Abstract
Background/Objectives: Complete rupture of the Achilles tendon is a common and challenging injury, specifically for individuals engaged in high-demand activities such as sports. Surgical repair is often required, but conventional methods, including direct suture repair, may fail to address the biological limitations associated with tendon healing, especially in cases involving chronic degeneration or extensive tissue damage. Methods: This case report explains how bioinductive implants, such as the Regeneten collagen-based scaffold, have gained attention as an innovative approach to augment tendon repair. Results: These implants not only provide mechanical stabilization but also promote the regeneration of tendon-like tissue by enhancing the biological healing environment. Conclusions: The use of bioinductive implants, such as the Regeneten scaffold, improves outcomes in tendon repair by augmenting both mechanical stabilization and biological healing. This approach represents a valuable alternative to improve clinical outcomes, particularly in patients with poor prognostic factors.
1. Introduction
The Achilles tendon—the strongest and largest tendon in the human body—plays a crucial role in locomotion by transmitting forces generated by the calf muscles to the calcaneus. Despite its robust structure, the Achilles tendon is susceptible to a variety of injuries, which can result in significant functional impairment and long-term morbidity.
Achilles tendon injuries predominantly occur due to excessive mechanical stress exceeding the tensile capacity of the tendon. These injuries are commonly seen in athletes engaged in activities involving repetitive jumping, sprinting, or abrupt directional changes. Non-athletic populations, particularly middle-aged individuals, may also be at risk due to tendon degenerative changes associated with age-related collagen disorganization and reduced vascularity. Both intrinsic factors, such as overpronation, limited ankle dorsiflexion, and muscle imbalances, and extrinsic factors, including inappropriate footwear and overtraining, contribute to the risk of injury. Systemic conditions such as diabetes, obesity, and chronic corticosteroid use also play a role in predisposing individuals to Achilles tendon injuries.
Patients with acute Achilles tendon rupture often report a sudden, sharp pain in the posterior ankle accompanied by a loud “pop” and an immediate loss of plantarflexion strength. Chronic or degenerative injuries may present with an insidious onset of pain, rigidity, and swelling, which are exacerbated by activity. During physical examination, hallmark findings are tenderness along the tendon, joint stiffness, and a palpable gap due to complete rupture.
The treatment of Achilles tendon injuries ranges from conservative approaches to surgical interventions. The choice depends on the type and severity of the injury, patient activity level, and overall health status. Conservative management is often reserved for tendinopathy and partial tears and includes rest, physical therapy, eccentric strengthening exercises, and non-invasive treatments such as shockwave therapy. Orthotics and bracing can offload the tendon and facilitate healing. Surgical repair is indicated for acute complete ruptures, particularly in young, active individuals, and aims to restore tendon continuity and optimize biomechanical properties. Techniques include open repair, mini-open approaches, and percutaneous suturing.
Alternative therapies, such as Platelet-Rich Plasma (PRP), promote healing through the release of growth factors, while stem cell therapy is under investigation for its regenerative potential. Recent progress includes the use of collagen-based bioinductive implants, such as Regeneten, designed to augment tendon repair by stimulating natural healing and promoting tissue regeneration. Regeneten has shown promise in accelerating recovery and reducing the risk of re-rupture in cases of chronic tendinopathy or partial tears, where native healing is compromised.
The efficacy of Achilles tendon repair is influenced by multiple factors, including the therapeutic strategy employed. The success of postoperative recovery and symptom alleviation is partially attributed to the physiological determinants of tendon degeneration, such as vascular supply and tissue integrity. As a result, there has been growing interest in biological repair to improve clinical outcomes [1]. Bioinductive implants, constructed from collagen-based scaffolds, represent an innovative approach to fostering tendon regeneration. By harnessing the intrinsic healing mechanisms of the host tissue, these implants have gained prominence in the management of chronic and degenerative tendon injuries, offering supplemental advantages to conventional repair techniques. They promote cellular infiltration and extracellular matrix remodeling, thereby establishing an optimal milieu for tissue repair [2].
