Use of Pyrolyzed Soybean Hulls as Fillers in Polypropylene and Linear Low Density Polyethylene
Abstract
:1. Introduction
- Add pyrolyzed soybean hull (PSBH) fillers to two polyolefins, linear low-density polyethylene (LLDPE) and polypropylene (PP), in weight percentages of 10%, 20%, 30%, 40%, and 50%.
- Analyze the resulting plastic/PSBH composites for their mechanical, thermal, and water absorption properties.
- Observe the effects that pyrolysis temperature and particle size of the filler have on the properties of the composites.
- Lower the overall cost of products.
- Enhance material properties.
- Lower resource use and environmental impact.
2. Materials and Experimental Procedures
2.1. Materials Used
2.1.1. Polyolefins
2.1.2. Pyrolyzed Soybean Hull (PSBH) Filler
2.2. Experimental Procedures
2.2.1. Particle Size of As-Received PSBH
2.2.2. Particle Size Reduction of PSBH Using Jar Mill
2.2.3. Particle Size of Ground SBH (GSBH)
2.2.4. Sample Preparation for Mechanical Testing
2.2.5. Mechanical Testing
2.2.6. Thickness Swelling and Liquid Uptake
2.2.7. Dynamic Mechanical Analyses (DMA)
2.2.8. Differential Scanning Calorimetry (DSC)
2.2.9. Thermo Gravimetric Analyses (TGA)
