Verification of the Semiquantitative Assessment of Vertebral Deformity for Subsequent Vertebral Body Fracture Prediction and Screening for the Initiation of Osteoporosis Treatment: A Case-Control Study Using a Clinical-Based Setting
Abstract
1. Introduction
2. Materials and Methods
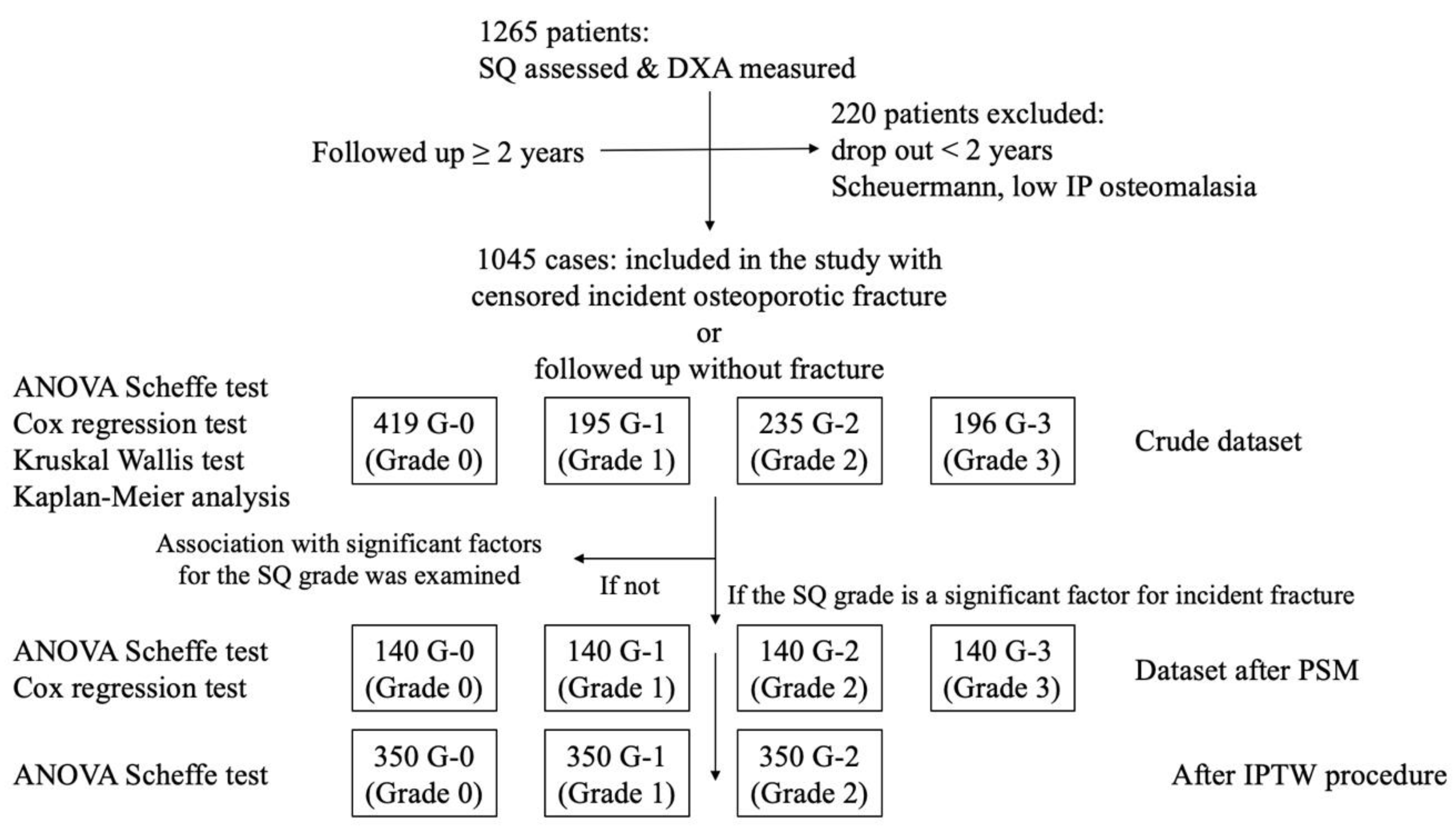
2.1. Patient Inclusion and Exclusion
2.2. Primary Vertebral Deformity Defining Using the SQ Method
2.3. Other Factors Extraction
2.4. Statistical Analyses
2.4.1. In the Crude Dataset
Group Comparison
Risk Factor Analysis
Differences in the Variables Among the SQ Grade Groups and the Likelihood of the Incident Clinical VFs in These Variables Analysis
Cox Regression Analysis When the SQ Grading Is Modified, Whether Grade 3 or Not
2.4.2. In the Dataset After PSM
Group Comparison After PSM
Risk Factor Analysis in the Dataset After PSM
Cox Regression Analysis When the SQ Grading Is Modified, Whether Grade 3 or Not
2.4.3. Association Between the Significant Factor and the SQ Grade
2.4.4. In the Dataset After IPTW
Group Comparison After IPTW
Risk Factor Analysis in the Dataset After IPTW
- Statistical procedures and software
- Ethical considerations
3. Results
3.1. In the Crude Dataset
3.1.1. Group Comparison
3.1.2. Risk Factor Analysis
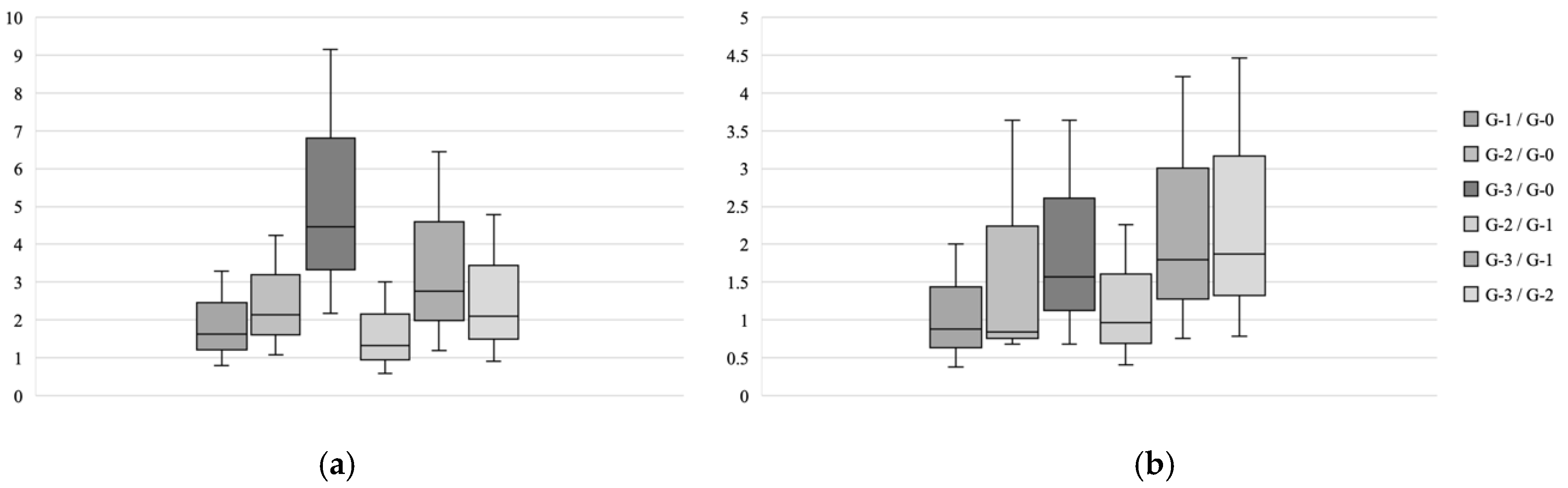
3.1.3. Differences in the Variables Among the SQ Grade Groups and the Likelihood of the Incident VFs in These Variables Analysis
3.1.4. Cox Regression Analysis When the SQ Grading Is Modified, Whether Grade 3 or Not
3.2. In the Dataset After PSM
3.2.1. Group Comparison After PSM
3.2.2. Risk Factor Analysis in the Dataset After PSM
3.2.3. Cox Regression Analysis When the SQ Grading Is Modified, Whether Grade 3 or Not
3.3. Association Between the Significant Factor and the SQ Grade
3.4. In the Dataset After IPTW
3.4.1. Group Comparison After IPTW
3.4.2. Risk Factor Analysis in the Dataset After IPTW
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cummings, S.R.; Melton, L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002, 359, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005, 16 (Suppl. S2), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P. Bone density, geometry, and fracture in older men. Curr. Osteoporos. Rep. 2006, 4, 57–63. [Google Scholar] [CrossRef]
- Sugimoto, T.; Sato, M.; Dehle, F.C.; Brnabic, A.J.; Weston, A.; Burge, R. Lifestyle-Related Metabolic Disorders, Osteoporosis, and Fracture Risk in Asia: A Systematic Review. Value Health Reg. Issues 2016, 9, 49–56. [Google Scholar] [CrossRef]
- Otonari, J.; Ikezaki, H.; Furusyo, N.; Sudo, N. Association of lifestyle factors with osteoporosis and fracture in postmenopausal women: A Japanese cohort study. Menopause 2021, 28, 1254–1263. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Ara, I. Fragility fracture risk and skeletal muscle function. Climacteric 2016, 19, 37–41. [Google Scholar] [CrossRef]
- Peeters, G.; van Schoor, N.M.; Lips, P. Fall risk: The clinical relevance of falls and how to integrate fall risk with fracture risk. Best. Pract. Res. Clin. Rheumatol. 2009, 23, 797–804. [Google Scholar] [CrossRef]
- Morin, S.N.; Lix, L.M.; Leslie, W.D. The importance of previous fracture site on osteoporosis diagnosis and incident fractures in women. J. Bone Miner. Res. 2014, 29, 1675–1680. [Google Scholar] [CrossRef]
- Aspray, T.J.; Hill, T.R. Osteoporosis and the Ageing Skeleton. Subcell. Biochem. 2019, 91, 453–476. [Google Scholar]
- Yoshii, I.; Chijiwa, T.; Sawada, N.; Kokei, S. Musculoskeletal ambulation disability symptom complex as a risk factor of incident bone fragility fracture. Osteoporos. Sarcopenia 2021, 7, 115–120. [Google Scholar] [CrossRef]
- Ginsberg, C.; Ix, J.H. Diagnosis and Management of Osteoporosis in Advanced Kidney Disease: A Review. Am. J. Kidney Dis. 2022, 79, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Biver, E.; Brennan-Speranza, T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021, 9, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Grauer, A.; Curtis, J.R.; Wenkert, D.; Balasubramanian, A.; Daigle, S.G.; Zhang, J.; Chen, L. Risk of subsequent fracture after prior fracture among older women. Osteoporos. Int. 2019, 30, 79–92. [Google Scholar]
