic compounds, in the ILs’ micelle core. The yields obtained with these ILs is significantly higher than those achieved with water or with aqueous solutions of hydrotrope ILs. Thus, the importance of the surface activity of the IL applied and micelles formation is evident when attempting chlorophyll extraction from spinach leaves. When using IL with surfactant characteristics, solubility and extraction yield enhancement occur due to IL self-aggregation and the possibility of incorporating hydrophobic solutes in the micelle core, being usually more pronounced at low concentrations of IL, close to CMC. This behavior is in agreement with that observed by Faria et al. [35] and Martins et al. [38] in the extraction of triterpenic acids from apples and chlorophyll a from Spirulina maxima, respectively.
In order to evaluate extraction selectivity, the ratio between chlorophyll a and chlorophyll b is also shown in Figure 3. Taking into account that the ratio of chlorophyll a and b in plants is around 3:1, the extraction process is selective if the calculated ratio is higher than 3. The results obtained reveal that ILs [C16py]Cl and [C16py]Br are able to isolate higher amounts of chlorophyll a, where ratios of 3.31 and 3.22, respectively, were achieved. Compared with pure water, where this ratio is 1.77, it is possible to conclude that the addition of IL is advantageous in terms of selectivity towards chlorophyll a.
Overall, ILs [P44414]Cl, [C16py]Br and [C16py]Cl are the most promising ILs for the extraction of chlorophylls from spinach leaves. Despite the results obtained with [P44414]Cl, they are slightly better than those obtained with [C16py]Cl, and the latter presents a higher selectivity value; as such, it was chosen for optimizing the operating conditions to improve extraction process performance. Although IL [N44414]Br was efficient in the extraction of chlorophyll a from Spirulina, resulting in an increased yield of 25% compared to conventional methodology [38], in our work pyridinium-based and phosphonium-based ILs promoted better yields in the extraction of chlorophylls from spinach leaves, revealing that IL performance largely depends on biomass nature.
3.2. Optimization of the Operating Conditions by Response Surface Methodology
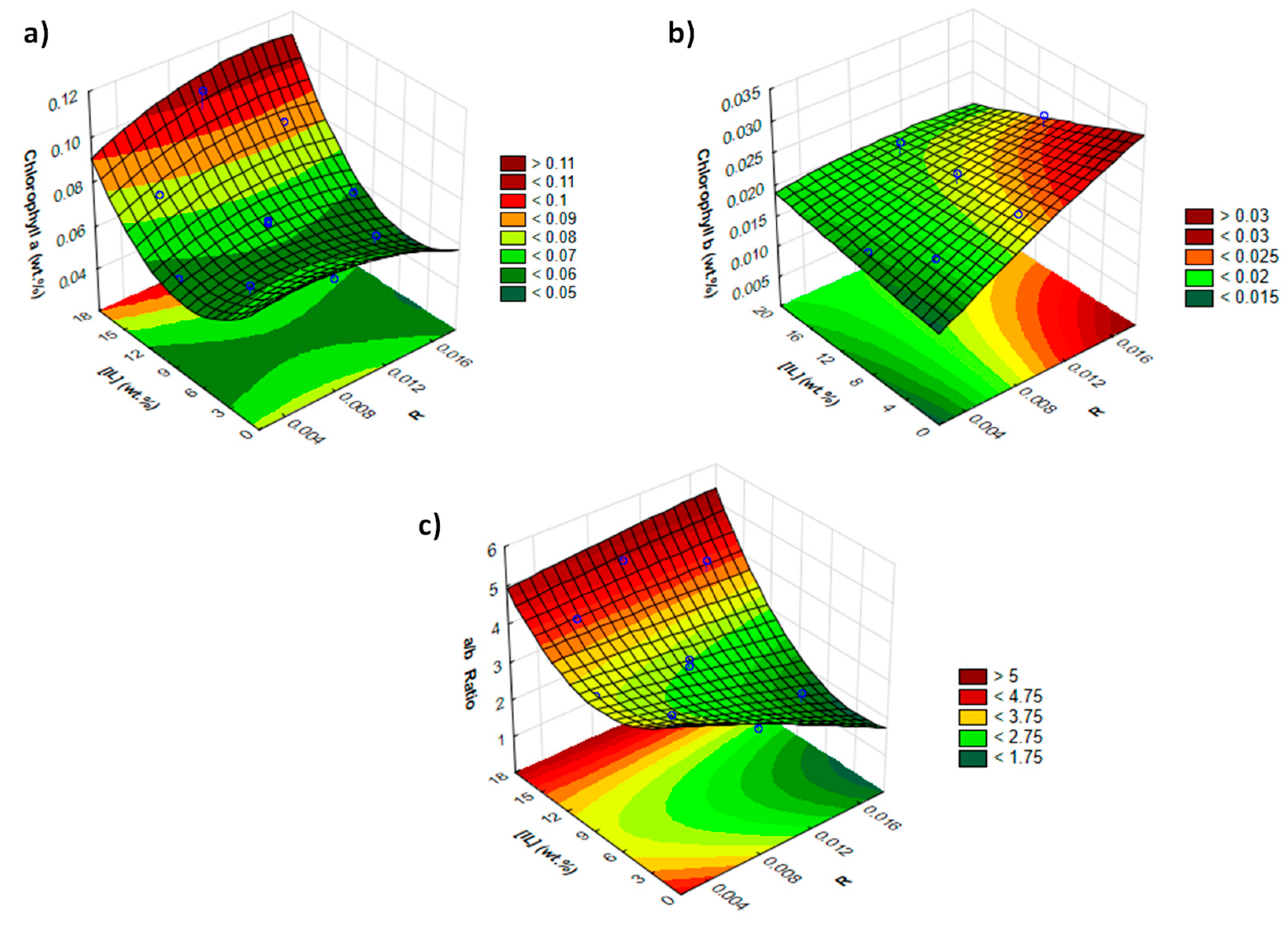
