Abstract
Background/Objectives: To prevent potential complications for patients with metal hypersensitivity requiring total knee arthroplasty (TKA), implant coatings have been developed. Thermal nitriding of the titanium surface creates a TiN layer that increases hardness and wear resistance while preventing release of cobalt and chromium ions. The aim of this study was to evaluate the clinical safety and performance of an anatomic implant system comprised of thermally nitrided Ti-6Al-4V. Methods: This is an ongoing prospective, multicenter observational cohort study of primary and revision TKA patients. Patient-reported outcome measures including the Oxford Knee Score (OKS), Knee Society Score (KSS) Expectations subscale, EQ-5D-5L, physical exams, and radiographic assessments to document abnormalities were investigated in 94 patients who provided at least two years of follow-up data. The primary endpoint was improvement in the Oxford Knee Score (OKS), defined as the minimal clinically important difference (MCID, 7.0 points). Results: All outcome measures including patient-reported function (OKS) demonstrated significant improvements (19.4–22.6 points) exceeding the MCID with no between-group differences by bearing types utilized. Health-related quality of life as measured by EQ-5D-5L improved over the cohort and was maintained at 2-years post-operative. In total, three (1.4%) radiographic abnormalities were observed, all of which resolved at two-year follow-up. 12 (5.3%) serious complications were reported, none of which were related to the device. Two revisions have occurred, one due to infection and one due to a fall, in the ultracongruent bearing cohort (survivorship 98.1%, 95%CI 87.4–99.7). Implant survivorship was 100% in all other bearing cohorts. Conclusions: This anatomically designed, thermally nitrided titanium alloy implant demonstrated clinically significant improvements in function, PROMs, and quality of life in patients undergoing TKA regardless of bearing type. Excellent two-year implant survivorship between 98.1% and 100% across cohorts were observed, with no radiographic abnormalities at 2 years.
1. Introduction
Total knee arthroplasty (TKA) is the definitive treatment for end-stage osteoarthritis (OA) to provide relief of pain, restore function and mobility, and improve quality of life. The prevalence of OA has continued to increase, due to several factors including an aging population and rise in incidence of obesity [1,2]. The volume of TKA increased by 156% between the years 2000 and 2019 in the United States and is projected to increase to nearly 3 million procedures annually by 2060 [3]. National registry reports suggest that the revision burden of total joint procedures has remained between 8–10%, where infection and inflammatory reactions account for about a third of all TKA revision procedures [4]. The second most common diagnosis leading to revision, mechanical loosening, accounts for nearly a quarter of these procedures [4]. Analysis of multiple national European registries suggests revision procedures are 76% more expensive than primary procedures [5]. The economic burden in the US has been anticipated to exceed $13 billion annually by 2030 [6].
A significant proportion of the general population self-reports cutaneous allergy or sensitivity to at least one metal, most often nickel. Within the arthroplasty population, metal hypersensitivity has been estimated between 4.1 and 21.7% [7,8,9]. There is conflicting evidence regarding the connection between cutaneous sensitivity and the potential for deep tissue response to metal, as well as outcomes following arthroplasty in patients with metal hypersensitivity who receive traditional implants [10,11]. It has been suggested that cases reporting continued symptoms may be related to fear-driven perception due to known metal sensitivity [12]. Although it has been suggested that metal implants may induce metal hypersensitivity, little evidence supports this assertion [7,13,14].
Any metal implant that is continually exposed to biological conditions will undergo corrosion. Surrounding inflammatory cell mechanisms have been hypothesized as a contributor to biologically-induced surface damage. Studies from multi-institutional retrieval programs have shown that even contemporary implant designs and advanced manufacturing processes continue to demonstrate surface damage. This may be microscale (confirmed by digital optical microscopy) or visually apparent, with mild to severe damage noted in more than half of retrieved implants in a recent report [15]. Authors have also suggested damage may be imparted by surgical tools during implantation or explantation (particularly electrocautery devices), as well as third party damage (trapped metal debris, bone chips, cement or polyethylene particles), and wear at articulation surfaces [16]. All these processes contribute to the release of metal ions into local tissues and systemically.
In the first year after implantation, patients who undergo total knee arthroplasty (TKA) exhibit increased cobalt, chromium, and titanium serum ion levels [17,18,19]. Most patients’ levels remain within safe ranges and no adverse effects are noted. However, in some cases the release of metal ions has been linked to pain, metallosis, osteolysis, and loosening of prosthesis components after total knee arthroplasty [19,20,21]. Most reports detailing the impact of metal ion release after arthroplasty have focused on metal-on-metal hip implants, however, local tissue reactions and the formation of pseudotumors after TKA have been reported [22,23,24]. This has not been limited to hinged or revision knee devices [16], which contain more modular junctions at risk of fretting and corrosion. It is unknown whether patients with self-reported metal hypersensitivity are at greater risk of these events, though research has shown that that degree of damage to retrieved implants correlates with metal ion levels in tissue and blood [25]. Additionally, the incidence of metal reactions leading to revision are unknown, as symptoms often mimic those of joint infection.
While the components of hip implants have been largely replaced with ceramic materials, CoCr alloys are still widely used in the manufacture of knee implants. Given the possibility of metal ion release and the proportion of the population reporting metal hypersensitivity, hypoallergenic solutions are being developed. These are typically separated into two categories: implants that are made of cobalt and chromium and coated with zirconium or titanium by physical vapor deposition (PVD) methods, and those that are made entirely of hypoallergenic materials such as ceramics or polyetheretherketone (PEEK) [12,26].