- Kwok, A.W.L.; Gong, J.S.; Wang, Y.J.; Leung, J.C.S.; Kwok, T.; Griffith, J.F.; Leung, P.C. Prevalence and risk factors of radiographic vertebral fractures in elderly Chinese men and women: Results of Mr. OS (Hong Kong) and Ms. OS (Hong Kong) studies. Osteoporos. Int. 2013, 24, 877–885. [Google Scholar] [CrossRef]
- Koo, K.-H.; Yeom, J.S.; Park, S.-M.; Park, J.W.; Han, H.; Go, S.J.; Kim, H.-J.; Lee, Y.-K. Risk factors for subsequent vertebral fractures following a previous hip fracture. J. Bone Miner. Metab. 2021, 39, 193–200. [Google Scholar]
- Nakamura, H.; Takahata, M.; Yoshii, T.; Otani, K.; Haro, H.; Okawa, A.; Tsuji, T.; Ohba, T.; Hirano, T.; Sato, K.; et al. Risk factors for subsequent vertebral fracture after acute osteoporotic vertebral fractures. Eur. Spine J. 2021, 30, 2698–2707. [Google Scholar]
- Genant, H.K.; Wu, C.Y.; van Kuijk, C.; Nevitt, M.C. Vertebral fracture assessment using a semiquantitative technique. J. Bone Miner. Res. 1993, 8, 1137–1148. [Google Scholar] [CrossRef]
- El Maghraoui, A.; Mounach, A.; Gassim, S.; Ghazi, M. Vertebral fracture assessment in healthy men: Prevalence and risk factors. Bone 2008, 43, 544–548. [Google Scholar] [CrossRef]
- Uemura, Y.; Miyakawa, N.; Orimo, H.; Shiraki, M.; Nakamura, T.; Mori, S. Comparison of expert and nonexpert physicians in assessing vertebral fractures using the semiquantitative method in Japan. J. Bone Miner. Metab. 2015, 33, 642–650. [Google Scholar] [CrossRef]
- Diacinti, D.; Guglielmi, G. How to define an osteoporotic vertebral fracture? Quant. Imaging Med. Surg. 2019, 9, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Aboudiab, M.; Grados, F.; Batteux, B.; Henry-Desailly, I.; Fardellone, P.; Goëb, V. Vertebral fracture assessment (VFA) in patients over 50 years of age with a non-severe peripheral fracture. Osteoporos. Int. 2020, 31, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Akune, T.; Kawaguchi, H.; Tsutsui, S.; Asai, Y.; Oshima, Y.; Oka, H.; Muraki, S.; Iidaka, T.; Hashizume, H.; et al. The cumulative incidence of and risk factors for morphometric severe vertebral fractures in Japanese men and women: The ROAD study third and fourth surveys. Osteoporos. Int. 2022, 33, 889–899. [Google Scholar]
- Akune, T.; Oka, H.; Yoshida, M.; Kawaguchi, H.; Muraki, S.; Tsutsui, S.; Asai, Y.; Iidaka, T.; Tanaka, S.; Yamada, H.; et al. Differences in prevalence and associated factors between mild and severe vertebral fractures in Japanese men and women: The third survey of the ROAD study. J. Bone Miner. Metab. 2019, 37, 844–853. [Google Scholar]
- Yu, W.; Lin, Q.; Zhou, X.; Shao, H.; Sun, P. Reconsideration of the relevance of mild wedge or short vertebral height deformities across a broad age distribution. Osteoporos. Int. 2014, 25, 2609–2615. [Google Scholar] [CrossRef]
- Zhu, T.; Tang, X.-L.; Li, E.; Griffith, J.; Leung, P.-C.; Au, S.-K.; Tam, L.-S.; Kwok, A. Incidence of and risk factors for non-vertebral and vertebral fracture in female Chinese patients with systemic lupus erythematosus: A five-year cohort study. Lupus 2014, 23, 854–861. [Google Scholar] [CrossRef]
- Zoccali, C.; Tripepi, G.; Dekker, F.W.; Jager, K.J.; Stel, V.S.; Chesnaye, N.C.; Fu, E.L. An introduction to inverse probability of treatment weighting in observational research. Clin. Kidney J. 2021, 15, 14–20. [Google Scholar]
- Klotzbuecher, C.M.; Ross, P.D.; Landsman, P.B.; Abbott, T.A., 3rd; Berger, M. Patients with prior fractures have an increased risk of future fractures: A summary of the literature and statistical synthesis. J. Bone Miner. Res. 2000, 15, 721–739. [Google Scholar] [CrossRef]