The enhanced extraction yields and selectivity obtained with surface-active IL aqueous solutions motivated the optimization of process operating conditions. The effect of extraction time (from 5 to 60 min) was initially evaluated (see Figure S1 in the Supplementary Materials) using a 10 wt.% [C16py]Cl solution. Results showed that the extraction time does not seem to affect the chlorophylls extraction after 30 min, and this time was then used in the following experiments. With the aim of optimizing the extraction process of chlorophylls from spinach leaves, a response surface methodology was then used. Two responses were investigated, namely extraction yield and selectivity. To this end, factorial planning 22 (2 factors and 2 levels) was performed. Based on the results obtained in the initial screening, the first factorial planning was carried out with [C16py]Cl, where the IL concentration and spinach–solvent ratio were optimized. At this stage, extraction time was maintained at 30 min, and temperature was maintained at 25 °C. The response-surface graphs obtained are depicted in Figure 4. Variance analysis (ANOVA) was used to estimate the statistical significance of variables and the interactions between them. The experimental points, model equation, the yield of chlorophylls experimentally obtained and the respective calculated values, as well as the complete statistical analysis, are provided in Supplementary Materials (Tables S5–S11 and Figures S2 and S3).
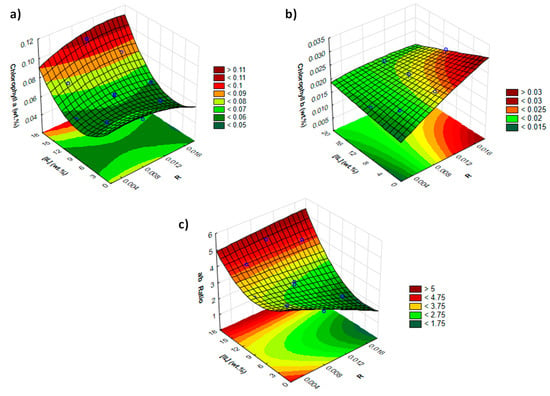
Figure 4.
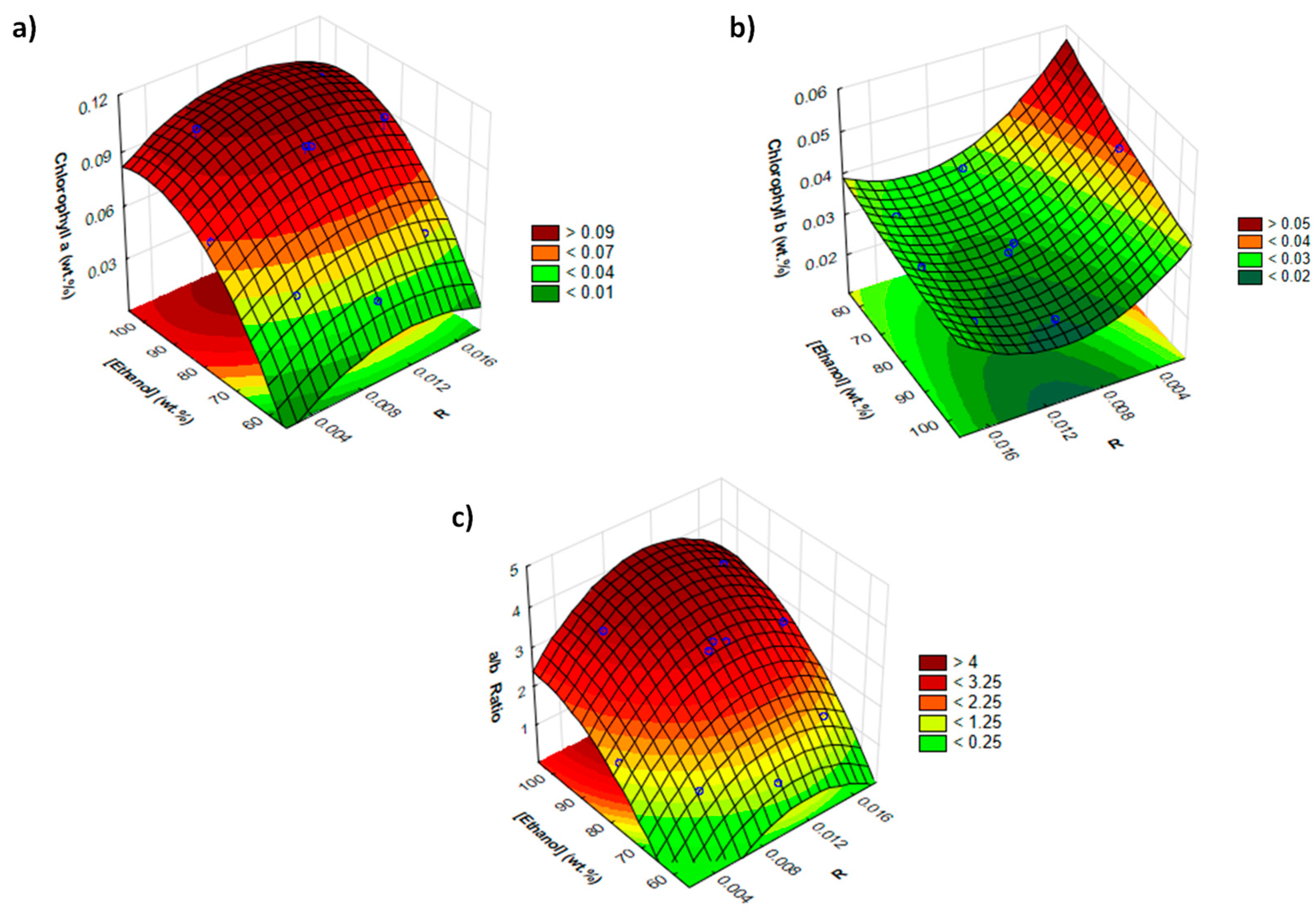
Response surface with combined effects of solid–liquid ratio (R) and [C16py]Cl concentration ([IL] wt.%) on the (a) extraction yield of chlorophyll a (wt.%); (b) extraction yield of chlorophyll b (wt.%); and (c) chlorophyll a/b ratio.
From the presented factorial planning results, obtained by using the response surface methodology, three distinct behaviors occur according to the response under study. When using high IL concentrations and at any solid–liquid ratio, higher chlorophyll a yields were obtained as well as a higher selectivity (Figure 4a,c). On the other hand, in order to obtain a higher chlorophyll b yield, it is necessary to use low IL concentrations in aqueous solutions and a high solid–liquid