1. Introduction
The rhizome
Zingiber officinale Roscoe (generally known as ginger) has been used as a spice all over the world for centuries. Apart from its applications in food flavoring, it has been termed “superfood” due to its medicinal properties. The main bioactive constituents of ginger are its phenolic compounds, such as gingerols and shogaols. Gingerols of various alkyl chain lengths are the most abundant pungent components of ginger and have been proved to possess a variety of pharmacological activities, such as antioxidant, anti-inflammatory, anticancer, antimicrobial, analgesic, antiemetic, antithrombotic and anti-metabolic syndrome activity, while offering neuroprotection [
1,
2,
3,
4,
5,
6,
7,
8]. These compounds are thermally labile, they easily undergo dehydration reactions and can be converted to their corresponding shogaols [
9]. Among gingerols, 6-gingerol is the major compound of the rhizome responsible for the pungency in fresh ginger and, according to several studies [
10,
11,
12,
13], exhibits biological activity against gastric carcinoma, as well as anticancer, anti-endocrine and free-radical scavenging effects etc. Shogaols impart the characteristic pungent and spicy-sweet fragrance to dried ginger [
9,
14]. Further, 6-shogaol is the most common dehydration product and in many cases is characterized by better biological activities than its precursor 6-gingerol. In addition, 6-shogaol, possesses a number of pharmacological activities such as anti-inflammatory activity [
15], antioxidant [
16], antitumor properties etc. [
17]. Both gingerols and shogaols have the ability to scavenge free radicals and various other damaging oxidants, reduce reactive oxygen species’ (ROS) production [
2] and can be considered as an antidote for various toxic agents [
18].
Since the knowledge about the safety of synthetic medicinal compounds (e.g., synthetic antioxidants) is insufficient, bioactive substances from natural sources are generally preferred by most consumers. The phenolic compounds contained in ginger are of great pharmacological and financial importance, so their extraction from this plant material is extremely appealing. However, it is known that gingerols are thermally sensitive and undergo significant dehydration and degradation at temperatures higher than 60 °C [
1,
14]. For instance, the thermal instability of 6-gingerol is attributed to the presence of the β-ketohydroxyl moiety in the alkyl chain. Therefore, in case the extraction temperature is high, decomposition of the bioactive molecules and loss of the valuable phenolic compounds might take place. As a consequence, traditional extraction methods, such as Soxhlet and percolation extraction, tend to be less efficient because of either high process temperature or long extraction times, which are usually required by them. Large amounts of common volatile organic solvents (VOCs) also need to be consumed most of the time, resulting in increasing process risks, because the majority of them are toxic, volatile, flammable, explosive, involved in environmental concerns and therefore their isolation from the extracts is obligatory. It is apparent that the conventional extraction methods are incompatible with the principles of “green” extraction techniques [
1,
3,
19,
20,
21,
22].
The term deep eutectic solvents (DESs) describes a novel and advanced class of green and sustainable solvents first introduced in 2003 by Abbott et al. [
23]. DES is defined as a eutectic liquid mixture of minimum two solid or liquid components, a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA), in specific molar ratios which are capable of forming intermolecular hydrogen bonds and van der Waals interactions. In case the components of DESs are of natural origin, the mixtures are termed natural deep eutectic solvents (NaDESs). Usually, HBA are nontoxic quaternary ammonium salts or amino acids (e.g., alanine, proline, glycine, betaine), whereas HBD are natural plant-based organic acids (e.g., oxalic acid, lactic acid, malic acid, amino acids etc.) or carbohydrates (e.g., glucose, fructose, maltose, etc.). Hydrogen bonding between HBA and HBD and van der Waals interactions cause charge delocalization, which interferes with the ability of the individual components to crystallize and, as a consequence, the melting point of the NaDES is significantly depressed compared to that of each initial compound. The possibility to design NaDESs for specific purposes, offered by the unlimited number of HBA/HBD combinations and molar ratios, makes them tailor-made solvents [
22,
24,
25,
26,
27,
28].
Recently, the scientific community has been focusing on NaDESs, because they display a broad range of advantages, including negligible volatility, non-flammability, ability to dissolve a variety of compounds, chemical and thermal stability, adjustable viscosity, remaining at liquid state within wide temperature range and cheap, easy and fast preparation procedures with high purity and without waste generation. Owing to these superior characteristics and depending on their physicochemical properties, NaDESs have been extensively used in extractions of a large variety of secondary metabolites from natural sources, such as phenolic compounds, alkaloids, saponines, anthraquinones, essential oils, terpenoids, proteins, carbohydrates, polyunsaturated fatty acids and photosynthetic pigments. Some recent reviews indicate the growing interest for the development and implementation of innovative extraction techniques for the extraction of bioactive compounds from natural products using as green, environmentally friendly solvents the DESs and NaDESs [
28,
29,
30,
31]. The strong hydrogen bonds between the components of NaDES and the extracted compounds increase the process yield and this might become even higher than those with traditional organic solvents [
19,
21,
22,
24,
26,
27,
28].
In addition, since NaDESs are generally composed of naturally occurring substances, they may be directly incorporated in the final products without additional time- and cost-consuming purification steps in order to recover the bioactive compounds from the solvent, provided that the necessary toxicity and biocompatibility tests have been carried out. Furthermore, due to the intermolecular interactions, the NaDES used as extraction solvent acts as a stabilizing agent against oxidative degradation and as a storage medium for the desirable but also sensitive extracted bioactive molecules [
26,
28]. Due to NaDESs’ major advantages over hazardous VOSs, the formers are considered a safe, environmentally friendlier and efficient alternative to conventional solvents and their applications in a wide spectrum of fields such as pharmaceuticals (as drug delivery systems), electrochemistry (as electrolytes and for the electrochemical detection of phenolics) [
19,
24], catalysis [
19], lignocellulosic biomass processing [
24], extractions of bioactive compounds [
21,
22,
25,
26,
27], organic synthesis [
32] and many others, have gained a lot of attention lately.
NaDES extractions are frequently combined with high-energy non-conventional extraction techniques such as ultrasound- (UAE) and microwave-assisted extraction (MAE) technologies. As for UAE, it is a sonochemical approach of great interest for the phytopharmaceutical industry that can be easily performed both on laboratory and industrial scale. The main driving force in UAE is the acoustic cavitation, which generates microjets, shock waves and other strong stretching forces that destroy the plant cell walls, increase the matrix-solvent contact surface, enhance the penetrability of the solvent and subsequently facilitate the mass transfer and the release of the target molecules into the solvent. The method is characterized by simplicity in use, affordable instrumentation, shorter extraction times, no need for high temperatures, improved extraction yields and often better extract quality, absence of undesirable environmental effects and, as a result, it is regarded as a promising green extraction technique [
1,
3,
19,
24,
25,
26,
33,
34,
35,
36].
Response surface methodology (RSM) is a statistical tool often used in order to determine the optimal extraction conditions with a minimum of experiments. Box–Behnken design (BBD) is an efficient three-level design which allows the development of mathematical models that describe the correlations between the obtained responses and the experimental conditions and it also demonstrates the statistical significance of the factor effects [
26,
37].
To evaluate the use of ginger as a natural source of bioactive compounds, several studies have reported the various extraction methods using common VOCs, in various temperatures and extraction times [
1,
3,
38,
39]. However, to our knowledge, there is only one study by Hsieh et al. [
19] investigating the application of greener techniques such as UAE (using an ultrasonic bath) in combination with NaDESs as extraction media for the extraction of valuable compounds from dry ginger as well as for the optimization of the proposed method.
In the research work presented herein, ultrasound probe and different NaDESs have been used, whereas different extraction parameters and responses have been under consideration for the method optimization. Regarding the antioxidant activity, to our knowledge it is the first time that the time-dependence of the NaDES-containing extracts’ antioxidant activity is investigated, proving the protective character of the NaDESs to the extracted phytochemicals. Finally, the proposed greener method is compared with the extraction process of dry ginger with commonly used solvents such as water, ethanol and the ethanol/water system.
