Modified Medial Meniscectomy (MMM) Model to Assess Post-Traumatic Knee Osteoarthritis in Mouse
Abstract
1. Introduction
2. Methods
2.1. Animal Experiments
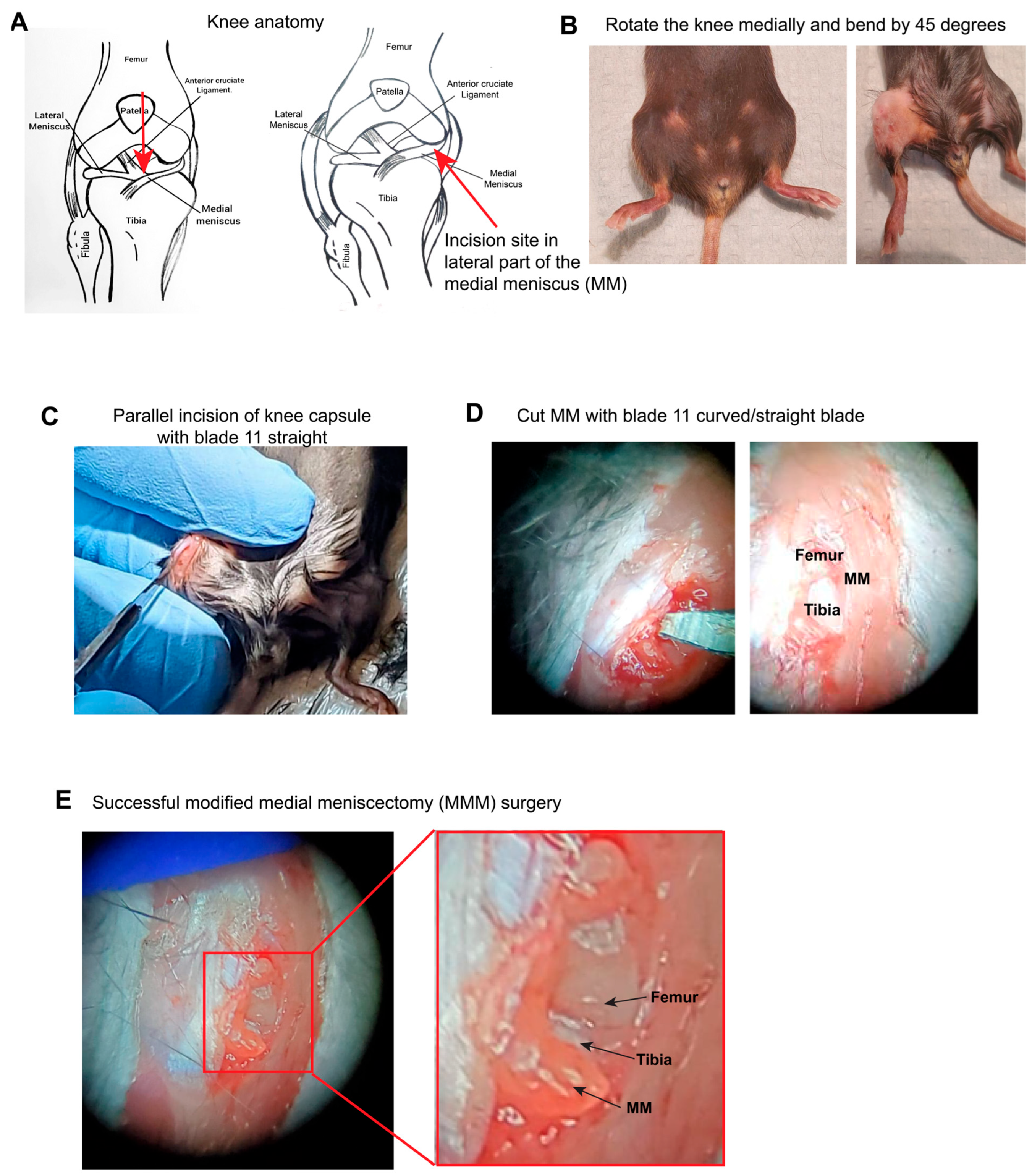
2.2. Surgical Procedure for Modified Medial Meniscectomy (MMM)
2.2.1. Treadmill Exhaustion Test
2.2.2. Allodynia Test
2.3. Habituation Protocol
2.4. Histology
2.5. Sample Collection and RNA Extraction
2.6. RNA Sequencing and Bioinformatics Analyses
3. Differential Gene Expression (DGE) Analysis
4. Results
4.1. The MMM Model Is Methodologically Innovative and Less Invasive
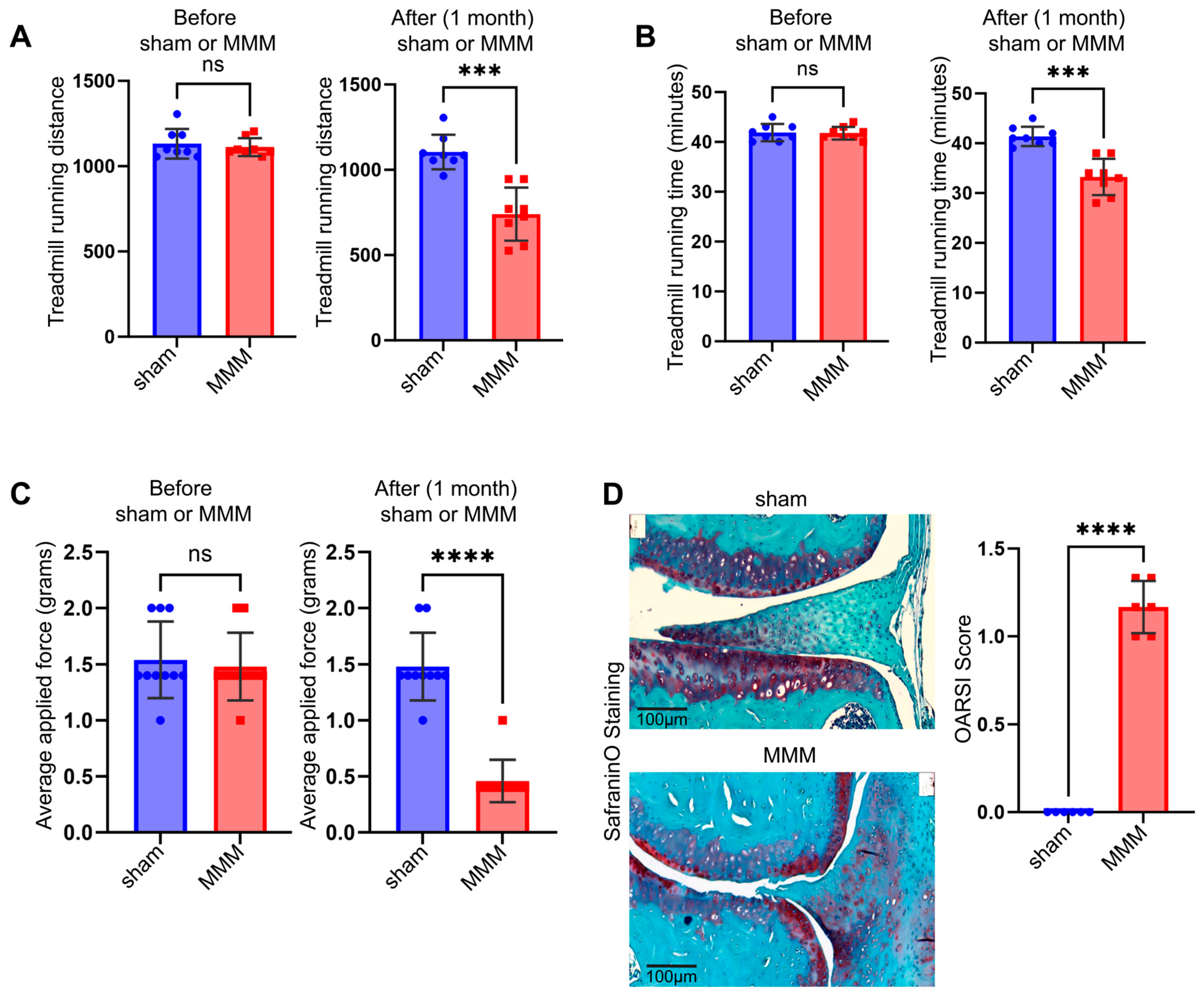
4.2. The MMM Model Could Be Used to Study Joint Biomechanics, Pain, and PTOA
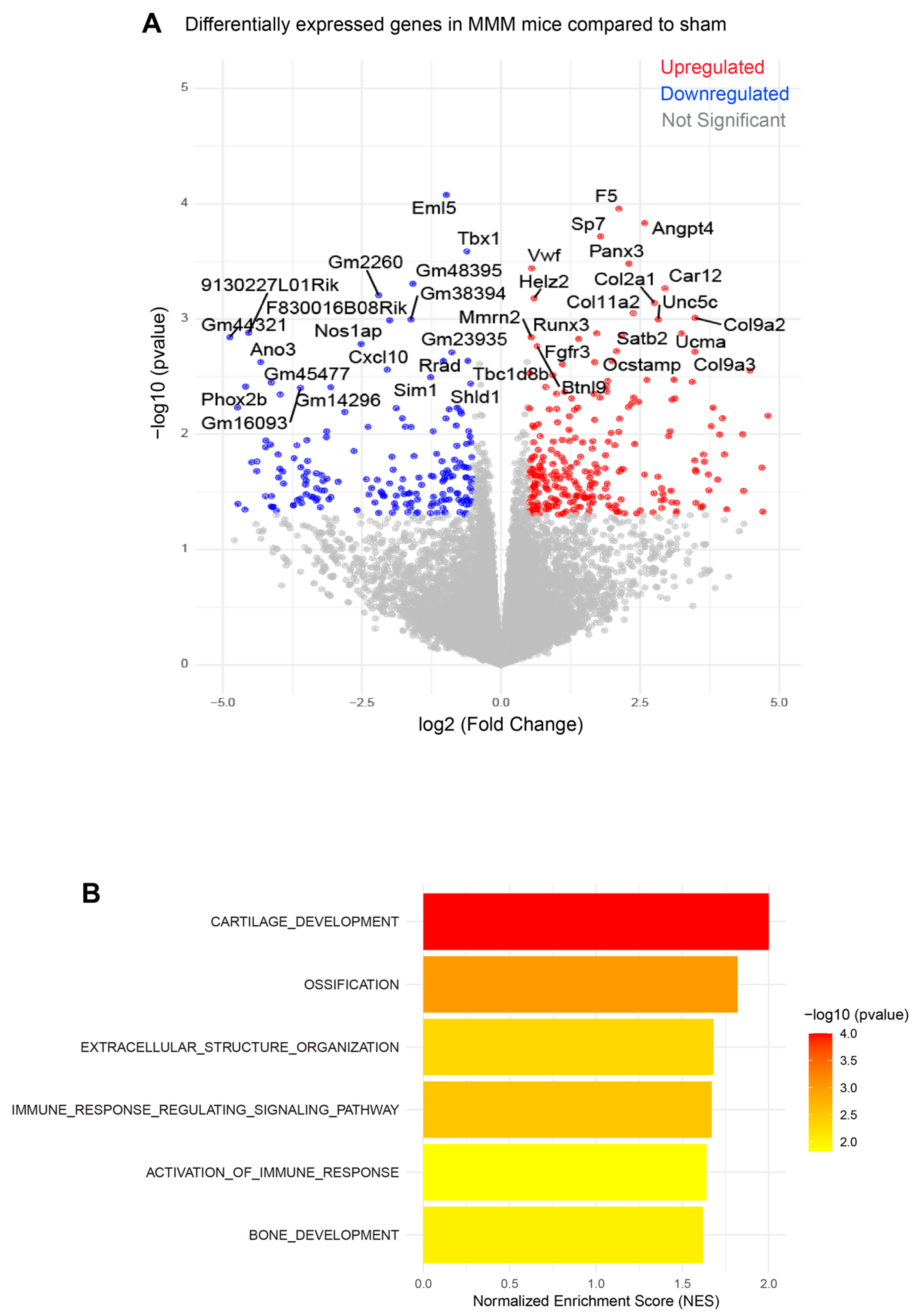
4.3. The MMM Model Shows OA- and Inflammation-Related Gene Expression
5. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Losina, E.; Weinstein, A.M.; Reichmann, W.M.; Burbine, S.A.; Solomon, D.H.; Daigle, M.E.; Rome, B.N.; Chen, S.P.; Hunter, D.J.; Suter, L.G.; et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res. 2013, 65, 703–711. [Google Scholar] [CrossRef]
- Wang, C.; Iversen, M.D.; McAlindon, T.; Harvey, W.F.; Wong, J.B.; Fielding, R.A.; Driban, J.B.; Price, L.L.; Rones, R.; Gamache, T.; et al. Assessing the comparative effectiveness of Tai Chi versus physical therapy for knee osteoarthritis: Design and rationale for a randomized trial. BMC Complement. Altern. Med. 2014, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.S.; Zhang, Y.; Zhu, Y.; Niu, J.; Zhang, B.; Felson, D.T. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: Survey and cohort data. Ann. Intern. Med. 2011, 155, 725–732. [Google Scholar] [CrossRef]
- Geraghty, T.; Ishihara, S.; Obeidat, A.M.; Adamczyk, N.S.; Hunter, R.S.; Li, J.; Wang, L.; Lee, H.; Ko, F.C.; Malfait, A.M.; et al. Acute systemic macrophage depletion in osteoarthritic mice alleviates pain-related behaviors and does not affect joint damage. Arthritis Res. Ther. 2024, 26, 224. [Google Scholar] [CrossRef]