2.2.10. Scanning Electron Microscopy (SEM)
2.2.11. Fourier Transform Infrared Spectroscopy (FT-IR)
3. Results and Discussion
3.1. Particle Sizes of As-Received PSBH
3.2. Particle Sizes of GSBH
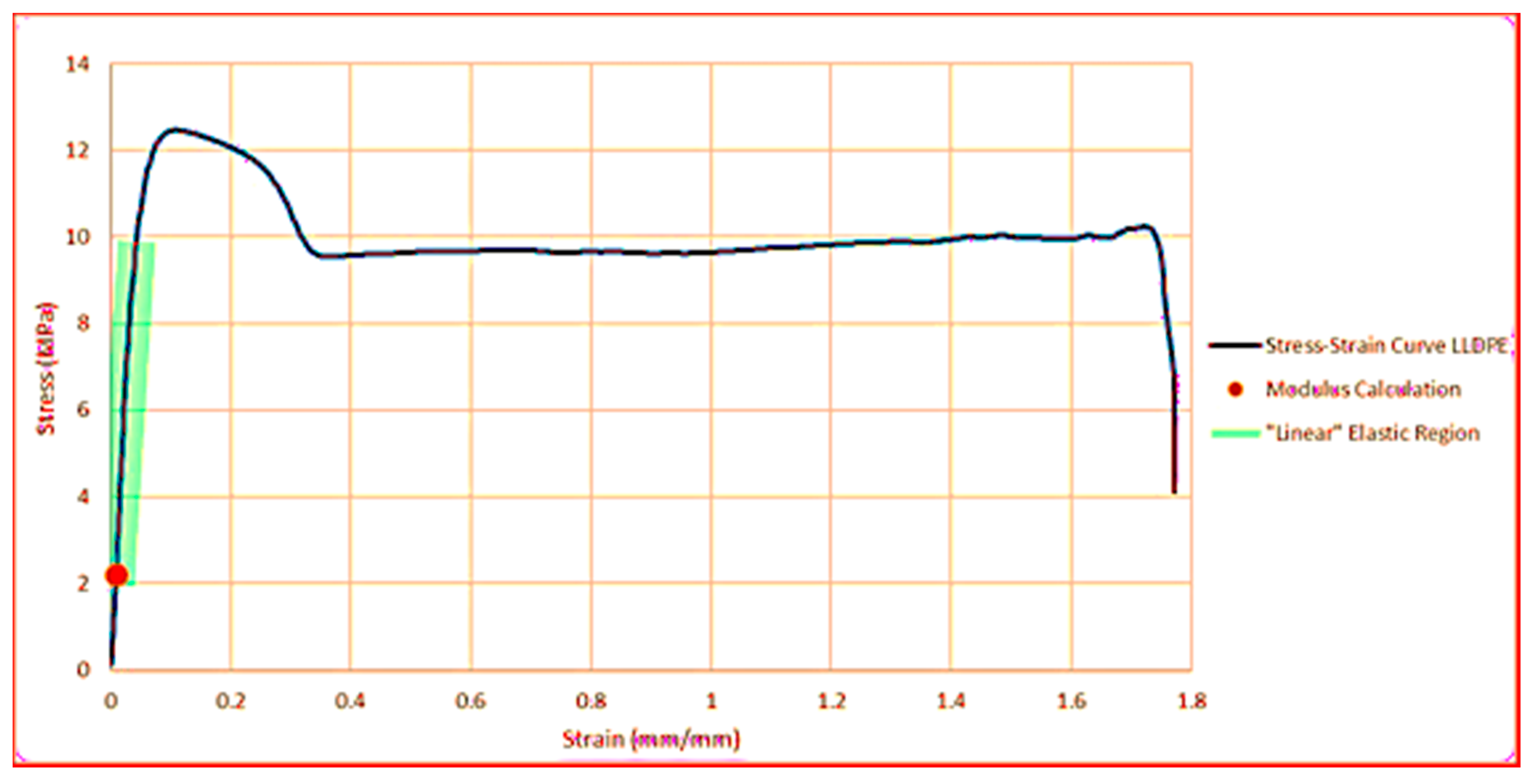
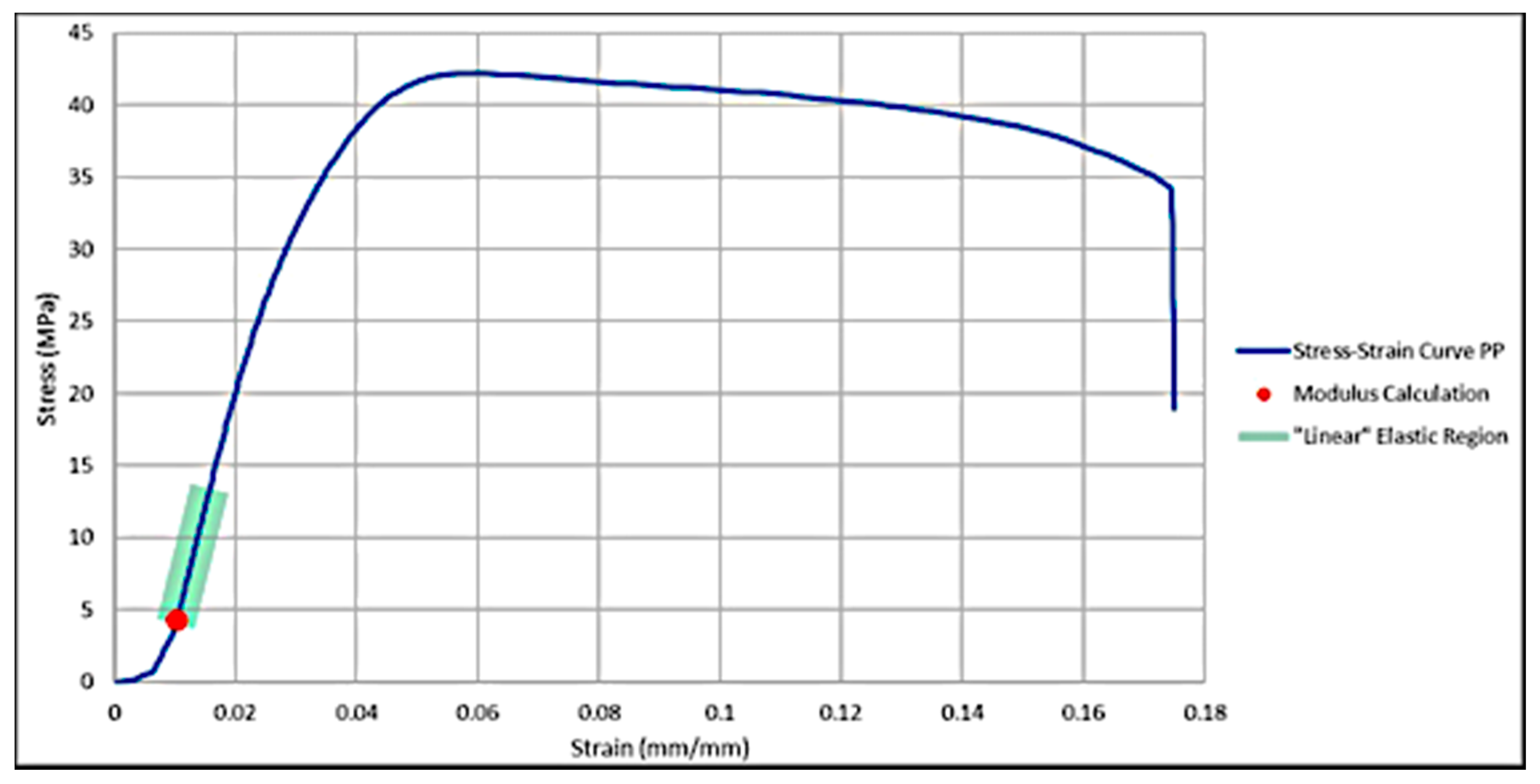
3.3. Mechanical Properties
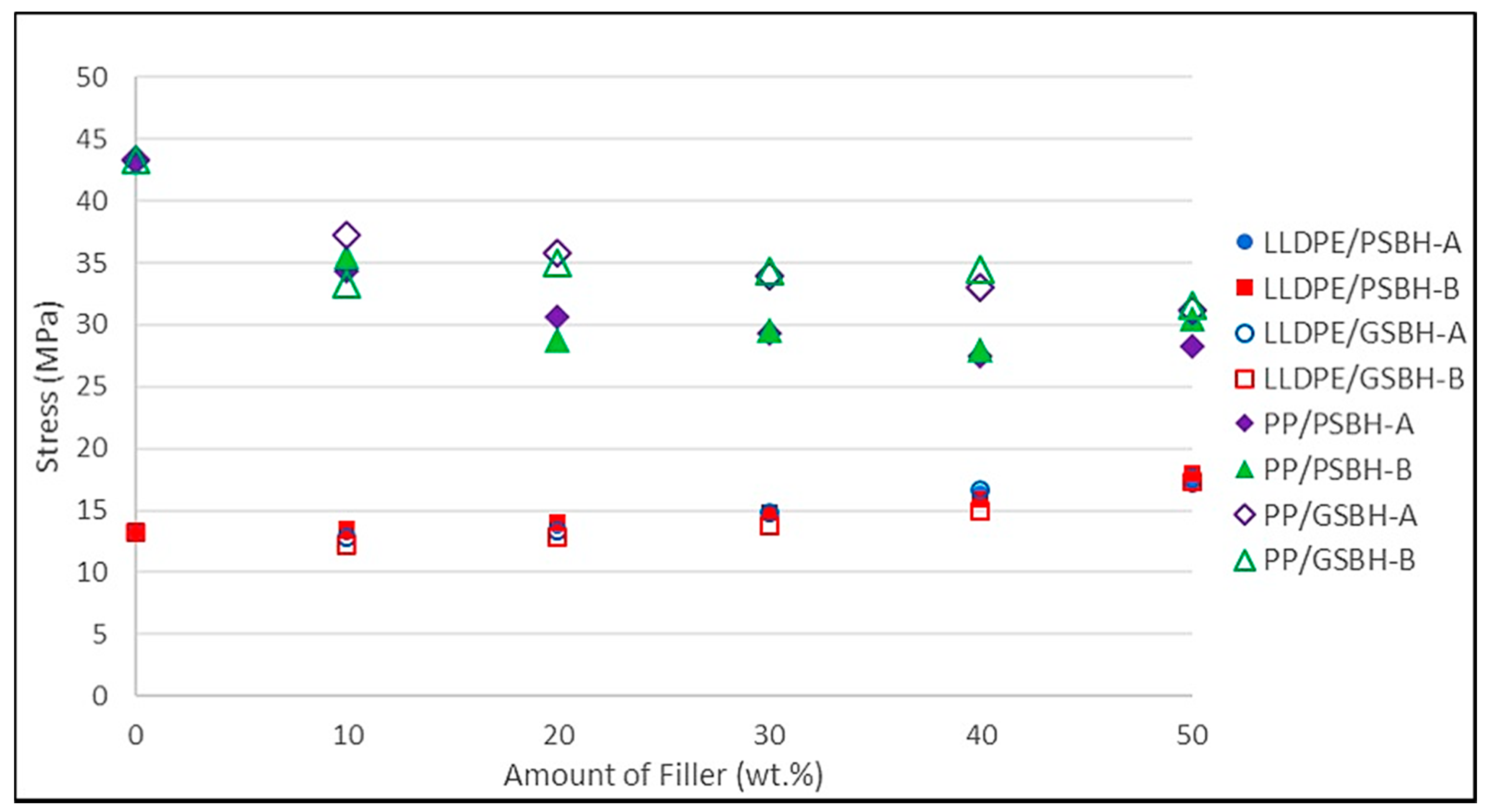
3.3.1. Maximum Tensile Stress
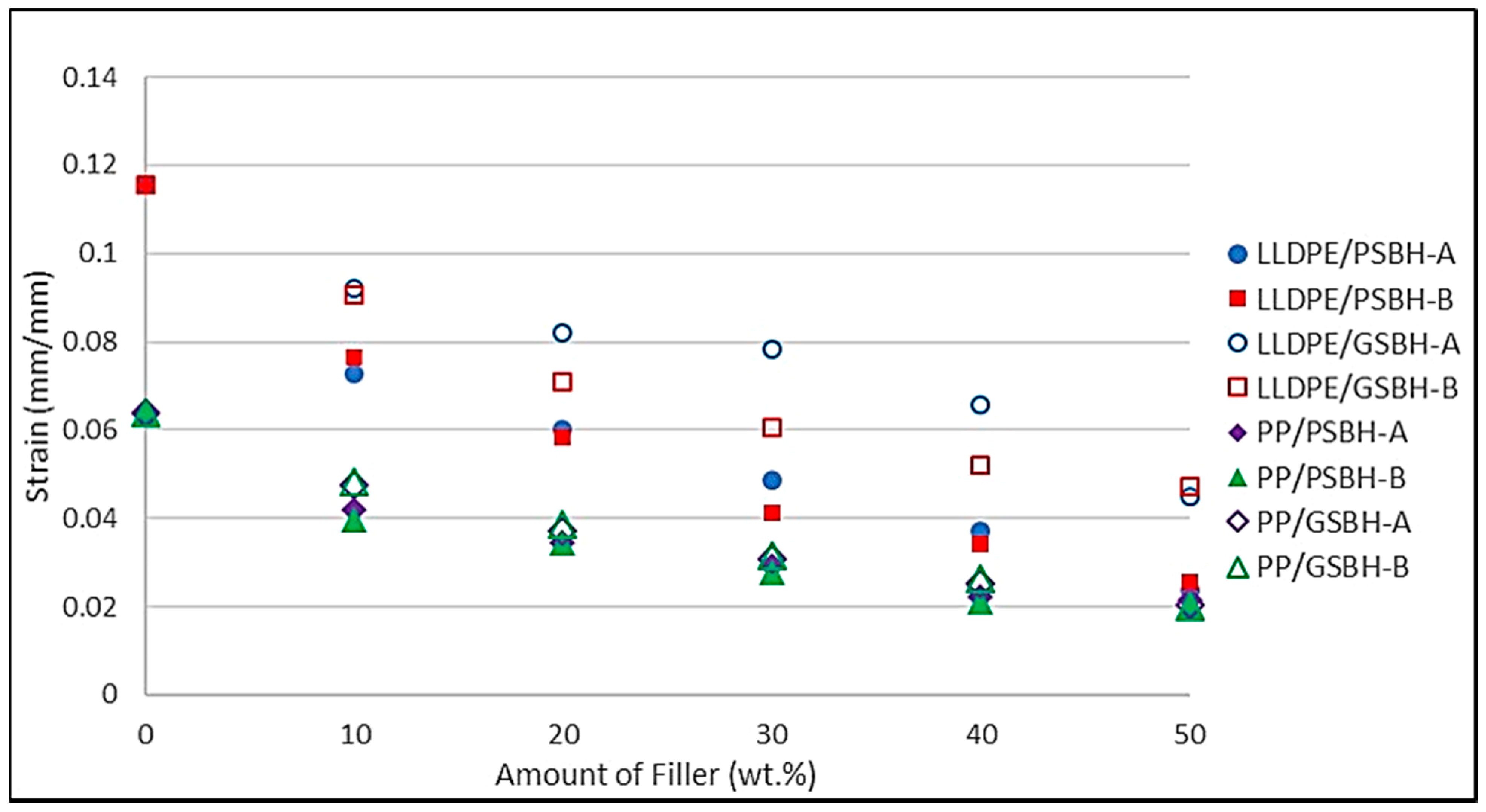
3.3.2. Strain at Maximum Tensile Stress
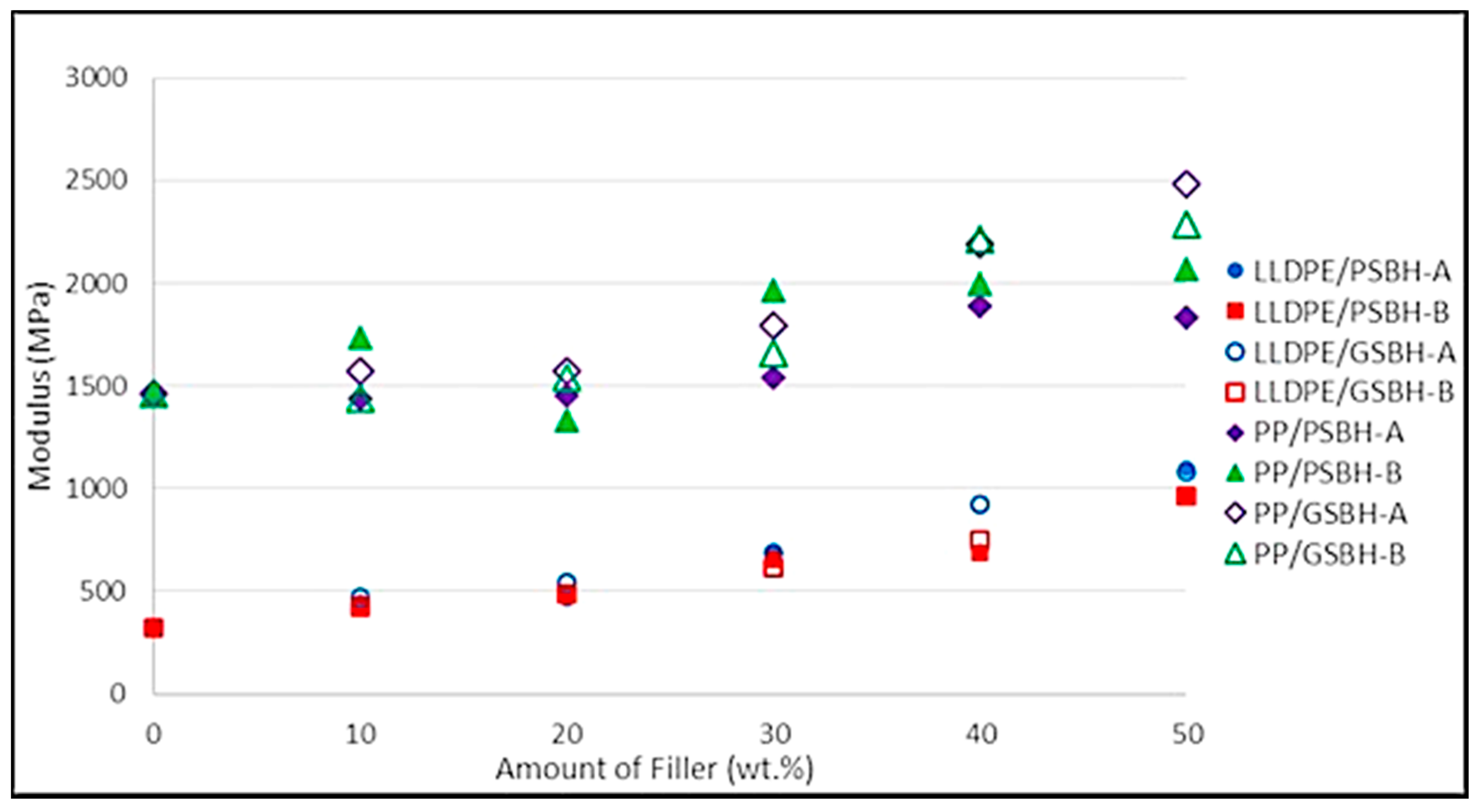
3.3.3. Modulus of Elasticity
3.4. Failure Pattern
3.5. Thermal Properties