The interaction of small molecules with DNA, which is the primary intracellular target for the development of novel therapeutic agents, has always received much attention by the scientific community [
40]. The interaction of plant extracts, rich in bioactive phytochemicals, with DNA has also been studied in effort to further exploit the extracts as obtained, without isolating the natural products. For example, the grape-seed extract prevents oxidation-induced DNA damage [
41], whereas the olive leaf extract [
42] and fresh-cut yam slices [
43] interact with calf Thymus DNA (ctDNA). In order to develop new drugs which specifically target DNA, it is necessary to understand the different binding ways in which a molecule is capable of binding. Molecules interact with DNA in either a non-covalent or a covalent way. The first way includes three binding modes: intercalation, groove binding and external static electronic effects. Among these interactions, intercalation is one of the most important DNA binding modes as it invariably leads to cellular degradation. In addition, the coordination geometry and the type of ligand donor atom also play key roles in determining the binding extent of DNA [
42,
44]. Haris et al. [
45] recently reported the interaction of 6-gingerol with ctDNA using experimental as well as molecular modeling techniques. In addition, 6-gingerol was found to bind in the minor groove of ctDNA. In the present research work, we set out to investigate for the first time in the literature, the ability of the NaDES-ginger extract and pure 6-shogaol (as the major phytochemical isolated from dry ginger) to bind to calf thymus DNA (ctDNA).
Overall, the aim of the present work is the development of a green extraction process of bioactive compounds from ginger powder using NaDESs as green and alternative extraction media with the remarkable ability to also act as storage media for the valuable and often thermally sensitive and easily oxidized substances of ginger and ultrasound as a high-energy technique. The optimization process accomplished through a 3-factor, 3-level Box–Behnken experimental design (BBD) and statistical analysis of the results in order to obtain an extract rich in bioactive components with good antioxidant activity. The antioxidant activity and the total phenolic content (TPC) were selected as responses of the BBD and have been determined using the DPPH scavenging assay and the Folin–Ciocalteu (F-C) method respectively. Although the Folin–Ciocalteu reagent is also possible to react with some non-phenolic compounds [
46], the TPC method has been used as one of the most commonly used methods for the determination of TPC in extracts from various natural sources [
46,
47,
48] including ginger [
49]. The proposed methodology is also compared with conventional extraction processes and the obtained extracts are evaluated for their antioxidant activity and TPC, while the extract derived by the optimum extraction conditions was investigated for its ability to bind to ctDNA.
2. Materials and Methods
2.1. Materials and Reagents
Anhydrous betaine and D,L-lactic acid (80–85% aq. soln.) were obtained from Fluorochem (Hadfield, UK) and Alfa Aesar (Ward Hill, MA, USA). Folin-Ciocalteu reagent was purchased from Merck Millipore and 2,2-diphenyl-1-picrylhydrazyl (DPPH) was purchased from Aldrich. The ginger powder was obtained from a local pharmacy in Athens, Greece. All solvents and reagents were analytical grade and used without further purification. For all the experiments double-deionized water was used.
2.2. Structural Characterization
For the NMR characterization of NaDESs and of the isolated 6-shogaol, a Varian V 600 MHz NMR spectrometer (National Hellenic Research Foundation, Institute of Chemical Biology) was used.
The isolated compounds of ginger (using the conventional methods) were characterized by 1H NMR spectroscopy using CDCl3 as a solvent, while the NaDESs were characterized by NMR spectroscopy using as solvents DMSO-d6 or CDCl3. For the NMR experiments of the NaDESs, 20 μL of NaDES were dissolved in DMSO-d6 or CDCl3 (5% v/v) and the solution was transferred in 5 mm NMR tubes.
FTIR spectroscopy was performed both for the NaDES derived by betaine and D,L-lactic acid (Bet/La/W) and for the ginger extract derived by the optimum extraction conditions, operated on a JASCO FT/IR-4200 spectrometer in attenuated total reflectance (ATR) mode, in the range from 4000 to 400 cm−1.
Thermal properties of the NaDES Bet/La/W were studied via thermogravimetric analysis (TGA). The experiments were conducted from 25 to 700 °C, with a temperature step of 10 °C/min, under inert N2 atmosphere, in a METTLER TOLEDO TGA/DSC 1 STARe System.
2.3. NaDES Synthesis
The NaDESs were prepared using the heating and stirring method, as described in our previous work with slight modifications [
32]. In a round-bottom flask equipped with a magnetic stirrer the appropriate amounts of the starting materials were added and the mixture was stirred for 30 min to 2 h at temperatures between 50–70 °C under inert conditions (nitrogen atmosphere). When a homogenous, without any precipitates, transparent liquid is formed, the NaDES is transferred in glass hermetically closed vessels and stored at room temperature in absence of light until their further use.
2.3.1. NaDES Betaine/D,L-Lactic Acid (Bet/La/W) (1:2:2.5)
For the NaDES derived from betaine and D,L-lactic acid, which is the NaDES that was used for the optimization studies of the extraction process, anhydrous betaine was mixed with D,L-lactic acid (80% aq. soln.) in a 1:2 molar ratio (the 2.5 eq of water (W) refers to the amount of water that is present in the commercially available D,L-lactic acid). The mixture was stirred at 50 °C under inert atmosphere, until a colorless transparent liquid was formed (1 h).
1H NMR (25 °C, 600 MHz, DMSO-d6), δ (ppm): 4.03 (q, 2H, J = 6.0 Hz, -CH D,L-lactic acid), 3.70 (s, 2H, CH2COO-), 3.15 (s, 9H, -NCH3 (x3)), 1.22 (d, J = 6.7 Hz, 6H, -CH3 D,L-lactic acid (x2)).
13C NMR (25 °C, 600 MHz, DMSO-d6), δ (ppm): 177.96, 167.74, 66.94, 66.18, 53.58, 21.13
2.3.2. NaDES Betaine/Glycerol (Bet/Gly) (1:3)
For the NaDES derived from betaine and glycerol (Bet/Gly), anhydrous betaine was mixed with glycerol in a 1:3 molar ratio. The mixture was stirred at 70 °C under inert atmosphere, until a colorless transparent liquid was formed (3 h).
1H NMR (25 °C, 600 MHz, CDCl3), δ (ppm): 5.24 (brs, 9H, 9xOH), 3.92–3.51 (m, 17H), 4.21–4.09 (m, 1H), 3.28 (brs, 9H, -NCH3 (x3)).
2.3.3. NaDES D-Glucose/D,L-Lactic Acid (Glu/La/W) (1:5:6.2)
For the NaDES derived from D-glucose and D,L-lactic acid (Glu/La), anhydrous D-(+)-glucose was mixed with DL-lactic acid (80% aq. soln.) in a 1:5 molar ratio (the 6.2 eq of water (W) refers to the amount of water that is present in the D,L-lactic acid). The mixture was stirred at 65 °C under inert atmosphere, until a colorless transparent liquid was formed (2 h).
1H NMR (25 °C, 600 MHz, DMSO-d6), δ (ppm): 4.95–4.88 (m, 1H), 4.32–4.24 (m, 4H), 4.21–4.09 (m, 1H), 4.03 (q, 5H, J = 6.0 Hz, -CH D,L-lactic acid (x5)), 3.80–3.30 (m, 4H), 1.39 (d, J = 7.0 Hz, 2H), 1.27 (d, J = 6.9 Hz, 1H), 1.22 (d, J = 6.9 Hz, 15H, -CH3 D,L-lactic acid (x5)).
2.4. Extraction of Ginger Powder Using a Conventional Technique for the Isolation of 6-shogaol
In order to isolate 6-shogaol (
Figure 1) from dry ginger, a slightly modified conventional extraction method was carried out [
50]. First, 50 g of dry ginger powder were submitted in three 24-h extractions using each time 300 mL of ethanol diluted 50% in water as a solvent. Then, after filtration under pressure, the filtrate was subjected to extraction using ethyl acetate (150 mL) and water (150 mL) and the organic phase was condensed by evaporation under pressure. Finally, column chromatography in a system of n-hexane/ethyl acetate in a ratio 7:3 was performed in order to successfully isolate 6-shogaol in satisfying yields (2.6 mg/g dry ginger).