- Kanis, J.A.; Harvey, N.C.; Johansson, H.; Odén, A.; Leslie, W.D.; McCloskey, E.V. FRAX Update. J. Clin. Densitom. 2017, 20, 360–367. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johansson, H.; Harvey, N.C.; McCloskey, E.V. A brief history of FRAX. Arch. Osteoporos. 2018, 13, 118. [Google Scholar] [CrossRef]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Tsukutani, Y.; Hagino, H.; Ito, Y.; Nagashima, H. Epidemiology of fragility fractures in Sakaiminato, Japan: Incidence, secular trends, and prognosis. Osteoporos. Int. 2015, 26, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Shoji, A.; Gao, Z.; Arai, K.; Yoshimura, N. 30-year trends of hip and vertebral fracture incidence in Japan: A systematic review and meta-analysis. J Bone Miner Metab 2022, 40, 327–336. [Google Scholar] [CrossRef]
- Lu, C.; Niu, M.; Kong, C.; Zhu, M.; Yang, Z.; Li, Z.; Li, Q.; Li, H.; Li, J.; Kang, P. Prevalence and risk factors of osteoporotic fracture among the elderly population in China: A multicenter cross-sectional study. Int. Orthop. 2024, 48, 1323–1330. [Google Scholar]
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e80–e92. [Google Scholar]
- Himič, V.; Syrmos, N.; Ligarotti, G.K.I.; Kato, S.; Fehlings, M.G.; Ganau, M. The role of genetic and epigenetic factors in determining the risk of spinal fragility fractures: New insights in the management of spinal osteoporosis. Quant. Imaging Med. Surg. 2023, 13, 7632–7645. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakamura, T.; Nakano, T.; Takaoka, K.; Shiraki, M.; Hagino, H.; Fukunaga, M.; Matsumoto, T. Number and severity of prevalent vertebral fractures and the risk of subsequent vertebral fractures in Japanese women with osteoporosis: Results from the minodronate trial. J. Bone Miner. Metab. 2013, 31, 544–550. [Google Scholar]
- Genant, H.K.; Cheng, X.; Kim, Y.M.; Bouxsein, M.L.; Yu, W.; Kiel, D.P.; Demissie, S.; Samelson, E.J. Identification of prevalent vertebral fractures using CT lateral scout views: A comparison of semi-automated quantitative vertebral morphometry and radiologist semi-quantitative grading. Osteoporos. Int. 2012, 23, 1007–1101. [Google Scholar]
| All (N = 1045) | Grade 0 (N = 419) | Grade 1 (N = 195) | Grade 2 (N = 235) | Grade 3 (N = 196) | p-Value in ANOVA | Statistical Significance in Scheffé Test | |
|---|---|---|---|---|---|---|---|
| male:female (%) | 13.7:86.3 | 15.8:85.8 | 16.4:83.6 | 12.3:87.7 | 8.2:91.8 | <0.05 | n.s. |
| age, years | 78.3 (10.8) | 72.6 (11.7) | 80.2 (8.4) | 82.4 (8.5) | 84.0 (7.0) | <0.001 | Grade-0 <<< Grade-1 Grade-0 <<< Grade-2 Grade-0 <<< Grade-3 Grade-1 << Grade-3 |
| incident VF | 59 (5.6%) | 13 (3.1%) | 9 (4.6%) | 13 (5.5%) | 24 (12.2%) | <0.001 | Grade-0 <<< Grade-3 Grade-1 < Grade-3 Grade-2 < Grade-3 |
| incident NVF | 111 (10.6%) | 22 (5.3%) | 25 (12.8%) | 27 (11.5%) | 37 (18.9%) | <0.001 | Grade-0 < Grade-1 Grade-0 <<< Grade-3 |
| current smoke | 25 (2.4%) | 15 (3.6%) | 4 (2.1%) | 2 (0.9%) | 4 (2.0%) | 0.16 | n.s. |
| alcohol habitat | 14 (1.3%) | 8 (1.9%) | 2 (1.0%) | 3 (1.3%) | 1 (0.5%) | 0.53 | n.s. |
| parent’s fracture | 16 (1.5%) | 5 (1.2%) | 3 (1.5%) | 4 (1.7%) | 4 (2.0%) | 0.11 | n.s. |
| prevalent VF | 217 (20.8%) | 38 (9.1%) | 28 (17.0%) | 65 (24.6%) | 86 (43.7%) | <0.001 | Grade-0 <<< Grade-2 Grade-0 <<< Grade-3 Grade-1 <<< Grade-3 Grade-2 <<< Grade-3 |
| prevalent NVF | 100 (9.6%) | 30 (7.2%) | 21 (12.7%) | 19 (7.2%) | 30 (15.2%) | <0.01 | Grade-0 < Grade-3 Grade-2 < Grade-3 |
| VEC | 329 (31.5%) | 38 (9.1%) | 31 (15.9%) | 64 (27.2%) | 196 (100%) | <0.001 | Grade-0 <<< Grade-2 Grade-0 <<< Grade-3 Grade-1 << Grade-2 Grade-1 <<< Grade-3 Grade-2 <<< Grade-3 |
| AAC | 784 (75.0%) | 202 (48.2%) | 174 (89.2%) | 226 (96.1%) | 182 (92.9%) | <0.001 | Grade-0 <<< Grade-1 Grade-0 <<< Grade-2 Grade-0 <<< Grade-3 |
| Diabetes mellitus | 209 (20.0%) | 94 (22.4%) | 37 (19.0%) | 44 (18.7%) | 34 (17.3%) | 0.43 | n.s. |
| COPD | 79 (7.6%) | 26 (6.2%) | 8 (4.1%) | 24 (10.2%) | 21 (10.7%) | <0.05 | n.s. |
| hypertension | 467 (44.7%) | 160 (38.2%) | 81 (41.5%) | 113 (48.1%) | 113 (57.7%) | <0.001 | Grade-0 <<< Grade-3 Grade-1 < Grade-3 |
| hyperlipidemia | 247 (23.6%) | 99 (23.6%) | 49 (25.1%) | 54 (23.0%) | 45 (23.0%) | 0.95 | n.s. |
| insomnia | 196 (18.8%) | 75 (17.9%) | 39 (20.0%) | 42 (17.9%) | 40 (20.4%) | 0.83 | n.s. |
| Cognitive Impairment | 146 (14.0%) | 25 (6.0%) | 24 (12.3%) | 47 (20.0%) | 50 (25.5%) | <0.001 | Grade-0 <<< Grade-2 Grade-0 <<< Grade-3 Grade-1 << Grade-3 |
