Analysis of Sustainable Methods to Recover Neodymium
Abstract
1. Introduction
2. Recovery of Nd via Hydrometallurgy
3. Recovery of Nd via Pyrometallurgy
4. Recovery of Nd via Electrodeposition
5. Remarks and Future Suggestions
Author Contributions
Funding
Conflicts of Interest
References
- Omodara, L.; Pitkäaho, S.; Turpeinen, E.M.; Saavalainen, P.; Oravisjärvi, K.; Keiski, R.L. Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications—A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Chen, C. Global potential of rare earth resources and rare earth demand from clean technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Reilly, J.F. Mineral Commodity Summaries 2020; USGS: Reston, VA, USA, 2020.
- Pell, R.S.; Wall, F.; Yan, X.; Bailey, G. Applying and advancing the economic resource scarcity potential (ESP) method for rare earth elements. Resour. Policy 2019, 62, 472–481. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Müller, T.; Yurramendi, L. Rare Earths and the Balance Problem: How to Deal with Changing Markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Reimer, M.V.; Schenk-Mathes, H.Y.; Hoffmann, M.F.; Elwert, T. Recycling decisions in 2020, 2030, and 2040—when can substantial NdFeB extraction be expected in the EU? Metals 2018, 8, 867. [Google Scholar] [CrossRef]
- Rollat, A.; Guyonnet, D.; Planchon, M.; Tuduri, J. Prospective analysis of the flows of certain rare earths in Europe at the 2020 horizon. Waste Manag. 2016, 49, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.A.P.; Huda, N.; Farjana, S.H.; Lang, C. Comparative life cycle environmental impact analysis of lithium-ion (Liio) and nickel-metal hydride (nimh) batteries. Batteries 2019, 5, 22. [Google Scholar] [CrossRef]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating rare earth element availability: A case with revolutionary demand from clean technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.T.; Du, X.; Graedel, T.E. Criticality of the Rare Earth Elements. J. Ind. Ecol. 2015, 19, 1044–1054. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Firdaus, M.; Rhamdhani, M.A.; Durandet, Y.; Rankin, W.J.; McGregor, K. Review of High-Temperature Recovery of Rare Earth (Nd/Dy) from Magnet Waste. J. Sustain. Metall. 2016, 2, 276–295. [Google Scholar] [CrossRef]
- Chen, J.P.; Lim, L.L. Recovery of precious metals by an electrochemical deposition method. Chemosphere 2005, 60, 1384–1392. [Google Scholar] [CrossRef]
- Endres, F. Ionic liquids: Solvents for the electrodeposition of metals and semiconductors. ChemPhysChem 2002, 3, 144–154. [Google Scholar] [CrossRef]
- Halli, P.; Heikkinen, J.J.; Elomaa, H.; Wilson, B.P.; Jokinen, V.; Yliniemi, K.; Franssila, S.; Lundström, M. Platinum Recovery from Industrial Process Solutions by Electrodeposition-Redox Replacement. ACS Sustain. Chem. Eng. 2018, 6, 14631–14640. [Google Scholar] [CrossRef]