The following case report examines the use of a bioinductive implant in the repair of a full-thickness Achilles tendon rupture. The outcomes highlight the transformative potential of this method, proposing a biologically enriched strategy that augments traditional mechanical repair practices and may redefine the therapeutic approach to such injuries.
2. Case Presentation
A 22-year-old man presented with acute onset of pain and functional limitation in the right ankle that occurred during a basketball game. The patient reported a sudden “pop” sensation, followed by an inability to bear weight or plantarflex the ankle. The patient’s medical history was unremarkable, with no prior musculoskeletal injuries.
The patient sought outpatient evaluation nine days post-injury, reporting an inability to bear weight on the right lower limb and walking with crutches. On physical examination, the Thompson test was positive, indicating an Achilles tendon rupture. Visible swelling and ecchymosis were noted over the posterior ankle, and palpation revealed a palpable gap in the tendon, about 4 cm proximal to its calcaneal insertion.
Ultrasound and MRI confirmed a complete rupture of the Achilles tendon at the proximal third, with a defect measuring around 35 mm and partial retraction of the proximal limb. The patient underwent a brief period of immobilization without improvement.
After discussing available treatment options with the patient and considering his active lifestyle as well as the potential benefits of enhanced healing using the Regeneten implant, surgical repair augmented with this bioinductive scaffold was selected as the preferred treatment approach.
While awaiting surgical treatment, the patient continued ambulating with non-weight-bearing on the right lower limb, wearing an immobilizer boot in maximum equinus, and adhered to antithromboembolic prophylaxis.
3. Surgical Technique
Surgery was performed 24 days after the injury. The procedure was performed under regional anesthesia with the patient in a prone position. A longitudinal incision was made over the posteromedial aspect of the Achilles tendon (Figure 1), followed by dissection through the superficial and deep fascia, to expose the rupture site (Figure 2).
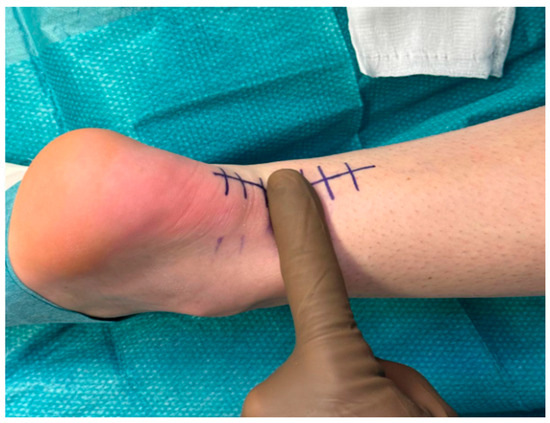
Figure 1.
Preoperative condition of the Achilles tendon showing a clear deficit between the proximal and distal tendon stumps.
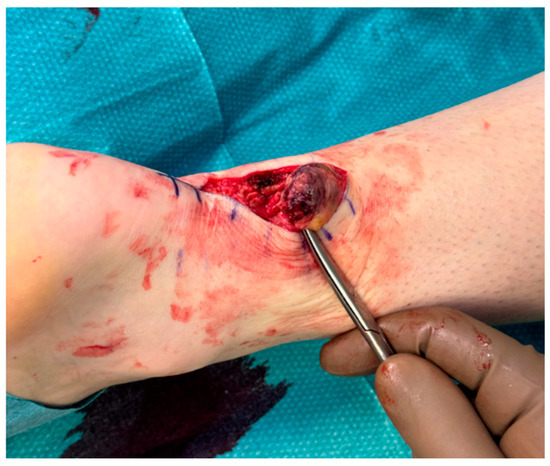
Figure 2.
Exposure of the proximal stump, which appears completely detached from its distal portion.
The retracted tendon ends were debrided to remove necrotic tissue and optimize the repair surface. The tendon was repaired using an open, end-to-end Krackow suture technique, utilizing high-tensile absorbable sutures. The tendon was reduced in a dependent equinus position, and the sutures were tied with excellent approximation (Figure 3).