Titanium has excellent tribological characteristics, however, its wear profile does not lend itself to use as the material for the implant itself without additional surface treatment [27]. To avoid the use of metals that may induce or exacerbate metal hypersensitivity, nitriding, a thermal process in a high nitrogen environment that allows intercalation of nitrogen molecules into the titanium surface, can be utilized [28]. The process has been used over a range of industrial and manufacturing processes and has been commercially available since the 1970s. The altered microstructure of the alloy with intercalated nitrogen creates a TiN layer with improved hardness and wear resistance while providing a homogeneous surface structure and antibacterial properties [29,30,31]. The conditions of the nitriding process can be varied, where time and temperature are used to control the surface roughness and wear properties [21].
Traditionally, coatings consisting of TiN, TiNbN, or ZnO2 have been layered upon CoCr or Ti-6Al-4V alloys by PVD, to improve surface hardness, decrease wear on the implant and bearing surface, impart antibacterial properties, and prevent the release of metal ions [12,32]. Early results suggest similar performance and survivorship of some hypoallergenic designs [10,33,34,35]. Moreover, patients undergoing revision with hypoallergenic implants due to metal sensitivity-induced failure have demonstrated resolution of symptoms [36,37]. However, there have been concerns regarding delamination of the coated layers due to adhesion failure [38,39]. Few studies have reported mid- to long-term performance of these types of implants, such that coating failures in the long-term are still of potential concern [27,28]. Given this potential failure mechanism, authors have suggested caution in use of these implants in patients with metal hypersensitivity [38].
Tivanium® Ti-6Al-4V alloy has been used successfully in the manufacturing of femoral components with excellent clinical performance [35]. This material can be used to create the entire implant component, followed by thermal nitriding for surface hardening [40] to avoid the use of cobalt and chromium completely. Creating the entire prosthesis from this alloy and treating the surface imparts the benefits of TiN coatings (increased hardness and wear resistance), while avoiding the potential for coating failure. Used in combination with highly crosslinked polyethylene (HXLPE) bearings infused with antioxidants such as Vitamin E, these prostheses present the potential for excellent outcomes, preventing metal hypersensitivity reactions and providing minimal wear to prevent complications such as aseptic loosening, one of the most common indications for revision following total knee arthroplasty (TKA). The purpose of this study was to evaluate the clinical performance of thermally nitrided Ti-6Al-4V alloy combined with a personalized anatomical implant system by assessing patient-reported outcome measures, clinically measured functional range of motion (ROM), radiographic findings, and incidence of adverse events (AEs).
2. Methods
2.1. Study Design
This is a prospective, multi-center observational cohort study (Clinicaltrials.gov identifier: NCT04817969) to confirm the long-term performance, and clinical benefits of the Persona® Ti-Nidium Total Knee System and instrumentation (Zimmer Biomet, Warsaw, IN, USA) in primary and revision TKA. This system builds on the clinical legacy of the NexGen® LPS Flex Tivanium® Knee System (Zimmer Biomet, Warsaw IN, USA), combining the Persona Knee instrument platform and anatomical implants with nitrided Ti-6Al-4V alloy [40] and Vivacit-E®, Vitamin E infused HXLPE bearings (Zimmer Biomet, Warsaw, IN, USA). The study was designed to enroll a total of 240 consecutive patients at seven sites within the United States, to include 60 patients into each of the following variants: posterior stabilized/constrained posterior stabilized (PS/CPS), cruciate retaining (CR), ultra congruent (UC), and medial congruent (MC) femoral components. The primary endpoint was an improvement in the Oxford Knee Score (OKS), defined as the minimal clinically important difference (MCID, 7.0 points) [41]. The sample size required was based on the overall group mean improvement of the OKS, assuming an expected change of 12.2 ± 8.4 points in each group. Considering 80% power and alpha set at 0.05, 23 patients per variant would be required; assuming a 40% attrition rate, 39 patients would be required per variant. To better support secondary endpoints within the investigation, this was expanded to 60 patients to be enrolled within each articular surface combination group. The study was designed to obtain long-term follow-up information up to 10 years after implantation in all eligible participants. This report describes the 2-year results of the study; 230 patients have been enrolled; enrollment continues in the cruciate retaining cohort at the time of writing. Patient flowchart is shown in Figure 1.
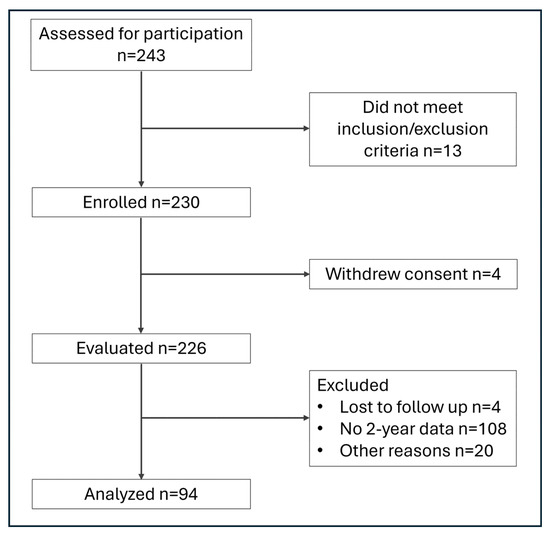
Figure 1.
Patient flowchart.