- Obeidat, A.M.; Kim, S.Y.; Burt, K.G.; Hu, B.; Li, J.; Ishihara, S.; Xiao, R.; Miller, R.E.; Little, C.; Malfait, A.M.; et al. Recommendations For a Standardized Approach to Histopathologic Evaluation of Synovial Membrane in Murine Models of Experimental Osteoarthritis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tsai, L.C.; Cooper, E.S.; Hetzendorfer, K.M.; Warren, G.L.; Chang, Y.H.; Willett, N.J. Effects of treadmill running and limb immobilization on knee cartilage degeneration and locomotor joint kinematics in rats following knee meniscal transection. Osteoarthr. Cartil. 2019, 27, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.P.; Lee, Y.S.; Choe, Y.; Kim, K.T.; Song, G.J.; Hwang, S.C. Knee osteoarthritis accelerates amyloid beta deposition and neurodegeneration in a mouse model of Alzheimer’s disease. Mol. Brain 2023, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.C.; Jain, L.; Dalbeth, N. Re-thinking osteoarthritis pathogenesis: What can we learn (and what do we need to unlearn) from mouse models about the mechanisms involved in disease development. Arthritis Res. Ther. 2023, 25, 59. [Google Scholar] [CrossRef]
- Butterfield, N.C.; Curry, K.F.; Steinberg, J.; Dewhurst, H.; Komla-Ebri, D.; Mannan, N.S.; Adoum, A.T.; Leitch, V.D.; Logan, J.G.; Waung, J.A.; et al. Accelerating functional gene discovery in osteoarthritis. Nat. Commun. 2021, 12, 467. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Xu, R.; Wang, Y.; Jian, F.; Long, H.; Lai, W. Delay in articular cartilage degeneration of the knee joint by the conditional removal of discoidin domain receptor 2 in a spontaneous mouse model of osteoarthritis. Ann. Transl. Med. 2020, 8, 1178. [Google Scholar] [CrossRef]
- Tashkandi, M.M.; Alsaqer, S.F.; Alhousami, T.; Ali, F.; Wu, Y.C.; Shin, J.; Mehra, P.; Wolford, L.M.; Gerstenfeld, L.C.; Goldring, M.B.; et al. LOXL2 promotes aggrecan and gender-specific anabolic differences to TMJ cartilage. Sci. Rep. 2020, 10, 20179. [Google Scholar] [CrossRef]