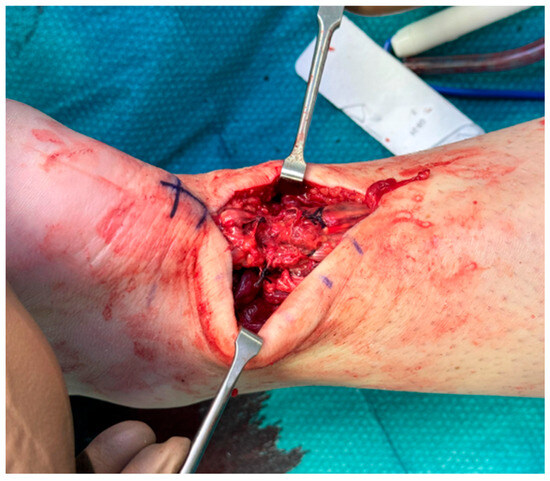
Figure 3.
Reduction in the tendon gap with end-to-end suture in a dependent equinus position.
Given the location of the myotendinous lesion and the patient’s status as a young individual with high functional demands, the repair was performed with the bioinductive collagen patch Regeneten (Smith+Nephew, Andover, MA, USA), in order to enhance biological healing. The collagen scaffold was secured to the tendon with absorbable sutures placed along the tendon edges, ensuring firm contact between the implant and tendon tissue. Additional sutures were used to better cover the repair construct within the scaffold, promoting cellular infiltration and matrix deposition (Figure 4).
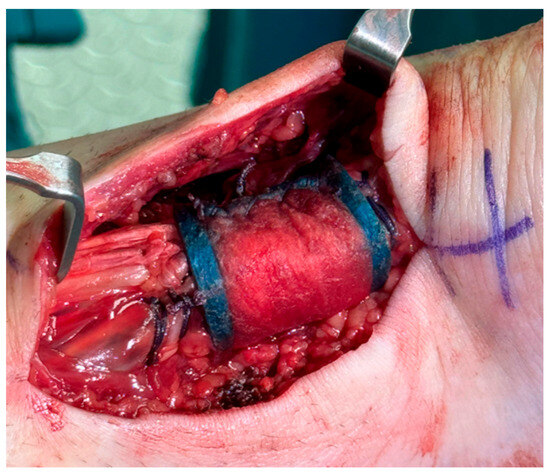
Figure 4.
A bioinductive collagen patch (Regeneten) is placed over the Achilles tendon repaired with end-to-end suture.
The wound was irrigated and closed in layers, first with a subcutaneous suture with absorbable sutures, followed by a superficial cutaneous suture using Nylon threads. The ankle was immobilized in a well-padded short-leg splint in maximum equinus to minimize tension on the repair.
4. Postoperative Rehabilitation
The patient was closely monitored with follow-up visits at one, two, and three weeks, and then subsequently evaluated once a month.
The patient followed a structured and specific rehabilitation protocol for augmented Achilles tendon repair. The cast was maintained for three weeks in a position of equinus, initially non-weight-bearing for the first ten days, followed by weight-bearing as tolerated. During this period, the patient ambulated with the assistance of two crutches, kept the limb elevated during rest periods, and performed isometric exercises to strengthen the quadriceps muscle in order to maintain good muscle trophism.
Approximately 20 days after the surgical procedure, the cast was removed, and the patient began wearing an immobilizer boot with wedges, starting from a position of maximum equinus and removing one wedge every two weeks. The immobilizer boot was removed four months after the surgical procedure. The patient then began the physiotherapy rehabilitation protocol.
5. Follow-Up
At the three-month follow-up, the patient reported significant improvement in pain and functional mobility. Physical examination revealed a well-healed incision and no palpable defect in the Achilles tendon.
An MRI was performed between 3 and 4 months postoperatively to assess the repair and confirm radiological healing. The images showed signs of tendon healing, with a significant increase in tendon thickness compared to the preoperative condition (Figure 5).
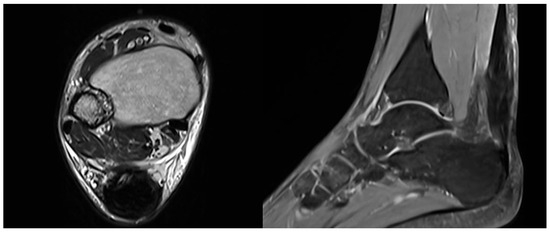
Figure 5.