2.2. Inclusion/Exclusion Criteria
Patients were eligible for inclusion if over the age of 18 years and skeletally mature, qualified for total knee arthroplasty based upon physical exam and medical history, meeting the indications of use of the Persona Ti-Nidium Knee System with Vivacit-E polyethylene articulating surface independent of study participation (according to commercially available use indications), and willing to provide written informed consent and complete scheduled follow-up evaluations. Patients were excluded from participation if currently participating in any other surgical intervention or pain management study or presenting with: a mental or neurologic condition preventing the capability of following post-operative care instructions; a history of infection of the affected joint or other local or systemic infection that may affect the prosthetic joint; insufficient bone stock on femoral or tibial surfaces to support the prosthesis; neuropathic arthropathy, osteoporosis or loss of musculature or neuromuscular disease compromising the affected limb; a stable, painless arthrodesis in satisfactory functional position; severe instability secondary to absence of collateral ligament integrity; rheumatoid arthritis accompanied by an ulcer of the skin or a history of recurrent breakdown of the skin; kinematic alignment surgical technique in those with ≥5° valgus deformity with MCL insufficiency. Surgical procedures were performed according to the device’s instructions for use; cemented fixation was utilized in all procedures.
2.3. Assessments
Pre-operative assessment data included demographic data collection, physical examination, Knee Society Knee Score (KSS) objective knee assessment, KSS pre-operative expectations, Oxford Knee Score, and EuroQol 5-Dimension 5-Level (EQ-5D-5L) general quality of life (QoL) score. Questionnaires and physical exams were also completed at 6 months and at 1-, 2-, 3-, 5-, 7- and 10-year follow-up. Intra-operative information and early post-operative data were collected. Radiographic views, including standing anteroposterior (AP) and standard medial lateral (ML) views of the operative knee were captured pre-operatively, immediately post-operative, at 6 months and annually through 5 years post-operative, and at 7- and 10-years post-operative.
3. Results
Baseline patient demographics are summarized in Table 1. Significant differences were observed among the four implant groups for sex distribution (p = 0.011) and baseline BMI (p = 0.005). Knee range of motion (ROM) showed significant improvement (p < 0.05) from baseline across all follow-up visits (6 months, 1 year, and 2 years). A between-group difference in knee ROM at the 1-year follow-up was observed (p = 0.028), where ROM was lowest in the UC group and did not demonstrate significant improvement over baseline at this timepoint, however, no other between-group differences in ROM were detected.

Table 1.
Patient demographics.
Patient-reported outcome measures (PROMs) from baseline through the 2-year follow-up are detailed in Table 2. Both the Oxford Knee Score (OKS) and EQ-5D-5L scores significantly improved (p < 0.05) from baseline to all follow-up time points across all four cohorts. There were no between-group differences at any time point for either questionnaire. Baseline KSS Expectations scores differed significantly among the groups (p < 0.001), but no between-group differences were noted at any follow-up visit.

Table 2.
Patient reported outcome measures.
Abnormal radiographic findings are outlined in Table 3. Across all patients, four radiographic abnormalities were reported from early postoperative through the 2-year visit. In the CR group, one case of heterotopic ossification along the anterior cortex of the femur was reported at the early postoperative visit. At 6 months, the PS/CPS group had one instance of patella baja. At one year, the MC group reported one longitudinal patellar fracture, which did not affect motion. This was managed conservatively and healed without additional treatment. No abnormal radiographic findings were observed in the UC group.

Table 3.
Radiographic Abnormalities Observed.
Adverse events (AEs) and serious adverse events (SAEs) are reported in Table 4. Of the 127 reported AEs, seven were deemed to have a possible relationship to the device, while none of the 12 SAEs were device-related. No events were attributed to instrumentation or cement, and no device deficiencies were reported. Additionally, there were no significant differences in the frequency of AEs or SAEs among the four implant groups. Two revisions have occurred within the UC cohort at 2 years of follow-up; one was due to infection and required debridement and polyethylene bearing exchange, the other was revised following a fall and evidence of hyperextension and instability such that the 2-year survival estimate within that cohort is 98.1% (95%CI 87.4–99.7). No revisions occurred in any of the remaining cohorts (survival estimate 100%).

Table 4.
Summary of Adverse Events by Type or Relationship to Device.
4. Discussion
In this study, we present 2-year clinical safety and performance data of a new prosthesis composed completely of a titanium alloy that has undergone thermal nitriding to create a hard TiN surface layer that is not subject to delamination or adhesion concerns. Our results suggest an excellent performance of the device, as ROM improved significantly from baseline across all articulation surface cohorts. Additionally, OKS scores and patient-reported health-related quality of life significantly improved at 6 months compared to pre-operative values. Few radiographic abnormalities were observed across all cohorts. We also report an excellent safety profile across cruciate retaining, medial congruent, posterior stabilized, constrained posterior stabilized, and ultracongruent articular surfaces, as no serious adverse event observed was device-related. Finally, only two revisions have occurred, both limited to the ultracongruent articular surface group.
Metal hypersensitivity has been a subject of contention within the arthroplasty community. Its prevalence within the general population is suggested to be approximately 25% [7], though some reports have suggested ranges from 10–48%, with nickel typically being the most common offender [42,43,44]. Sensitivity to cobalt and chromium have also been reported, though at lower rates, 2% and 1% of the general population, respectively. The prevalence within patients tested for metal sensitivities before undergoing arthroplasty has been lower, with reports typically between 1 and 3%, but as high as 16% [13,45]. Testing for metal sensitivity has been an additional source of controversy. Blood tests such as lymphocyte transformation testing (LTT) and lymphocyte proliferation testing (LPT) are believed to be of greater sensitivity and more representative of expected reactions to implants, however cutaneous patch testing is more easily accessible and cost-effective. The agreement between the results of the different types of tests is generally low, and cutaneous patch testing has the potential to induce metal hypersensitivity after exposure [46]. A consensus study amongst surgeon experts concluded that routine metal allergy screening prior to arthroplasty is not necessary and suggests the use of traditional CoCr or stainless steel implants regardless of allergy status [47].