- Lindsay, S.J.; Welch, B.J. A Review: Understanding the Science and the Impacts of Impurities upon the Electrolytic Bath of Hall–Héroult Reduction Cells. J. Miner. Met. Mater. Soc. 2021, 73, 1196–1209. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, T.H.; Kim, D.Y.; Nersisyan, H.H.; Han, M.H.; Kang, K.S.; Bae, K.K.; Shin, Y.J.; Lee, J.H. Microstructural and corrosion characteristics of tantalum coatings prepared by molten salt electrodeposition. Surf. Coat. Technol. 2013, 235, 819–826. [Google Scholar] [CrossRef][Green Version]
- Izadpanah, S.M.; Aboutalebi, M.R.; Adeli, M. Molten salt electrodeposition of aluminum on mild steel: Effect of process parameters on surface morphology and corrosion properties. Mater. Res. Express 2021, 8, 046518. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G.; Ryder, K.S. Electroplating using ionic liquids. Annu. Rev. Mater. Res. 2013, 43, 335–358. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G.; Ryder, K.S. Metal complexation in ionic liquids. Annu. Rep. Prog. Chem.—Sect. A 2008, 104, 21–45. [Google Scholar] [CrossRef]
- Abbott, A.P.; McKenzie, K.J. Application of ionic liquids to the electrodeposition of metals. Phys. Chem. Chem. Phys. 2006, 8, 4265–4279. [Google Scholar] [CrossRef]
- Bernasconi, R.; Panzeri, G.; Accogli, A.; Liberale, F.; Nobili, L.; Magagnin, L. Chapter 11—Electrodeposition from Deep Eutectic Solvents. In Progress and Developments in Ionic Liquids; IntechOpen: London, UK, 2017; pp. 235–261. [Google Scholar]
- AliAkbari, R.; Marfavi, Y.; Kowsari, E.; Ramakrishna, S. Recent Studies on Ionic Liquids in Metal Recovery from E-Waste and Secondary Sources by Liquid-Liquid Extraction and Electrodeposition: A Review. Mater. Circ. Econ. 2020, 2, 10. [Google Scholar] [CrossRef]
- Tunsu, C. Hydrometallurgy in the recycling of spent NdFeB permanent magnets. In Waste Electrical and Electronic Equipment Recycling: Aqueous Recovery Methods; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 175–211. ISBN 9780081020579. [Google Scholar]
- Erust, C.; Akcil, A.; Tuncuk, A.; Deveci, H.; Yazici, E.Y. A Multi-stage Process for Recovery of Neodymium (Nd) and Dysprosium (Dy) from Spent Hard Disc Drives (HDDs). Miner. Process. Extr. Metall. Rev. 2019, 42, 90–101. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Process optimization and kinetics for leaching of rare earth metals from the spent Ni-metal hydride batteries. Waste Manag. 2016, 51, 196–203. [Google Scholar] [CrossRef]
- Pietrelli, L.; Bellomo, B.; Fontana, D.; Montereali, M.R. Rare earths recovery from NiMH spent batteries. Hydrometallurgy 2002, 66, 135–139. [Google Scholar] [CrossRef]
- Parhi, P.K.; Sethy, T.R.; Rout, P.C.; Sarangi, K. Separation and recovery of neodymium and praseodymium from permanent magnet scrap through the hydrometallurgical route. Sep. Sci. Technol. 2016, 51, 2232–2241. [Google Scholar] [CrossRef]
