Tuberculosis in Portugal: Intertwining History and Public Health Development
Abstract
1. Introduction
2. Materials and Methods
3. From the First Epidemics to the Emergence of Public Health
4. Industrialization and the Arrival of the White Plague
5. Tuberculosis and Political Shifts in the Early 20th Century
6. The Role of TB’s Private Initiative in Public Health Development
7. The TB Fight at the End of the 20th Century
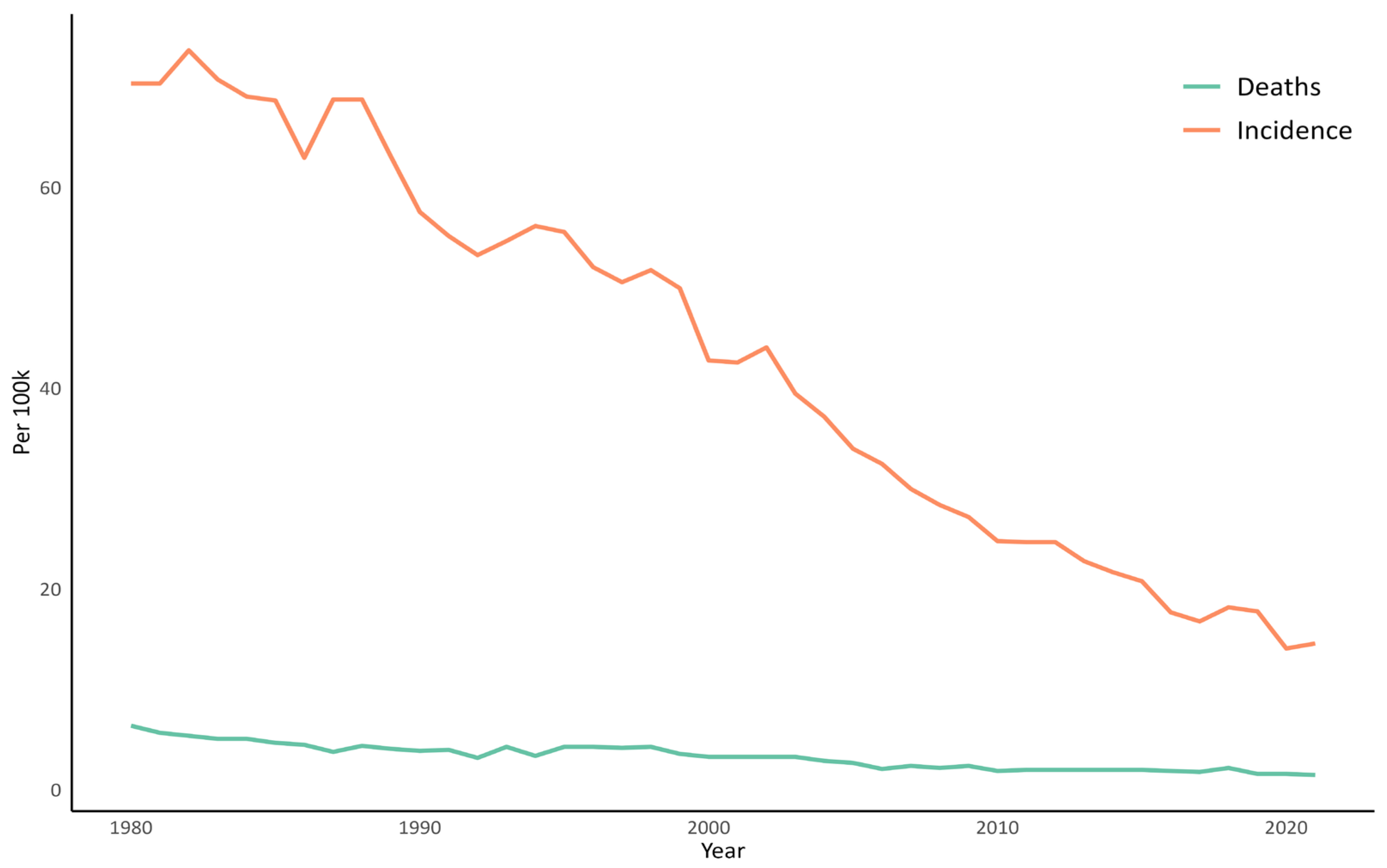
8. TB in Current Portugal
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TB | Tuberculosis |
| WHO | World Health Organization |
| MTB | Mycobacterium tuberculosis |
| HPLC | High-performance liquid chromatography |
| INE | National Institute of Statistics |
| LPSS | Portuguese League for Social Prophylaxis |
| LNCT | National League Against Tuberculosis |
| ANT | National Assistance for Tuberculosis |
| IANT | National Institute for Assistance to Tuberculosis |
| CIAS | Research Centre for Anthropology and Health |
| CBMA | Centre of Molecular and Environmental Biology |
| FCT | Fundação para a Ciência e a Tecnologia |
| PNT | National Program to Combat Tuberculosis |
References
- WHO Global Tuberculosis Report; WHO: Geneva, Switzerland, 2023.