6-shogaol: 1H NMR (600 MHz, CDCl3), δ (ppm): 6.84–6.79 (m, 2H, H-5 & H-3′), 6.71 (s, 1H, H-6′), 6.68 (br, 1H, H-2′), 6.09 (d, J = 15.6 Hz, 1H, H-4), 5.50 (s, 1H, OH), 3.87 (s, 3H, OCH3), 2.88–2.82 (m, 4H, H-1 & H-2), 2.19 (q, J = 7.8 Hz, 2H, H-6), 1.44 (quint, J = 7.2 Hz, 2H, H-7), 1.33–1.25 (m, 4H, H-8 & H-9), 0.89 (t, J = 7.2 Hz, 3H, H-10)
2.5. NaDES—Ultrasound Assisted Extraction process
For NaDES-UAE, a Sonics VC 400 High Intensity Processor (Sonics and Materials Inc.), equipped with a piezoelectric converter and a 13 mm diameter titanium alloy (Ti-6Al-4V) probe was used.
Powdered ginger samples were firstly suspended in the selected extraction medium and then introduced in an ice bath in order to avoid the sample overheating during the UAE. The pulse sequence of sonication during the extraction was set at 2 s on–2 s off.
After the UAE, the supernatant was recovered from the solid material by centrifugation of the samples at 10,000 rpm for 20 min and by filtration under vacuum. The extracts were stored in the dark at 4 °C until further analysis. Total phenolic content (TPC) as well as the ability of the extracts to scavenge the DPPH radical were determined by standard colorimetric procedures.
The extraction process was firstly optimized via a series of experiments using as an extraction medium the NaDES Bet/La/W. After the optimization of the process, the scope of this study was expanded by using two more NaDESs, the Bet/Gly and Glu/La/W. Moreover, an extraction using conventional solvents (Ethanol/Water, 70:30) was performed for the sake of comparison.
2.6. Box–Behnken Experimental Design—Response Surface Methodology
The optimal conditions of NaDES-UAE for the extraction of phenolic compounds from ginger were identified by response surface methodology (RSM). A three-level, 17-run Box–Behnken design (BBD) including five replicates at the center point was applied. The coded factor levels for the BBD of the three-variable system studied are: −1, 0 and +1.
The impact of the factors (A) extraction time (min), (B) ultrasound power (Watt) and (C) NaDES-to-dry ginger ratio (
w/w) on the responses (R
1) total phenolic content (TPC) and (R
2) antioxidant activity using the DPPH radical scavenging assay (IC
50) of the extracts was investigated. The levels, as well as the conditions of the conducted experiments can be seen in
Table 1.
The experimental data of NaDES-UAE were fitted to a second-order polynomial equation, which correlated each response to the factors. Each equation had the following form:
where
Ri is the dependent variable, i (=1, 2) is the index corresponding to each response, x
0,i is the constant term, x
j,i, y
j,i, z
j,i (j = 1, 2, 3) are the linear, interaction and quadratic regression coefficients respectively, which are calculated by the statistical analysis of the experimental results and A, B and C are the independent variables.
2.7. Data Analysis
The statistical analysis of the experimental data and the 3D RSM plots were performed with the Design-Expert 12.0 software package (Stat-Ease Inc., Minneapolis, MN, USA—Trial Version) using the analysis of variance (ANOVA). The level of statistical significance was set at p < 0.05.
2.8. Colorimetric Determination of Total Phenolic Content of Extracts (TPC)
TPC of ginger extracts was determined through the reducing capacity of the Folin–Ciocalteu reagent, as described by Singleton et al. [
51], with slight modifications. The ginger extracts were first 50% diluted in distilled water. A total of 50 μL of the solutions were mixed with 3 mL of water and 250 μL of the Folin–Ciocalteu reagent, and the mixture was stirred using a Vortex mixer before being incubated in the dark for 2 min. Then, 750 μL of saturated aqueous Na
2CO
3 solution were added for pH control, the mixture was stirred again and the final volume of the solution was adjusted to 5 mL by adding distilled H
2O. After 1 h incubation in the dark at room temperature, the absorbance was measured at 755 nm using a Jasco V-770 UV-Vis/NIR spectrometer. The above-mentioned procedure was repeated for a blank sample, which contained water, and its absorbance was subtracted from that of each sample. The experiments were performed in triplicate.
The TPC of the extracts was calculated as gallic acid equivalents (GAE) from a calibration curve, using gallic acid as a standard and it was expressed as mg GAE per g of ginger.
where
CGA is the calculated GAE by calibration curve,
D is the dilution factor,
Vext is the solvent volume used for the extraction and
mginger is the mass of ginger used for the extraction.
2.9. Colorimetric Determination of DPPH Radical Scavenging Ability of the Extracts
The antioxidant capacity of the ginger extracts was studied using the procedure described by Boly et al. [
52] with slight modifications. According to this method, the stable DPPH radical reacts with the antioxidant molecules of the extract and it is reduced. A total of 5 mg of 2,2-diphenyl-1-picrylhydrazyl (DPPH) was accurately weighed and dissolved in pure ethanol, so that the final volume of the solution was 50 mL. The DPPH solution was kept at 4 °C in the dark until further analysis but its storage never lasted for more than a couple of hours, to avoid the self-reduction of the radical.
In a 96-well plate, 100 μL DPPH solution were added to 100 μL of ginger extract diluted to 2% v/v in ethanol (initial concentration C) and then the same procedure was followed for samples diluted in ethanol, with concentrations 0.8C, 0.6C, 0.4C and 0.2C. The samples were incubated for 15, 30, 60, 90 and 120 min, in the dark at room temperature and then the absorbance was measured at 515 nm using a Molecular Devices SpectraMax 250 Microplate UV/Vis Reader. The above-mentioned procedure was repeated for a blank sample, which contained 100 μL ethanol instead of ginger extract. All the experiments were performed in triplicate. The isolated 6-shogaol as well as curcumin and Trolox were used as reference antioxidants.
The graph demonstrating the linear correlation between the % inhibition of the DPPH radical and the extract concentration was created. The percentage of inhibition was calculated using the following formula:
where
Ablank is the absorbance of the blank samples containing ethanol/DPPH and
Asample is the absorbance of each sample containing extract.
The DPPH radical scavenging activity of the samples was quantified by the IC50 value, which stands for the extract concentration that is required to reduce 50% of the initial DPPH absorbance. Therefore, IC50 was calculated graphically, and the results were finally expressed as mg of ginger extract per ml of solution.
Moreover, the DPPH radical scavenging activity of the extracts was expressed as Trolox equivalent antioxidant capacity (TEAC). For this reason, the standard curve of Trolox was prepared (starting with a stock solution of 0.01 mg/mL) following the same procedure as described before.
TEAC was calculated as follows [
53]:
The higher TEAC value means the higher DPPH radical scavenging activity.
2.10. DNA Binding Studies Using UV-Vis Spectroscopy
1 mg of lyophilized calf-thymus DNA (ctDNA) was dissolved in 1 mL of Tris-HCl buffer solution of concentration 10 mM and pH 7.4 and left overnight at 4 °C. The concentration was determined from the absorbance at 260 nm using an extinction coefficient of 6600 M−1cm−1. The NaDES Bet/La/W, ginger extract, shogaol and rhodamine B were dissolved in the buffer to a concentration of 1 mg/mL, which were then used as the stock solution for the preparation of the concentration of 100 μg/mL. Afterwards, various concentrations (0–400 μM) of ctDNA were added to the prepared solutions which were incubated for 5 min at 37 °C.
3. Results and Discussion
3.1. Synthesis and Structural characterization of NaDES
The components of the NaDES used in this study have been task-specifically selected on the basis of their biocompatibility and safety that will render it a privileged medium for extraction and storage of the ginger phytochemicals.
Betaine (trimethylglycine) is a naturally occurring amino acid that can be found in microorganisms, plants and animals. As a zwitterion, it interacts with water and other similar molecules forming hydrogen bonds. Betaine is generally recognized as safe (GRAS), is non-toxic and is listed by the European commission in the glossary of the common ingredients used in cosmetic products [
54]. Betaine and its derivatives are used in cosmetics since they are considered as antistatic/viscosity controlling agents, skin and hair conditioning agents and a gentle hydrating ingredient, etc. Lactic acid (2-hydroxypropanoic acid) is an organic acid widely distributed in nature. It is used extensively in the food industry [
55] for the production of dairy products but it also finds use in the pharmaceutical [
56] and cosmetics industries [
57].