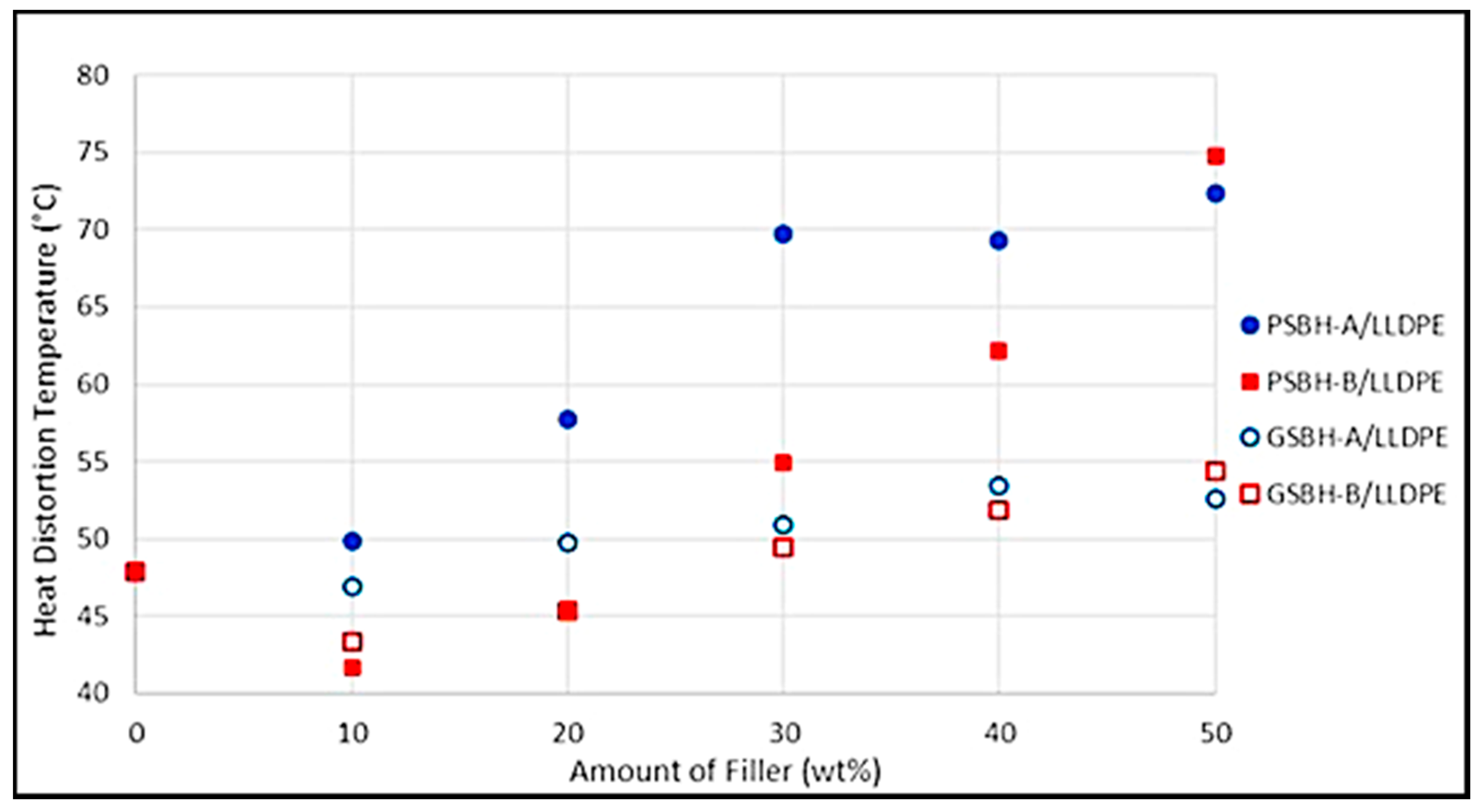
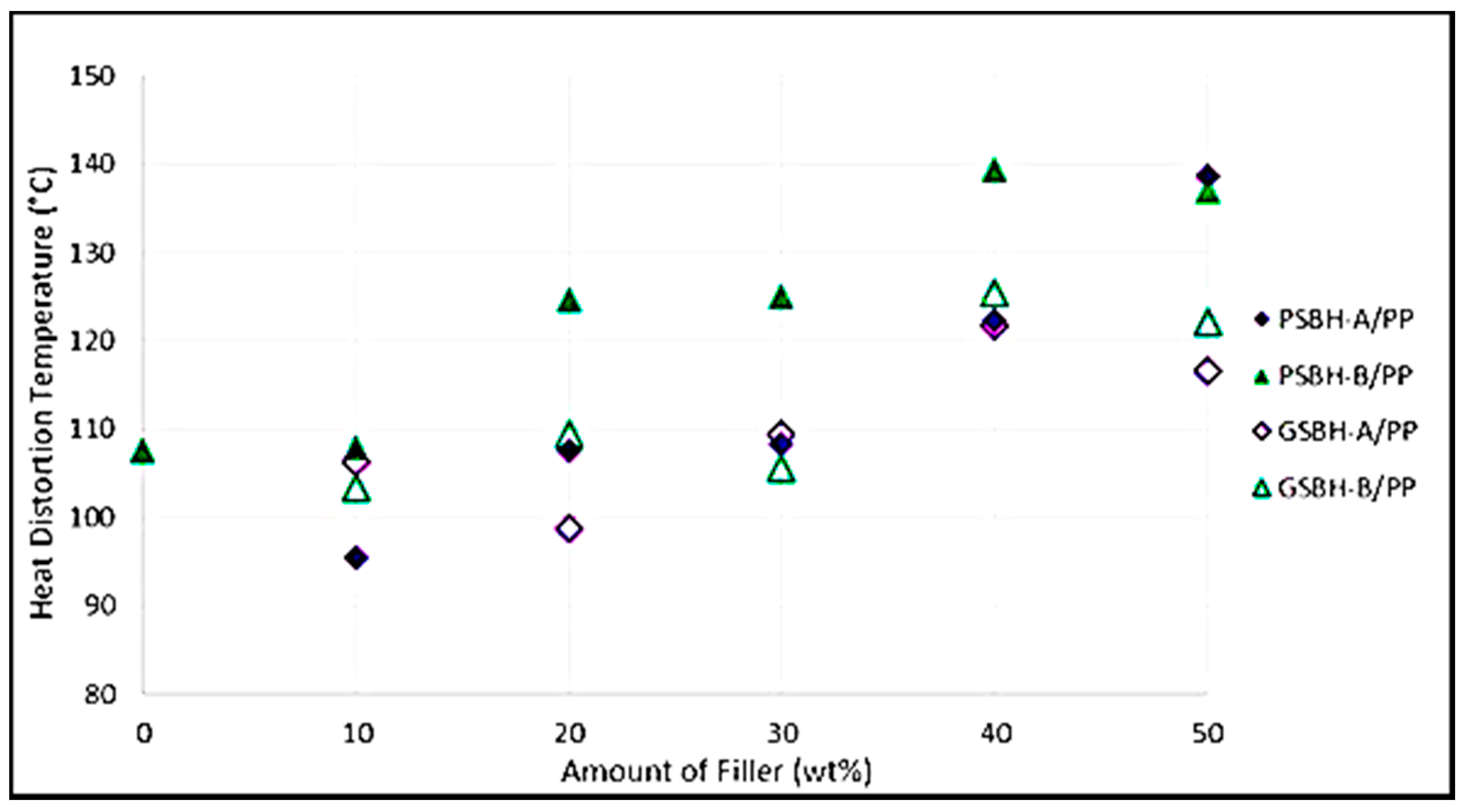
3.5.1. Heat Distortion Temperature
3.5.2. Melting Temperature and Heat of Fusion
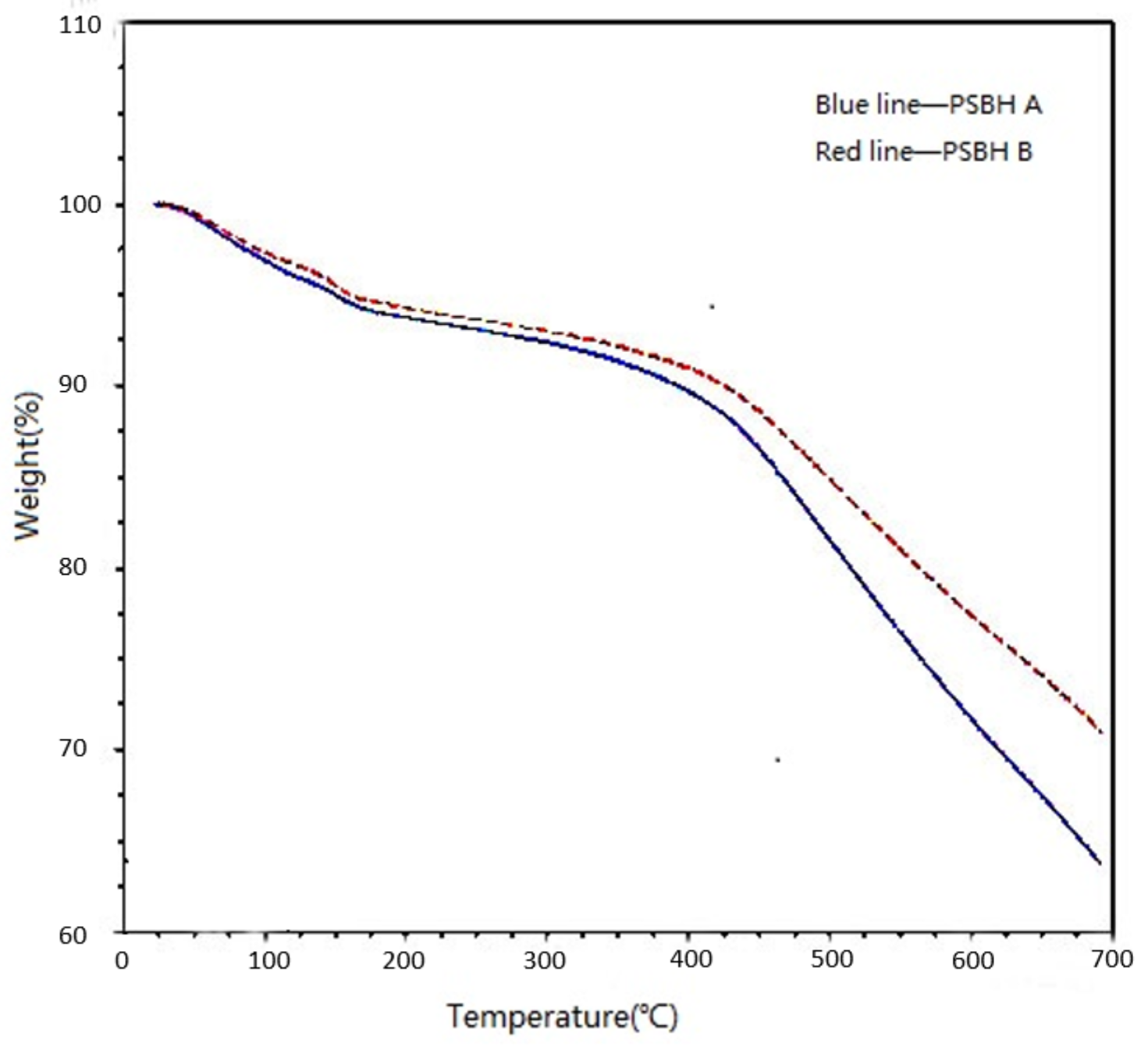
3.5.3. Thermal Stability
3.6. Thickness Swelling and Liquid Uptake
3.6.1. Thickness Swelling and Liquid Uptake in Water
3.6.2. Thickness Swelling and Liquid Uptake in 0.1 M HCl
3.6.3. Thickness Swelling and Liquid Uptake in 0.1 M NaOH
3.6.4. Comparison of Water, 0.1 M HCl, and 0.1 M NaOH Results
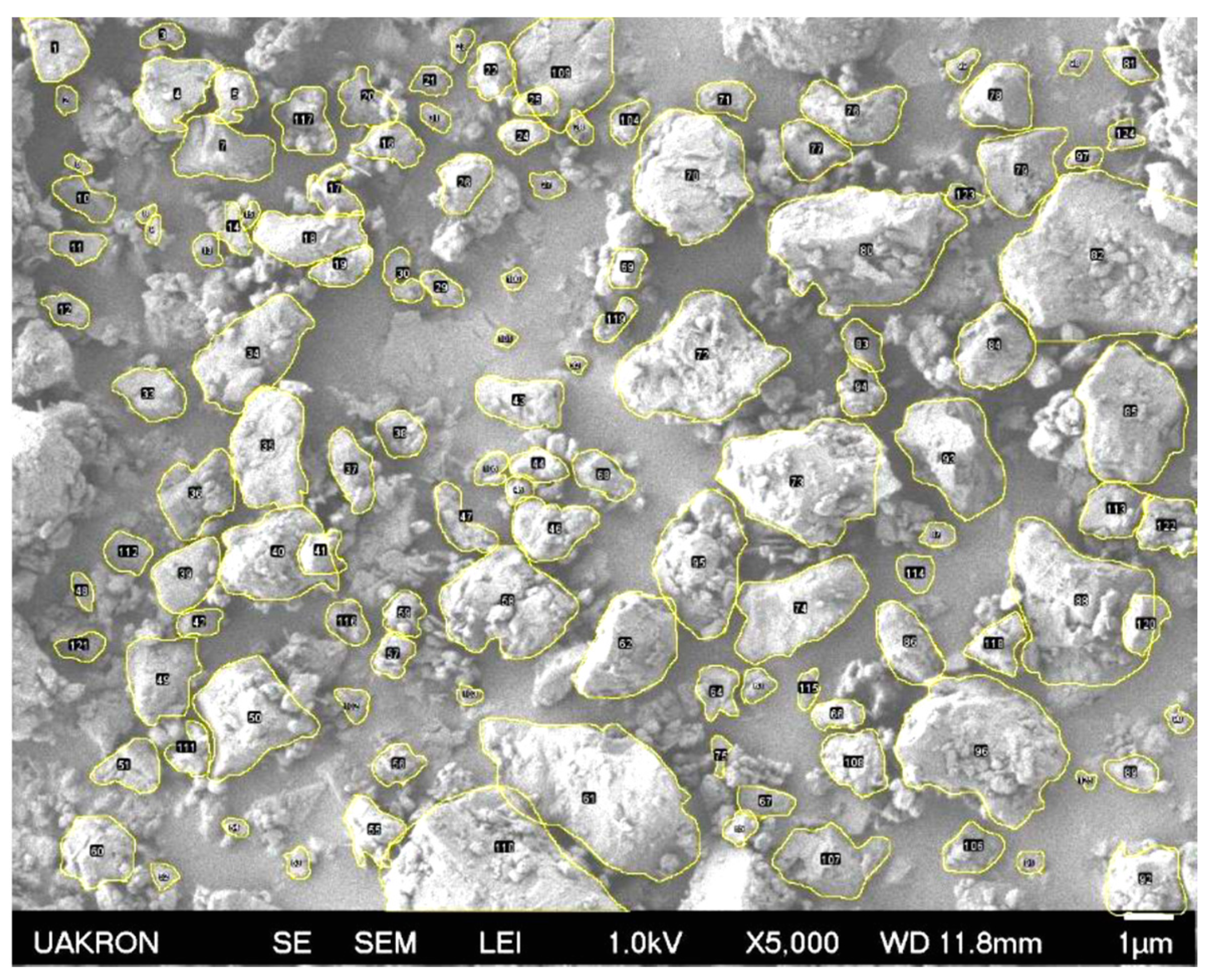
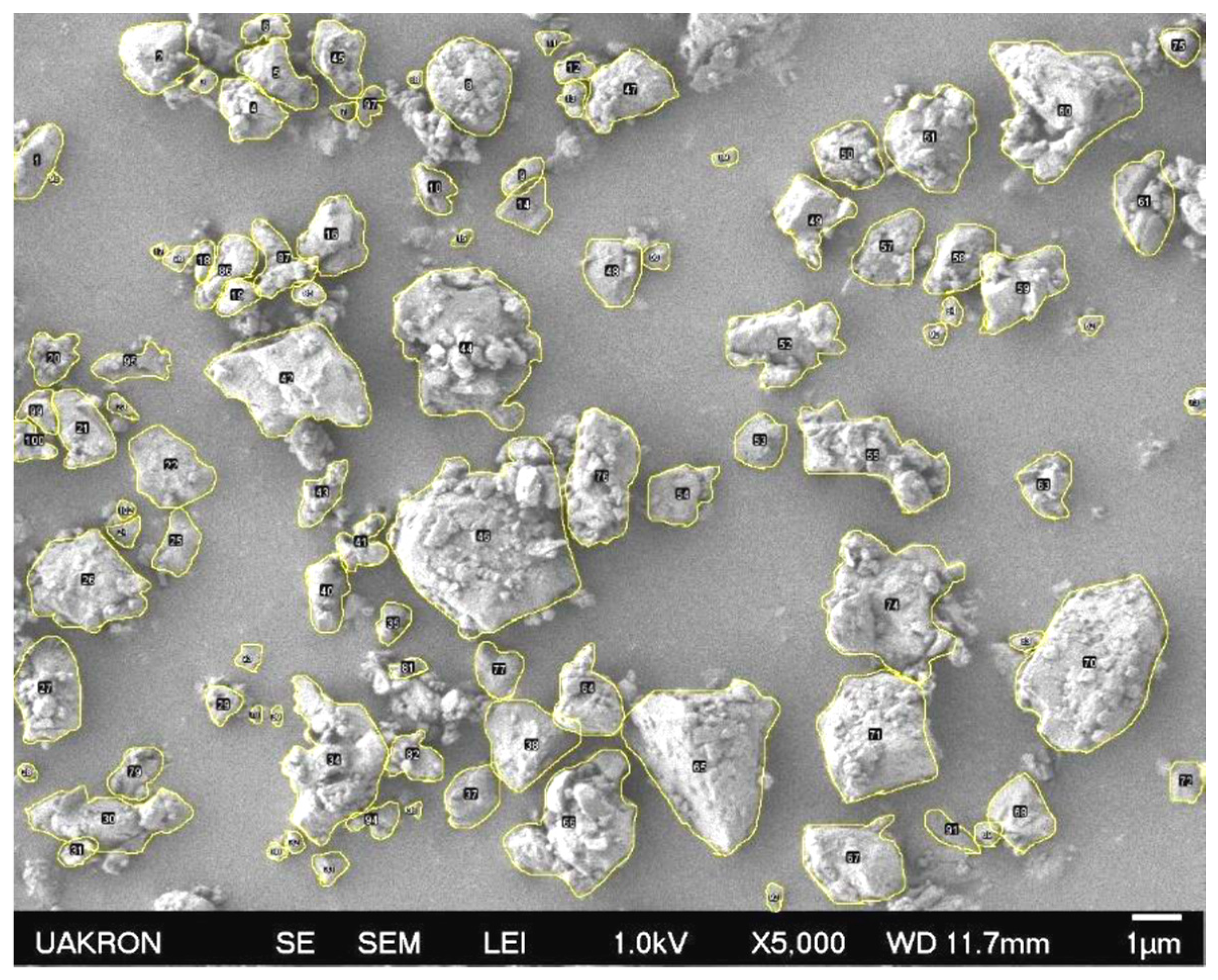