- PNT Relatório de Vigilância e Monitorização da Tuberculose Em Portugal—Dados Definitivos 2022; Direção-Geral da Saúde: Lisboa, Portugal, 2024.
- Kanabalan, R.D.; Lee, L.J.; Lee, T.Y.; Chong, P.P.; Hassan, L.; Ismail, R.; Chin, V.K. Human Tuberculosis and Mycobacterium Tuberculosis Complex: A Review on Genetic Diversity, Pathogenesis and Omics Approaches in Host Biomarkers Discovery. Microbiol. Res. 2021, 246, 126674. [Google Scholar] [CrossRef] [PubMed]
- Rito, T.; Inlamea, O.; Oliveira, O.; Duarte, R.; Soares, P.; Correia-Neves, M. Evolution and Molecular Characteristics of Mycobacterium Tuberculosis and Mycobacterium Bovis BT—Tuberculosis: Integrated Studies for a Complex Disease. In Tuberculosis; Rezaei, N., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 847–865. ISBN 978-3-031-15955-8. [Google Scholar]
- Delogu, G.; Sali, M.; Fadda, G. The Biology of Mycobacterium Tuberculosis Infection. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013070. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Alves, C.; Booty, M.G.; Carpenter, S.M.; Jayaraman, P.; Rothchild, A.C.; Behar, S.M. In Search of a New Paradigm for Protective Immunity to TB. Nat. Rev. Microbiol. 2014, 12, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Ryndak, M.B.; Laal, S. Mycobacterium Tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells. Front. Cell. Infect. Microbiol. 2019, 9, 299. [Google Scholar] [CrossRef]
- Narasimhan, P.; Wood, J.; Macintyre, C.R.; Mathai, D. Risk Factors for Tuberculosis. Pulm. Med. 2013, 2013, 828939. [Google Scholar] [CrossRef]
- Grange, J.M.; Yates, M.D. Zoonotic Aspects of Mycobacterium Bovis Infection. Vet. Microbiol. 1994, 40, 137–151. [Google Scholar] [CrossRef]
- Grange, J.M. Mycobacterium Bovis Infection in Human Beings. Tuberculosis 2001, 81, 71–77. [Google Scholar] [CrossRef]
- Machado, A.; Rito, T.; Ghebremichael, S.; Muhate, N.; Maxhuza, G.; Macuamule, C.; Moiane, I.; Macucule, B.; Marranangumbe, A.S.; Baptista, J.; et al. Genetic Diversity and Potential Routes of Transmission of Mycobacterium Bovis in Mozambique. PLoS Negl. Trop. Dis. 2018, 12, e0006147. [Google Scholar] [CrossRef]
- Inlamea, O.F.; Soares, P.; Ikuta, C.Y.; Heinemann, M.B.; Achá, S.J.; Machado, A.; Neto, J.S.F.; Correia-Neves, M.; Rito, T. Evolutionary Analysis of Mycobacterium Bovis Genotypes across Africa Suggests Co-Evolution with Livestock and Humans. PLoS Negl. Trop. Dis. 2020, 14, e0008081. [Google Scholar] [CrossRef]
- Galagan, J.E. Genomic Insights into Tuberculosis. Nat. Rev. Genet. 2014, 15, 307–320. [Google Scholar] [CrossRef]
- Santos, A.L.; Matos, V.M.J. Contribution of Paleopathology to the Knowledge of the Origin and Spread of Tuberculosis: Evidence from Portugal. Antropol. Port. 2019, 36, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Hershkovitz, I.; Donoghue, H.D.; Minnikin, D.E.; May, H.; Lee, O.Y.C.; Feldman, M.; Galili, E.; Spigelman, M.; Rothschild, B.M.; Bar-Gal, G.K. Tuberculosis Origin: The Neolithic Scenario. Tuberculosis 2015, 95, S122–S126. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.D.S. Insights on the History of Tuberculosis: Novalis and the Romantic Idealization. Antropol. Port. 2019, 36, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I. Doutrinas e Profilaxia Da Tuberculose Em Portugal Nos Finais Do Século XIX. Rev. História Soc. E Cult. 2013, 13, 335–359. [Google Scholar] [CrossRef]