In this study, the Bet/La/W was chosen as a promising green solvent for the implementation of the extraction process and its optimization through the experimental design. In order to expand the scope of this work, two more NaDESs were also synthesized, namely Bet/Gly and Glu/La/W. These NaDESs were chosen since they are also derived from GRAS and inexpensive starting materials (Glycerol, D-Glucose) that are common constituents of end-user products such as foods, pharmaceuticals and cosmetics. Glycerol and D-glucose have been used and studied in the literature as the most representative polyol- and sugar-based DESs, respectively. Glycerol is a natural, inexpensive and non-toxic liquid that over the past decade has been used as an alternative solvent [
58]. Furthermore, glycerol is a the third most common ingredient (after water and fragrance) in cosmetics [
58]. Regarding D-glucose, it was selected since it is the most abundant monosaccharide and it has been studied lately for the preparation of NaDESs.
The NaDES Bet/La/W was structurally characterized by
1H and
13C NMR using DMSO-d
6 as solvent (
Figure 2). At 4.02 ppm appears the quartet owed to the protons of position 3′ of the two molecules of D,L-lactic acid. The singlet at 3.70 ppm is owed to the methylene protons of betaine, whereas the protons of the methyl groups of betaines appear as a singlet at 3.15 ppm. Finally, the doublet at 1.21 ppm is owed to the six protons of the methyl groups of lactic acid.
Regarding the
13C NMR spectrum (
Figure 2b), the chemical shifts at 177.96 ppm and 167.75 ppm can be attributed to the carbonyl groups of D,L-lactic acid (C-1′) and betaine (C-1) respectively. At 66.94 ppm and 66.18 ppm are presented the peaks of the C-2′ and C-2 respectively, while in higher magnetic field, the signals at 53.58 ppm and 21.13 ppm are given by the methyl groups of position 3 of the betaine skeleton and the carbons C-3′ of the lactic acid skeleton.
3.2. Thermogravimetric Analysis (TGA) of the NaDES Bet/LA/W
The TGA plot can be used to identify the actual decomposition points, based on the maximum weight loss rate. In this work, a TGA analysis of the Bet/LA/W has been conducted (
Figure 3), and the thermogram reveals single-stage degradation at 243 °C. This high degradation temperature ensures that the solvent is suitable for the selected extraction process without any concern about its thermal stability.
3.3. Preliminary Extraction Experiments
A series of extraction experiments using an ultrasound probe were carried out so as to select the extraction parameters and the margins of the parameters that should be used in the experimental design.
In
Table 2 are presented the results of the preliminary experiments of the UAE using the Sonics VC 400 High Intensity Processor equipped with a piezoelectric converter and a probe.
The parameters under investigation were the extraction time (min), the NaDES-to-ginger powder ratio (w/w) as well as the amount of water added on the NaDES Bet/LA/W. Ultrasound power of 120 Watts was selected.
According to the presented data, it can be concluded that the parameter of NaDES-to-water ratio in the investigated range (10–30% water) does not significantly affect the antioxidant activity and the phenolic content of the extracts. In many cases, water is added in NaDES extractions in order to improve the extraction performance due to the significant reduction of NaDES viscosity [
19]. In this study, the viscosity of the used NaDES Bet/LA/W was not a problem, possibly since water is present in the system due to the water content of the D,L-lactic acid.
Moreover, due to the fact that important bioactive compounds of ginger are insoluble in water, we set out to proceed to the next experiments without the addition of extra water. Thus, regarding our study, this parameter was not considered as significant for further evaluation. Contrariwise, the parameter of ultrasound power was selected to be further investigated. The selected extraction times (10, 15 and 20 min) and the NaDES-to-ginger powder ratio (w/w) (15 and 20 w/w) do not lead to significant changes regarding to the under-investigation responses, thus, it can be concluded that the boundaries of these parameters should be broader in the experimental design.
3.4. Experimental Design
The extraction of secondary metabolites strongly depends on the extraction method as well as the conditions of the extraction process. The NaDES Bet/LA/W was selected as a green solvent and UAE as an alternative to the conventional heating, aiming to shorten the extraction time [
33,
59].
The preliminary experiments indicated that the extraction time, ultrasound power and NaDES-to-ginger powder ratio play a significant role in the phytochemical profile of the extract. Implementing experimental design and statistical analysis, the optimal conditions were identified, aiming to maximize the total phenolic content (TPC) of the extracts and their ability to scavenge the stable free DPPH radical.
The responses TPC (R
1), expressed as mg GAE/g of ginger powder and antioxidant activity expressed as the IC
50 value (R
2) obtained from each extraction are presented in
Table 3.
The statistical analysis of the 17 runs revealed that the TPC is best described by the reduced quadratic model (Equation (1)):
The proposed model was significant with the F-value equal to 37.42 and the
p-value less than 0.0001. Moreover, the coefficient R
2 was calculated 0.9574, indicating accuracy and a good fit of the experimental data with the calculated ones. The adjusted determination coefficient R
2adj verified the adequacy of the model, as its value was 0.9318 (
Table 4).
The results clearly show that the NaDES-to-ginger powder ratio is the factor that contributes the most to the efficient extraction of phenols. This observation is in agreement with analogous works reported in the literature and can be attributed to the higher mass transfer that is succeeded when a higher volume of solvent is added [
25,
59,
60].
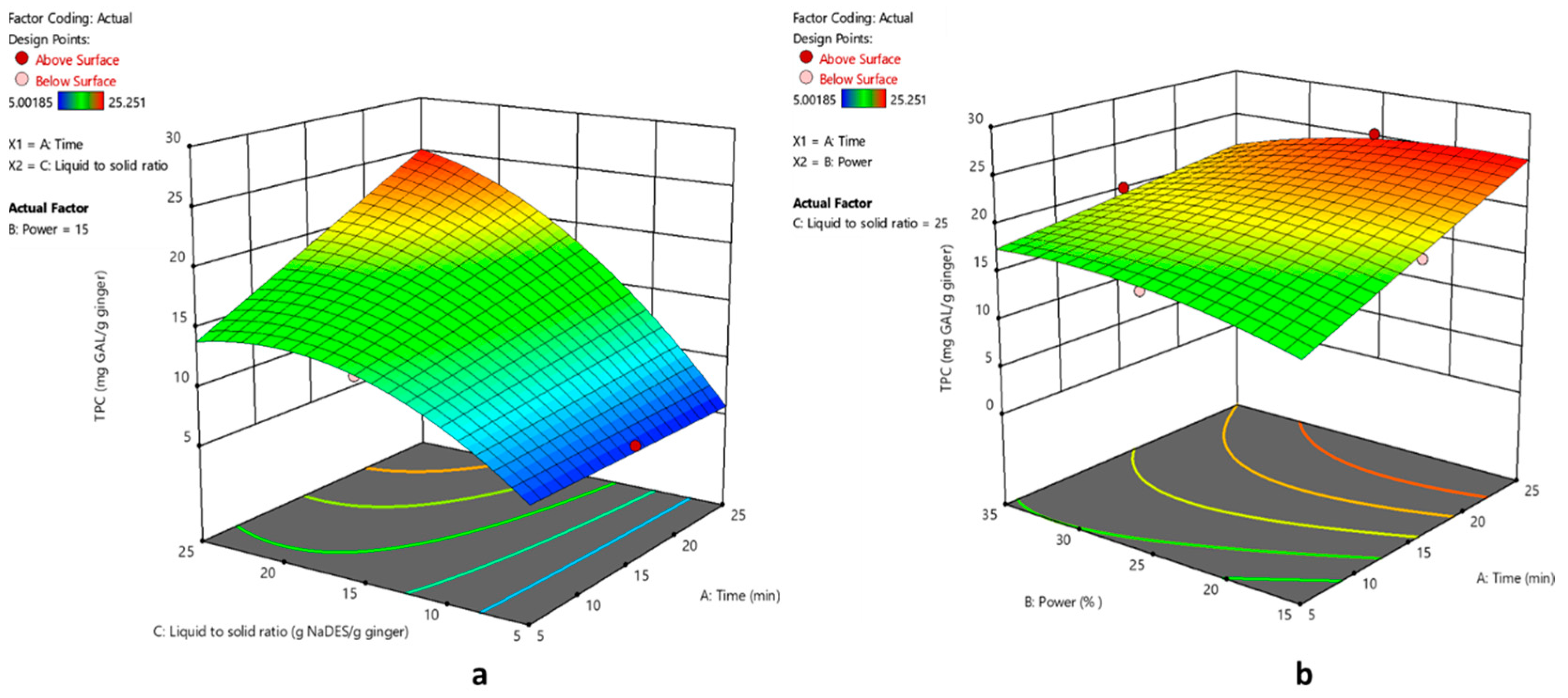
The 3D surface response plots depict the correlation between the studied factors and the TPC of the extract obtained (
Figure 4).