Postoperative MRI at 3/4 months after surgery showing progressive formation of new tendon-like tissue with improvement of tendon thickness.
Passive dorsiflexion to neutral was achieved, and physical therapy and full weight-bearing activities were initiated. At 4 months, the patient regained full ankle range of motion, including 20° of dorsiflexion, and could perform a two-leg heel raise. At about 5 months, he maintained full range of motion and progressed to performing a single-leg heel raise.
By six months postoperatively, the patient had resumed light recreational activities, including jogging, without pain or instability. MRI at this stage showed full integration of the Regeneten implant and thickened tendon tissue consistent with improved structural integrity.
6. Discussion
The use of bioinductive implants in the management of Achilles tendon injuries represents a paradigm shift from conventional repair techniques to a biologically enhanced approach. This case report explores the potential of the Regeneten implant not only to meet the mechanical demands of tendon repair but also to address the biological deficiencies that often hinder successful healing. Achilles tendon ruptures, particularly those that are chronic or associated with degenerative changes, pose significant challenges due to the tendon’s limited regenerative capacity. Effectively managing these injuries requires innovative solutions that integrate mechanical stabilization with the promotion of intrinsic tissue healing [3].
As the largest and strongest tendon in the human body, the Achilles tendon withstands substantial forces during activities such as walking, running, and jumping. Despite its resilience, it remains vulnerable to injury, particularly in individuals with risk factors such as aging, chronic overuse, or metabolic disorders like diabetes. Tendon healing is inherently complex, relying on a finely regulated sequence of inflammation, cellular proliferation, and extracellular matrix remodeling. However, in cases of chronic injury or degeneration, this process is often impaired by factors such as reduced vascularity, fibrosis, and diminished native cellular activity. These limitations highlight the need for adjunctive strategies that extend beyond mechanical repair to actively support the biological environment [4].
Conventional surgical techniques for Achilles tendon repair involving direct suture repair primarily focus on restoring the tendon’s biomechanical integrity. While these approaches provide immediate mechanical stability, they often fail to address the underlying biological deficiencies. Chronic tears are associated with significant tendon degeneration, including collagen disorganization, fatty infiltration, and reduced cellularity, all of which impair the tendon’s healing capacity. Consequently, these pathological changes contribute to high rates of re-rupture or incomplete functional recovery [5].
Furthermore, the mechanical properties of repaired tendons often remain inferior to those of healthy tendons, even after extended healing periods. Research has shown that traditional repair techniques frequently lead to scar tissue formation, which lacks the biomechanical strength and elasticity of native tendon tissue. This limitation is particularly problematic in high-demand individuals, where the tendon must withstand repetitive mechanical loading. These challenges have driven the development of biologically augmented repair strategies aimed at improving both the quality and quantity of regenerated tissue [6].
Current surgical options for Achilles tendon reconstruction include direct repair, autologous grafts, alloplastic materials, and tendon transfer. Direct repair is the preferred approach for acute injuries when the remaining tendon tissue is of good quality. However, in chronic cases—often characterized by tendon retraction and significant degeneration—direct repair is typically not feasible due to extensive tissue loss.
The use of autologous grafts, such as the plantaris tendon or semitendinosus tendon bundles, provides a viable alternative for addressing moderate tendon deficits. While these approaches offer excellent biological integration, they are associated with potential drawbacks, including donor site morbidity and increased tendon stiffness. Synthetic materials, such as polyester fibers or decellularized dermal grafts, present another option for complex reconstructions, but they come with limitations such as a higher risk of infection and inconsistent biological integration [7].
Among advanced surgical techniques, flexor hallucis longus (FHL) transfer is particularly effective for chronic Achilles tendon injuries involving tissue loss greater than 5 cm. This procedure utilizes the biomechanical properties of the FHL, whose contractile strength closely approximates that of the Achilles tendon. The transfer can be performed through either an open or endoscopic approach, with the latter being preferred due to its reduced soft tissue disruption, lower postoperative pain, and faster recovery times [8].