- Vieira, I. Conhecer, Tratar e Combater a “Peste Branca”. A Tisiologia e a Luta Contra a Tuberculose Em Portugal (1853–1975). Ph.D. Thesis, Faculdade de Letras da Universidade do Porto, Porto, Portugal, 2012; 500p. [Google Scholar]
- Vieira, I. O Primeiro Congresso Nacional de Tuberculose Em Portugal (1895). Rev. Humanidades Médicas Estud. Soc. Cienc. Tecnol. 2014, 6, 1–28. [Google Scholar]
- Saraiva, P.C.S.; Doria, J.L.; Duarte, J.M.C.; Saraiva, P.C.S. Tuberculose: A História e o Património. An. Inst. Hig. Med. Trop. (Lisb.) 2017, 16, 89–101. [Google Scholar] [CrossRef]
- Ramalho de Almeida, A. A Tuberculose—Doença Do Passado, Do Presente e Do Futuro; Laboratórios Bial: Porto, Portugal, 1995. [Google Scholar]
- Fiolhais, C. Uma Breve História Da Tuberculose Em Portugal. Revistamultidisciplinar 2022, 4, 41–55. [Google Scholar] [CrossRef]
- Millheiro, F. A Tuberculose Desde Os Seus Primórdios Em Portugal e No Mundo. Master’s Thesis, Universsidade do Porto, Porto, Portugal, 2016. [Google Scholar]
- Santos, A.F.C.P. O Combate à Tuberculose: Uma Abordagem Demográfico-Epidemiológica O Hospital De Repouso De Lisboa (1882–1975). Master’s Thesis, Universidade de Lisboa, Lisbon, Portugal, 2010. [Google Scholar]
- Matos, V.M.J.; Santos, A.L. Trends in Mortality from Pulmonary Tuberculosis before and after Antibiotics in the Portuguese Sanatorium Carlos Vasconcelos Porto (1918–1991): Archival Evidence and Its Paleopathological Relevance. Tuberculosis 2015, 95, S101–S104. [Google Scholar] [CrossRef]
- Matos, V.M.J.; Santos, A.L. On the Trail of Pulmonary Tuberculosis Based on Rib Lesions: Results from the Human Identified Skeletal Collection from the Museu Bocage (Lisbon, Portugal). Am. J. Phys. Anthropol. 2006, 130, 190–200. [Google Scholar] [CrossRef]
- Santos, A.L.; Roberts, C.A. Anatomy of a Serial Killer: Differential Diagnosis of Tuberculosis Based on Rib Lesions of Adult Individuals from the Coimbra Identified Skeletal Collection, Portugal. Am. J. Phys. Anthropol. 2006, 130, 38–49. [Google Scholar] [CrossRef]
- Santos, A.L. Archives and Skeletons: An Interdisciplinary Approach to the Study of Paleopathology of Tuberculosis. Tuberculosis 2015, 95, S109–S111. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Roberts, C.A. A Picture of Tuberculosis in Young Portuguese People in the Early 20th Century: A Multidisciplinary Study of the Skeletal and Historical Evidence. Am. J. Phys. Anthropol. 2001, 115, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L. A Skeletal Picture of Tuberculosis: Macroscopic, Radiological, Biomolecular, and Historical Evidence from the Coimbra Identified Skeletal Collection. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 2000. [Google Scholar]
- Ortner, D.J. Identification of Pathological Conditions in Human Skeletal Remains; Academic Press: San Diego, CA, USA, 2003; ISBN 978-0-12-528628-2. [Google Scholar]
- Bastos, M. A Realeza e a Saúde Pública Em Portugal (Séculos XIV–XVI). Passagens Rev. Int. História Política Cult. Juríd. 2013, 5, 29–51. [Google Scholar] [CrossRef]
- Sousa, J.; Costa, R. Regimento Proveitoso Contra a Pestilência (c. 1496)—Uma Apresentação. História Ciênc. Saúde—Manguinhos 2005, 12, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Longo, C. Epidemias: Perspectiva de Portugal Com Principal Enfoque Em Lisboa e Na Peste Branca (Tuberculose). Cad. Cult. Med. Na Beira Inter. Pré-História Ao Século XXI 2015, 29, 109–120. Available online: http://hdl.handle.net/10400.10/1555 (accessed on 5 January 2025).