The coded mathematical equation describing the relation between the DPPH radical scavenging ability of the extract and the extraction conditions is (Equation (2)):
The reduced proposed model presented an F-value of 7.73 and
p-value of 0.0027, indicating a significant model. The R
2 was calculated 0.8226 which shows good fit of the experimental and the predicted results, and the R
2adj was 0.7162, verifying the adequacy of the model (
Table 5).
For the DPPH radical scavenging ability response, the extraction time was found to be the most significant factor. The 3D surface response plots depict the correlation between the studied factors and the antioxidant activity of the extract obtained (
Figure 5).
The desirability equation was set to maximize the response R1 and simultaneously minimize the response R2. The analysis of the results concluded that the optimal extraction conditions inside the studied area of parameters are 23.8 min extraction time, 60 Watt (15% of the maximum ultrasound power) and 25:1 w/w NaDES/dry ginger.
In order to confirm the validity of the proposed models, three additional experiments were conducted. Each experiment was run once. The experimental conditions, the observed responses and the 95% prediction intervals (PI) according to the estimated models are presented in the following table (
Table 5).
Since the experimental values of TPC are contained in the prediction intervals generated from the additional experiments, the validity of the model estimated for TPC is verified. As far as the ability of the extracts to scavenge the stable free radical DPPH is concerned, only one of the three experimentally determined DPPH scavenging ability values deviates 0.6% from the boundary set.
3.5. Quality Assessment of the NaDES-Extract
In this study, NaDESs were selected as a green extraction alternative to conventional VOCs. However, the proposed methodology is considered advantageous not only because of the green character of the solvents but also because of their ability to act as “storage” and “protective” media [
61,
62] for the sensitive extracted compounds and the extracts can be used “as obtained”, without the need of any further energy and time consuming procedures. Due to the NaDESs’ character, the extracts are supposed to be stable regarding their quality characteristics, thus, this is a parameter of high importance that should be examined.
The shelf-life of the obtained extract is an important feature for both manufacturers and consumers and the shelf-life studies can ensure the quality of the extract during the storage period.
In this context, in order to examine the extracts’ quality after a long enough period of time such as six months, their antioxidant activity and total phenolic content were evaluated. The extracts were analyzed at the first day of their production, and then, they were stored in the dark at 4 °C until their further analysis after 6 months. In this context, Extracts 2 and 3 (
Table 6) were selected as indicative extracts (
Table 6).
According to the results, it is clear that the quality of the extracts is stable in respect of the examined parameters (TPC and antioxidant activity), and it can be concluded that the NaDESs have a “protective” effect for the extracted phytochemicals.
3.6. Total Phenolic Content of the Ginger Extracts
The highest content in phenolic compounds was found in extract 4 (25.3 mg GAE/g of ginger powder) which was obtained after 25 min extraction, using 100 W ultrasound power and NaDES/ginger powder ratio 25:1. Extract 2 (20.8 mg GAE/g of ginger powder) and extract 5 (20.1 mg GAE/g of ginger powder) also showed satisfactory TPC. The lowest TPC was observed for extract 12 (5.0 mg of gallic acid/g of ginger powder), which was performed using the lowest NADES/ginger powder ratio (5:1).
The abovementioned results could be considered as important since the TPC of the extracts is considered satisfactory and, in some cases, higher compared to other techniques. Shan et al. [
63] have reported that a methanolic extract of dried ginger, did not possess a very high phenol content as compared to other spices (6.3 mg GAE/1 g of dry ginger). In a recent report by Contreras-López et al. [
38], which involved the ultrasound-assisted extraction of whole ginger rhizomes using water as solvent, the authors found low TPC values (ranging from 0.08 to 0.17 mg GAE/g of dry material) due to the effect of the ultrasonic energy which could promote side-reactions between the extracted phenolic compounds, which does not allow their efficient extraction in water.
Another asset of the proposed methodology, is the presence of NaDES in the produced extracts, which provides special advantages since NaDESs can act as “protective” media for the desirable and sensitive, in many cases, bioactive compounds. The extracted natural products are “stored” inside the NaDES leading to a stable extract which retains its TPC and antioxidant activity for a long period of time. A possible explanation of the NaDESs’ protective character is given by Guo et al. [
62] who explain how NaDESs behave as protective agents for anthocyanins extracted from fresh mulberry since it possibly acts as an antioxidant for the sensitive and unstable compounds. In the same study, they describe that the protection is based on the possible interaction between the hydrogen bond donor and the hydrogen bond acceptor, which can behave as a substitute in the anion or cation exchange when the compounds are exposed to extreme conditions.
3.7. Antioxidant Activity of the Ginger Extracts
The Bet/LA/W NaDES showed very low DPPH radical scavenging ability, with an IC50,t = 30 min of 328 mg/mL. All the obtained extracts showed remarkably higher antioxidant activity than the NaDES, with IC50,t = 30 min values ranging from 14.5 to 27.1 mg/mL, therefore this activity is owed exclusively to the extracted phytochemicals from the ginger powder. The best DPPH radical scavenging ability was shown by extract 7 (IC50,t = 30 min 14.5 mg of extract/mL) and the extract 1 (IC50,t = 30 min 14.9 mg of extract/mL), which were both obtained using the highest ultrasound power (140 W).
It is generally thought that the antioxidant activity of plant extracts is owed, up to a point, to the phenolic phytochemicals present in the extract [
64]. However, this does not seem to be the case in the present work: Extract 7 has one of the lowest TPC (6.9 mg GAE/g of ginger powder) whereas extract 1 has a TPC of 17.5 mg GAE/g of ginger powder. Extract 1, which was obtained after 5 min of extraction using NaDES/ginger powder ratio of 15:1 shows almost the same antioxidant activity with Extract 7, which was obtained after 15 min extraction using NaDES/ginger powder ratio of 5:1. However, Extract 1 possesses 2.5 times higher TPC than extract 7. Based on this observation, it can be postulated that prolonged extraction time and low NaDES/ginger ratio at 140 W leads to the extraction of phytochemicals with high DPPH radical scavenging ability but at the same time, does not favor the extraction of phenolic compounds (or their stability).
Moreover, the correlation of the antioxidant with the total phenolic content is not a trivial issue. The DPPH assay used in this work is a measure of the ability of a compound to transfer hydrogen and this is only one of the possible mechanisms that the antioxidant activity can be exerted. Thus, it is possible that the extracts with a high TPC do not show a very high DPPH radical scavenging ability because the extracted components act via a different antioxidant mechanism.
3.8. Expand the Scope of the NaDES-UA Ginger Extraction
In an effort to expand the scope of the present study, two different NaDESs were selected as extraction media, Glu/LA/W and Bet/Gly. Furthermore, for comparison reasons, conventional solvents were also used for the ginger extraction. The extraction process was conducted under the optimum conditions for simultaneous maximization of TPC and antioxidant activity of the extract.
The results of this study are presented in
Table 7. The TPC of the Bet/LA/W-extract is significantly higher (20.10 ± 0.26 mg GAE/g ginger) compared to the NaDESs as well as the conventional solvents. The lowest TPC values obtained using the conventional solvents were given when water was used as the extraction medium (5.12 ± 0.77 mg GAE/g ginger) possibly due to the high amount of insoluble to water bioactive compounds of ginger. Comparing the NaDESs, the lowest TPC values were found when Bet/Gly was used as the extraction medium. This result could be attributed to the high viscosity of the solvent that led to poor mass-transfer rate of the phenolic compounds from ginger to the solvent. Moreover, another possible factor that affects the extraction of the phenolic compounds is the pH of the solvent. In respect of pH, Bet/LA/W and Glu/LA/W are acidic solvents while Bet/Gly possesses a pH close to neutral.
Regarding the antioxidant activity of the extracts, the IC
50 values for DPPH scavenging activity in relation to time (
Table 7), has been studied. Regarding the NaDES extracts the activities seem to be constantly increasing during the 120 min of observation and measurement. More specifically, the difference in the IC
50 values between the first 15 and 30 min was higher, while from 30 min to 120 min, the values decreased following a slower rate.