- Alshenibr, W.; Tashkandi, M.M.; Alsaqer, S.F.; Alkheriji, Y.; Wise, A.; Fulzele, S.; Mehra, P.; Goldring, M.B.; Gerstenfeld, L.C.; Bais, M.V. Anabolic role of lysyl oxidase like-2 in cartilage of knee and temporomandibular joints with osteoarthritis. Arthritis Res. Ther. 2017, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, M.; Ali, F.; Alsaqer, S.; Alhousami, T.; Cano, A.; Martin, A.; Salvador, F.; Portillo, F.; Gerstenfeld, L.C.; Goldring, M.B.; et al. Lysyl Oxidase-Like 2 Protects against Progressive and Aging Related Knee Joint Osteoarthritis in Mice. Int. J. Mol. Sci. 2019, 20, 4798. [Google Scholar] [CrossRef] [PubMed]
- Gil Alabarse, P.; Chen, L.Y.; Oliveira, P.; Qin, H.; Liu-Bryan, R. Targeting CD38 to Suppress Osteoarthritis Development and Associated Pain After Joint Injury in Mice. Arthritis Rheumatol. 2023, 75, 364–374. [Google Scholar] [CrossRef]
- Willcockson, H.; Ozkan, H.; Arbeeva, L.; Mucahit, E.; Musawwir, L.; Longobardi, L. Early ablation of Ccr2 in aggrecan-expressing cells following knee injury ameliorates joint damage and pain during post-traumatic osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1616–1630. [Google Scholar] [CrossRef]
- Shin, Y.; Cho, D.; Kim, S.K.; Chun, J.S. STING mediates experimental osteoarthritis and mechanical allodynia in mouse. Arthritis Res. Ther. 2023, 25, 90. [Google Scholar] [CrossRef]
- Miller, R.E.; Tran, P.B.; Ishihara, S.; Syx, D.; Ren, D.; Miller, R.J.; Valdes, A.M.; Malfait, A.M. Microarray analyses of the dorsal root ganglia support a role for innate neuro-immune pathways in persistent pain in experimental osteoarthritis. Osteoarthr. Cartil. 2020, 28, 581–592. [Google Scholar] [CrossRef]
- Macfarlane, E.; Cavanagh, L.; Fong-Yee, C.; Tuckermann, J.; Chen, D.; Little, C.B.; Seibel, M.J.; Zhou, H. Deletion of the chondrocyte glucocorticoid receptor attenuates cartilage degradation through suppression of early synovial activation in murine posttraumatic osteoarthritis. Osteoarthr. Cartil. 2023, 31, 1189–1201. [Google Scholar] [CrossRef]