Several studies have evaluated the efficacy of these surgical techniques. While direct repairs are technically straightforward, they are associated with a high recurrence rate in chronic injuries. Autologous grafts have demonstrated clinical success rates of 85–90%, although they carry a 15% risk of tendon stiffness. Synthetic grafts, despite eliminating donor site morbidity, have shown complication rates as high as 25%, including infections and foreign body reactions [7]. In contrast, FHL transfer—particularly via an endoscopic approach—has produced excellent outcomes. Sammarco and Taylor (2018) reported a clinical success rate of 95%, with a significantly reduced time to return to sport activity [9]. Additionally, FHL transfer provides superior tendon strength and functionality, making it one of the most effective options for managing chronic injuries with extensive tendon loss.
Recently, bioinductive implants such as the Regeneten collagen-based scaffold have emerged as a promising solution to the challenges associated with tendon repair. These implants harness the body’s intrinsic healing mechanisms by creating a microenvironment conducive to cellular infiltration, vascular ingrowth, and extracellular matrix deposition. Unlike traditional augmentation methods that rely on external grafts or synthetic materials, bioinductive implants are designed to integrate seamlessly with host tissue, promoting the regeneration of tendon-like tissue that closely mimics the native structure [10].
In the present case, the Regeneten implant was used to augment the repair of a full-thickness Achilles tendon tear. By serving as a scaffold for cellular infiltration and matrix remodeling, the implant facilitated the formation of new, biologically active tendon tissue, thereby enhancing the overall healing process. Histological studies have demonstrated that these implants are fully resorbed within six months, leaving behind regenerated tissue that, on imaging studies, is indistinguishable from native tendon. This seamless integration represents a key advantage over traditional grafts, which may provoke foreign body reactions or fail to achieve adequate biological incorporation [11].
In this case, the bioinductive implant addressed several critical challenges. First, it compensated for the poor quality of the residual tendon tissue by providing a structural framework that enhanced the mechanical stability of the repair. Second, it mitigated biological deficiencies by stimulating cellular activity and promoting vascular ingrowth. Postoperative imaging revealed progressive formation of tendon-like tissue, confirming the implant’s efficacy in facilitating biologically mediated healing. At the six-month follow-up, the patient demonstrated significant improvements in pain, strength, and functional performance, consistent with expected outcomes for augmented tendon repair [12].
The findings of this case align with the existing literature on the use of bioinductive implants in tendon repair. Studies have shown that these implants not only enhance the structural integrity of the repaired tendon but also improve clinical outcomes, including pain reduction, increased range of motion, and enhanced functional recovery. For instance, Longo et al. reported significant improvements in ROM and in Constant-Murley and ASES scores in patients treated with the Regeneten implant for rotator cuff injuries, findings that are particularly relevant to Achilles tendon repair. Furthermore, the implant’s ability to promote neotendon formation without altering the native tendon footprint is especially advantageous in preserving the tendon’s natural biomechanics [13].
7. Conclusions
Achilles tendon ruptures pose significant challenges due to the high biomechanical demands placed on the tendon and the variability in tissue quality following injury, both of which can severely impact mobility and quality of life [14]. Chronic or degenerative ruptures are particularly difficult to manage, as poor vascularization and impaired cellular activity hinder the healing process [15]. Advances in diagnostic techniques and treatment modalities have improved the management of these injuries [16]. While traditional repair methods remain the mainstay of treatment, emerging therapies such as bioinductive implants offer promising alternatives for enhancing outcomes in complex cases [17].
The Regeneten bioinductive implant addresses these challenges by providing a scaffold that promotes cellular infiltration, vascular ingrowth, and extracellular matrix production [18]. This approach enhances mechanical repair by creating a biologically favorable environment for tendon regeneration [19].
In the present case, the application of the Regeneten implant led to the formation of tendon-like tissue, as confirmed by histological analysis and imaging studies [20]. These findings align with the existing literature demonstrating the efficacy of bioinductive implants in enhancing tendon thickness, reducing re-rupture rates, and supporting long-term functional recovery [14].
This case highlights the potential of bioinductive implants to transform the treatment of Achilles tendon ruptures, particularly in patients with compromised tissue quality. By integrating mechanical repair with biological augmentation, this approach overcomes the limitations of traditional techniques and represents a promising strategy for improving tendon repair outcomes [21].