| MADS | 197 (18.9%) | 52 (12.4%) | 35 (17.9%) | 50 (21.3%) | 60 (30.6%) | <0.001 | Grade-0 <<< Grade-2 Grade-0 <<< Grade-3 Grade-1 << Grade-3 |
| rheumatoid arthritis | 303 (29.0%) | 156 (37.2%) | 60 (30.8%) | 48 (20.4%) | 39 (19.9%) | <0.001 | Grade-0 <<< Grade-2 Grade-0 <<< Grade-3 |
| osteoarthritis | 526 (50.3%) | 197 (47.0%) | 106 (54.4%) | 120 (51.1%) | 103 (50.1%) | 0.32 | n.s. |
| Contracture | 91 (8.7%) | 37 (8.8%) | 20 (10.3%) | 19 (8.1%) | 15 (7.7%) | 0.81 | n.s. |
| Disuse | 55 (6.2%) | 17 (4.1%) | 10 (5.1%) | 17 (7.2%) | 21 (10.7%) | <0.05 | Grade-0 < Grade-3 |
| Parkinsonism | 25 (2.4%) | 4 (1.0%) | 7 (3.6%) | 6 (2.6%) | 8 (4.1%) | 0.06 | n.s. |
| T-score in the LS | −2.3 (1.7) | −1.7 (1.8) | −2.2 (1.6) | −2.7 (1.5) | −3.1 (1.5) | <0.001 | Grade-0 >> Grade-1 Grade-0 >>> Grade-2 Grade-0 >>> Grade-3 Grade-1 > Grade-2 Grade-1 >>> Grade-3 Grade-2 > Grade-3 |
| T-score in the FN | −2.0 (1.2) | −1.6 (1.2) | −2.0 (1.0) | −2.3 (1.1) | −2.7 (1.0) | <0.001 | Grade-0 >>> Grade-1 Grade-0 >>> Grade-2 Grade-0 >>> Grade-3 Grade-1 >>> Grade-3 Grade-2 >> Grade-3 |
| GCs | 180 (17.2%) | 73 (17.4%) | 46 (23.6%) | 31 (13.2%) | 30 (15.3%) | <0.05 | Grade-1 > Grade-2 |
| V-D | 621 (59.4%) | 215 (50.0%) | 137 (70.3%) | 147 (62.6%) | 122 (48.6%) | <0.001 | Grade-0 <<< Grade-1 |
| OPD † | 212 (20.3%) | 57 (13.6%) | 49 (25.1%) | 39 (16.7%) | 67 (34.2%) | <0.001 | Grade-0 < Grade-1 Grade-0 <<< Grade-3 Grade-2 <<< Grade-3 |
| polypharmacy | 168 (16.1%) | 64 (15.3%) | 33 (16.9%) | 38 (16.2%) | 33 (16.8%) | 0.94 | n.s. |
| eGFR | 58.9 (21.2) | 67.1 (21.8) | 56.6 (18.8) | 52.2 (20.2) | 49.9 (16.1) | <0.001 | Grade-0 >>> Grade-1 Grade-0 >>> Grade-2 Grade-0 >>> Grade-3 |
| ALB | 4.0 (0.4) | 4.1 (0.4) | 4.0 (0.3) | 3.9 (0.4) | 3.9 (0.4) | <0.001 | Grade-0 >>> Grade-2 Grade-0 >>> Grade-3 Grade-1 > Grade-2 Grade-1 >> Grade-3 |
| P1NP | 61.9 (54.4) | 62.0 (56.4) | 57.4 (48.2) | 58.8 (49.6) | 69.9 (60.5) | 0.13 | n.s. |
| TRACP-5b | 505.9 (244.2) | 491.1 (218.1) | 483.0 (196.4) | 542.1 (313.7) | 516.2 (240.6) | <0.05 | n.s. |
| Hgb | 12.2 (1.6) | 12.6 (1.5) | 12.3 (1.5) | 11.9 (1.8) | 11.7 (1.4) | <0.001 | Grade-0 >>> Grade-2 Grade-0 >>> Grade-3 Grade-1 >> Grade-3 |
| Lymph | 1563 (660) | 1637 (652) | 1636 (686) | 1508 (670) | 1401 (608) | <0.001 | Grade-0 >>> Grade-3 Grade-1 >> Grade-3 |
| Mono | 344 (141) | 342 (141) | 344 (117) | 351 (157) | 341 (144) | 0.88 | n.s. |
| Univariate Mode | Multivariate Mode | ||||
|---|---|---|---|---|---|
| p-Value | Risk Ratio (95% CI) | β-Value | p-Value | Risk Ratio (95% CI) | |
| female | 0.18 | 2.21 (0.69–7.07) | |||
| age, years | <0.001 | 1.05 (1.02–1.07) | −0.014 | 0.59 | 0.99 (0.94–1.04) |
| current smoke | 0.78 | 0.82 (0.20–3.36) | |||
| alcohol habitat | 0.98 | 0.00 (0.00–INF) | |||
| parent’s fracture | 0.98 | 0.00 (0.00–INF) | |||
| SQ Grade | <0.001 | 1.82 (1.46–2.27) | 0.546 | <0.01 | 1.73 (1.22–2.44) |
| prevalent VF | 0.98 | 2.6 × 108 (0.00–INF) | |||
| prevalent NVF | 0.37 | 1.52 (0.60–3.84) | |||
| VEC | 0.98 | 3.2 × 107 (0.00–INF) | |||
| AAC | <0.05 | 1.88 (1.01–3.52) | −0.158 | 0.76 | 0.85 (0.30–2.40) |
| Diabetes mellitus | 0.28 | 0.70 (0.37–1.33) | |||
| COPD | 0.17 | 0.44 (0.14–1.41) | |||
| hypertension | 0.08 | 1.61 (0.94–2.75) | |||
| hyperlipidemia | 0.98 | 0.99 (0.58–1.70) | |||
| chronic heart failure | <0.01 | 2.21 (1.28–3.80) | 0.407 | 0.29 | 1.50 (0.71–3.19) |
| insomnia | 0.37 | 0.76 (0.41–1.39) | |||
| Cognitive Impairment | <0.05 | 2.01 (1.10–3.68) | 0.141 | 0.76 | 1.15 (0.47–3.82) |
| MADS | <0.05 | 1.96 (1.15–3.34) | −0.204 | 0.58 | 0.82 (0.40–1.68) |
| rheumatoid arthritis | 0.06 | 0.57 (0.32–1.02) | |||
| osteoarthritis | 0.71 | 1.11 (0.65–1.90) | |||
| Contracture | 0.72 | 1.14 (0.57–2.25) | |||
| Disuse | 0.15 | 1.75 (0.82–3.70) | |||
| Parkinsonism | 0.84 | 0.82 (0.11–5.94) | |||
| T-score in the LS | <0.01 | 0.78 (0.66–0.92) | 0.012 | 0.93 | 1.01 (0.77–1.32) |
| T-score in the FN | <0.001 | 0.64 (0.51–0.82) | −0.326 | 0.11 | 0.72 (0.48–1.08) |
| GCs | 0.24 | 0.68 (0.35–1.31) | |||
| V-D | 0.62 | 1.15 (0.66–1.98) | |||
| OPD † | 0.65 | 1.14 (0.66–1.96) | |||