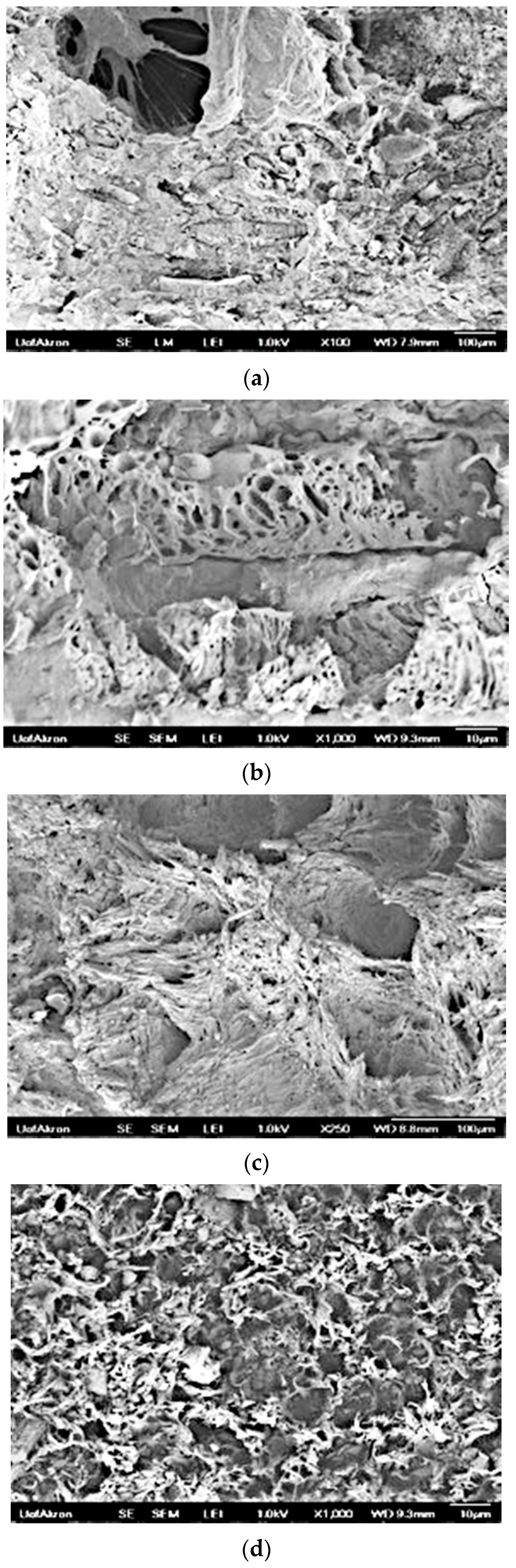
3.7. Morphology, Roughness, and Compatibility of PSBH in LLDPE and PP
3.7.1. SEM Images of PSBH-A/LLDPE and GSBH-A/LLDPE Composites
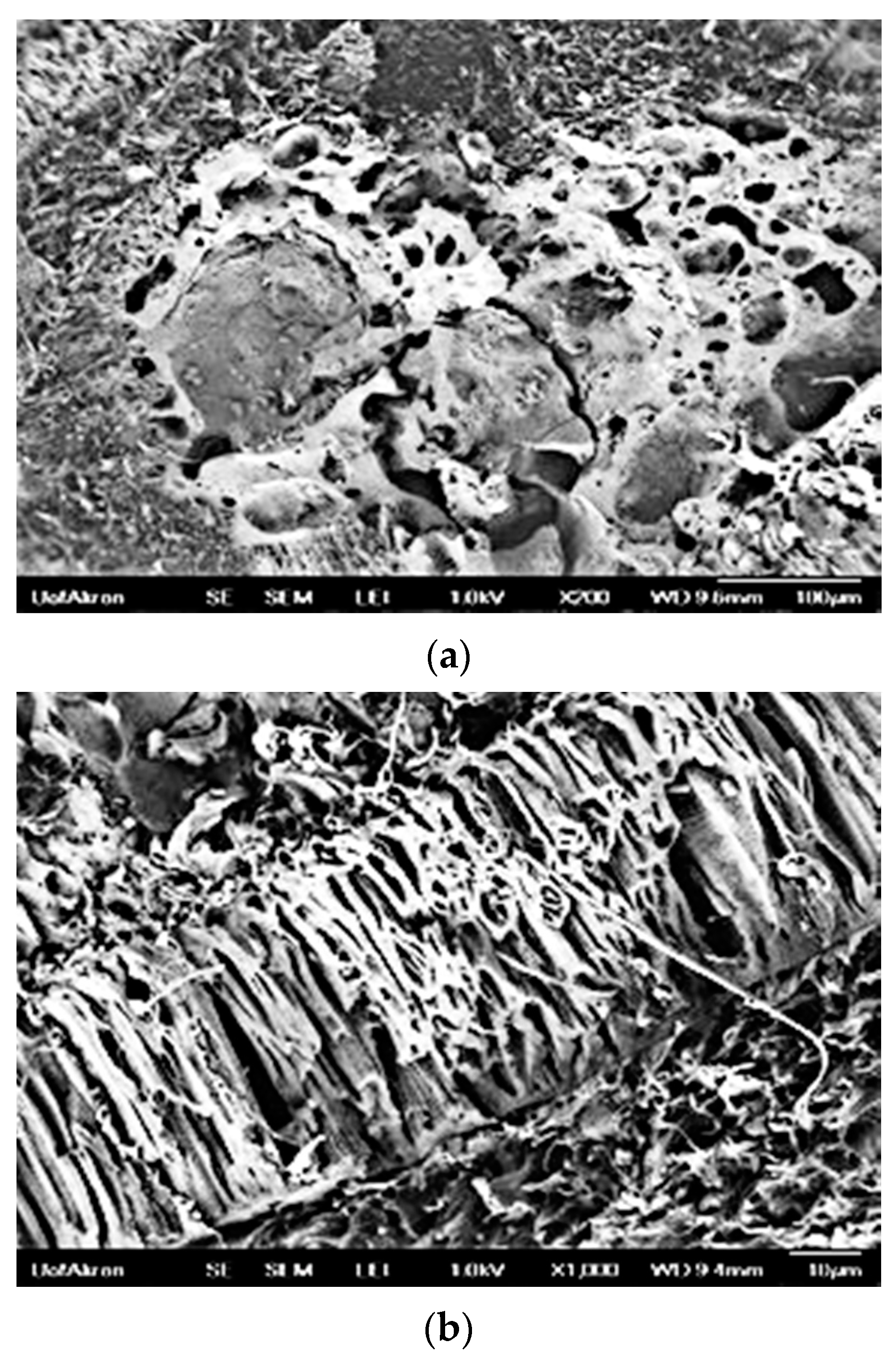
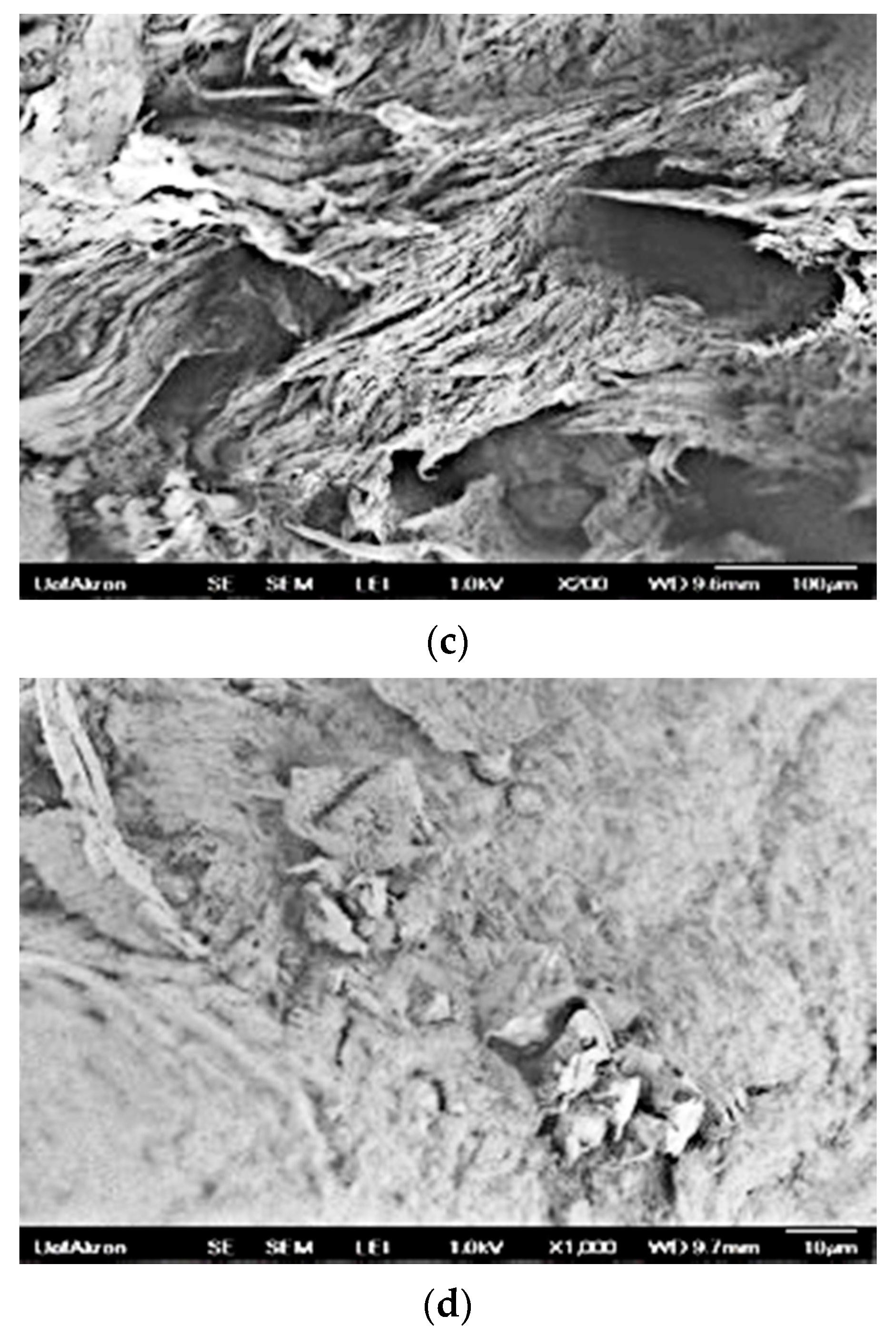
3.7.2. SEM Images of PSBH-A/PP and GSBH-A/PP Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polypropylene Production Capacity, Market and Price. Available online: https://www.plasticsinsight.com/resin-intelligence/resin-prices/polypropylene/ (accessed on 20 January 2020).