ratio (Figure 4b). The respective statistical data also show different behaviors, as observed in Pareto charts shown in Supplementary Materials (Figure S2). For chlorophyll a, IL concentration is the only significant parameter with a positive effect. On the other hand, for chlorophyll b, the solid–liquid ratio is the only significant parameter that also has a positive effect. Finally, for the chlorophyll a/b ratio, both variables (solid–liquid ratio and IL concentration) have a significant impact, which is expected since these results are counterbalanced by the effects ruling the extraction of each type of chlorophyll.
Overall, extraction yields of 0.105 and 0.027 wt.% for chlorophyll a and b, respectively (Figure 4a,b), were obtained. For chlorophyll a, these values were obtained by using an aqueous solution of 17.1 wt.% of [C16py]Cl and a solid–liquid ratio of 0.010 at 25 °C, with 30 min of extraction. For chlorophyll b, an aqueous solution of 5.0 wt.% of [C16py]Cl and a solid–liquid ratio of 0.015 at 25 °C during 30 min was used. Leite et al. [41] also studied the extraction of chlorophylls from spinach using non-ionic surfactants, with the results related to the extraction yield of chlorophylls being similar to ours. The authors reported a total chlorophyll extraction yield of 0.094 wt.% under optimal operating conditions (C11-C13 9EO’s at 12.4 mM, a solid–liquid ratio of 0.007, a temperature of 41 °C and 30 min of extraction time) [41].
In the current study, high values of chlorophyll a/b ratio or of selectivity towards chlorophyll a were achieved, i.e., a value of 4.68 (Figure 4c), by using the extraction conditions that result in the best yield of chlorophyll a. Leite et al. [41] did not optimize solvent selectivity but commented on the relevance of hydrophilic–lipophilic balance (HLB) of the solvent (non-ionic surfactant aqueous solutions) on selectivity. Surfactant Brij 98 (with an HLB of 15.3) allowed obtaining an extract rich in chlorophyll a (chlorophyll a/b ratio of 4.71) but with low performance in the yield of chlorophylls extracted (0.007 wt.% of chlorophyll a and 0.003 wt.% of chlorophyll b). Thus, aqueous solutions of surface-active ILs appear as more promising solvents when high yields combined with selectivity towards chlorophyll a is aimed.