However, conflicting evidence continues to be reported in the literature regarding outcomes for patients with metal allergies who receive traditional implants. Chimento et al. found [42] no difference in patient-reported functional outcomes for those with nickel sensitivity with traditional metal implants and reported no correlation between reactivity and OKS. Tille and colleagues [11] showed no increase in inflammatory markers after receipt of metal implants, suggesting no systemic impact. Despite this, there has been evidence of deleterious effects of metal implants in those with allergies. Hallab et al. [48] found a higher prevalence of metal hypersensitivity in patients with poorly performing implants (60%) compared to those with well-performing prostheses (25%). Granchi et al. [49] also reported that the frequency of positive hypersensitivity tests increased after joint replacement, supporting the assertion that implants may induce sensitivity, particularly in metal-on-metal prosthesis groups. Other authors have reported less improvement and worse outcomes in patients with any allergies, including metal allergies [50]. Bracey et al. [46] concluded that patients with metal allergies were predisposed to worse outcomes even when hypoallergenic implants were used, while other studies have suggested that those who self-reported metal sensitivity benefited from receipt of hypoallergenic prostheses in the short term [8]. Still, other authors have reported higher failure rates in patients receiving titanium nitride-coated devices [51,52].
While evidence conflicts, revision to hypoallergenic implants has been shown to resolve symptoms, lending credence to the assertion that some patients are impacted by metal implants [36,37]. In addition, corrosion and fretting of articulating surfaces may result in the release of debris, most often from the polyethylene liner, which contributes to aseptic loosening and osteolysis. It should be noted that increased levels of metal ions are relatively rare. Lutzner et al. [7] found no increase in plasma metal ion levels in patients without a history of metal hypersensitivity in either coated or non-coated implant recipients. However, Postler et al. [53] did find an increase in metal ion levels in patients with confirmed metal hypersensitivity.
Though routine metal allergy testing is not recommended in all patients, the evidence suggests that in those with known or suspected metal allergy, hypoallergenic implants are likely warranted to prevent metal-associated complications. In addition to preventing the leaching of metal ions [21], coatings and treatments that prevent allergic reactions have also been suggested to improve osteogenic, antibacterial, and anti-inflammatory properties of implants, as well as biomechanical properties such as hardness and corrosion resistance [29,31,32]. Studies comparing hypoallergenic implants to their exact CoCr counterparts have demonstrated similar results regarding functional outcomes and survivorship. Deroche et al. reported no difference in PROMs or revision rates between a CoCr implant and the TiN-coated version of the prosthesis at 5 years post-operative [34], while others have reported similar rates of achievement of MCID and patient acceptable symptom state (PASS) post-operatively [10]. Similar long-term results have been reported comparing a completely metal-free ceramic implant to a conventional implant, where clinical scores improved compared to baseline but did not differ between groups [33].
The thermally nitrided titanium alloy in the current report has previously demonstrated excellent clinical results. Rossi and colleagues [35] reported 97.2% and 95.1% survival at 5- and 10-years respectively, in patients with a metal allergy who received the NexGen LPS Tivanium prosthesis with a highly crosslinked polyethylene bearing. The authors also reported significant improvement in objective functional scores and all patient-reported outcome measures. Importantly, the three removed components in that study showed no signs of macroscopic damage or polyethylene wear, and no incidence of complications associated with metal hypersensitivity. Previous reports have also demonstrated excellent clinical outcomes of the Persona anatomic implant, with 2- and 5-year survivorship reported at 99.0% [54,55,56,57]. Significant improvements in range of motion, objective function, and patient-reported outcomes with the personalized knee system [54,55,56,57,58], with patient satisfaction reaching 95–99% have been reported [54,55]. In this study, the average improvement on OKS was greater than the MCID for this instrument, supporting evidence of clinically significant improvement in function. Additionally, ROM improved significantly across all variants by 6 months post-operatively in the current analysis. In a propensity-matched study of the current implant with its antecedent, radiologic assessments showed better fit to the native femur and tibia, with superior sparing of bone tissue. Omari and colleagues [59] also reported fewer outliers regarding femoral flexion and tibial slope on radiologic assessment compared to the previous standard implants used within their institution. Their radiologic assessments align with our results, as only three radiologic abnormalities were observed.
While this study is strengthened by the fact that it was a multicenter investigation and is the first clinical report on the use of the TiNidium Persona Knee System, it is subject to several limitations. This is an early report of results; additional follow-up is needed to confirm the long-term safety and effectiveness of this implant system. The cohort sizes, particularly those with 2-year follow-up information available, are relatively small, and must therefore be interpreted with some caution particularly in comparisons of use of bearing types. However, the study continues to enroll patients and collect longer-term data. This study did not include only patients with metal sensitivity on enrollment, such that additional studies to confirm safety in this population, including metal ion levels over long-term follow-up, are needed. We did not measure cobalt and chromium in this study, as they are not included in the implant’s materials, however we did not monitor titanium or aluminum serum ion concentrations, which should be an additional area of future study. Additionally, there is no control group, however, we can compare our results to the clinical performance of other hypoallergenic devices, and to the predecessor device composed of the same thermally nitrided Tivanium alloy, where our results are similar in terms of implant survivorship, objective functional and patient-reported improvements. Finally, as a multi-surgeon observational study, we did not dictate techniques beyond approved labeled instruction for use and thus could not control for variations in surgical or cementation techniques.