- Behera, S.S.; Parhi, P.K. Leaching kinetics study of neodymium from the scrap magnet using acetic acid. Sep. Purif. Technol. 2016, 160, 59–66. [Google Scholar] [CrossRef]
- Meshram, P.; Somani, H.; Pandey, B.D.; Mankhand, T.R.; Deveci, H. Abhilash Two stage leaching process for selective metal extraction from spent nickel metal hydride batteries. J. Clean. Prod. 2017, 157, 322–332. [Google Scholar] [CrossRef]
- Lee, C.H.; Yen, H.Y.; Liao, C.H.; Popuri, S.R.; Cadogan, E.I.; Hsu, C.J. Hydrometallurgical processing of Nd–Fe–B magnets for Nd purification. J. Mater. Cycles Waste Manag. 2017, 19, 102–110. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, C.J.; Chung, K.W.; Kim, S.D.; Lee, J.Y.; Kumar, J.R. Solvent extraction, separation and recovery of dysprosium (Dy) and neodymium (Nd) from aqueous solutions: Waste recycling strategies for permanent magnet processing. Hydrometallurgy 2016, 165, 27–43. [Google Scholar] [CrossRef]
- Ryu, H.Y.; Lee, J.H.; Kim, W.G.; Nersisyan, H.H.; Lee, G.G.; Jo, S.K.; Lee, H.H.; Hwang, I.S. Electrochemical behavior of Nd in its pyrometallurgical recovery from waste magnet. Rare Met. 2015, 34, 111–117. [Google Scholar] [CrossRef]
- Öhl, J. Challenges in electrolysis of neodymium in chloride melts at 500 °C. J. Appl. Electrochem. 2018, 48, 765–772. [Google Scholar] [CrossRef]
- Tsubota, T.; Ohtaki, M.; Eguchi, K. Thermoelectric Properties of Chevrel-Type Sulfides AMo6S8 (A = Fe, Ni, Ag, Zn, Sn, Pb, Cu). J. Ceram. Soc. Jpn. 1999, 107, 697–701. [Google Scholar] [CrossRef]
- Shirayama, S.; Okabe, T.H. Selective Extraction and Recovery of Nd and Dy from Nd-Fe-B Magnet Scrap by Utilizing Molten MgCl2. Metall. Mater. Trans. B 2018, 49, 1067–1077. [Google Scholar] [CrossRef]
- Akahori, T.; Miyamoto, Y.; Saeki, T.; Okamoto, M.; Okabe, T.H. Optimum conditions for extracting rare earth metals from waste magnets by using molten magnesium. J. Alloys Compd. 2017, 703, 337–343. [Google Scholar] [CrossRef]
- Bian, Y.Y.; Guo, S.Q.; Xu, Y.L.; Tang, K.; Lu, X.G.; Ding, W.Z. Recovery of rare earth elements from permanent magnet scraps by pyrometallurgical process. Rare Met. 2015, 239–248. [Google Scholar] [CrossRef]
- Maroufi, S.; Khayyam Nekouei, R.; Sahajwalla, V. Thermal Isolation of Rare Earth Oxides from Nd-Fe-B Magnets Using Carbon from Waste Tyres. ACS Sustain. Chem. Eng. 2017, 5, 6201–6208. [Google Scholar] [CrossRef]
- Maroufi, S.; Nekouei, R.K.; Hossain, R.; Assefi, M.; Sahajwalla, V. Recovery of Rare Earth (i.e., La, Ce, Nd, and Pr) Oxides from End-of-Life Ni-MH Battery via Thermal Isolation. ACS Sustain. Chem. Eng. 2018, 6, 11811–11818. [Google Scholar] [CrossRef]