For comparison purposes, a factorial design using ethanol as solvent was also applied. The choice of this solvent is related to the fact that it is one of the used conventional organic solvents for the extraction of chlorophylls [42,43], being the most “environmental friendly” one. The results obtained with ethanol result in a maximum yield of chlorophyll a and b of 0.099 and 0.044 wt.%, respectively, and a maximum value of chlorophyll a/b ratio of 3.92, as depicted in Figure 5 (cf. Tables S12–S18 and Figures S4 and S5 in the Supplementary Materials), under similar operating extraction conditions (i.e., solid–liquid ratio, extraction temperature and extraction time). Similar patterns were observed by Derrien et al. [44] in the extraction of chlorophylls from dried spinach leaves using a mixture of ethanol–water mixtures at 93%, during 258 min at 43 °C, achieving values of 0.104 wt.% of total chlorophyll.
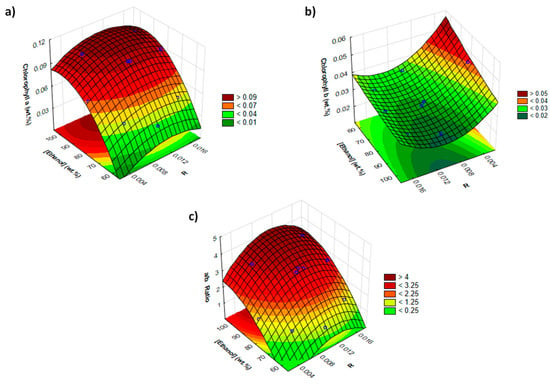
Figure 5.
Response surface on the (a) extraction yield of chlorophyll a (wt.%); (b) extraction yield of chlorophyll b (wt.%) and (c) chlorophyll a/b ratio combined effects of solid–liquid ratio (R) and ethanol concentration ((ethanol) wt.%].
Although the chlorophyll yields obtained with ethanol are similar to those achieved with aqueous solutions of the best surface-active IL investigated, the advantage of the approach here proposed is the selectivity found for chlorophyll a. Taking into account factorial planning results, the optimum extraction conditions identified in this study with the IL aqueous solution correspond to the following: [C16py]Cl at 17.1 wt.% and solid–liquid ratio of 0.017 (see Figure S6 in Supplementary Materials), resulting in a yield of (0.104 ± 0.009) wt.% of chlorophyll a and 4.79 ± 0.10 of chlorophyll a/b ratio. Thus, the results obtained highlight the potential of IL aqueous solutions to selectively extract chlorophyll a from spinach at mild conditions when compared to conventional solvents, as summarized in Table 1.

Table 1.
Summary of the results obtained in this study and in the literature on the extraction of chlorophylls.
The stability of chlorophylls in the extract (obtained under the optimized extraction conditions) was evaluated. More specifically, the extract was stored at 4 °C and protected from light for one month, and its chlorophyll content was measured once a week. The results showed that no significant losses in chlorophyll content occurred during 3 weeks, with visible losses only appearing after 4 weeks of storage. The respective results are provided in Figure S7 and Table S19 in Supplementary Materials.
3.3. Chlorophylls Recovery and Solvent Reuse
After demonstrating that aqueous solutions of surface-active ILs are promising solvents for extracting chlorophylls from biomass, we further investigated their recovery while envisaging the reuse of the IL aqueous solution. The recovery of the chlorophylls was performed by back-extraction using a renewable solvent, namely ethyl acetate, that was placed in contact with the IL aqueous solution containing chlorophylls in a volume ratio of 1:1, as depicted in Figure 6. After chlorophyll liquid–liquid extraction, the concentration of chlorophyll a and b in the IL aqueous solution was reduced from 18.53 mg/L to 0.09 mg/L and 2.38 mg/L to 0.06 mg/L, respectively, allowing its reuse into a new cycle of extraction. We carried out three further cycles of extraction with the recovered IL under optimum extraction conditions ([C16py]Cl at 17.1 wt.%, solid–liquid ratio of 0.017, during 30 min at 25 °C), where the yields of extraction of chlorophyll a and b and chlorophyll ratio a/b were shown to be maintained. Furthermore, the possibility of reusing the solvent in new extraction cycles, as shown above, and the fact that the process employs an aqueous solution of IL instead of a pure organic solvent demonstrate the higher sustainable character of the developed process. Although it may raise the moderate toxicity of [C16py]Cl, which obviously depends on IL amount and organism or cell line being appraised, it should be mentioned that it is currently used in cosmetic products under European regulation [45]. Furthermore, this IL is applied in aqueous solution in a low content and is eliminated from the chlorophylls’ extract, as shown before, further permitting the recovery and reuse of the IL.