- Arnold, K.M.; Weaver, S.R.; Zars, E.L.; Tschumperlin, D.J.; Westendorf, J.J. Inhibition of Phlpp1 preserves the mechanical integrity of articular cartilage in a murine model of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2024, 32, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Luo, L.; Gui, T.; Yu, F.; Yan, L.; Yao, L.; Zhong, L.; Yu, W.; Han, B.; Patel, J.M.; et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci. Transl. Med. 2021, 13, eabb3946. [Google Scholar] [CrossRef]
- Akkiraju, H.; Srinivasan, P.P.; Xu, X.; Jia, X.; Safran, C.B.K.; Nohe, A. CK2.1, a bone morphogenetic protein receptor type Ia mimetic peptide, repairs cartilage in mice with destabilized medial meniscus. Stem Cell Res. Ther. 2017, 8, 82. [Google Scholar] [CrossRef]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef]
- Fang, H.; Huang, L.; Welch, I.; Norley, C.; Holdsworth, D.W.; Beier, F.; Cai, D. Early Changes of Articular Cartilage and Subchondral Bone in The DMM Mouse Model of Osteoarthritis. Sci. Rep. 2018, 8, 2855. [Google Scholar] [CrossRef]
- Christiansen, B.A.; Guilak, F.; Lockwood, K.A.; Olson, S.A.; Pitsillides, A.A.; Sandell, L.J.; Silva, M.J.; van der Meulen, M.C.; Haudenschild, D.R. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Park, I.Y.; Hong, J.I.; Kim, J.R.; Kim, H.A. Comparison of joint degeneration and pain in male and female mice in DMM model of osteoarthritis. Osteoarthr. Cartil. 2021, 29, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.L.; Blanchet, T.J.; Peluso, D.; Hopkins, B.; Morris, E.A.; Glasson, S.S. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthr. Cartil. 2007, 15, 695–700. [Google Scholar] [CrossRef]
- Gowler, P.R.W.; Mapp, P.I.; Burston, J.J.; Shahtaheri, M.; Walsh, D.A.; Chapman, V. Refining surgical models of osteoarthritis in mice and rats alters pain phenotype but not joint pathology. PLoS ONE 2020, 15, e0239663. [Google Scholar] [CrossRef]