- Cieslik, A.I. Evidence of Tuberculosis among Children in Medieval (13th–15th Century) Wrocław: A Case Study of Hip Joint Tuberculosis in a Juvenile Skeleton Excavated from the Crypt of the St. Elizabeth Church. Anthropol. Rev. 2017, 80, 219–231. [Google Scholar] [CrossRef]
- Cooper, C.; Fellner, R.; Heubi, O.; Maixner, F.; Zink, A.; Lösch, S. Tuberculosis in Early Medieval Switzerland—Osteological and Molecular Evidence. Swiss Med. Wkly. 2016, 146, w14269. [Google Scholar] [CrossRef]
- Paja, L.; Coqueugniot, H.; Dutour, O.; Willmon, R.; Farkas, G.L.; Palkó, A.; Pálfi, G. Knee Ankyloses Associated with Tuberculosis from the Medieval Hungary—Differential Diagnosis Based on Medical Imaging Techniques. Int. J. Osteoarchaeol. 2015, 25, 352–360. [Google Scholar] [CrossRef]
- Taylor, G.M.; Goyal, M.; Legge, A.J.; Shaw, R.J.; Young, D. Genotypic Analysis of Mycobacterium Tuberculosis from Medieval Human Remains. Microbiology 1999, 145, 899–904. [Google Scholar] [CrossRef]
- Weber, J.; Czarnetzki, A.; Pusch, C.M. Paleopathological Examination of Medieval Spines with Exceptional Thoracic Kyphosis Most Likely Secondary to Spinal Tuberculosis. Historical Vignette. J. Neurosurg. Spine 2004, 1, 238–242. [Google Scholar] [CrossRef]
- Fernandes, T.; Granja, R.; Thillaud, P.L. Spectrometric Analysis and Scanning Electronic Microscopy of Two Pleural Plaques from Mediaeval Portuguese Period. Rev. Port. Pneumol. 2014, 20, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Matos, V.M.J.; Marques, C.; Lopes, C. Severe Vertebral Collapse in a Juvenile from the Graveyard (13th/14th–19th Centuries) of the São Miguel Church (Castelo Branco, Portugal): Differential Palaeopathological Diagnosis. Int. J. Osteoarchaeol. 2011, 21, 208–217. [Google Scholar] [CrossRef]
- Barbosa, M.H.V. Crises de Mortalidade Em Portugal—Desde Meados Do Século XVI Até Ao Início Do Século XX. J. Phys. Math. Theor. 2011, 44, 085201. [Google Scholar]
- Pires, S.C. Junta de Inspecção de Providências Contra a Peste: Contributos para o estudo da administração da Saúde nas comarcas portuguesas no início do século XIX. Cad. Arq. Munic. 2020, 14, 137–161. [Google Scholar] [CrossRef]
- Abreu, L. A Luta Contra as Invasões Epidémicas Em Portugal: Políticas e Agentes, Séculos XVI–XIX. Ler História 2018, 73, 93–120. [Google Scholar] [CrossRef]
- Bicho, F. Organização Dos Serviços Sanitários Em Portugal. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 1926. [Google Scholar]
- Subtil, J. O Antigo Regime Da Saúde Pública Entre O Reino E O Brasil. Rev. Ultramares 2015, I, 39–66. [Google Scholar]
- Daniel, T.M. The History of Tuberculosis. Respir. Med. 2006, 100, 1862–1870. [Google Scholar] [CrossRef]
- Barberis, I.; Bragazzi, N.L.; Galluzzo, L.; Martini, M. The History of Tuberculosis: From the First Historical Records to the Isolation of Koch’s Bacillus. J. Prev. Med. Hyg. 2017, 58, E9–E12. [Google Scholar] [CrossRef]
- Reis, J. A Industrialização Num País de Desenvolvimento Lento e Tardio: Portugal, 1870–1913. Análise Soc. 1987, 23, 207–227. [Google Scholar]