| polypharmacy | 0.27 | 0.67 (0.33– 1.36) | |||
| eGFR | <0.01 | 0.97 (0.96–0.99) | −0.017 | 0.12 | 0.98 (0.96–1.00) |
| ALB | 0.21 | 0.60 (0.27–1.33) | |||
| Calcium | 0.14 | 1.17 (0.95–1.45) | |||
| P1NP | 0.23 | 1.00 (0.99–1.01) | |||
| TRACP-5b | 0.16 | 1.00 (1.00–1.00) | |||
| Hgb | <0.01 | 0.78 (0.67–0.92) | −0.195 | 0.14 | 0.82 (0.64–1.07) |
| Lymph | <0.001 | 1.00 (1.00–1.00) | −0.000 | 0.99 | 1.00 (1.00–1.00) |
| Mono | 0.06 | 1.00 (1.00–1.00) | |||
| Variables | Incident VF (ROC and Kaplan-Meier) | |||||
|---|---|---|---|---|---|---|
| Kruskal–Wallis | ROC | Kaplan-Meier | ||||
| p-Value | COI (†) | Positive | Negative | Hazard Ratio | p-Value | |
| gender | 0.43 | female | 56/902 (6.2%) | 3/143 (2.1%) | 2.80 | 0.07 |
| age | <0.001 | >86 (#) | 51/788 (6.5%) | 8/249 (3.1%) | 1.69 | 0.16 |
| current smoke | 0.95 | smokes | 2/25 (8.0%) | 57/1020 (5.6%) | 1.14 | 0.85 |
| alcohol habitat | 0.99 | positive | 59/1031 (5.7%) | 0/14 (0.0%) | INF | n/a |
| parent’s fracture | 0.98 | positive | 59/1029 (5.7%) | 0/16 (0.0%) | INF | n/a |
| SQ Grade | ≥Grade-2 (#) | 37/431 (8.6%) | 22/614 (3.6%) | 3.5 | <0.001 | |
| prevalent VF | <0.001 | positive (#) | 59/217 (27.2%) | 0/828 (0.0%) | INF | <0.001 |
| prevalent NVF | 0.38 | positive | 16/111 (14.4%) | 43/934 (4.6%) | 2.60 | <0.001 |
| VEC | <0.001 | positive (#) | 59/329 (17.9%) | 0/716 (0.0%) | INF | <0.001 |
| AAC | <0.001 | positive (#) | 46/784 (5.9%) | 13/261 (5.0%) | 1.33 | 0.36 |
| Diabetes mellitus | 0.71 | positive | 12/209 (5.7%) | 47/836 (5.6%) | 0.87 | 0.68 |
| COPD | 0.57 | positive | 56/906 (5.8%) | 3/79 (3.8%) | 1.89 | 0.27 |
| hypertension | <0.001 | positive (#) | 34/467 (7.3%) | 25/578 (4.3%) | 1.43 | 0.17 |
| hyperlipidemia | 0.98 | positive | 21/247 (8.5%) | 38/798 (4.8%) | 1.42 | 0.19 |
| CHF | <0.001 | positive (#) | 21/209 (10.0%) | 38/836 (4.5%) | 2.06 | <0.01 |
| insomnia | 0.94 | positive | 14/196 (7.1%) | 45/849 (5.3%) | 1.13 | 0.69 |
| Cognitive Impairment | <0.001 | positive (#) | 14/146 (9.6%) | 45/899 (5.0%) | 1.96 | <0.05 |
| MADS | <0.01 | positive (#) | 21/197 (10.7%) | 38/848 (4.5%) | 2.09 | <0.01 |
| rheumatoid arthritis | <0.001 | negative (#) | 43/742 (5.8%) | 16/303 (5.3%) | 1.42 | 0.22 |
| osteoarthritis | 0.47 | positive | 38/526 (7.2%) | 21/519 (4.0%) | 1.59 | 0.08 |
| Contracture | 0.97 | positive | 10/91 (11.0%) | 49/954 (5.1%) | 1.69 | 0.13 |
| Disuse | 0.59 | positive | 8/65 (12.3%) | 51/980 (5.2%) | 1.98 | 0.07 |
| Parkinsonism | 0.92 | negative | 58/1020 (5.7%) | 1/25 (4.0%) | 1.50 | 0.69 |
| T-score in the LS | <0.001 | ≤−2.3 (#) | 41/577 (6.9%) | 18/468 (3.8%) | 1.97 | <0.05 |
| T-score in the FN | <0.001 | ≤−2.5 (#) | 27/421 (6.4%) | 32/624 (5.1%) | 1.43 | 0.16 |
| GCs | 0.30 | positive | 11/180 (6.1%) | 48/865 (5.5%) | 0.88 | 0.70 |
| V-D | <0.001 | positive (#) | 39/621 (6.3%) | 20/424 (4.7%) | 1.30 | 0.34 |
| OPD † | <0.001 | positive (#) | 20/212 (9.4%) | 39/832 (4.7%) | 1.70 | 0.05 |
| polypharmacy | 0.99 | negative | 50/877 (5.7%) | 9/168 (5.4%) | 1.22 | 0.58 |
| eGFR | <0.001 | <42.0 (#) | 24/237 (10.1%) | 35/808 (4.3%) | 2.46 | <0.01 |
| ALB | <0.001 | ≤4.3 (#) | 56/824 (6.3%) | 3/151 (2.0%) | 2.89 | 0.05 |
| P1NP | 0.28 | ≤22.6 | 17/138 (12.3%) | 42/907 (4.6%) | 2.32 | <0.01 |
| TRACP-5b | 0.13 | ≤421 | 30/404 (7.4%) | 29/641 (4.5%) | 1.50 | 0.12 |
| Hgb | <0.001 | ≤12.0 (#) | 30/437 (6.9%) | 29/608 (4.8%) | 1.60 | 0.07 |
| Lymph | <0.001 | ≥1136 (#) | 50/751 (6.5%) | 9/270 (3.3%) | 1.80 | 0.10 |
| Mono | 0.86 | >271 | 46/704 (6.5%) | 13/330 (3.8%) | 1.81 | 0.05 |
| All (N = 560) | Grade 0 (N = 140) | Grade 1 (N = 140) | Grade 2 (N = 140) | Grade 3 (N = 140) | p-Value in ANOVA | Statistical Significance in Scheffé Test | |
|---|---|---|---|---|---|---|---|
| male:female (%) | 11.1:88.9 | 11.7:89.3 | 12.9:87.1 | 10.0:90.0 | 10.7:89.3 | 0.89 | n.s. |
| age, years | 80.6 (9.0) | 77.0 (9.9) | 80.1 (8.4) | 82.0 (9.2) | 83.1 (7.2) | <0.001 | Grade-0 < Grade-1 Grade-0 <<< Grade-2 Grade-0 <<< Grade-3 Grade-1 < Grade-3 |
| Time length of follow-up, months | 45.4 (29.1) | 51.8 (32.0) | 46.6 (30.0) | 42.0 (27.4) | 41.0 (26.0) | <0.01 | Grade-0 > Grade-3 |