- Müller, T.; Friedrich, B. Development of a recycling process for nickel-metal hydride batteries. J. Power Sources 2006, 158, 1498–1509. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, Y.; Bu, W. Pyrometallurgical Extraction of Valuable Elements in Ni-Metal Hydride Battery Electrode Materials. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2015, 46, 2153–2157. [Google Scholar] [CrossRef]
- Huang, C.; Liu, X.; Gao, Y.; Liu, S.; Li, B. Cathodic processes of neodymium(III) in LiF-NdF3-Nd2O3 melts. Faraday Discuss. 2016, 190, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Pesic, B. Electrochemistry and the mechanisms of nucleation and growth of neodymium during electroreduction from LiCl-KCl eutectic salts on Mo substrate. J. Nucl. Mater. 2015, 458, 37–44. [Google Scholar] [CrossRef]
- Hamel, C.; Chamelot, P.; Taxil, P. Neodymium(III) cathodic processes in molten fluorides. Electrochim. Acta 2004, 49, 4467–4476. [Google Scholar] [CrossRef]
- Novoselova, A.; Smolenski, V. Electrochemical behavior of neodymium compounds in molten chlorides. Electrochim. Acta 2013, 87, 657–662. [Google Scholar] [CrossRef]
- Stefanidaki, E.; Hasiotis, C.; Kontoyannis, C. Electrodeposition of neodymium from LiF-NdF3-Nd2O3 melts. Electrochim. Acta 2001, 46, 2665–2670. [Google Scholar] [CrossRef]
- Bourbos, E.; Karantonis, A.; Sygellou, L.; Paspaliaris, I.; Panias, D. Study of Nd electrodeposition from the aprotic organic solvent dimethyl sulfoxide. Metals 2018, 8, 803. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Patrice, S.; Austen, A.C. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef]
- Endres, F.; Zein El Abedin, S. Air and water stable ionic liquids in physical chemistry. Phys. Chem. Chem. Phys. 2006, 8, 2101–2116. [Google Scholar] [CrossRef]
- Kondo, H.; Matsumiya, M.; Tsunashima, K.; Kodama, S. Attempts to the electrodeposition of Nd from ionic liquids at elevated temperatures. Electrochim. Acta 2012, 66, 313–319. [Google Scholar] [CrossRef]
- Sanchez-Cupido, L.; Pringle, J.M.; Siriwardana, A.L.; Unzurrunzaga, A.; Hilder, M.; Forsyth, M.; Pozo-Gonzalo, C. Water-Facilitated Electrodeposition of Neodymium in a Phosphonium-Based Ionic Liquid. J. Phys. Chem. Lett. 2019, 10, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Bourbos, E.; Giannopoulou, I.; Karantonis, A.; Paspaliaris, I.; Panias, D. Reduction of Light Rare Earths and a Proposed Process for Nd Electrorecovery Based on Ionic Liquids. J. Sustain. Metall. 2018, 4, 395–406. [Google Scholar] [CrossRef]
- Matsumiya, M.; Ishii, M.; Kazama, R.; Kawakami, S. Electrochemical analyses of diffusion behaviors and nucleation mechanisms for neodymium complexes in [DEME][TFSA] ionic liquid. Electrochim. Acta 2014, 146, 371–377. [Google Scholar] [CrossRef]
- Matsumiya, M.; Kikuchi, Y.; Yamada, T.; Kawakami, S. Extraction of rare earth ions by tri-n-butylphosphate/phosphonium ionic liquids and the feasibility of recovery by direct electrodeposition. Sep. Purif. Technol. 2014, 130, 91–101. [Google Scholar] [CrossRef]