- Santos, A.L.; Magalhães, B.M. Changes in Mortality in a Non-Industrialized Portugal: Coimbra Municipal Cemetery Records (1861–1914) and Identified Osteological Collections. Int. J. Paleopathol. 2022, 37, 77–86. [Google Scholar] [CrossRef]
- Veiga, T. A População Portuguesa No Século XIX; CEPESE e Afrontamento, E., Ed.; Rainho & Neves Lda: Santa Maria da Feira, Portugal, 2004; ISBN 972-36-0700-X. [Google Scholar]
- Subtil, C.L. O Conselho de Saúde Pública, Uma Imanência da Revolução de 1820. Cad. Arq. Munic. 2021, 15, 139–158. [Google Scholar]
- Fee, E.; Brown, T.M. The Public Health Act of 1848. Bull. World Health Organ. 2005, 83, 866–867. [Google Scholar] [PubMed]
- Shaw-Taylor, L. An Introduction to the History of Infectious Diseases, Epidemics and the Early Phases of the Long-run Decline in Mortality. Econ. Hist. Rev. 2020, 73, E1–E19. [Google Scholar] [CrossRef] [PubMed]
- Dutour, O.; Colombo, A.; Coqueugniot, H. Was the Rise of TB Contemporaneous with the Industrial Revolution? Epidemiological Evolution of TB in France (17th–20th Centuries) Inferred from Osteoarchaeological and Historical Archives. Int. J. Paleopathol. 2021, 34, 130–133. [Google Scholar] [CrossRef]
- Ministério das Obras Públicas, Comércio e Indústria; Direção Geral de Agricultura; 1.ª Repartição dos Serviços Agrícolas. Portaria, de 28 de Outubro de 1886, Diário do Governo, n.º 217, 29 de Outubro de 1886; Ministério das Obras Públicas: Lisbon, Portugal, 1886. [Google Scholar]
- Vieira, I. How an Epidemic Was Prevented: Prophylaxis against Tuberculosis in the First Half of the 20th Century in Portugal. Rev. Port. Hist. 2021, 52, 55–76. [Google Scholar] [CrossRef]
- Jorge, R. A Luta Contra a Tuberculose. Arq. Inst. Cent. Hig. 1914, 1, 203–212. [Google Scholar]
- Boletim Mensal de Estatística Sanitária; Serviço Municipal de Saúde e Higiene da Cidade do Porto: Porto, Portugal, 1897.
- Wilson, L.G. The Historical Decline of Tuberculosis in Europe and America: Its Causes and Significance. J. Hist. Med. Allied Sci. 1990, 45, 366–396. [Google Scholar] [CrossRef]
- Boletim Mensal de Estatística Demográfico-Sanitária da Cidade de Lisboa. Instituto Central de Higiene: Lisboa, Portugal; Imprensa Nacional: Lisboa, Portugal, 1892.
- Ferreira, M.d.L.d.C. A Doença Do Peito—Contributo Para o Estudo Histórico da Tuberculose; Faculdade de Letras da Universidade do Porto: Porto, Portugal, 2005. [Google Scholar]
- Alves, J.F.; Carneiro, M. Saúde Pública E Política: Do «Código Sanitário» Ao Regulamento Geral De 1901. CEM Cult. Espaço Mem. 2014, 5, 27–43. [Google Scholar]
- Marques, A. O Instituto Bacteriológico Câmara Pestana: Ciência Médica e Cuidados de Saúde (1892–1030). Ph.D. Thesis, Universidade de Évora, Évora, Portugal, 2020. [Google Scholar]
- de Carvalho, L. A Luta Contra a Tuberculose em Portugal; Sep. Lisboa Médica; Imprensa Libânio da Silva: Lisboa, Portugal, 1934; Volume 11, p. 873. Available online: https://am.uc.pt/item/85449 (accessed on 5 January 2025).