5. Conclusions
This anatomically designed thermally nitrided titanium implant demonstrated improved functional outcomes and PROMs in patients undergoing total knee arthroplasty at 6 months and were maintained through 2 years post-operatively. Only two revisions have occurred, with no device-related serious adverse events, supporting the safety and effectiveness of the device. While the cobalt and chromium are eliminated in this implant design, additional research to determine whether this device is an effective option specifically in patients with metal sensitivity and investigate the long-term clinical safety of the device is needed.
Author Contributions
Conceptualization—D.J., P.M.C., H.B. and K.R.T. Methodology—D.J., P.M.C., H.B., E.K., R.E.R. and K.R.T. Formal Analysis—E.K. and K.R.T. Investigation—D.J., P.M.C., H.B., E.K., R.E.R. and K.R.T. Resources—E.K. and R.E.R. Writing—Original Draft Preparation Writing—R.E.R. and E.K. Reviewing and Editing—D.J., P.M.C., H.B., E.K., R.E.R. and K.R.T. Supervision—E.K. and K.R.T. Project Administration—E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This prospective study was funded by Zimmer Biomet, CMG2020-05K.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the WCG Institutional Review Board (20211371) on 3 September 2021.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are not publicly available due to commercial and privacy restrictions.
Conflicts of Interest
D.J.—Paid speaker or presenter Zimmer Biomet, ConforMIS Inc.; Paid consultant—Zimmer Biomet, ConforMIS Inc.; Stock or stock option, ConforMIS Inc.; Research support, ConforMIS Inc., Corin USA, Medacta, Zimmer Biomet P.M.C.—Paid speaker or presenter Zimmer Biomet, ConforMIS Inc., Paid consultant—Zimmer Biomet, Stryker, Smith & Nephew; Stock or stock options, Parvizi Surgical Innovation; Editorial/governing board, Journal of Arthroplasty; Board committee/member, AAHKS, AAOS H.B.—Royalties, Innomed; Paid consultant, Globus Medical E.K.—Employed, Zimmer Biomet; Stock or stock options, Zimmer Biomet R.E.R.—Employed, Zimmer Biomet; Stock or stock options, Zimmer Biomet K.R.T.—Stock or stock options, Alio, Sparta Biomedical; Research support, Zimmer Biomet; Editorial/governing board, Journal of Arthroplasty, Arthroplasty Today.
References
- Li, Z.; Chen, Y.; Shen, Z. Global Shifts in Osteoarthritis Subtype Trends among Older Adults Due to Elevated Bmi: An Age-Period-Cohort Analysis Based on the Global Burden of Disease Database. Front. Public Health 2025, 13, 1518572. [Google Scholar] [CrossRef]
- Nguyen, A.; Lee, P.; Rodriguez, E.K.; Chahal, K.; Freedman, B.R.; Nazarian, A. Addressing the Growing Burden of Musculoskeletal Diseases in the Ageing Us Population: Challenges and Innovations. Lancet Healthy Longev. 2025, 6, 100707. [Google Scholar] [CrossRef] [PubMed]
- Shichman, I.; Roof, M.; Askew, N.; Nherera, L.; Rozell, J.C.; Seyler, T.M.; Schwarzkopf, R. Projections and Epidemiology of Primary Hip and Knee Arthroplasty in Medicare Patients to 2040–2060. JBJS Open Access 2023, 8, e22.00112. [Google Scholar] [CrossRef]
- National Joint Registry: 21st Annual Report 2024; National Joint Registry: London, UK, 2024.
- Sadoghi, P.; Koutp, A.; Prieto, D.P.; Clauss, M.; Kayaalp, M.E.; Hirschmann, M.T. The Projected Economic Burden and Complications of Revision Hip and Knee Arthroplasties: Insights from National Registry Studies. Knee Surg. Sports Traumatol. Arthrosc. 2025; online ahead of print. [Google Scholar]
- Bhandari, M.; Smith, J.; Miller, L.E.; Block, J.E. Clinical and Economic Burden of Revision Knee Arthroplasty. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2012, 5, 89–94. [Google Scholar] [CrossRef]
- Lutzner, J.; Hartmann, A.; Dinnebier, G.; Spornraft-Ragaller, P.; Hamann, C.; Kirschner, S. Metal Hypersensitivity and Metal Ion Levels in Patients with Coated or Uncoated Total Knee Arthroplasty: A Randomised Controlled Study. Int. Orthop. 2013, 37, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Matar, H.E.; Porter, P.J.; Porter, M.L. Metal Allergy in Primary and Revision Total Knee Arthroplasty: A Scoping Review and Evidence-Based Practical Approach. Bone Jt. Open 2021, 2, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Siljander, B.R.; Chandi, S.K.; Cororaton, A.D.; Debbi, E.M.; McLawhorn, A.S.; Sculco, P.K.; Chalmers, B.P. A Comparison of Clinical Outcomes after Total Knee Arthroplasty in Patients Who Have and Do Not Have Self-Reported Nickel Allergy: Matched and Unmatched Cohort Comparisons. J. Arthroplast. 2024, 39, 2490–2495. [Google Scholar] [CrossRef]
