Improvement of the Obliteration of Non-Critical Size Defects by Using a Mixture of Bone Dust and Bone Replacement Material (Bioactive Glass S53P4)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Animals
2.1.2. Harvesting Bone Dust
2.1.3. Bioactive Glass S53P4
2.2. Methods
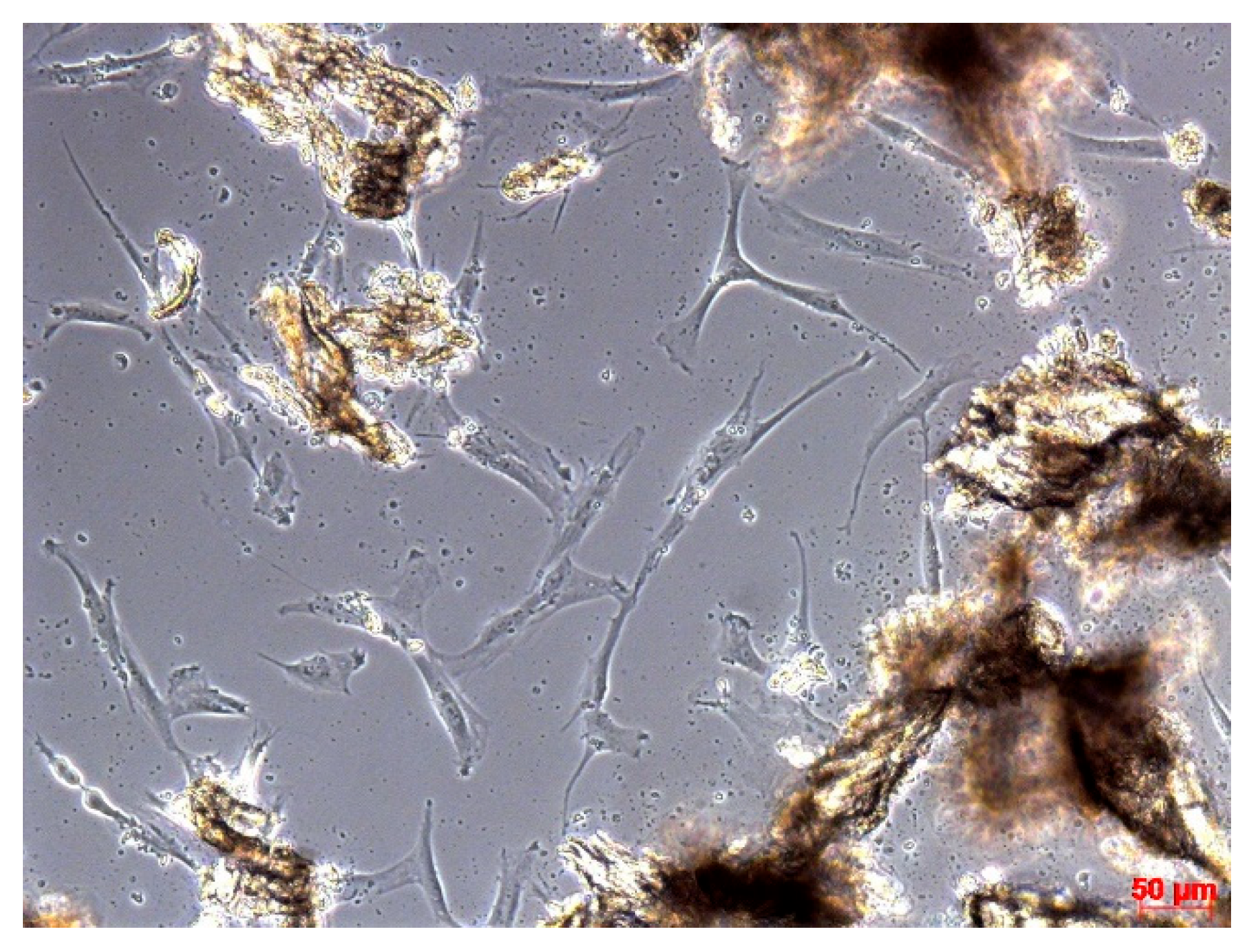