It is noteworthy that in the case of the extract obtained using Bet/Gly (
Table 7, Entry 3), the difference in the antioxidant activity in relation to time was higher. A plausible explanation for this observation is that the Bet/Gly has a higher viscosity than the other two NaDESs used in this study. The high viscosity probably prevents the immediate interaction of the DPPH radical with the extracted phytochemicals that are entrapped in the solvent. As the time passes, the compounds are “released” from the NaDES and interact with DPPH leading to an increase of the antioxidant activity. On the other hand, when conventional solvents were used for the extraction (
Table 7, Entries 4, 5 and 6), the DPPH scavenging ability of the extracts did not change significantly, indicating that the results are not significantly time dependent (from 30 to 60 min).
This behavior reinforces the belief that the NaDESs act as “protective” and “storage” media for the extracted antioxidant compounds.
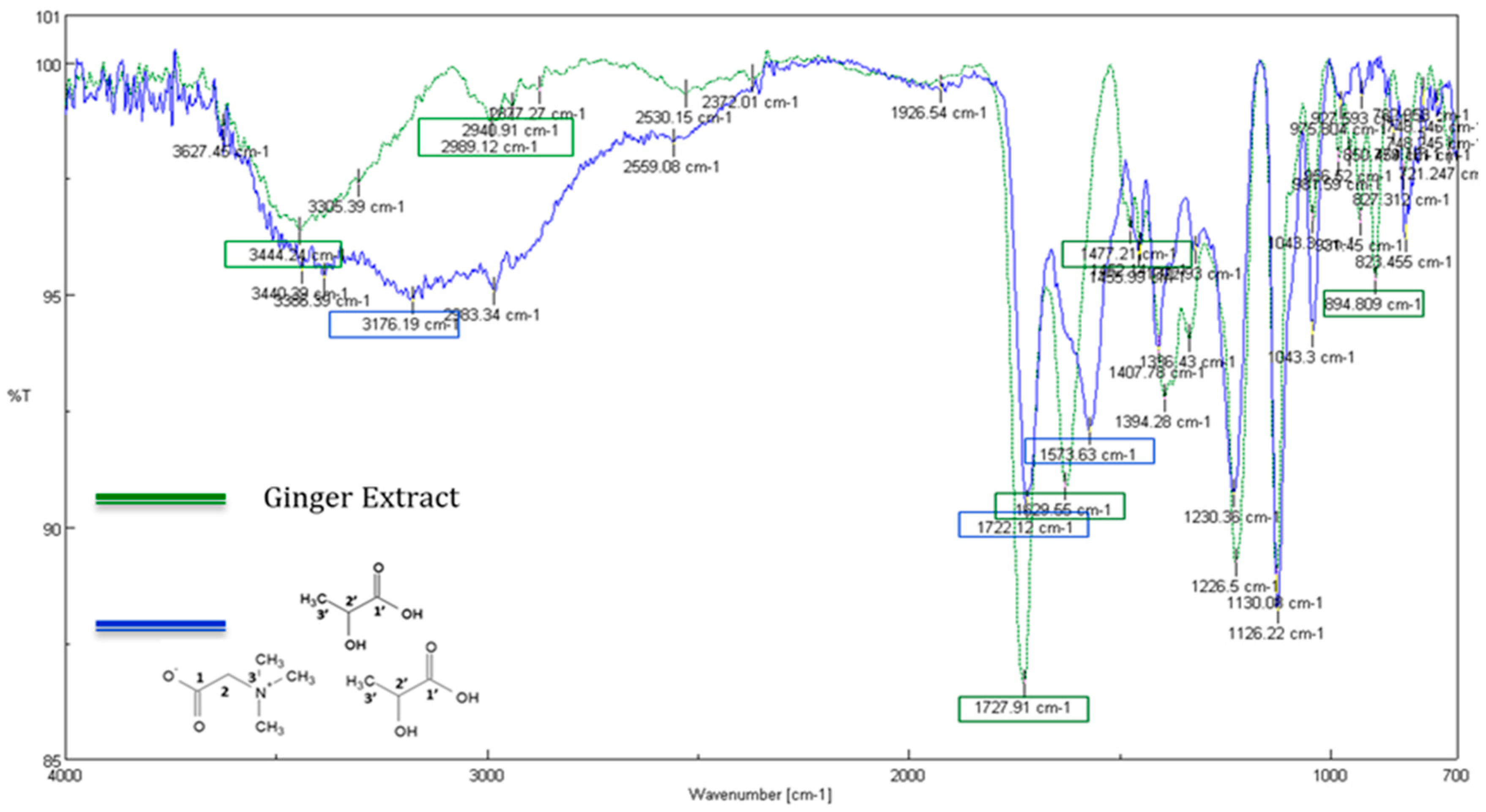
3.9. FTIR Characterization of the Bet/LA/W-Ginger Extract
Figure 6 shows the FTIR spectra of Bet/LA/W (blue) and the ginger extract derived from the extraction using the Bet/LA/W NaDES under the optimum conditions (green) as obtained by the proposed UAE methodology. The most important peaks in the Bet/LA/W spectrum are the broad absorption at around 3176 cm
−1 that is assigned to the
v-OH stretching and the
v-COO
− asymmetrical stretching vibration at 1722.12 cm
−1. The absorbance band at the 1573.63 cm
−1 is owed to the COO
- asymmetric stretch and the band at 1230 cm
−1 is attributed to the C-N stretching of the amine group of betaines. In addition, the peak at 1407.78 cm
−1 is characteristic for the COO
- symmetric stretch, and the C-O stretch of the carboxylic acid appears at 1126.22 cm
−1. Finally, the absorption band at 1043.3 cm
−1 can be assigned to the
v-(C–CH
3) stretching.
Regarding the ginger extract spectrum, the peaks at 2989.12 and 2940.91 cm
−1 could be attributed to the OH groups that are involved in hydrogen bonds. In the spectrum of the extract, the slightly shifted and more intense peak at 1727.91 cm
−1 provides the indication that the components of the extract interact with the NaDES and cause shifting of the COO
- absorption due to hydrogen bonding. The characteristic peaks at the 1573.63 cm
−1 and 1407.28 cm
−1 for the CO
2- asymmetric and symmetric stretch [
65] of the betaine molecule are shifted at 1629.55 cm
−1 and 1394.28 cm
−1 respectively, indicating the interaction of NaDES with the extracted components. Similarly, a slighter shift is also observed from 1126.22 cm
−1 (NaDES spectrum) to 1130.08 cm
−1 (extract spectrum) probably indicates the interaction of the C-O group of lactic acid with extracted compounds.
In the range of wavelengths 1477–1043 cm−1 discrete peaks appear, which in comparison with those of the Bet/LA spectrum, are more and shifted. This region is typical for the absorptions of C=C stretch of the aromatic ring as well as C-O vibration, so it is possible that their intensity is enhanced in the presence of the extracted components (such as phenolic compounds).
Finally, a new peak at wavelength of 894.809 cm
−1 (extract spectrum) could be attributed to the bending vibrations R
2C=CH
2 of alkenes, to the O-H deformation, C=O deformation or to the C–H deformation of cellulose (-anomeric linkage) [
66], indications of the presence of the extracted valuable compounds of ginger.