- Tidd, J.L.; Gudapati, L.S.; Simmons, H.L.; Klika, A.K.; Pasqualini, I.; Group Cleveland Clinic Arthroplasty; Piuzzi, N.S. Do Patients with Hypoallergenic Total Knee Arthroplasty Implants for Metal Allergy Do Worse? An Analysis of Health Care Utilizations and Patient-Reported Outcome Measures. J. Arthroplast. 2024, 39, 103–110. [Google Scholar] [CrossRef]
- Tille, E.; Beyer, F.; Lutzner, C.; Postler, A.; Thomas, P.; Summer, B.; Lutzner, J. No Difference in Patient Reported Outcome and Inflammatory Response after Coated and Uncoated Total Knee Arthroplasty—A Randomized Controlled Study. BMC Musculoskelet. Disord. 2023, 24, 968. [Google Scholar] [CrossRef]
- Xie, F.; Sheng, S.; Ram, V.; Pandit, H. Hypoallergenic Knee Implant Usage and Clinical Outcomes: Are They Safe and Effective? Arthroplast. Today 2024, 28, 101399. [Google Scholar] [CrossRef]
- Nam, D.; Li, K.; Riegler, V.; Barrack, R.L. Patient-Reported Metal Allergy: A Risk Factor for Poor Outcomes after Total Joint Arthroplasty? J. Arthroplast. 2016, 31, 1910–1915. [Google Scholar] [CrossRef]
- Peacock, C.J.H.; Fu, H.; Asopa, V.; Clement, N.D.; Kader, D.; Sochart, D.H. The Effect of Nickel Hypersensitivity on the Outcome of Total Knee Arthroplasty and the Value of Skin Patch Testing: A Systematic Review. Arthroplasty 2022, 4, 40. [Google Scholar] [CrossRef]
- Arnholt, C.M.; MacDonald, D.W.; Klein, G.R.; Cates, H.E.; Rimnac, C.M.; Kurtz, S.M.; Writing, C.I.R.C.; Kocagoz, S.; Chen, A.F. What Is the Incidence of Cobalt-Chromium Damage Modes on the Bearing Surface of Contemporary Femoral Component Designs for Total Knee Arthroplasty? J. Arthroplast. 2018, 33, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, M.A.; Hallab, N.J.; Rainey, J.P.; Pelt, C.E.; Mihalko, W.M.; Piuzzi, N.S.; Mont, M.A.; Spece, H.; Kurtz, S.M. Metal Release in Total Knee Arthroplasty: A Review of Mechanisms, Adverse Local Tissue Reactions, and Biological Effects. J. Arthroplast. 2025; in press. [Google Scholar]
- Luetzner, J.; Krummenauer, F.; Lengel, A.M.; Ziegler, J.; Witzleb, W.C. Serum Metal Ion Exposure after Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2007, 461, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Lons, A.; Putman, S.; Pasquier, G.; Migaud, H.; Drumez, E.; Girard, J. Metallic Ion Release after Knee Prosthesis Implantation: A Prospective Study. Int. Orthop. 2017, 41, 2503–2508. [Google Scholar] [CrossRef] [PubMed]
- Reiner, T.; Sorbi, R.; Muller, M.; Nees, T.; Kretzer, J.P.; Rickert, M.; Moradi, B. Blood Metal Ion Release after Primary Total Knee Arthroplasty: A Prospective Study. Orthop. Surg. 2020, 12, 396–403. [Google Scholar] [CrossRef]
- Vivegananthan, B.; Shah, R.; Karuppiah, A.S.; Karuppiah, S.V. Metallosis in a Total Knee Arthroplasty. BMJ Case Rep. 2014, 2014, bcr2013202801. [Google Scholar] [CrossRef]
- AbuAlia, M.; Fullam, S.; Cinotti, F.; Manninen, N.; Wimmer, M.A. Titanium Nitride Coatings on Cocrmo and Ti6al4v Alloys: Effects Onwear and Ion Release. Lubricants 2024, 12, 96. [Google Scholar] [CrossRef]
- Harvie, P.; Torres-Grau, J.; Beaver, R.J. Common Peroneal Nerve Palsy Associated with Pseudotumour after Total Knee Arthroplasty. Knee 2012, 19, 148–150. [Google Scholar] [CrossRef]
- Rainey, J.P.; Gililland, J.M.; Peters, C.L.; Archibeck, M.J.; Anderson, L.A.; Pelt, C.E. Metallosis and Corrosion Associated with Revision Total Knee Arthroplasties with Metaphyseal Sleeves. Arthroplast. Today 2023, 22, 101167. [Google Scholar] [CrossRef]
- Thakur, R.R.; Ast, M.P.; McGraw, M.; Bostrom, M.P.; Rodriguez, J.A.; Parks, M.L. Severe Persistent Synovitis after Cobalt-Chromium Total Knee Arthroplasty Requiring Revision. Orthopedics 2013, 36, e520–e524. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, P.W.; Aslani, S.; Kurtz, M.A.; Taylor, L.M.; Barnes, E.R.; MacDonald, D.W.; Piuzzi, N.S.; Mihalko, W.M.; Kurtz, S.M.; Gilbert, J.L. Cobalt-Chromium-Molybdenum Femoral Knee Implant Damage Correlates with Elevated Periprosthetic Metal Concentrations. J. Arthroplast. 2025, 40, S315–S323. [Google Scholar] [CrossRef] [PubMed]
- Post, C.E.; Bitter, T.; Briscoe, A.; Fluit, R.; Verdonschot, N.; Janssen, D. The Primary Stability of a Cementless Peek Femoral Component Is Sensitive to Bmi: A Population-Based Fe Study. J. Biomech. 2024, 168, 112061. [Google Scholar] [CrossRef] [PubMed]