- Krishna, G.M.; Rout, A.; Venkatesan, K.A. Voltammetric investigation of some lanthanides in neutral ligand-ionic liquid. J. Electroanal. Chem. 2020, 856, 113671. [Google Scholar] [CrossRef]
- Bagri, P.; Luo, H.; Popovs, I.; Thapaliya, B.P.; Dehaudt, J.; Dai, S. Trimethyl phosphate based neutral ligand room temperature ionic liquids for electrodeposition of rare earth elements. Electrochem. Commun. 2018, 96, 88–92. [Google Scholar] [CrossRef]
- Murakami, S.; Matsumiya, M.; Yamada, T.; Tsunashima, K. Extraction of Pr(III), Nd(III), and Dy(III) from HTFSA Aqueous Solution by TODGA/Phosphonium-Based Ionic Liquids. Solvent Extr. Ion Exch. 2016, 34, 172–187. [Google Scholar] [CrossRef]
- Ishii, M.; Matsumiya, M.; Kawakami, S. Development of Recycling Process for Rare Earth Magnets by Electrodeposition Using Ionic Liquids Media. ECS Trans. 2012, 50, 549–560. [Google Scholar] [CrossRef]
- Kondo, H.; Matsumiya, M.; Tsunashima, K.; Kodama, S. Investigation of Oxidation State of the Electrodeposited Neodymium Metal Related with the Water Content of Phosphonium Ionic Liquids. ECS Trans. 2013, 50, 529–538. [Google Scholar] [CrossRef]
- Sanchez-Cupido, L.; Pringle, J.M.; Siriwardana, A.I.; Hilder, M.; Forsyth, M.; Pozo-Gonzalo, C. Correlating Electrochemical Behavior and Speciation in Neodymium Ionic Liquid Electrolyte Mixtures in the Presence of Water. ACS Sustain. Chem. Eng. 2020, 8, 14047–14057. [Google Scholar] [CrossRef]
- Dong, Y. Effect of alkyl chain length on interfacial structure of imidazolium-based tetrafluoroborate ionic liquids on Au(100) electrodes. Mater. Res. Express 2020, 7, 075010. [Google Scholar] [CrossRef]
- Li, H.; Endres, F.; Atkin, R. Effect of alkyl chain length and anion species on the interfacial nanostructure of ionic liquids at the Au(111)-ionic liquid interface as a function of potential. Phys. Chem. Chem. Phys. 2013, 15, 14624–14633. [Google Scholar] [CrossRef]
- Ota, H.; Matsumiya, M.; Sasaya, N.; Nishihata, K.; Tsunashima, K. Investigation of electrodeposition behavior for Nd(III) in [P2225][TFSA] ionic liquid by EQCM methods with elevated temperatures. Electrochim. Acta 2016, 222, 20–26. [Google Scholar] [CrossRef]
- Razo-Negrete, M.; Ortega-Borges, R.; Zinovyeva, V.; Cannes, C.; Le Naour, C.; Trejo-Córdova, G.; Meas, Y. Comparison of the electrochemical behavior of some Rare Earth Elements in butyl methylpyrrolidinium dicyanamide ionic liquid. Int. J. Electrochem. Sci. 2019, 14, 10431–10447. [Google Scholar] [CrossRef]
- Sanchez-Cupido, L.; Pringle, J.M.; Siriwardana, A.; Pozo-Gonzalo, C.; Forsyth, M. Electrochemistry of Neodymium in Phosphonium Ionic Liquids: The Influence of Cation, Water Content, and Mixed Anions. Aust. J. Chem. 2020, 73, 1080–1087. [Google Scholar] [CrossRef]