The use of bioinductive implants in Achilles tendon repair marks a paradigm shift in the management of these complex injuries. By combining biological augmentation with conventional surgical repair, these implants address the multifaceted nature of tendon healing, leading to improved structural and functional outcomes [16]. The findings of this case further support the role of the Regeneten implant in optimizing the repair environment, offering a biologically active solution that complements existing surgical techniques [15]. As the field of tendon repair continues to advance, bioinductive implants are poised to play an increasingly central role in elevating the standard of care for patients with challenging tendon pathologies [14,20].
The primary limitation of this study lies in its case report nature, as it describes a single clinical case. Consequently, the observed outcomes cannot be easily generalized to a broader patient population. Individual factors such as age, activity level, and comorbidities can significantly influence surgical results, making it difficult to draw universally applicable conclusions. Further research, including larger clinical studies and long-term follow-up, is necessary to validate these findings and refine the role of bioinductive implants in tendon repair.
8. Future Perspectives
The success of this case highlights the broader potential of bioinductive implants to transform the treatment of tendon injuries, particularly in chronic and degenerative cases. By addressing both mechanical and biological aspects of tendon healing, these implants provide a comprehensive solution aligned with the principles of tissue engineering and regenerative medicine. However, several critical questions remain unanswered, necessitating further research. For instance, the long-term durability of regenerated tissue and its ability to withstand high-load activities require further investigation. Additionally, the cost-effectiveness of bioinductive implants compared to traditional repair techniques must be assessed to ensure their feasibility for widespread clinical adoption.
Future studies should also explore the potential of combining bioinductive implants with other regenerative therapies, such as platelet-rich plasma (PRP) or stem cell injections, to further enhance healing outcomes. Advances in biomaterials and scaffold design may lead to the development of next-generation implants with enhanced biocompatibility, mechanical properties, and bioactivity. Moreover, identifying patient-specific factors that influence the success of bioinductive repair—such as age, activity level, and the extent of tendon degeneration—will be essential for optimizing patient selection and tailoring treatment strategies.
Further research is required to refine patient selection criteria, optimize surgical techniques, and evaluate long-term outcomes associated with bioinductive implants in Achilles tendon repair. As biological augmentation becomes increasingly integrated into tendon surgery, its role in improving patient outcomes and reducing recurrence rates is expected to expand, marking a paradigm shift in the management of tendon injuries.
Author Contributions
Conceptualization, U.G.L.; methodology, A.C. (Alessandra Corradini) and A.C. (Alice Ceccaroli); software, A.C. (Alessandra Corradini) and A.C. (Alice Ceccaroli); validation, U.G.L., P.D. and A.d.S.; formal analysis, A.S. and G.M.; investigation, A.C. (Alessandra Corradini) and A.C. (Alice Ceccaroli); resources, A.S. and G.M.; data curation, A.S., G.M., A.C. (Alessandra Corradini) and A.C. (Alice Ceccaroli); writing—original draft preparation, A.S. and G.M.; writing—review and editing, A.S. and G.M.; visualization, A.S. and G.M.; supervision, U.G.L., P.D. and A.d.S.; project administration, U.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not application.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McIntyre, L.F.; McMillan, S.; Trenhaile, S.W.; Bishai, S.K.; Bushnell, B.D. Full-Thickness Rotator Cuff Tears Can Be Safely Treated with a Resorbable Bioinductive Bovine Collagen Implant: One-Year Results of a Prospective, Multicenter Registry. Arthrosc. Sports Med. Rehabil. 2021, 3, e1473–e1479. [Google Scholar] [CrossRef] [PubMed]