2.2.1. Cell Culture Studies of Human BD
2.2.2. Surgery
2.2.3. Fluorochrome Sequential Labelling
2.2.4. Digital Volume Tomography
2.2.5. Measurement of Bone Mineral Density
2.2.6. Histological Analysis
2.2.7. Statistical Analysis
3. Results
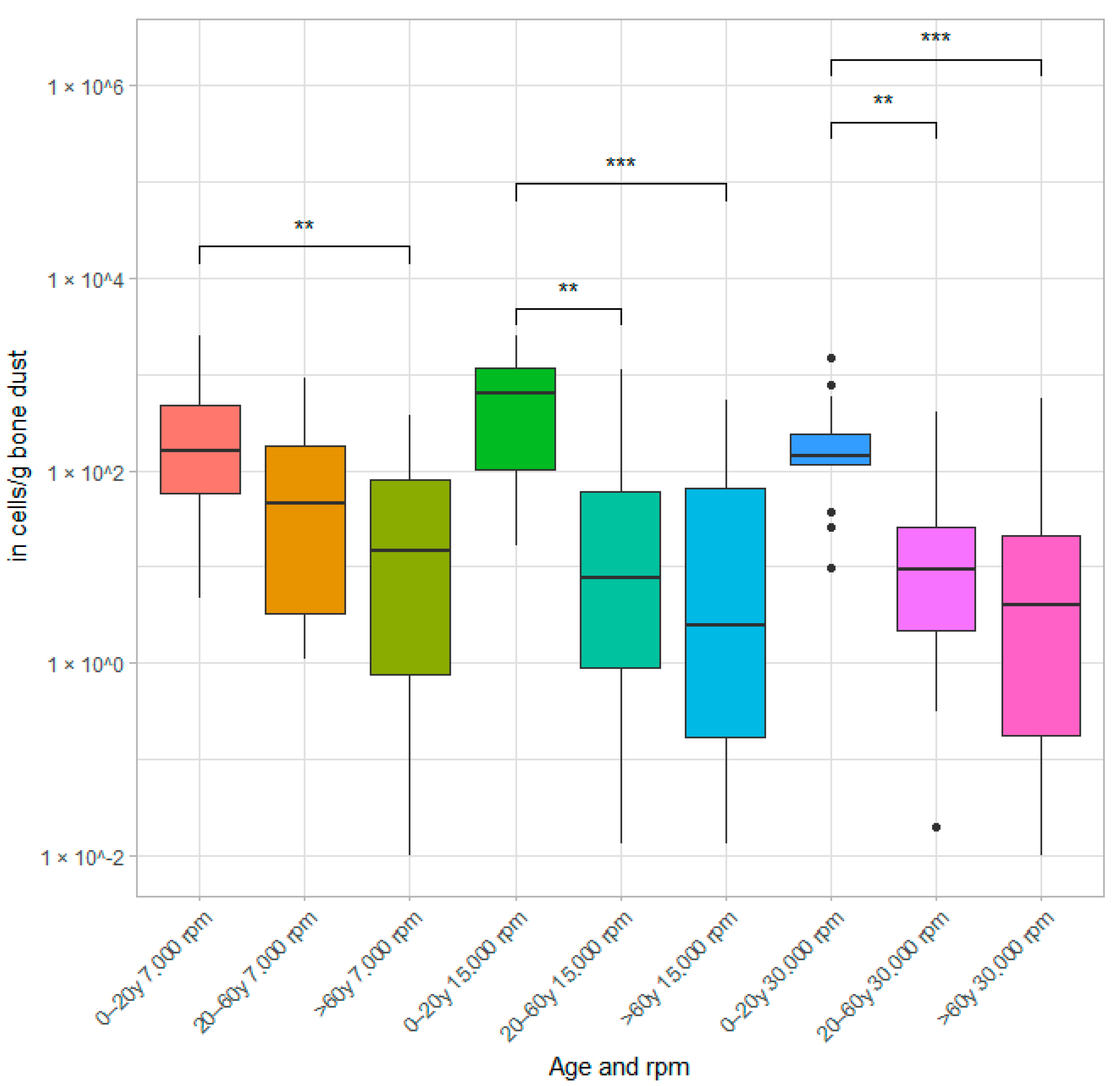
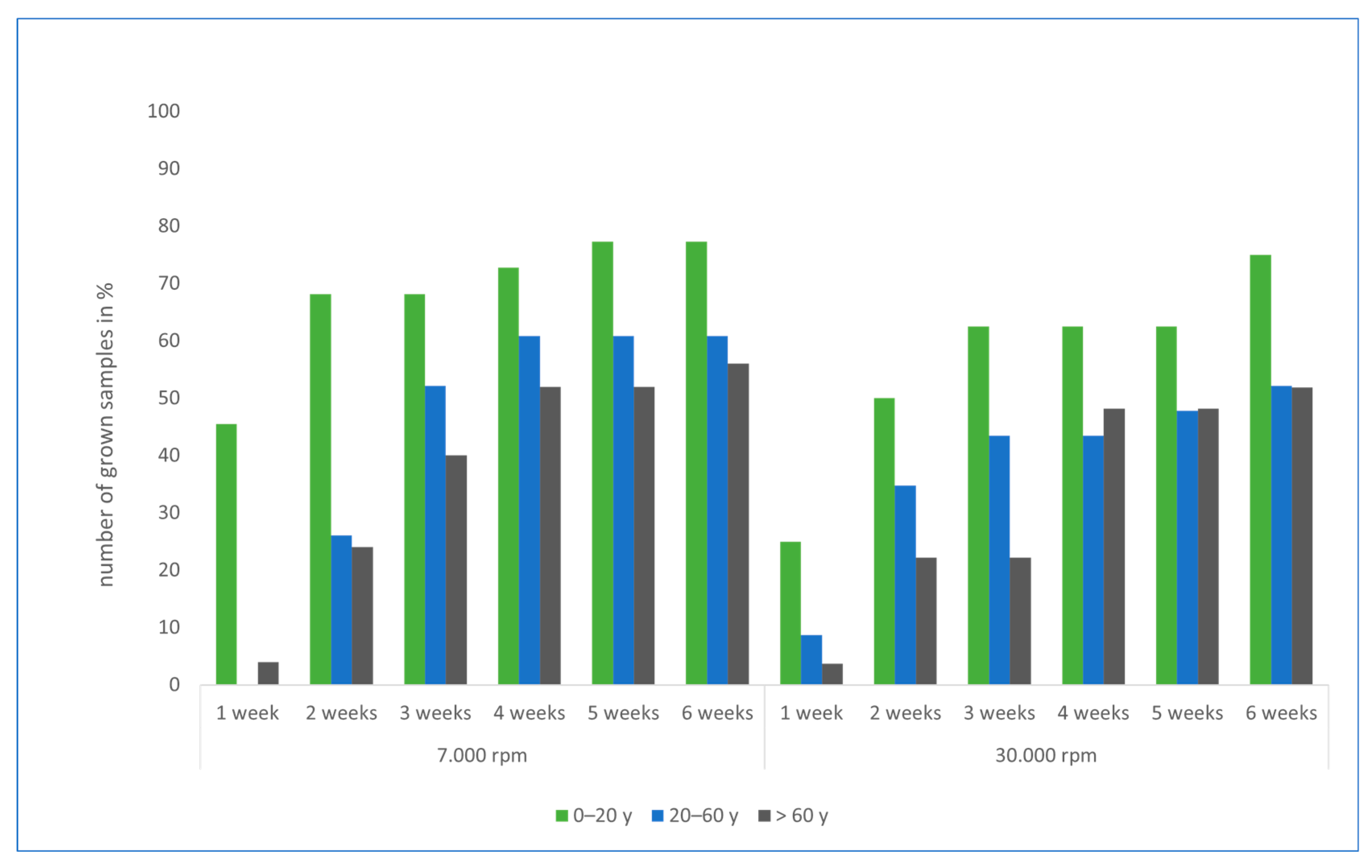