- Periyapperuma, K.; Pringle, J.M.; Sanchez-Cupido, L.; Forsyth, M.; Pozo-Gonzalo, C. Fluorine-free ionic liquid electrolytes for sustainable neodymium recovery using an electrochemical approach. Green Chem. 2021, 23, 3410–3419. [Google Scholar] [CrossRef]
- Costa, S.P.F.; Azevedo, A.M.O.; Pinto, P.C.A.G.; Saraiva, M.L.M.F.S. Environmental Impact of Ionic Liquids: Recent Advances in (Eco)toxicology and (Bio)degradability. ChemSusChem 2017, 10, 2321–2347. [Google Scholar] [CrossRef]
- Neuwald, I.J.; Zahn, D.; Knepper, T.P. Are (fluorinated) ionic liquids relevant environmental contaminants? High-resolution mass spectrometric screening for per- and polyfluoroalkyl substances in environmental water samples led to the detection of a fluorinated ionic liquid. Anal. Bioanal. Chem. 2020, 412, 4881–4892. [Google Scholar] [CrossRef]
- Vieira, N.S.M.; Stolte, S.; Araújo, J.M.M.; Rebelo, L.P.N.; Pereiro, A.B.; Markiewicz, M. Acute Aquatic Toxicity and Biodegradability of Fluorinated Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 3733–3741. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Ionic liquids are not always green: Hydrolysis of 1-butyl-3- methylimidazolium hexafluorophosphate. Green Chem. 2003, 5, 361–363. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Radošević, K.; Redovniković, I.R.; Halambek, J.; Srček, V.G. A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, S.A.; Golding, J.; Deacon, G.B. Ionic liquids based on imidazolium, ammonium and pyrrolidinium salts of the dicyanamide anion. Green Chem. 2002, 4, 444–448. [Google Scholar] [CrossRef]
- Dutta, B.; Ruhela, R.; Yadav, M.; Singh, A.K.; Sahu, K.K.; Padmanabhan, N.P.H.; Chakravartty, J.K. Liquid-liquid extraction studies of gadolinium with N-methyl-N,N,N-trioctyl ammonium-bis-(2-ethylhexyl) phosphonate—Task specific ionic liquid. Sep. Purif. Technol. 2017, 175, 158–163. [Google Scholar] [CrossRef]
- Zarrougui, R.; Mdimagh, R.; Raouafi, N. Highly efficient and eco-friendly extraction of neodymium using, undiluted and non-fluorinated ionic liquids. Direct electrochemical metal separation. Sep. Purif. Technol. 2017, 175, 87–98. [Google Scholar] [CrossRef]
- Chen, L.; Sharifzadeh, M.; Mac Dowell, N.; Welton, T.; Shah, N.; Hallett, J.P. Inexpensive ionic liquids: [HSO4]−-based solvent production at bulk scale. Green Chem. 2014, 16, 3098–3106. [Google Scholar] [CrossRef]
- Sousa, N.G.; Salgueira, J.F.S.; Sousa, C.P.; Campos, O.S.; Salazar-Banda, G.R.; Eguiluz, K.I.B.; de Lima-Neto, P.; Correia, A.N. Silver electrodeposition at room temperature protic ionic liquid 1-H-methylimidazolium hydrogen sulfate. J. Mol. Liq. 2020, 313, 113487. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, X.; Hua, Y.; Xue, W. The effect of quaternary ammonium-based ionic liquids on copper electrodeposition from acidic sulfate electrolyte. J. Appl. Electrochem. 2015, 45, 79–86. [Google Scholar] [CrossRef]