- Skjoldebrand, C.; Tipper, J.L.; Hatto, P.; Bryant, M.; Hall, R.M.; Persson, C. Current Status and Future Potential of Wear-Resistant Coatings and Articulating Surfaces for Hip and Knee Implants. Mater. Today Bio 2022, 15, 100270. [Google Scholar] [CrossRef]
- van Hove, R.P.; Sierevelt, I.N.; van Royen, B.J.; Nolte, P.A. Titanium-Nitride Coating of Orthopaedic Implants: A Review of the Literature. Biomed. Res. Int. 2015, 2015, 485975. [Google Scholar] [CrossRef]
- Tkachik, O.V.; Sheykin, S.E.; Lavrys, S.M.; Rostotskii, I.Y.; Danyliak, M.-O.M.; Pohrelyuk, I.M.; Proskurnyak, R.V. Effect of Stage Gas Nitriding on Corrosion and Wear Resistance of Ti6al4v. Vacuum 2024, 230, 113713. [Google Scholar] [CrossRef]
- Venugopalan, R.; Weimer, J.J.; George, M.A.; Lucas, L.C. The Effect of Nitrogen Diffusion Hardening on the Surface Chemistry and Scratch Resistance of Ti-6a1-4v Alloy. Biomaterials 2000, 21, 1669–1677. [Google Scholar] [CrossRef]
- Matijošius, T.; Pohrelyuk, I.; Lavrys, S.; Staišiūnas, L.; Selskienė, A.; Stičinskaitė, A.; Ragelienė, L.; Smailys, A.; Andriušis, A.; Padgurskas, J. Wear Resistance and Antibacterial Properties of 3d-Printed Ti6al4v Alloy after Gas Nitriding. Tribol. Int. 2024, 197, 109839. [Google Scholar] [CrossRef]
- Chan, C.-W.; Quinn, J.; Hussain, I.; Carson, L.; Smith, G.C.; Lee, S. A Promising Laser Nitriding Method for the Design of Next Generation Orthopaedic Implants: Cytotoxicity and Antibacterial Performance of Titanium Nitride (Tin) Wear Nano-Particles, and Enhanced Wear Properties of Laser-Nitrided Ti6al4v Surfaces. Surf. Coat. Technol. 2021, 405, 126714. [Google Scholar] [CrossRef]
- Breuer, R.; Fiala, R.; Hartenbach, F.; Pollok, F.; Huber, T.; Strasser-Kirchweger, B.; Rath, B.; Trieb, K. Long Term Follow-up of a Completely Metal Free Total Knee Endoprosthesis in Comparison to an Identical Metal Counterpart. Sci. Rep. 2024, 14, 20958. [Google Scholar] [CrossRef]
- Deroche, E.; Batailler, C.; Shatrov, J.; Gunst, S.; Servien, E.; Lustig, S. No Clinical Difference at Mid-Term Follow-up between Tin-Coated Versus Uncoated Cemented Mobile-Bearing Total Knee Arthroplasty: A Matched Cohort Study. SICOT J. 2023, 9, 5. [Google Scholar] [CrossRef]
- Rossi, S.M.P.; Perticarini, L.; Mosconi, M.; Ghiara, M.; Benazzo, F. Ten-Year Outcomes of a Nitrided Ti-6al-4v Titanium Alloy Fixed-Bearing Total Knee Replacement with a Highly Crosslinked Polyethylene-Bearing in Patients with Metal Allergy. Knee 2020, 27, 1519–1524. [Google Scholar] [CrossRef]
- Guenther, D.; Thomas, P.; Kendoff, D.; Omar, M.; Gehrke, T.; Haasper, C. Allergic Reactions in Arthroplasty: Myth or Serious Problem? Int. Orthop. 2016, 40, 239–244. [Google Scholar] [CrossRef]
- Zondervan, R.L.; Vaux, J.J.; Blackmer, M.J.; Brazier, B.G.; Taunt, C.J., Jr. Improved Outcomes in Patients with Positive Metal Sensitivity Following Revision Total Knee Arthroplasty. J. Orthop. Surg. Res. 2019, 14, 182. [Google Scholar] [CrossRef]
- Lapaj, L.; Rozwalka, J. Retrieval Analysis of Tin (Titanium Nitride) Coated Knee Replacements: Coating Wear and Degradation in Vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1251–1261. [Google Scholar] [CrossRef]
- Herbster, M.; Doring, J.; Nohava, J.; Lohmann, C.H.; Halle, T.; Bertrand, J. Retrieval Study of Commercially Available Knee Implant Coatings Tin, Tinbn and Zrn on Tial6v4 and Cocr28mo6. J. Mech. Behav. Biomed. Mater. 2020, 112, 104034. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.H. Surface Hardening of Orthopedic Implants. In Biomaterials Engineering and Devices: Human Applications: Orthopedic, Dental, and Bone Graft Applications; Wise, D.L., Trantolo, D.J., Lewandrowski, K.-U., Gresser, J.D., Cattaneo, M.V., Yaszemski, M.J., Eds.; Humana Press: Totowa, NJ, USA, 2000; Volume 2, pp. 191–202. [Google Scholar]
- Beard, D.J.; Harris, K.; Dawson, J.; Doll, H.; Murray, D.W.; Carr, A.J.; Price, A.J. Meaningful Changes for the Oxford Hip and Knee Scores after Joint Replacement Surgery. J. Clin. Epidemiol. 2015, 68, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Chimento, G.; Daher, J.; Desai, B.; Velasco-Gomez, C. Nickel Allergy Does Not Correlate with Function after Total Knee Arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2024, 33, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Bloemke, A.D.; Clarke, H.D. Prevalence of Self-Reported Metal Allergy in Patients Undergoing Primary Total Knee Arthroplasty. J. Knee Surg. 2015, 28, 243–246. [Google Scholar] [CrossRef]