- LLDPE Production Capacity by Region and Price. Available online: https://www.plasticsinsight.com/resin-intelligence/resin-prices/lldpe/ (accessed on 20 January 2020).
- Van Der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, H. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Liu, H.-M.; Li, H.-Y. Application and Conversion of Soybean Hulls. In Soybean: The Basis of Yield, Biomass and Productivity; Kasai, M., Ed.; IntechOpen: London, UK, 2017; ISBN 978-1-78985-374-2. [Google Scholar]
- United States Department of Agriculture Economic Research Service. Available online: https://www.ers.usda.gov/data-products/#!topicid=14829&subtopicid=14851 (accessed on 23 September 2019).
- Toro-trochez, J.L.; Carrillo-pedraza, E.S.; Bustos-martínez, D.; García-mateos, F.J.; Ruiz-rosas, R.R.; Rodríguez-mirasol, J. Thermogravimetric characterization and pyrolysis of soybean hulls. Bioresour. Technol. Reports 2018, 6, 183–189. [Google Scholar] [CrossRef]
- GES–Soy. Available online: http://greenearthsystems.com.au/commodities/soy/ (accessed on 9 March 2020).
- How to Remove a lot of Soybean Skin Easily? 3 Steps. Available online: https://www.nutmachines.com/blog/remove-soybean-skin.html (accessed on 9 March 2020).