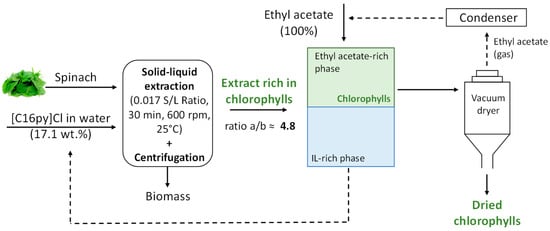
Figure 6.
Schematic process of extraction and recovery of chlorophylls developed.
Figure 6 summarizes the selective chlorophyll extraction method developed using aqueous solutions of surface-active ILs, with the goal of recovering natural chlorophylls, mainly chlorophyll a, that may be employed (at a cheaper cost) in food, nutraceutical, cosmetic or pharmaceutical applications.
4. Conclusions
Aiming at developing a more cost-effective and sustainable process for chlorophyll extraction, we report here the successful application of aqueous solutions of surface-active ionic liquids (ILs) as alternatives solvents. Compared to pure water, extraction with aqueous solutions of surface-active ILs results in a higher amount of extracted chlorophylls, and IL hexadecylpyridinium chloride ([C16py]Cl) is the one that allows achieving a higher yield of chlorophylls extraction, as well as a higher ratio of chlorophylls a/b. Then, response surface methodology was applied to assess the significance of the most important process variables, namely IL concentration and solid–liquid ratio, obtaining extraction yields of 0.104 and 0.022 wt.% for chlorophyll a and b, respectively, and a ratio of chlorophyll a/b of 4.79. These values were achieved by using an aqueous solution of 17.1 wt.% of [C16py]Cl and a solid–liquid ratio of 0.017 during 30 min at 25 °C. A response surface methodology was also applied to ethanol–water mixtures as solvent. Although extraction yields between both types of solvents are similar, improved results in terms of selectivity towards chlorophyll a were achieved with IL aqueous solutions. Finally, the recovery of chlorophylls and reuse of the IL aqueous solution were successfully demonstrated.
The results presented here produce new perspectives on using aqueous solution of surface-active ILs for the selective extraction of chlorophylls from biomass while replacing the traditionally used organic solvents.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/suschem2040040/s1, Figure S1. Yield of extracted chlorophyll a and chlorophyll b from spinach leaves using 10 wt.% [C16py]Cl solution in water and different extraction times (R = 0.02; T = 25 °C) and chlorophyll a/b ratio; Figure S2. Pareto charts for the standardized main effects in factorial planning using [C16py]Cl aqueous solutions for (i) chlorophyll a, (ii) chlorophyll b and (iii) chlorophyll a/b ratio. Vertical line indicates the statistical significance of the effects; Figure S3. Observed values vs. predicted values in factorial planning [C16py]Cl aqueous solutions (i) chlorophyll a, (ii) chlorophyll b and (iii) chlorophyll a/b ratio; Figure S4. Pareto charts for the standardized main effects in factorial planning using ethanol solutions for (i) chlorophyll a, (ii) chlorophyll b and (iii) chlorophyll a/b ratio. Vertical line indicates the statistical significance of the effects; Figure S5. Observed values vs. predicted values in factorial planning ethanol solutions (i) chlorophyll a, (ii) chlorophyll b and (iii) chlorophyll a/b ratio; Figure S6. Profile of prediction of the optimal operating conditions for obtaining the maximum yield of chlorophyll a and chlorophyll a/b ratio; Figure S7. Chlorophyll a (■) and b (■) contents in the extract during storage time, at 4 °C and protected from light; Table S1. 22 factorial planning; Table S2. Independent variables coded levels used in factorial planning using [C16py]Cl aqueous solutions; Table S3. Independent variables coded levels used in factorial planning using ethanol solutions; Table S4. Surface-active ionic liquids used in this study and their respective critical micellar concentrations (CMC) and IL concentration values (in mM) applied in chlorophyll extraction—IL screening; Table S5. Experimental data and response surface predicted values of the factorial planning using [C16py]Cl aqueous solutions; Table S6. Regression coefficients of the predicted second-order polynomial model for chlorophyll a, from factorial planning using [C16py]Cl aqueous solutions. R-sqr = 0.95436; Adj.:90871; Table S7. Regression coefficients of the predicted second-order polynomial model for chlorophyll b, from factorial planning using [C16py]Cl aqueous solutions. R-sqr = 0.78277; Adj.:56555; Table S8. Regression coefficients of the predicted second-order polynomial model for chlorophyll a/b ratio, from factorial planning using [C16py]Cl aqueous solutions. R-sqr = 0.94078; Adj.:88156; Table S9. ANOVA results for chlorophyll a extraction from factorial planning using [C16py]Cl aqueous solutions; Table S10. ANOVA results for chlorophyll b extraction from factorial planning using [C16py]Cl aqueous solutions; Table S11. ANOVA results for chlorophyll a/b ratio extraction from factorial planning using [C16py]Cl aqueous solutions; Table S12. Experimental data and response surface predicted values of factorial planning using ethanol aqueous solutions; Table S13. Regression coefficients of the predicted second-order polynomial model for chlorophyll a, from factorial planning using ethanol solutions. R-sqr = 0.96244; Adj.:92487; Table S14. Regression coefficients of the predicted second-order polynomial model for chlorophyll b, from factorial planning using ethanol solutions. R-sqr = 0.92847; Adj.:85693; Table S15. Regression coefficients of the predicted second-order polynomial model for chlorophyll a/b ratio, from factorial planning using ethanol solutions. R-sqr = 0.98187; Adj.:96374; Table S16. ANOVA results for chlorophyll a extraction from factorial planning using ethanol solutions; Table S17. ANOVA results for chlorophyll b extraction from factorial planning using ethanol solutions; Table S18. ANOVA results for chlorophyll a/b ratio extraction from factorial planning using ethanol solutions; Table S19. Chlorophyll a and b contents in the extract during storage time at 4 °C and protected from light.