- D’Ambrosi, R.; Nuara, A.; Mariani, I.; Di Feo, F.; Ursino, N.; Hirschmann, M. Titanium Niobium Nitride Mobile-Bearing Unicompartmental Knee Arthroplasty Results in Good to Excellent Clinical and Radiographic Outcomes in Metal Allergy Patients with Medial Knee Osteoarthritis. J. Arthroplast. 2021, 36, 140–147.e2. [Google Scholar] [CrossRef]
- Desai, M.M.; Shah, K.A.; Mohapatra, A.; Patel, D.C. Prevalence of Metal Hypersensitivity in Total Knee Replacement. J. Orthop. 2019, 16, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Bracey, D.N.; Hegde, V.; Johnson, R.; Kleeman-Forsthuber, L.; Jennings, J.; Dennis, D. Poor Correlation among Metal Hypersensitivity Testing Modalities and Inferior Patient-Reported Outcomes after Primary and Revision Total Knee Arthroplasties. Arthroplast. Today 2022, 18, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Razak, A.; Ebinesan, A.D.; Charalambous, C.P. Metal Allergy Screening Prior to Joint Arthroplasty and Its Influence on Implant Choice: A Delphi Consensus Study Amongst Orthopaedic Arthroplasty Surgeons. Knee Surg. Relat. Res. 2013, 25, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.; Merritt, K.; Jacobs, J.J. Metal Sensitivity in Patients with Orthopaedic Implants. J. Bone Jt. Surg. Am. 2001, 83, 428–436. [Google Scholar] [CrossRef]
- Granchi, D.; Cenni, E.; Giunti, A.; Baldini, N. Metal Hypersensitivity Testing in Patients Undergoing Joint Replacement: A Systematic Review. J. Bone Jt. Surg. Br. 2012, 94, 1126–1134. [Google Scholar] [CrossRef]
- Soler, F.; Murcia, A.; Benlloch, M.; Mariscal, G. The Impact of Allergies on Patient-Reported Outcomes after Total Hip and Knee Arthroplasty: A Systematic Review and Meta-Analysis. Arch. Orthop. Trauma Surg. 2024, 144, 3755–3765. [Google Scholar] [CrossRef]
- Lionberger, D.; Conlon, C.; Wattenbarger, L.; Walker, T.J. Unacceptable Failure Rate of a Ceramic-Coated Posterior Cruciate-Substituting Total Knee Arthroplasty. Arthroplast. Today 2019, 5, 187–192. [Google Scholar] [CrossRef]
- Song, S.J.; Lee, H.W.; Bae, D.K.; Park, C.H. High Incidence of Tibial Component Loosening after Total Knee Arthroplasty Using Ceramic Titanium-Nitride-Coated Mobile Bearing Prosthesis in Moderate to Severe Varus Deformity: A Matched-Pair Study between Ceramic-Coated Mobile Bearing and Fixed Bearing Prostheses. J. Arthroplast. 2020, 35, 1003–1008. [Google Scholar]
- Postler, A.; Beyer, F.; Lutzner, C.; Tille, E.; Lutzner, J. Similar Outcome During Short-Term Follow-up after Coated and Uncoated Total Knee Arthroplasty: A Randomized Controlled Study. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3459–3467. [Google Scholar] [CrossRef]
- Kim, S.E.; Ro, D.H.; Lee, M.C.; Cholewa, J.M. Early- to Mid-Term Review of a Prospective, Multi-Center, International, Outcomes Study of an Anatomically Designed Implant with Posterior-Stabilized Bearing in Total Knee Arthroplasty. Medicina 2023, 59, 2105. [Google Scholar] [CrossRef]
- Mahmood, F.; Rae, F.; Rae, S.; Ewen, A.; Holloway, N.; Clarke, J. Mid-Term Results of an Anatomic Total Knee Replacement Design. Arch. Orthop. Trauma Surg. 2024, 144, 2239–2247. [Google Scholar] [CrossRef]
- de Villeneuve, F.B.; Jacquet, C.; Puech, S.; Parratte, S.; Ollivier, M.; Argenson, J.-N. Minimum Five Years Follow-up of Total Knee Arthroplasty Using Morphometric Implants in Patients with Osteoarthritis. J. Arthroplast. 2021, 36, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Mathijssen, N.M.C.; Verburg, H.; London, N.J.; Landsiedl, M.; Dominkus, M. Patient Reported Outcomes and Implant Survivorship after Total Knee Arthroplasty with the Persona Knee Implant System: Two Year Follow Up. BMC Musculoskelet. Disord. 2019, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Dauder Gallego, C.; Fenoll, I.B.M.; Contreras, J.L.P.; Coronas, F.J.M.; de la Cal, M.D.C.T.; Martin, J.M. Midterm Results of a New Personalized Knee Implant for Total Knee Arthroplasty: Implant Survivorship and Patient-Reported Outcome after Five Years’ Follow-Up. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 257–262. [Google Scholar] [CrossRef]
- Omari, A.; Troelsen, A.; Husted, H.; Nielsen, C.S.; Gromov, K. Early Clinical Outcome and Learning Curve Following Unilateral Primary Total Knee Arthroplasty after Introduction of a Novel Total Knee Arthroplasty System. World J. Orthop. 2020, 11, 431–441. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).