- Liu, T.; Vilar, R.; Eugénio, S.; Grondin, J.; Danten, Y. Electrodeposition of nanocrystalline copper thin films from 1-ethyl-3-methylimidazolium ethylsulphate ionic liquid. J. Appl. Electrochem. 2014, 44, 189–198. [Google Scholar] [CrossRef]
| Name | Acronym | Structure |
|---|---|---|
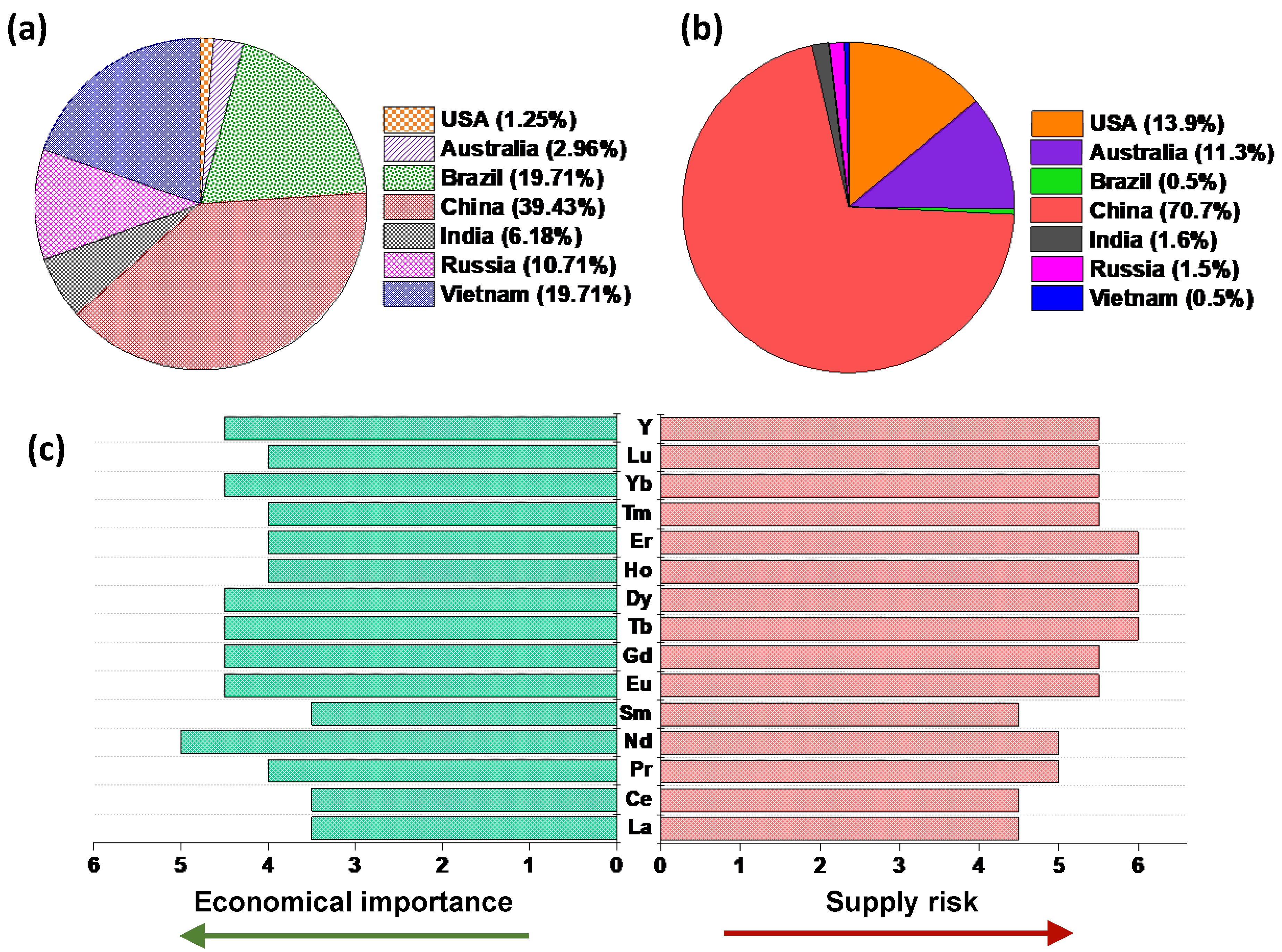
| Bis(trifluoromethylsulfonyl)imide | [TFSI]− | 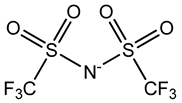 |
| Triethylpentylphosphonium | [P2225]+ | 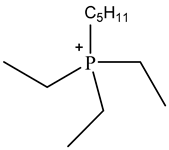 |
| Trihexyltetradecylphosphonium | [P66614]+ | 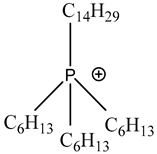 |
| Trimethylbutylammonium | [N1114]+ |  |
| N,N-diethyl-N-methyl-N-(2-methoxyethyl)ammonium | [DEME]+ |  |
| Trimethylhexylammonium | [N1116]+ | 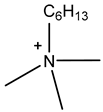 |
| 2-hydroxyethyltrimethylammonium | Ch+ | 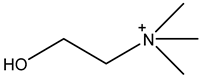 |
| Ionic Liquid | Nd Salt | [Nd3+] | Temp. (°C) | Scan Rate (mV s−1) | jpeak reduction (mA cm−2) | Ref. |
|---|---|---|---|---|---|---|
| [C4mpyr][TFSI] | Nd(NO3)3 | 0.06 M | 25 | 20 | −4 | [57] |
| [P2225][TFSI] | Nd(TFSI)3 | 0.5 M | 50 | 100 | −0.25 × 10−4 | [55] |
| 75 | −0.35 × 10−4 | |||||
| 90 | −0.4 × 10−4 | |||||
| 105 | −0.4 × 10−4 | |||||
| [P2225][TFSI] | Nd(TFSI)3 | 0.05 M | 90 | 2 | −0.12 | [68] |
| 100 | −0.15 | |||||
| DMSO | NdCl3 | 0.1 M | R.T. | 20 | −2 | [51] |
| [DEME][TFSI] | Nd(TFSI)3 | 0.5 M | 80 | 10 | −0.8 | [58] |
| [P66614][TFSI] + 0.4 wt% H2O | Nd(TFSI)3 | 0.1 m | 75 | 100 | −5 | [56] |
| [N1114][TFSI] | Nd(NO3)3.H2O | 0.06 M | 25 | 20 | −5 | [57] |
| [C4mpyr][DCA] | Nd(TFSI)3 | 0.03 M | 25 | 100 | −0.1 (+IL reduction) | [69] |
| [P66614][DCA] | Nd(OTf)3 | 0.1 m | 75 | 10 | −0.25 | [70] |
| [C4mpyr][DCA] | Nd(OTf)3 | 0.2 m | 50 | 100 | −12 | [71] |
| [C4mpyr][DCA] | Nd(NO3)3.6H2O | 0.2 m | 50 | 100 | −38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periyapperuma, K.; Sanchez-Cupido, L.; Pringle, J.M.; Pozo-Gonzalo, C. Analysis of Sustainable Methods to Recover Neodymium. Sustain. Chem. 2021, 2, 550-563. https://doi.org/10.3390/suschem2030030
Periyapperuma K, Sanchez-Cupido L, Pringle JM, Pozo-Gonzalo C. Analysis of Sustainable Methods to Recover Neodymium. Sustainable Chemistry. 2021; 2(3):550-563. https://doi.org/10.3390/suschem2030030
Chicago/Turabian StylePeriyapperuma, Kalani, Laura Sanchez-Cupido, Jennifer M. Pringle, and Cristina Pozo-Gonzalo. 2021. "Analysis of Sustainable Methods to Recover Neodymium" Sustainable Chemistry 2, no. 3: 550-563. https://doi.org/10.3390/suschem2030030
APA StylePeriyapperuma, K., Sanchez-Cupido, L., Pringle, J. M., & Pozo-Gonzalo, C. (2021). Analysis of Sustainable Methods to Recover Neodymium. Sustainable Chemistry, 2(3), 550-563. https://doi.org/10.3390/suschem2030030