| incident VF | 43 (7.7%) | 11 (7.9%) | 9 (6.4%) | 8 (5.7%) | 15 (10.7%) | 0.41 | n.s. |
| incident NVF | 65 (11.6%) | 12 (8.6%) | 19 (13.6%) | 15 (10.7%) | 19 (13.6%) | 0.49 | n.s. |
| current smoke | 11 (2.0%) | 6 (4.3%) | 2 (1.4%) | 1 (0.7%) | 2 (1.4%) | 0.14 | n.s. |
| alcohol habitat | 2 (0.4%) | 1 (0.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | 0.57 | n.s. |
| parent’s fracture | 4 (0.7%) | 1 (0.7%) | 1 (0.7%) | 1 (0.7%) | 1 (0.7%) | 1.00 | n.s. |
| prevalent VF | 159 (28.4%) | 33 (23.6%) | 23 (19.8%) | 42 (26.3%) | 61 (42.4%) | <0.001 | Grade-0 << Grade-3 Grade-1 <<< Grade-3 Grade-2 << Grade-3 |
| prevalent NVF | 63 (11.3%) | 14 (10.0%) | 18 (15.5%) | 12 (7.5%) | 19 (13.2%) | 0.16 | n.s. |
| VEC | 241 (38.4%) | 33 (23.6%) | 31 (22.1%) | 37 (26.4%) | 140 (100%) | <0.001 | Grade-0 <<< Grade-3 Grade-1 <<< Grade-3 Grade-2 <<< Grade-3 |
| AAC | 469 (83.8%) | 76 (54.3%) | 125 (89.3%) | 136 (97.1%) | 132 (94.3%) | <0.001 | Grade-0 <<< Grade-1 Grade-0 <<< Grade-2 Grade-0 <<< Grade-3 |
| Diabetes mellitus | 102 (18.2%) | 40 (28.6%) | 25 (17.9%) | 19 (13.6%) | 18 (12.9%) | <0.01 | Grade-0 > Grade-2 Grade-0 >> Grade-3 |
| COPD | 44 (7.9%) | 10 (7.1%) | 6 (4.3%) | 15 (10.7%) | 13 (9.3%) | 0.21 | n.s. |
| hypertension | 241 (43.0%) | 66 (47.1%) | 54 (38.6%) | 50 (35.7%) | 71 (50.7%) | <0.05 | n.s. |
| hyperlipidemia | 135 (24.1%) | 45 (32.1%) | 38 (27.1%) | 30 (21.4%) | 22 (15.7%) | <0.01 | Grade-0 < Grade-3 |
| chronic heart failure | 102 (18.2%) | 24 (17.1%) | 22 (15.7%) | 23 (16.4%) | 33 (23.6%) | 0.09 | n.s. |
| insomnia | 99 (17.7%) | 31 (22.1%) | 25 (17.9%) | 20 (14.3%) | 23 (16.4%) | 0.37 | n.s. |
| Cognitive Impairment | 66 (11.8%) | 17 (12.1%) | 17 (12.1%) | 16 (11.4%) | 16 (11.4%) | 1.00 | n.s. |
| MADS | 93 (16.6%) | 20 (14.3%) | 25 (17.9%) | 24 (17.1%) | 24 (17.1%) | 0.86 | n.s. |
| rheumatoid arthritis | 143 (25.5%) | 46 (32.9%) | 40 (28.6%) | 30 (21.4%) | 27 (19.3%) | <0.05 | n.s. |
| osteoarthritis | 299 (53.4%) | 74 (52.9%) | 77 (55.0%) | 76 (54.3%) | 72 (51.4%) | 0.94 | n.s. |
| Contracture | 54 (9.6%) | 17 (12.1%) | 14 (10.0%) | 13 (9.3%) | 10 (7.1%) | 0.56 | n.s. |
| Disuse | 34 (6.1%) | 11 (7.9%) | 6 (4.3%) | 9 (6.4%) | 8 (5.7%) | 0.65 | n.s. |
| Parkinsonism | 10 (1.8%) | 0 (0.0%) | 5 (3.6%) | 0 (0.0%) | 5 (3.6%) | <0.05 | n.s. |
| T-score in the LS | −2.8 (1.2) | −2.8 (1.3) | −2.8 (1.1) | −2.7 (1.1) | −2.8 (1.4) | 0.96 | n.s. |
| T-score in the FN | −2.3 (1.0) | −2.2 (1.0) | −2.2 (0.9) | −2.3 (0.9) | −2.5 (1.0) | <0.05 | n.s. |
| GCs | 95 (17.0%) | 24 (17.1%) | 31 (22.1%) | 20 (14.3%) | 20 (14.3%) | 0.55 | n.s. |
| V-D | 374 (66.8%) | 92 (65.7%) | 104 (74.3%) | 93 (66.4%) | 85 (60.7%) | 0.11 | n.s. |
| OPD † | 128 (22.9%) | 28 (20.0%) | 37 (26.4%) | 21 (15.0%) | 42 (30.0%) | <0.05 | Grade-2 < Grade-3 |
| polypharmacy | 84 (15.0%) | 25 (17.9%) | 20 (14.3%) | 19 (13.6%) | 20 (14.3%) | 0.75 | n.s. |
| eGFR | 56.8 (17.4) | 58.4 (15.4) | 58.5 (18.4) | 57.2 (20.1) | 52.2 (151) | 0.12 | n.s. |
| ALB | 4.0 (0.4) | 4.0 (0.4) | 4.1 (0.3) | 3.9 (0.4) | 3.9 (0.4) | <0.01 | Grade-1 > Grade-2 Grade-1 >> Grade-3 |
| P1NP | 59.8 (51.2) | 57.5 (39.8) | 50.5 (30.6) | 62.0 (57.9) | 69.4 (64.3) | <0.05 | Grade-1 < Grade-3 |
| TRACP-5b | 507.7 (264.3) | 523.8 (227.0) | 469.8 (186.4) | 528.9 (366.5) | 508.7 (245.3) | 0.25 | n.s. |
| Hgb | 12.1 (1.6) | 12.3 (1.4) | 12.4 (1.4) | 12.0 (2.0) | 11.8 (1.4) | <0.01 | Grade-0 >> Grade-3 Grade-1 >> Grade-3 |
| Lymph | 1518 (656) | 1510 (619) | 1649 (715) | 1523 (677) | 1390 (590) | <0.05 | Grade-1 > Grade-3 |
| Mono | 349 (144) | 340 (142) | 352 (119) | 361 (158) | 345 (154) | 0.66 | n.s. |
| Univariate Mode | Multivariate Mode | ||||
|---|---|---|---|---|---|
| p-Value | Risk Ratio (95% CI) | β-Value | p-Value | Risk Ratio (95% CI) | |
| female | 0.49 | 1.51 (0.47–4.88) | |||
| age, years | <0.05 | 0.97 (0.94–1.00) | −0.03 | 0.08 | 0.97 (0.94–1.00) |
| current smoke | 0.97 | 1.04 (0.14–7.55) | |||
| alcohol habitat | 0.99 | 0.00 (0.00–INF) | |||
| parent’s fracture | 0.99 | 0.00 (0.00–INF) | |||
| SQ Grade | 0.26 | 1.16 (0.89–1.52) | |||