- Basu, P. Biomass Gasification and Pyrolysis: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2010; ISBN 978-0-12-374988-8. [Google Scholar]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Chen, W.H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Pabst, W.; Gregorov, E. Characterization of Particles and Particle Systems; Institute of Chemical Technology: Prague, Czech Republic, 2007; Available online: http://old.vscht.cz/sil/keramika/Characterization_of_particles/CPPS%20_English%20version_.pdf (accessed on 15 September 2021).
- ASTM Designation: D638-14. Standard Test Method for Tensile Properties of Plastics; ASTM International: Miami, FL, USA, 2014; Volume 82, pp. 1–15. [Google Scholar] [CrossRef]
- ASTM Designation: D648-18. Standard Test Method for Deflection Temperature of Plastics Under Flexural Load in the Edgewise Position; ASTM International: Miami, FL, USA, 2001; Volume 8, pp. 1–15. [Google Scholar] [CrossRef]
- Wadud, S.E.B.; Ullbrich, R.R. Using the DMA Q800 for ASTM International D 648 Deflection Temperature Under Load; TA Instruments: New Castle, DE, USA; Available online: http://www.tainstruments.com/pdf/literature/RH086_Using_Q800_for_ASTM_D468.pdf (accessed on 15 September 2021).
- Baş, G.S.; Sancaktar, E.; Karadurmuş, E. Physical Properties of LLDPE and PP Filled with Wood Flours. In Proceedings of the ASME 2015 International Design Engineering Technical Conferences & Computers and Information in Engineering Conference, New York, NY, USA, 2–5 August 2015. [Google Scholar] [CrossRef]
- Beyler, C.L.; Hirschler, M.M. Thermal decomposition of polymer. Fire Prot. Eng. 2002, 2, 110–131. [Google Scholar] [CrossRef]
- Pǎrpǎriţǎ, E.; Nistor, M.T.; Popescu, M.C.; Vasile, C. TG/FT-IR/MS study on thermal decomposition of polypropylene/biomass composites. Polym. Degrad. Stab. 2014, 109, 13–20. [Google Scholar] [CrossRef]


| Temperature (°C) | Feed Reduction | Carbon Content |
|---|---|---|
| 275 | 52% | 61% |
| 325 | 65% | 68% |
| 450 (Lot A) | 80% | 77% |
| 500 (Lot B) | 91% | 85% |
| Sample Name | Melting Temperature (°C) | ΔH (J/g) | ΔH Rank |
|---|---|---|---|
| Neat LLDPE | 123.40 | 86.06 | 100.00 |
| 10%wt PSBH-A 90%wt LLDPE | 124.27 | 66.85 | 77.68 |
| 20%wt PSBH-A 80%wt LLDPE | 125.83 | 67.53 | 78.47 |
| 30%wt PSBH-A 70%wt LLDPE | 126.17 | 53.23 | 61.85 |
| 40%wt PSBH-A 60%wt LLDPE | 125.95 | 48.07 | 55.85 |
| 50%wt PSBH-A 50%wt LLDPE | 125.61 | 44.13 | 51.28 |
| 10%wt PSBH-A 90%wt LLDPE | 127.13 | 78.82 | 91.59 |
| 20%wt PSBH-A 80%wt LLDPE | 126.10 | 64.97 | 75.49 |
| 30%wt PSBH-A 70%wt LLDPE | 126.83 | 52.07 | 60.50 |
| 40%wt PSBH-A 60%wt LLDPE | 124.79 | 42.52 | 49.41 |
| 50%wt PSBH-A 50%wt LLDPE | 126.32 | 28.50 | 33.12 |
| 10%wt PSBH-A 90%wt LLDPE | 127.78 | 68.10 | 79.13 |
| 20%wt PSBH-A 80%wt LLDPE | 127.83 | 66.57 | 77.35 |
| 30%wt PSBH-A 70%wt LLDPE | 126.04 | 49.47 | 57.48 |
| 40%wt PSBH-A 60%wt LLDPE | 126.61 | 46.99 | 54.60 |
| 50%wt PSBH-A 50%wt LLDPE | 126.61 | 37.47 | 43.54 |
| 10%wt PSBH-A 90%wt LLDPE | 126.67 | 73.95 | 85.93 |
| 20%wt PSBH-A 80%wt LLDPE | 123.36 | 64.90 | 75.41 |
| 30%wt PSBH-A 70%wt LLDPE | 125.82 | 51.43 | 59.76 |
| 40%wt PSBH-A 60%wt LLDPE | 125.68 | 50.48 | 58.66 |
| 50%wt PSBH-A 50%wt LLDPE | 125.51 | 29.64 | 34.44 |
| Sample Name | Melting Temperature (°C) | ΔH (J/g) | ΔH Rank |
|---|---|---|---|
| Neat PP | 169.65 | 75.46 | 100.00 |
| 10%wt PSBH-A 90%wt PP | 165.20 | 72.39 | 95.93 |
| 20%wt PSBH-A 80%wt PP | 165.95 | 61.50 | 81.50 |
| 30%wt PSBH-A 70%wt PP | 163.58 | 55.34 | 73.34 |
| 40%wt PSBH-A 60%wt PP | 164.26 | 59.82 | 79.27 |
| 50%wt PSBH-A 50%wt PP | 163.38 | 38.79 | 51.40 |
| 10%wt PSBH-A 90%wt PP | 165.61 | 74.32 | 98.49 |
| 20%wt PSBH-A 80%wt PP | 165.21 | 70.53 | 93.47 |
| 30%wt PSBH-A 70%wt PP | 162.88 | 55.11 | 73.03 |
| 40%wt PSBH-A 60%wt PP | 163.69 | 54.70 | 72.49 |
| 50%wt PSBH-A 50%wt PP | 164.31 | 48.24 | 63.93 |
| 10%wt PSBH-A 90%wt PP | 162.61 | 60.11 | 79.66 |
| 20%wt PSBH-A 80%wt PP | 164.59 | 70.41 | 93.31 |
| 30%wt PSBH-A 70%wt PP | 163.55 | 60.75 | 80.51 |
| 40%wt PSBH-A 60%wt PP | 164.00 | 48.95 | 64.87 |
| 50%wt PSBH-A 50%wt PP | 163.52 | 37.20 | 49.30 |
| 10%wt PSBH-A 90%wt PP | 166.21 | 73.55 | 97.47 |
| 20%wt PSBH-A 80%wt PP | 168.21 | 71.10 | 94.22 |
| 30%wt PSBH-A 70%wt PP | 164.77 | 54.17 | 71.79 |
| 40%wt PSBH-A 60%wt PP | 164.82 | 48.69 | 64.52 |
| 50%wt PSBH-A 50%wt PP | 163.08 | 46.71 | 61.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coben, C.; Sancaktar, E. Use of Pyrolyzed Soybean Hulls as Fillers in Polypropylene and Linear Low Density Polyethylene. Sustain. Chem. 2021, 2, 622-644. https://doi.org/10.3390/suschem2040035
Coben C, Sancaktar E. Use of Pyrolyzed Soybean Hulls as Fillers in Polypropylene and Linear Low Density Polyethylene. Sustainable Chemistry. 2021; 2(4):622-644. https://doi.org/10.3390/suschem2040035
Chicago/Turabian StyleCoben, Collin, and Erol Sancaktar. 2021. "Use of Pyrolyzed Soybean Hulls as Fillers in Polypropylene and Linear Low Density Polyethylene" Sustainable Chemistry 2, no. 4: 622-644. https://doi.org/10.3390/suschem2040035
APA StyleCoben, C., & Sancaktar, E. (2021). Use of Pyrolyzed Soybean Hulls as Fillers in Polypropylene and Linear Low Density Polyethylene. Sustainable Chemistry, 2(4), 622-644. https://doi.org/10.3390/suschem2040035