3.10. Binding Studies with ctDNA Using UV Spectroscopy
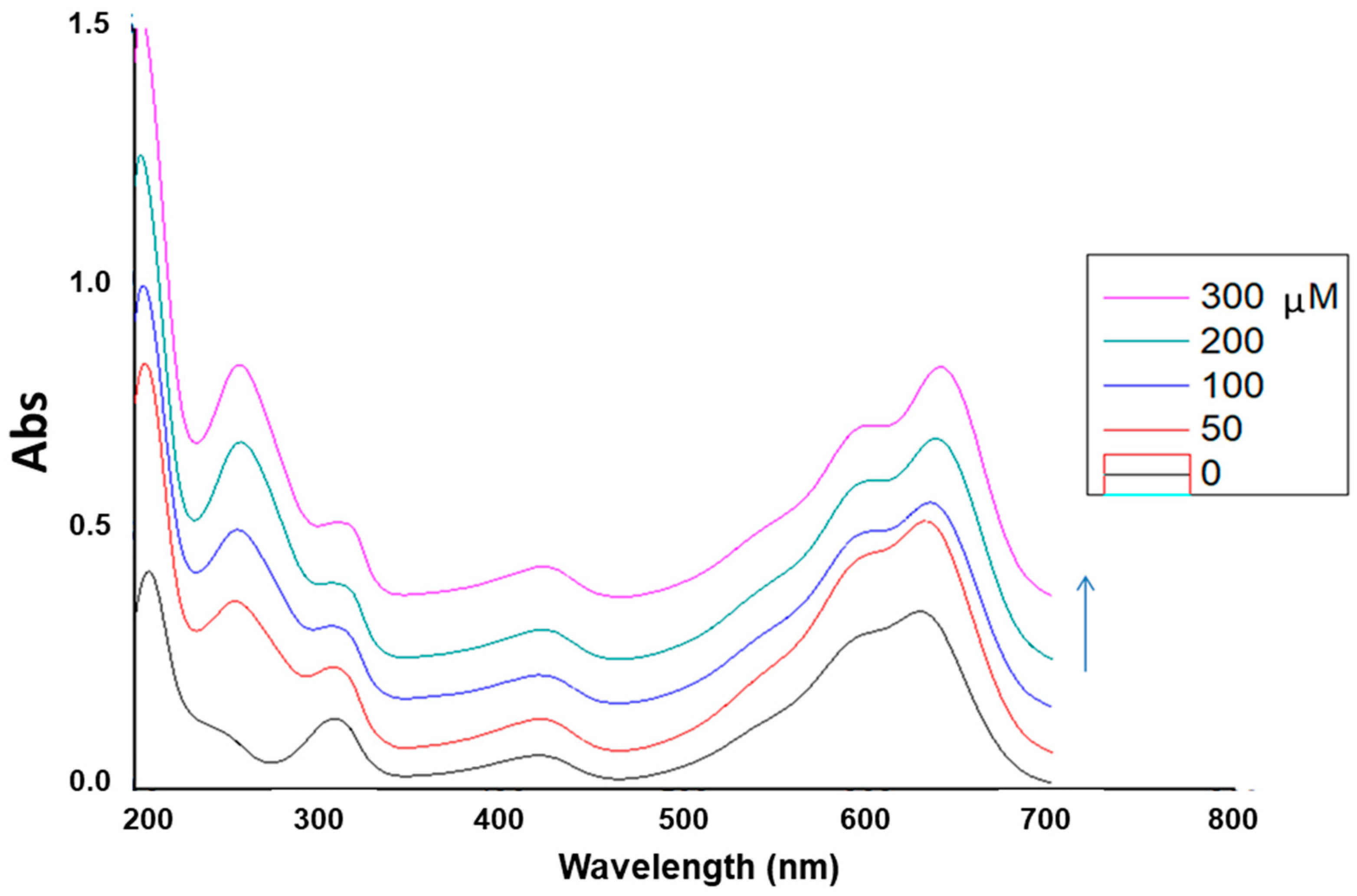
The interaction of the ginger extract obtained using Bet/LA/W at the optimum extraction conditions (
Table 7, Entry 1) with ctDNA was studied by UV spectroscopy. An interaction between a chemical entity or an extract and DNA can disrupt the ctDNA band located at 260–280 nm in the presence of increasing amounts of ctDNA.
In absorption spectroscopy, hypochromism and hyperchromism are important spectral features for the study of changes in the structure of DNA. Due to the strong interactions between a molecule and the DNA bases, a change in absorption is observed, showing the proximity of the molecule to the DNA bases. Based on the interaction of compounds with DNA, the binding constant k
b for ligand–DNA binding was determined in the present work, using the Benesi–Hildebrand plot [
67].
For the sake of comparison, the binding of two well-known dyes, was also studied: Rhodamine B, which has a non-intercalating ctDNA binder in the DNA minor groove [
68], and methyl green, which is a major groove binder, showing a weak to moderate interaction with DNA, depending on the binding ratio [
69,
70].
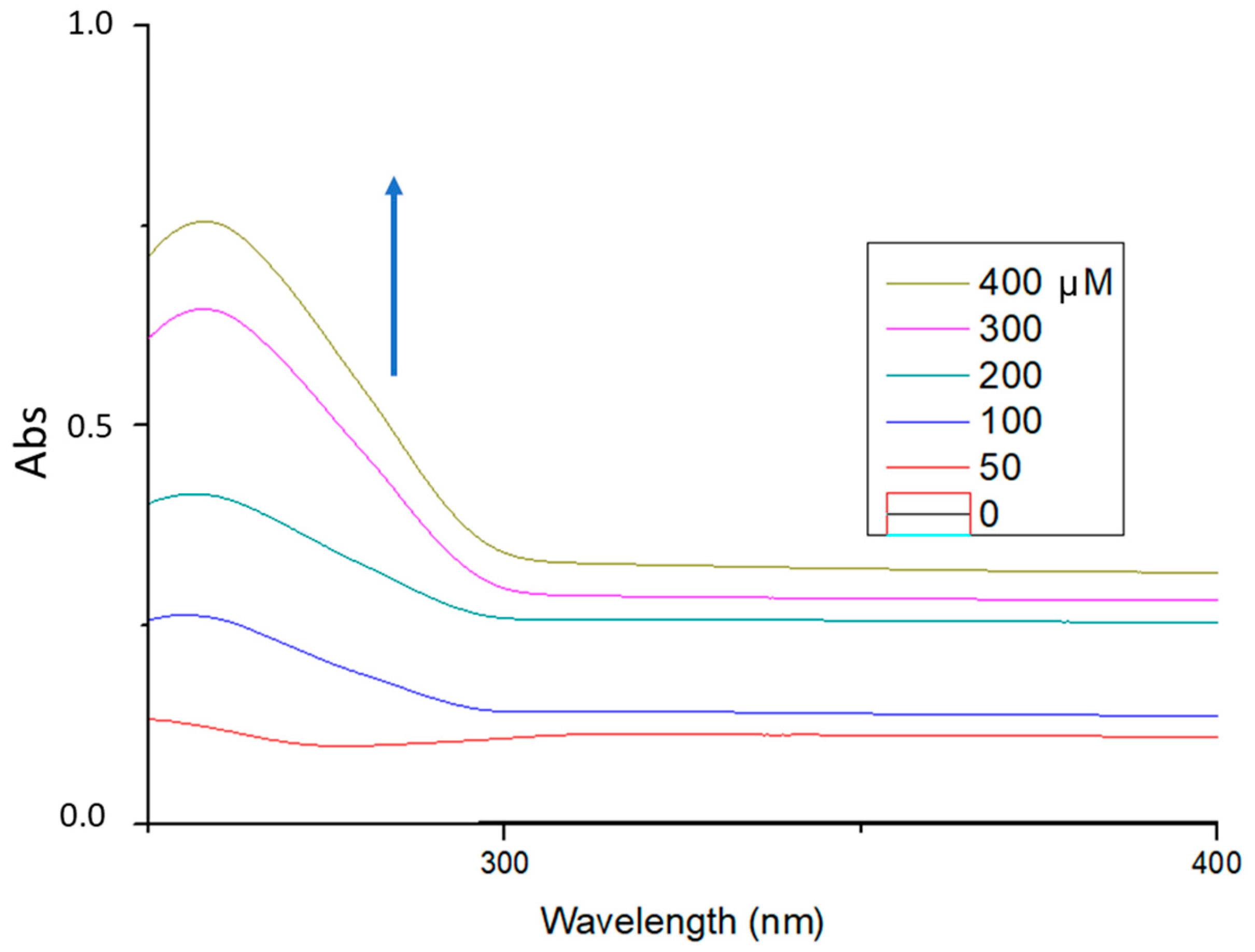
As shown in
Figure 7, the ginger extract (100 μg/mL, pH = 7.4) showed absorption maxima at 254.8 nm. With incremental addition of ctDNA to the solution of the extract, an increase in the absorption intensity at 254.8 nm was observed with concomitant red shift of the λ
max at 257.8 nm. The binding constant (k
b) of the extract was calculated from the ratio of the intercept to the slope and was found to be k
b = 2.0 × 10
3 M
−1. This hyperchromism suggests that the studied ginger extract binds to ctDNA in the major groove.