- Cobos, E.J.; Ghasemlou, N.; Araldi, D.; Segal, D.; Duong, K.; Woolf, C.J. Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain 2012, 153, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Fentz, J.; Kjobsted, R.; Birk, J.B.; Jordy, A.B.; Jeppesen, J.; Thorsen, K.; Schjerling, P.; Kiens, B.; Jessen, N.; Viollet, B.; et al. AMPKalpha is critical for enhancing skeletal muscle fatty acid utilization during in vivo exercise in mice. FASEB J. 2015, 29, 1725–1738. [Google Scholar] [CrossRef]
- Tsai, H.C.; Chen, T.L.; Chen, Y.P.; Chen, R.M. Traumatic osteoarthritis-induced persistent mechanical hyperalgesia in a rat model of anterior cruciate ligament transection plus a medial meniscectomy. J. Pain. Res. 2018, 11, 41–50. [Google Scholar] [CrossRef]
- Stockl, S.; Taheri, S.; Maier, V.; Asid, A.; Toelge, M.; Clausen-Schaumann, H.; Schilling, A.; Grassel, S. Effects of intra-articular applied rat BMSCs expressing alpha-calcitonin gene-related peptide or substance P on osteoarthritis pathogenesis in a murine surgical osteoarthritis model. Stem Cell Res. Ther. 2025, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Muschter, D.; Fleischhauer, L.; Taheri, S.; Schilling, A.F.; Clausen-Schaumann, H.; Grassel, S. Sensory neuropeptides are required for bone and cartilage homeostasis in a murine destabilization-induced osteoarthritis model. Bone 2020, 133, 115181. [Google Scholar] [CrossRef]
- Bansal, S.; Miller, L.M.; Patel, J.M.; Meadows, K.D.; Eby, M.R.; Saleh, K.S.; Martin, A.R.; Stoeckl, B.D.; Hast, M.W.; Elliott, D.M.; et al. Transection of the medial meniscus anterior horn results in cartilage degeneration and meniscus remodeling in a large animal model. J. Orthop. Res. 2020, 38, 2696–2708. [Google Scholar] [CrossRef]