- Instituto Central de Higiene. Tabelas do Movimento Fisiológico da População de Portugal: 1901–1910; Instituto Central de Higiene: Lisbon, Portugal, 1916. [Google Scholar]
- Direcção Geral de Estatística. Anuário Estatístico de Portugal; Imprensa Nacional: Lisboa, Portugal, 1926. [Google Scholar]
- Instituto Nacional de Estatística. Estatísticas Da Saúde: Continente e Ilhas Adjacentes; Ramos, Afonso & Moita: Lisboa, Portugal, 1970. [Google Scholar]
- Rocha, A.; Marques, A.; Figueiredo, C.; Almeida, C.; Batista, I.; Almeida, J. Evolução Da Saúde Escolar Em Portugal: Revisão Legislativa No Âmbito Da Educação. Millenium 2011, 41, 69–87. [Google Scholar]
- Portuguese Studies Center. Instituto Nacional de Assistência ao Tuberculoso. 2017. Available online: https://portuguesestudiescenter.wordpress.com/2017/03/27/instituto-de-assistencia-nacional-aos-tuberculosos/ (accessed on 5 January 2025).
- Abrantes, L.G. A Introdução Da Vacina BCG Em Portugal: Campanha, Consensos e Resistências. Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, 2021. [Google Scholar]
- Gonçalves, J.H.D. A tuberculose: Concepção de um modelo econométrico para a taxa bruta de mortalidade; Revista de Estudos Demográficos, no 36; Instituto Nacional de Estatistica Portugal: Lisbon, Portugal, 2004; pp. 111–126. [Google Scholar]
- dos Campos, M.A.L. A mortalidade por tuberculose em Portugal, no período de 1985 a 2002 Parte I; Revista de Estudos Demográficos, no 36; Instituto Nacional de Estatistica Portugal: Lisbon, Portugal, 2004; pp. 29–39. [Google Scholar]
- Tuberculosis: A Global Emergency. World Health Forum 1993, 14, 438.
- Oliveira, O.; Gaio, R.; Carvalho, C.; Correia-Neves, M.; Duarte, R.; Rito, T. A Nationwide Study of Multidrug-Resistant Tuberculosis in Portugal 2014–2017 Using Epidemiological and Molecular Clustering Analyses. BMC Infect. Dis. 2019, 19, 567. [Google Scholar] [CrossRef] [PubMed]
- Norma 006/2016; Estratégia de Vacinação Contra a Tuberculose Com a Vacina BCG. Direção Geral de Saúde: Lisbon, Portugal, 2023.
- Rito, T.; Matos, C.; Carvalho, C.; Machado, H.; Rodrigues, G.; Oliveira, O.; Ferreira, E.; Gonçalves, J.; Maio, L.; Morais, C.; et al. A Complex Scenario of Tuberculosis Transmission Is Revealed through Genetic and Epidemiological Surveys in Porto. BMC Infect. Dis. 2018, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Togun, T.; Kampmann, B.; Stoker, N.G.; Lipman, M. Anticipating the Impact of the COVID-19 Pandemic on TB Patients and TB Control Programmes. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dara, M.; Kuchukhidze, G.; Yedilbayev, A.; Perehinets, I.; Schmidt, T.; Van Grinsven, W.L.; Boeree, M.J. Early COVID-19 Pandemic’s Toll on Tuberculosis Services, WHO European Region, January to June 2020. Eurosurveillance 2021, 26, 27–35. [Google Scholar] [CrossRef]
- Alves, F.; Duarte, R. Impact of COVID-19 on Extrapulmonary TB and the Benefit of Decentralised TB Services. Int. J. Tuberc. Lung Dis. 2022, 26, 178–180. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, F.M.; Soares, P.; Rito, T.; Silva, A.M. Tuberculosis in Portugal: Intertwining History and Public Health Development. World 2025, 6, 61. https://doi.org/10.3390/world6020061
Ribeiro FM, Soares P, Rito T, Silva AM. Tuberculosis in Portugal: Intertwining History and Public Health Development. World. 2025; 6(2):61. https://doi.org/10.3390/world6020061
Chicago/Turabian StyleRibeiro, Fabiana M., Pedro Soares, Teresa Rito, and Ana Maria Silva. 2025. "Tuberculosis in Portugal: Intertwining History and Public Health Development" World 6, no. 2: 61. https://doi.org/10.3390/world6020061
APA StyleRibeiro, F. M., Soares, P., Rito, T., & Silva, A. M. (2025). Tuberculosis in Portugal: Intertwining History and Public Health Development. World, 6(2), 61. https://doi.org/10.3390/world6020061