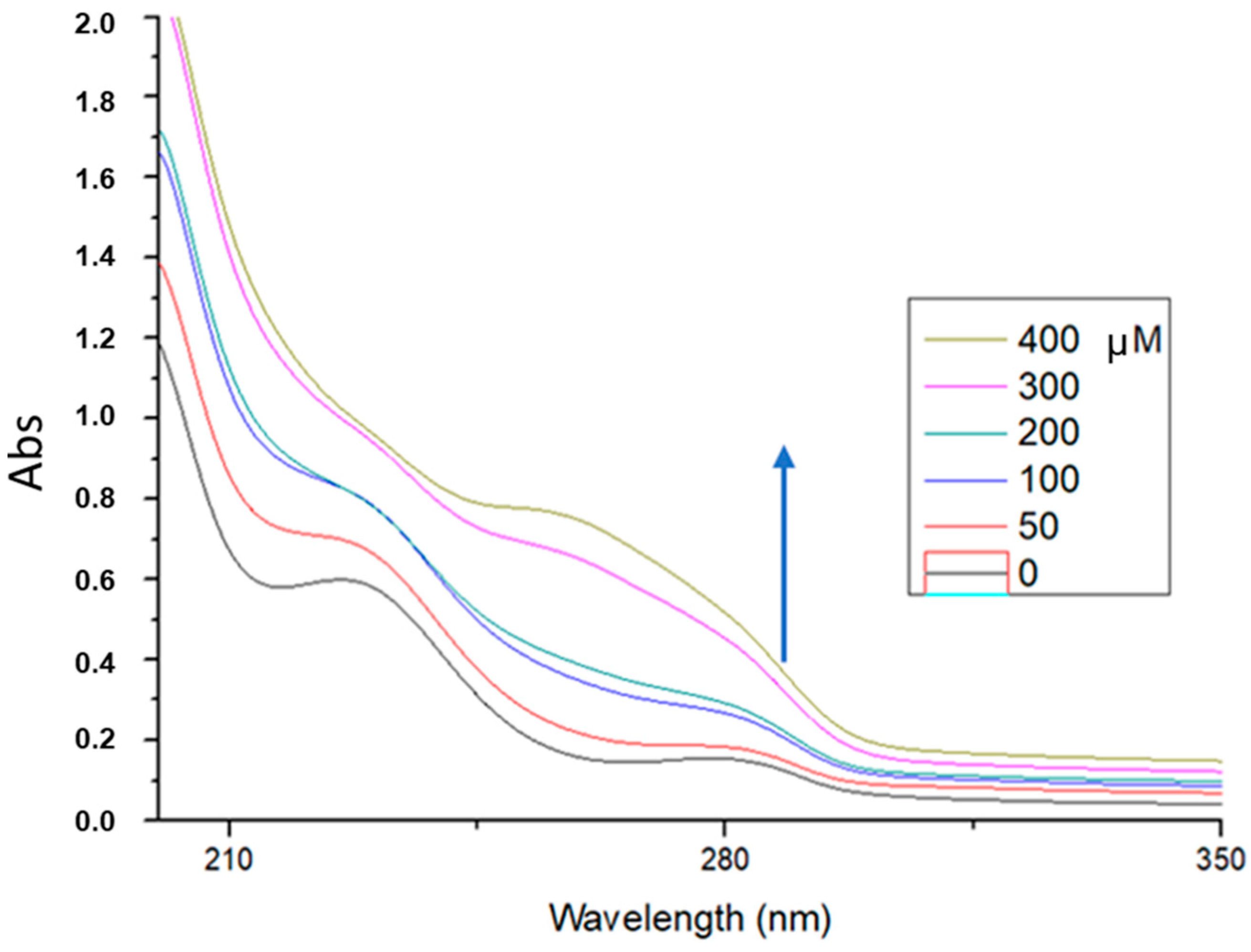
The phenolic compounds in ginger are mainly gingerols, shogaols and paradols. In fresh ginger, gingerols are the major polyphenols and with heat treatment or long-time storage, gingerols can be transformed into corresponding shogaols. Further, 6-shogaol isolated by conventional extraction from dry ginger, was also studied for its ability to bind to ctDNA. In addition, 6-shogaol (100 μg/mL, pH = 7.4) showed absorption maxima at 225.4 nm (
Figure 8). With incremental addition of ctDNA to the solution of 6-shogaol, an increase in the absorption intensity at 225.4 nm was observed with concomitant red shift of the λ
max at 260.0 nm. The binding constant (k
b) of the extract was calculated from the ratio of the intercept to the slope and was found to be k = 3.8 × 10
3 M
−1. This hyperchromism suggests that 6-shogaol binds inside the major groove of ctDNA.
The NaDES Bet/LA/W, pure betaine and pure D,L-lactic acid (80–85% aq. sol) were also studied, but no interaction with ctDNA could be observed.
The results from this study are summarized in
Table 8. According to the results, pure 6-shogaol binds to the major groove of ctDNA, showing an analogous behavior to methyl green (
Figure 9). The ginger extract obtained using Bet/LA/W under the optimal conditions (
Table 7, Entry 1) was also found to bind in the major groove of ctDNA and this binding can be considered to be owed exclusively to the extracted phytochemicals as the used NaDES does not interact with ctDNA.
4. Conclusions
The extraction of valuable phytochemicals from dry ginger has been widely investigated using conventional solvents and methods, even though to our knowledge the research regarding the implementation of high energy techniques such as ultrasound-assisted extraction (UAE) in combination with NaDESs is very limited for this plant material. This work presents the implementation and optimization of UAE of phytochemicals from ginger powder, using as extraction medium the NaDES Bet/LA/W (1:2:2.5). The implementation of an UAE technique using a NaDES for the extraction of dry ginger, demonstrated that the method could be considered as a practicable technique, alternative to the conventional harsh extraction techniques. RSM can be successfully employed in order to extract a satisfactory model for the optimization of the selected parameters (time, ultrasound % power, NaDES-to-dry ginger ratio). The data obtained from the experimental design indicated that the Bet/LA/W-to-dry ginger ratio contributes the most to the extraction of phenols while the extraction time was the most significant factor regarding the antioxidant activity of the extract. Finally, the applied BBD, indicated the following conditions as optimal within the experimental region for the simultaneous maximization of the TPC and the antioxidant activity: extraction time of 23.8 min, 60 Watt and the NaDES-to-dry ginger ratio at 25:1 w/w.
The quality of the extracts was also investigated, by measuring their TPC and antioxidant activity the first day of their production and after six months. No significant change on the extracts’ quality was observed, thus it can be concluded that the NaDES contained in the extract exerts a stabilization effect on the extracted phytochemicals.
Moreover, in order to expand the scope of this study, two more NaDESs were implemented for the extraction of ginger using the optimum conditions. From this study the NaDES Bet/LA/W was found to be the most effective medium, giving the extract with the higher amount of TPC and best antioxidant activity.
In order to compare the aforementioned results with conventional solvents such as ethanol, water and ethanol/water (70:30 v/v), the extractions with these solvents were implemented using the optimum conditions indicated by the experimental design. The Bet/LA/W extract presented the best TPC and antioxidant activity (TPC = 20.10 ± 0.26 mg GAE/g ginger, DPPH radical scavenging ability IC50,t = 120 min= 18.16 ± 1.80 mg extract/mL) among all the solvents tested (NaDESs and conventional). For the first time to our knowledge, the time-dependence of the antioxidant activity of NaDES-containing extracts is investigated. According to the findings of this research, the antioxidant activity of the obtained extracts is time dependent and shows an increase even after 120 min, proving once again that these solvents can act as protective agents for the extracted compounds.
Finally, this is the first study where the ctDNA binding studies are implemented in NaDES-containing extracts. The results of the ctDNA binding studies, suggest that the ginger extract obtained using Bet/LA/W under the optimal conditions (
Table 7, Entry 1) as well as pure 6-shogaol bind to the major groove of ctDNA, in an analogous mode to methyl green. Taking into account that no interaction of the NaDES or its separate components with ctDNA could be observed, we can conclude that the NaDES ginger extract binding to ctDNA is exclusively attributed to the extracted phytochemicals.
According to the finding of the present research, the NaDES-ginger extracts are characterized by time-dependent DPPH radical scavenging ability, as well as ctDNA binding ability, therefore, they could be exploited directly to the development of added-value end-products of interest in the pharmaceutical and cosmetic industry.