Author Contributions
Conceptualization, J.A.P.C. and M.G.F.; methodology, A.M.F. and A.C.L.; software, A.M.F. and A.C.L.; validation, A.M.F. and A.C.L.; formal analysis, A.M.F. and A.C.L.; investigation, A.M.F. and A.C.L.; writing—original draft preparation, A.M.F. and A.C.L.; writing—review and editing, A.M.F., J.A.P.C. and M.G.F.; supervision, J.A.P.C. and M.G.F.; funding acquisition, J.A.P.C. and M.G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was developed within the scope of project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 and UIDP/50011/2020, financed by national funds through FCT/MEC and, when appropriate, co-financed by FEDER under the PT2020 Partnership Agreement. This study was also financially supported by project POCI-01-0145-FEDER-030750 (PTDC/EQU-EPQ/30750/2017)—funded by FEDER, through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (OE), through FCT/MCTES.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hosikian, A.; Lim, S.-B.; Halim, R.; Danquah, M.K. Chlorophyll Extraction from Microalgae: A Review on the Process Engineering Aspects. Int. J. Chem. Eng. 2010, 2010, 391632. [Google Scholar] [CrossRef]
- Huang, S.C.; Hung, C.F.; Wu, W.B.; Chen, B.H. Determination of chlorophylls and their derivatives in Gynostemma pentaphyllum Makino by liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2008, 48, 105–112. [Google Scholar] [CrossRef]
- Makarska-Bialokoz, M.; Kaczor, A.A. Computational Analysis of Chlorophyll Structure and UV-Vis Spectra: A Student Research Project on the Spectroscopy of Natural Complexes. Spectrosc. Lett. 2014, 47, 147–152. [Google Scholar] [CrossRef]
- Cubas, C.; Gloria Lobo, M.; González, M. Optimization of the extraction of chlorophylls in green beans (Phaseolus vulgaris L.) by N,N-dimethylformamide using response surface methodology. J. Food Compos. Anal. 2008, 21, 125–133. [Google Scholar] [CrossRef]
- Schoefs, B. Determination of pigments in vegetables. J. Chromatogr. A 2004, 1054, 217–226. [Google Scholar] [CrossRef]
- Hojnik, M.; Škerget, M.; Knez, Ž. Isolation of chlorophylls from stinging nettle (Urtica dioica L.). Sep. Purif. Technol. 2007, 57, 37–46. [Google Scholar] [CrossRef]
- Kong, W.; Liu, N.; Zhang, J.; Yang, Q.; Hua, S.; Song, H.; Xia, C. Optimization of ultrasound-assisted extraction parameters of chlorophyll from Chlorella vulgaris residue after lipid separation using response surface methodology. J. Food Sci. Technol. 2014, 51, 2006–2013. [Google Scholar] [CrossRef]
- Damerum, A.; Selmes, S.L.; Biggi, G.F.; Clarkson, G.J.; Rothwell, S.D.; Truco, M.J.; Michelmore, R.W.; Hancock, R.D.; Shellcock, C.; Chapman, M.A.; et al. Elucidating the genetic basis of antioxidant status in lettuce (Lactuca sativa). Hortic. Res. 2015, 2, 15055. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.A.; Senge, M.O. How green is green chemistry? Chlorophylls as a bioresource from biorefineries and their commercial potential in medicine and photovoltaics. Photochem. Photobiol. Sci. 2015, 14, 638–660. [Google Scholar] [CrossRef]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.G.; Lee, D.U.; Pan, C.H. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef]
- Ballschmiter, K.; Cotton, T.M.; Strain, H.H.; Katz, J.J. Chlorophyll-water interactions Hydration, dehydration and hydrates of chlorophyll. Biochim. Biophys. Acta Bioenergy 1969, 180, 347–359. [Google Scholar] [CrossRef]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 8, 405–422. [Google Scholar]
- e Silva, F.A.; Pereira, J.F.B.; Kurnia, K.A.; Ventura, S.P.M.; Silva, A.M.S.; Rogers, R.D.; Coutinho, J.A.P.; Freire, M.G. Temperature dependency of aqueous biphasic systems: An alternative approach for exploring the differences between Coulombic-dominated salts and ionic liquids. Chem. Commun. 2017, 53, 7298–7301. [Google Scholar] [CrossRef]