- Doyran, B.; Tong, W.; Li, Q.; Jia, H.; Zhang, X.; Chen, C.; Enomoto-Iwamoto, M.; Lu, X.L.; Qin, L.; Han, L. Nanoindentation modulus of murine cartilage: A sensitive indicator of the initiation and progression of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2017, 25, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.; Chanalaris, A.; Knights, C.; Ismail, H.; Sacitharan, P.K.; Gentry, C.; Bevan, S.; Vincent, T.L. Nociceptive Sensitizers Are Regulated in Damaged Joint Tissues, Including Articular Cartilage, When Osteoarthritic Mice Display Pain Behavior. Arthritis Rheumatol. 2016, 68, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, G.W.; Mercer, H.; Cormier, J.; Dunbar, C.; Benoit, L.; Adams, C.; Jezierski, J.; Luginbuhl, A.; Bilsky, E.J. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: Implications for preclinical behavioral assessment of chronic pain. Pharmacol. Biochem. Behav. 2011, 98, 35–42. [Google Scholar] [CrossRef]
- Stulberg, J.J.; Huang, R.; Kreutzer, L.; Ban, K.; Champagne, B.J.; Steele, S.R.; Johnson, J.K.; Holl, J.L.; Greenberg, C.C.; Bilimoria, K.Y. Association Between Surgeon Technical Skills and Patient Outcomes. JAMA Surg. 2020, 155, 960–968. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bais, M.V.; Raut, R.D. Modified Medial Meniscectomy (MMM) Model to Assess Post-Traumatic Knee Osteoarthritis in Mouse. Osteology 2025, 5, 25. https://doi.org/10.3390/osteology5030025
Bais MV, Raut RD. Modified Medial Meniscectomy (MMM) Model to Assess Post-Traumatic Knee Osteoarthritis in Mouse. Osteology. 2025; 5(3):25. https://doi.org/10.3390/osteology5030025
Chicago/Turabian StyleBais, Manish V., and Rajnikant Dilip Raut. 2025. "Modified Medial Meniscectomy (MMM) Model to Assess Post-Traumatic Knee Osteoarthritis in Mouse" Osteology 5, no. 3: 25. https://doi.org/10.3390/osteology5030025
APA StyleBais, M. V., & Raut, R. D. (2025). Modified Medial Meniscectomy (MMM) Model to Assess Post-Traumatic Knee Osteoarthritis in Mouse. Osteology, 5(3), 25. https://doi.org/10.3390/osteology5030025