| prevalent NVF | 0.98 | 1.3 × 107 (0.00–INF) | |||
| VEC | 0.98 | 1.4 × 108 (0.00–INF) | |||
| AAC | 0.63 | 0.84 (0.40–1.75) | |||
| Diabetes mellitus | 0.75 | 0.88 (0.41–1.90) | |||
| COPD | 0.29 | 0.47 (0.11–1.93) | |||
| hypertension | 0.88 | 1.05 (0.57–1.91) | |||
| hyperlipidemia | 067 | 1.15 (0.60–2.20) | |||
| chronic heart failure | 0.61 | 1.20 (0.59–2.44) | |||
| insomnia | 0.71 | 1.15 (0.56–2.34) | |||
| Cognitive Impairment | 0.68 | 1.20 (0.51–2.84) | |||
| MADS | 0.12 | 1.70 (0.87–3.32) | |||
| rheumatoid arthritis | 0.70 | 0.87 (0.45–1.71) | |||
| osteoarthritis | 0.56 | 1.20 (0.65–2.21) | |||
| Contracture | 0.20 | 1.65 (0.76–3.59) | |||
| Disuse | 0.61 | 1.31 (0.47 –3.67) | |||
| Parkinsonism | 0.98 | 0.00 (0.00–INF) | |||
| T-score in the LS | 0.29 | 1.14 (0.90–1.44) | |||
| T-score in the FN | 0.44 | 1.13 (0.83–1.54) | |||
| GCs | 0.46 | 0.74 (0.33–1.66) | |||
| V-D | 0.75 | 0.58 (0.58–2.13) | |||
| OPD † | 0.20 | 1.52 (0.81–2.85) | |||
| polypharmacy | 0.91 | 0.96 (0.43– 2.15) | |||
| eGFR | 0.16 | 0.98 (0.96–1.01) | |||
| ALB | 0.34 | 1.60 (0.61–4.23) | |||
| P1NP | 0.54 | 1.00 (0.99–1.01) | |||
| TRACP-5b | 0.92 | 1.00 (1.00–1.00) | |||
| Hgb | 0.67 | 0.96 (0.79–1.17) | |||
| Lymph | <0.05 | 1.00 (1.00–1.00) | 1.2 × 10−4 | 0.73 | 1.00 (1.00–1.00) |
| Mono | 0.84 | 1.00 (1.00–1.00) | |||
| All (N = 1050) | Grade 0 (N = 350) | Grade 1 (N = 350) | Grade 2 (N = 350) | p-Value in ANOVA | Statistical Significance in Scheffé Test | |
|---|---|---|---|---|---|---|
| male:female (%) | 12.7:87.3 | 16.0:84.0 | 10.9:89.1 | 11.1:88.9 | 0.07 | |
| age, years | 81.8 (6.7) | 81.2 (6.3) | 81.6 (6.7) | 82.4 (7.1) | 0.06 | |
| Time length of follow-up, months | 45.3 (28.8) | 49.9 (30.7) | 43.4 (26.9) | 42.5 (28.2) | <0.001 | Grade-0 > Grade-1 Grade-0 >> Grade-2 |
| incident VF | 69 (6.6%) | 21 (6.0%) | 26 (7.4%) | 22 (6.3%) | 0.72 | |
| incident NVF | 99 (9.4%) | 28 (8.0%) | 35 (10.0%) | 36 (10.3%) | 0.53 | |
| current smoke | 14 (1.3%) | 8 (2.3%) | 4 (1.1%) | 2 (0.6%) | 0.13 | |
| alcohol habitat | 7 (0.7%) | 2 (0.6%) | 2 (0.6%) | 3 (0.9%) | 0.87 | |
| parent’s fracture | 10 (1.1%) | 3 (0.9%) | 4 (1.1%) | 3 (0.9%) | 0.9 | |
| prevalent VF | 166 (15.8%) | 38 (10.9%) | 56 (16.0%) | 72 (20.6%) | <0.01 | Grade-0 < Grade-1 Grade-0 > Grade-2 |
| prevalent NVF | 116 (11.0%) | 29 (8.3%) | 51 (14.6%) | 36 (10.3%) | <0.05 | Grade-0 < Grade-1 |
| VEC | 201 (19.1%) | 45 (12.9%) | 76 (21.7%) | 80 (22.9%) | <0.05 | Grade-0 < Grade-2 |
| AAC | 828 (78.9%) | 268 (76.6%) | 274 (78.3%) | 286 (81.7%) | 0.21 | |
| Diabetes mellitus | 192 (27.8%) | 121 (34.6%) | 105 (30.0%) | 66 (18.9%) | <0.001 | Grade-0 >>> Grade-2 Grade-1 >> Grade-2 |
| COPD | 84 (8.0%) | 30 (8.6%) | 28 (8.0%) | 26 (7.4%) | 0.86 | |
| hypertension | 531 (50.6%) | 198 (56.6%) | 171 (48.9%) | 162 (46.3%) | <0.05 | Grade-0 > Grade-2 Grade-1 >> Grade-2 |
| hyperlipidemia | 341 (32.5%) | 119 (34.0%) | 99 (28.3%) | 123 (35.1%) | 0.11 | |
| insomnia | 281 (26.8%) | 117 (33.4%) | 103 (29.4%) | 61 (17.4%) | <0.001 | Grade-0 >> Grade-2 Grade-1 >> Grade-2 |
| Cognitive Impairment | 210 (20.0%) | 70 (20.0%) | 72 (20.6%) | 68 (19.4%) | 0.93 | |
| MADS | 225 (21.4%) | 76 (21.7%) | 81 (23.1%) | 68 (19.4%) | 0.48 | |
| rheumatoid arthritis | 201 (19.1%) | 75 (21.4%) | 71 (21.4%) | 55 (15.7%) | 0.13 | |
| osteoarthritis | 570 (54.3%) | 207 (59.1%) | 203 (58.0%) | 160 (45.7%) | <0.001 | Grade-0 >> Grade-2 Grade-1 >> Grade-2 |
| Contracture | 112 (10.7%) | 74 (21.1%) | 22 (6.3%) | 16 (4.4%) | <0.001 | Grade-0 >>> Grade-1 Grade-0 >>> Grade-2 |
| Disuse | 57 (6.4%) | 30 (8.6%) | 18 (5.1%) | 19 (5.4%) | 0.12 | |
| Parkinsonism | 41 (3.9%) | 3 (0.9%) | 32 (9.1%) | 6 (1.7%) | <0.001 | Grade-0 <<< Grade-1 Grade-1 >>> Grade-2 |
| T-score in the LS | −2.3 (1.6) | −2.1 (1.7) | −2.2 (1.5) | −2.4 (1.6) | 0.05 | |
| T-score in the FN | −2.0 (1.1) | −2.0 (1.1) | −2.0 (0.9) | −2.0 (1.2) | 0.64 | |
| GCs | 119 (11.3%) | 35 (10.0%) | 45 (12.9%) | 39 (11.1%) | 0.49 | |
| V-D | 701 (66.8%) | 228 (65.1%) | 243 (69.4%) | 230 (65.7%) | 0.43 | |
| OPD † | 264 (25.1%) | 96 (27.4%) | 88 (25.1%) | 80 (22.8%) | 0.48 | |