- Mariani, A.; Bonomo, M.; Gao, X.; Centrella, B.; Nucara, A.; Buscaino, R.; Barge, A.; Barbero, N.; Gontrani, L.; Passerini, S. The unseen evidence of Reduced Ionicity: The elephant in (the) room temperature ionic liquids. J. Mol. Liq. 2021, 324, 115069. [Google Scholar] [CrossRef]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 10. [Google Scholar] [CrossRef]
- Gupta, H.; Chandrasekhar, M.; Krishna, T.S.; Sharma, V.K. Thermodynamic properties of mixtures containing 1-butyl-2,3-dimethylimidazolium tetrafluoroborate and cyclopentanone or cyclohexanone. J. Mol. Liq. 2017, 231, 225–237. [Google Scholar] [CrossRef]
- Philippi, F.; Welton, T. Targeted modifications in ionic liquids—from understanding to design. Phys. Chem. Chem. Phys. 2021, 23, 6993–7021. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity—Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; Marques, C.S.; Rosatella, A.A.; Afonso, C.A.M.; Gonçalves, F.; Coutinho, J.A.P. Toxicity assessment of various ionic liquid families towards Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2012, 76, 162–168. [Google Scholar] [CrossRef]
- Ventura, S.P.; Santos, L.D.; Saraiva, J.A.; Coutinho, J.A. Concentration effect of hydrophilic ionic liquids on the enzymatic activity of Candida antarctica lipase B. World J. Microbiol. Biotechnol. 2012, 28, 2303–2310. [Google Scholar] [CrossRef]
- Pernak, J.; Borucka, N.; Walkiewicz, F.; Markiewicz, B.; Fochtman, P.; Stolte, S.; Steudte, S.; Stepnowski, P. Synthesis, toxicity, biodegradability and physicochemical properties of 4-benzyl-4-methylmorpholinium-based ionic liquids. Green Chem. 2011, 13, 2901–2910. [Google Scholar] [CrossRef]
- Arning, J.; Stolte, S.; Böschen, A.; Stock, F.; Pitner, W.-R.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Qualitative and quantitative structure activity relationships for the inhibitory effects of cationic head groups, functionalised side chains and anions of ionic liquids on acetylcholinesterase. Green Chem. 2008, 10, 47–58. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Khorshid, M.; Renner, F.U. Interactions of Aqueous Imidazolium-Based Ionic Liquid Mixtures with Solid-Supported Phospholipid Vesicles. PLoS ONE 2016, 11, e0163518. [Google Scholar]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef]
- Oliveira, N.S.d.; Carlosa, A.L.d.S.; Mattedib, S.; Soaresa, C.M.F.; Souzaa, R.L.; Fricksa, A.T.; Lima, Á. Ionic Liquid-Based Ultrasonic-Assisted Extraction of Alkaloids from Cacao (Theobroma cacao). Chem. Eng. Trans. 2018, 64, 49–54. [Google Scholar]
- Syahmina, A.; Usuki, T. Ionic Liquid-Assisted Extraction of Essential Oils from Thujopsis dolobrata (Hiba). ACS Omega 2020, 5, 29618–29622. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, Q.; Liu, Q.; Tang, F.; Long, Y.; Yao, S. Ionic liquid surfactant-mediated ultrasonic-assisted extraction coupled to HPLC: Application to analysis of tanshinones in Salvia miltiorrhiza bunge. J. Sep. Sci. 2009, 32, 4220–4226. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gomes, H.M.D.; Coutinho, J.A.P.; Freire, M.G. Valorization of Spent Coffee by Caffeine Extraction Using Aqueous Solutions of Cholinium-Based Ionic Liquids. Sustainability 2021, 13, 7509. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Neves, M.C.; Shimizu, K.; Canongia Lopes, J.N.; Freire, M.G.; Coutinho, J.A.P. The magic of aqueous solutions of ionic liquids: Ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015, 17, 3948–3963. [Google Scholar] [CrossRef]
- de Faria, E.L.P.; Ferreira, A.M.; Cláudio, A.F.M.; Coutinho, J.A.P.; Silvestre, A.J.D.; Freire, M.G. Recovery of Syringic Acid from Industrial Food Waste with Aqueous Solutions of Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 14143–14152. [Google Scholar] [CrossRef]
- Abranches, D.O.; Benfica, J.; Soares, B.P.; Ferreira, A.M.; Sintra, T.E.; Shimizu, S.; Coutinho, J.A.P. The impact of the counterion in the performance of ionic hydrotropes. Chem. Commun. 2021, 57, 2951–2954. [Google Scholar] [CrossRef]