- Prabhath, A.; Vernekar, V.N.; Sanchez, E.; Laurencin, C.T. Growth factor delivery strategies for rotator cuff repair and regeneration. Int. J. Pharm. 2018, 544, 358–371. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Magnusson, S.P.; Kjaer, M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEB J. 2013, 27, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kumagai, K. Molecular dissection of tendon development and healing: Insights into tenogenic phenotypes and functions. J. Biol. Chem. 2025, 301, 108353. [Google Scholar] [CrossRef] [PubMed]
- Gil-Melgosa, L.; Grasa, J.; Urbiola, A.; Llombart, R.; Susaeta Ruiz, M.; Montiel, V.; Ederra, C. Muscular and Tendon Degeneration after Achilles Rupture: New Insights into Future Repair Strategies. Biomedicines 2021, 10, 19. [Google Scholar] [CrossRef]
- Graham, J.G.; Wang, M.L.; Rivlin, M.; Beredjiklian, P.K. Biologic and mechanical aspects of tendon fibrosis after injury and repair. Connect. Tissue Res. 2018, 60, 10–20. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Maffulli, G.D.; Rabitti, C.; Khanna, A.; Denaro, V. Marked pathological changes proximal and distal to the site of rupture in acute Achilles tendon ruptures. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 680–687. [Google Scholar] [CrossRef]
- Lui, T.H. Endoscopic flexor hallucis longus tendon transfer for chronic Achilles tendon rupture. Arthrosc. Technol. 2015, 4, e719–e723. [Google Scholar] [CrossRef][Green Version]
- Hockenbury, R.T.; Sammarco, G.J. Medial sliding calcaneal osteotomy with flexor hallucis longus transfer for the treatment of posterior tibial tendon insufficiency. Foot Ankle Clin. 2001, 6, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, A.; Marcello, G.; Mancuso, M.; Ceccaroli, A.; Corradini, A.; Mancini, L.; Schena, E. Bridging the Gap in Partial Repair of Full-Thickness Rotator Cuff Tears: A Case Report on the Rationale Behind Bioinductive Collagen Implants. Osteology 2025, 5, 12–20. [Google Scholar] [CrossRef]
- Camacho-Chacon, J.A.; Cuenca-Espierrez, J.; Roda-Rojo, V.; Martin-Martinez, A.; Calderon-Meza, J.M.; Alvarez-Alegret, R.; Martin-Hernandez, C. Bioinductive collagen implants facilitate tendon regeneration in rotator cuff tears. J. Exp. Orthop. 2022, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Benthami, K.M.; Verhaegen, F.; Debeer, P. The Clinical Efficacy of the Regeneten Bioinductive Implant in Rotator Cuff Repair: A Systematic Review. Acta. Orthop. Belgica. 2024, 90, 777–788. [Google Scholar] [CrossRef]
- Longo, U.G.; Marino, M.; Sire Ade Ruiz-Iban, M.A.; D’Hooghe, P. The bioinductive collagen implant yields positive histological, clinical and MRI outcomes in the management of rotator cuff tears: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2024, 33, 1070–1090. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Spiezia, F.; Testa, V.; Capasso, G.; Longo, U.G.; Denaro, V. Free gracilis tendon graft for reconstruction of chronic tears of the Achilles tendon. J. Bone Joint Surg. Am. 2012, 94, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Khan, K.M.; Purdam, C. Achilles tendinopathy. Man Ther. 2002, 7, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Iosifidis, M.I. Bioinductive implants in tendon regeneration. Sports Med. Arthrosc. Rev. 2015, 23, 91–97. [Google Scholar]
- Goswami, T.; Gokhale, S. Emerging therapies for tendon repair: Bioinductive implants. Curr. Treat. Options Orthop. 2020, 19, 243–256. [Google Scholar]
- Hutchison, A.M.; Topliss, C.; Beard, D.; Evans, R.M.; Williams, P. The treatment of a rupture of the Achilles tendon using a dedicated management programme. Bone Joint J. 2015, 97, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Pankovich, A.M. Neglected rupture of the Achilles tendon. Treatment by V-Y tendinous flap. J. Bone Joint Surg. Am. 1975, 57, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Yinger, K.; Mandelbaum, B.R.; Almekinders, L.C. Achilles rupture in the athlete. Current science and treatment. Clin. Podiatr. Med. Surg. 2002, 19, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Loppini, M.; Maffulli, N. Conservative management of tendinopathy: An evidence-based approach. Muscles Ligaments Tendons J. 2012, 1, 134–137. [Google Scholar] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).