| polypharmacy | 229 (12.4%) | 98 (28.0%) | 77 (22.0%) | 54 (15.4%) | <0.001 | Grade-0 >>> Grade-2 |
| eGFR | 52.6 (20.5) | 50.6 (20.5) | 51.7 (21.5) | 55.6 (19.2) | 0.05 | |
| ALB | 4.0 (0.4) | 4.0 (0.4) | 4.0 (0.3) | 4.0 (0.4) | 0.10 | |
| P1NP | 54.3 (44.4) | 57.5 (55.5) | 51.8 (42.2) | 53.4 (30.8) | 0.26 | |
| TRACP-5b | 493.7 (215.1) | 489.7 (234.6) | 488.6 (189.7) | 503.4 (219.2) | 0.62 | |
| Hgb | 12.0 (1.6) | 11.9 (1.5) | 12.1 (1.7) | 11.9 (1.5) | 0.21 | |
| Lymph | 1499 (607) | 1440 (586) | 1498 (642) | 1558 (585) | 0.05 | |
| Mono | 358 (153) | 340 (133) | 364 (123) | 369 (194) | 0.05 |
| Univariate Mode | Multivariate Mode | ||||
|---|---|---|---|---|---|
| p-Value | Risk Ratio (95% CI) | β-Value | p-Value | Risk Ratio (95% CI) | |
| female | 0.98 | 9.9 × 107 (0.00–INF) | |||
| age, years | <0.001 | 1.08 (1.04–1.12) | −0.08 | <0.001 | 0.92 (0.88–0.96) |
| current smoke | 0.87 | 0.91 (0.29–2.91) | |||
| alcohol habitat | 0.98 | 0.00 (0.00–INF) | |||
| parent’s fracture | 0.88 | 0.00 (0.00–INF) | |||
| SQ Grade | 0.69 | 1.06 (0.80–1.42) | |||
| prevalent VF | 0.86 | 9.9 × 107 (0.00–INF) | |||
| prevalent NVF | <0.05 | 2.01 (1.13–3.78) | 0.51 | 0.19 | 1.67 (0.78–3.57) |
| VEC | 0.98 | 9.9 × 107 (0.00–INF) | |||
| AAC | 0.26 | 0.73 (0.43–1.25) | |||
| Diabetes mellitus | 0.12 | 0.48 (0.18–1.23) | |||
| COPD | 0.98 | 0.00 (0.00–INF) | |||
| hypertension | 0.45 | 1.30 (0.66–2.57) | |||
| hyperlipidemia | 0.69 | 0.86 (0.42–1.77) | |||
| chronic heart failure | 0.40 | 1.41 (0.63–3.12) | |||
| insomnia | 0.18 | 0.56 (0.24–1.30) | |||
| Cognitive Impairment | <0.001 | 4.58 (2.86–7.36) | 1.83 | <0.001 | 6.32 (3.48–11.16) |
| MADS | 0.46 | 1.35 (0.61–2.97) | |||
| rheumatoid arthritis | <0.01 | 0.22 (0.08–0.61) | −1.91 | <0.001 | 0.15 (0.05–0.45) |
| osteoarthritis | 0.47 | 1.31 (0.64–2.70) | |||
| Contracture | 0.95 | 1.03 (0.43–2.50) | |||
| Disuse | 0.83 | 1.14 (0.35 –3.75) | |||
| Parkinsonism | 0.98 | 0.00 (0.00–INF) | |||
| T-score in the LS | 0.08 | 0.87 (0.74–1.02) | |||
| T-score in the FN | <0.001 | 0.59 (0.46–0.75) | −0.41 | <0.05 | 0.67 (0.49–0.92) |
| GCs | 0.06 | 0.33 (0.11–1.06) | |||
| V-D | <0.05 | 1.90 (1.06–3.41) | 1.01 | <0.01 | 2.73 (1.34–5.59) |
| OPD † | 0.92 | 1.04 (0.50–2.18) | |||
| polypharmacy | <0.001 | 0.09 (0.02– 0.36) | −2.90 | <0.001 | 0.06 (0.01–0.23) |
| eGFR | 0.24 | 0.99 (0.97–1.01) | |||
| ALB | 0.95 | 0.97 (0.33–2.84) | |||
| Calcium | 0.14 | 1.23 (0.93–1.63) | |||
| P1NP | 0.59 | 1.00 (0.99–1.01) | |||
| TRACP-5b | 0.69 | 1.00 (1.00–1.00) | |||
| Hgb | 0.63 | 0.94(0.75–1.19) | |||
| Lymph | 0.17 | 1.00 (1.00–1.00) | |||
| Mono | <0.001 | 1.00 (1.00–1.01) | 0.01 | <0.001 | 1.01 (1.00–1.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshii, I.; Sawada, N.; Chijiwa, T. Verification of the Semiquantitative Assessment of Vertebral Deformity for Subsequent Vertebral Body Fracture Prediction and Screening for the Initiation of Osteoporosis Treatment: A Case-Control Study Using a Clinical-Based Setting. Osteology 2025, 5, 19. https://doi.org/10.3390/osteology5030019
Yoshii I, Sawada N, Chijiwa T. Verification of the Semiquantitative Assessment of Vertebral Deformity for Subsequent Vertebral Body Fracture Prediction and Screening for the Initiation of Osteoporosis Treatment: A Case-Control Study Using a Clinical-Based Setting. Osteology. 2025; 5(3):19. https://doi.org/10.3390/osteology5030019
Chicago/Turabian StyleYoshii, Ichiro, Naoya Sawada, and Tatsumi Chijiwa. 2025. "Verification of the Semiquantitative Assessment of Vertebral Deformity for Subsequent Vertebral Body Fracture Prediction and Screening for the Initiation of Osteoporosis Treatment: A Case-Control Study Using a Clinical-Based Setting" Osteology 5, no. 3: 19. https://doi.org/10.3390/osteology5030019
APA StyleYoshii, I., Sawada, N., & Chijiwa, T. (2025). Verification of the Semiquantitative Assessment of Vertebral Deformity for Subsequent Vertebral Body Fracture Prediction and Screening for the Initiation of Osteoporosis Treatment: A Case-Control Study Using a Clinical-Based Setting. Osteology, 5(3), 19. https://doi.org/10.3390/osteology5030019