- Sales, I.; Abranches, D.O.; Costa, P.; Sintra, T.E.; Ventura, S.P.M.; Mattedi, S.; Coutinho, J.A.P.; Freire, M.G.; Pinho, S.P. Enhancing Artemisinin Solubility in Aqueous Solutions: Searching for Hydrotropes based on Ionic Liquids. Fluid Ph. Equilibria 2021, 534, 112961. [Google Scholar] [CrossRef]
- Ressmann, A.K.; Zirbs, R.; Pressler, M.; Gaertner, P.; Bica, K. Surface-active Ionic Liquids for Micellar Extraction of Piperine from Black Pepper. Z. Naturforsch. B 2013, 68, 1129–1137. [Google Scholar] [CrossRef]
- Jin, W.; Yang, Q.; Huang, B.; Bao, Z.; Su, B.; Ren, Q.; Yang, Y.; Xing, H. Enhanced solubilization and extraction of hydrophobic bioactive compounds using water/ionic liquid mixtures. Green Chem. 2016, 18, 3549–3557. [Google Scholar] [CrossRef]
- de Faria, E.L.P.; Shabudin, S.V.; Claúdio, A.F.M.; Válega, M.; Domingues, F.M.J.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. Aqueous Solutions of Surface-Active Ionic Liquids: Remarkable Alternative Solvents To Improve the Solubility of Triterpenic Acids and Their Extraction from Biomass. ACS Sustain. Chem. Eng. 2017, 5, 7344–7351. [Google Scholar] [CrossRef]
- De Faria, E.L.P.; Do Carmo, R.S.; Cláudio, A.F.M.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Deep Eutectic Solvents as Efficient Media for the Extraction and Recovery of Cynaropicrin from Cynara cardunculus L. Leaves. Int. J. Mol. Sci. 2017, 18, 2276. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Vieira, F.A.; Correia, I.; Ferreira, R.A.S.; Abreu, H.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of phycobiliproteins from the red macroalga Gracilaria sp. using ionic liquid aqueous solutions. Green Chem. 2016, 18, 4287–4296. [Google Scholar] [CrossRef]
- Martins, M.; Albuquerque, C.M.; Pereira, C.F.; Coutinho, J.A.P.; Neves, M.G.P.M.S.; GA Pinto, D.C.; Faustino, M.A.F.; Ventura, S.P.M. Recovery of Chlorophyll a Derivative from Spirulina maxima: Its Purification and Photosensitizing Potential. ACS Sustain. Chem. Eng. 2021, 9, 1772–1780. [Google Scholar] [CrossRef]
- Martins, M.; Fernandes, A.P.M.; Torres-Acosta, M.A.; Collén, P.N.; Abreu, M.H.; Ventura, S.P.M. Extraction of chlorophyll from wild and farmed Ulva spp. using aqueous solutions of ionic liquids. Sep. Purif. Technol. 2021, 254, 117589. [Google Scholar] [CrossRef]
- Goodchild, I.; Collier, L.; Millar, S.L.; Prokeš, I.; Lord, J.C.D.; Butts, C.P.; Bowers, J.; Webster, J.R.P.; Heenan, R.K. Structural studies of the phase, aggregation and surface behaviour of 1-alkyl-3-methylimidazolium halide + water mixtures. J. Colloid Interface Sci. 2007, 307, 455–468. [Google Scholar] [CrossRef]
- Leite, A.C.; Ferreira, A.M.; Morais, E.S.; Khan, I.; Freire, M.G.; Coutinho, J.A.P. Cloud Point Extraction of Chlorophylls from Spinach Leaves Using Aqueous Solutions of Nonionic Surfactants. ACS Sustain. Chem. Eng. 2018, 6, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zhou, Y.; Qin, J.G.; Yao, W.; Ma, Z. Optimization of the Method for Chlorophyll Extraction in Aquatic Plants. J. Freshw. Ecol. 2010, 25, 531–538. [Google Scholar] [CrossRef]
- Tsuji, T.; Mori, T.; Taniguchi, M.; Shimizu, K.; Kobayashi, T. Solvent extraction of plant pigments from leaf protein concentrate. J. Chem. Eng. Jpn. 1985, 18, 539–544. [Google Scholar] [CrossRef][Green Version]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a green process for the extraction of lutein and chlorophyll from spinach by-products using response surface methodology (RSM). LWT—Food Sci. Technol. 2017, 79, 170–177. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety. Opinion on Cetylpyridinium Chloride—Submission II ( COLIPA n° P97); European Union. 2015. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_171.pdf (accessed on 22 November 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).