3.1. Results of Cell Culture Studies
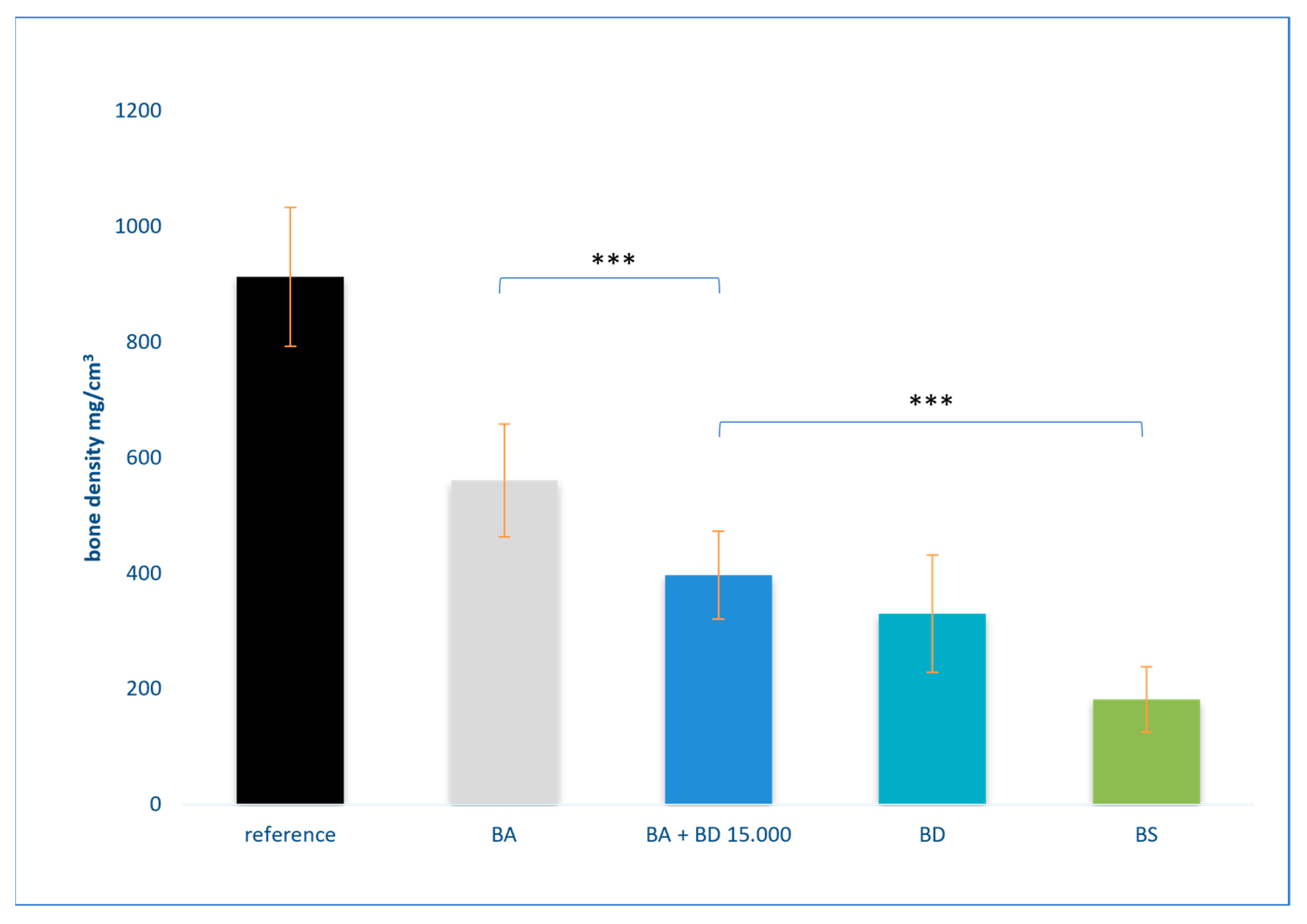
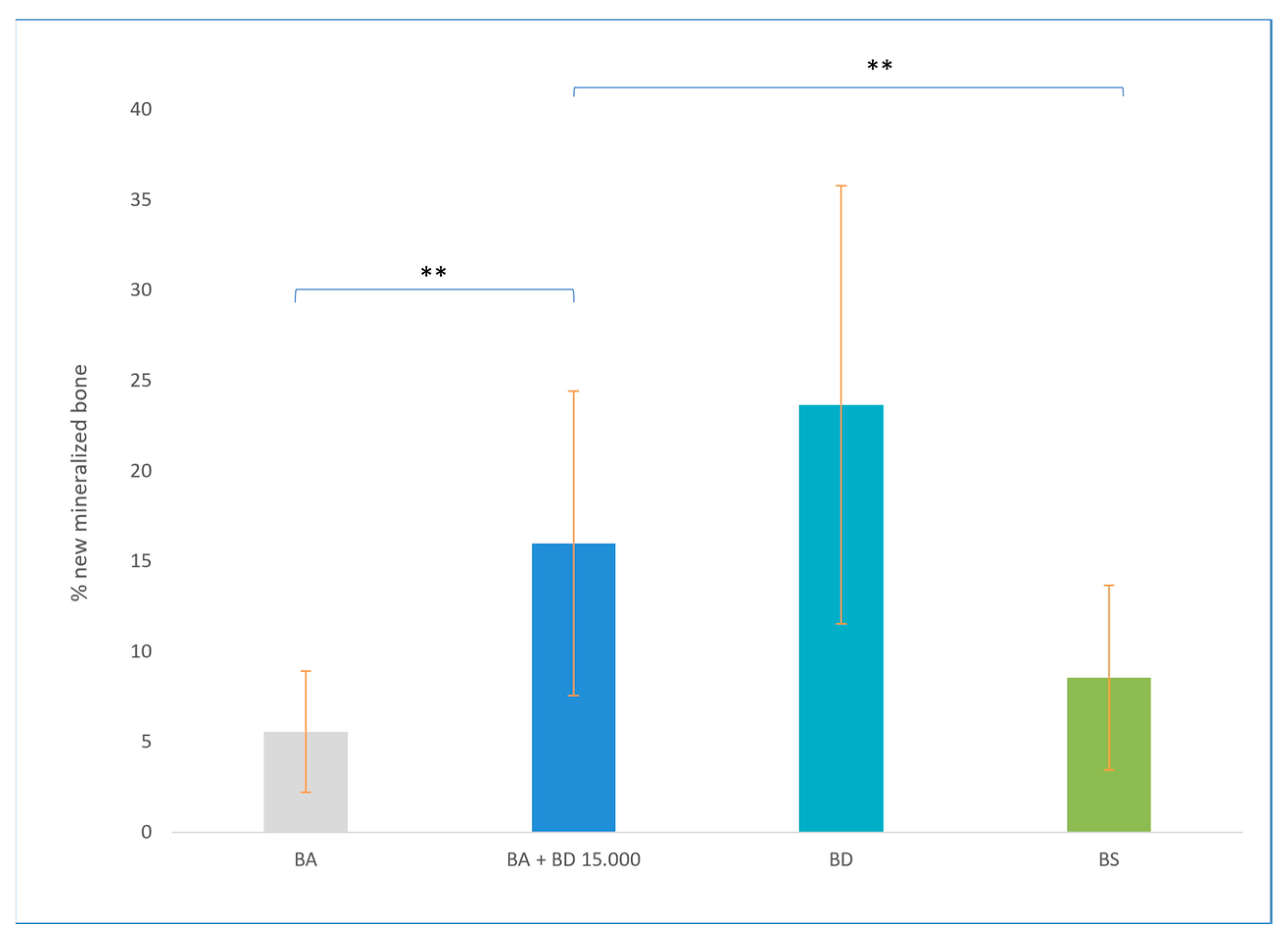
3.2. Results of Bone Density Measurement
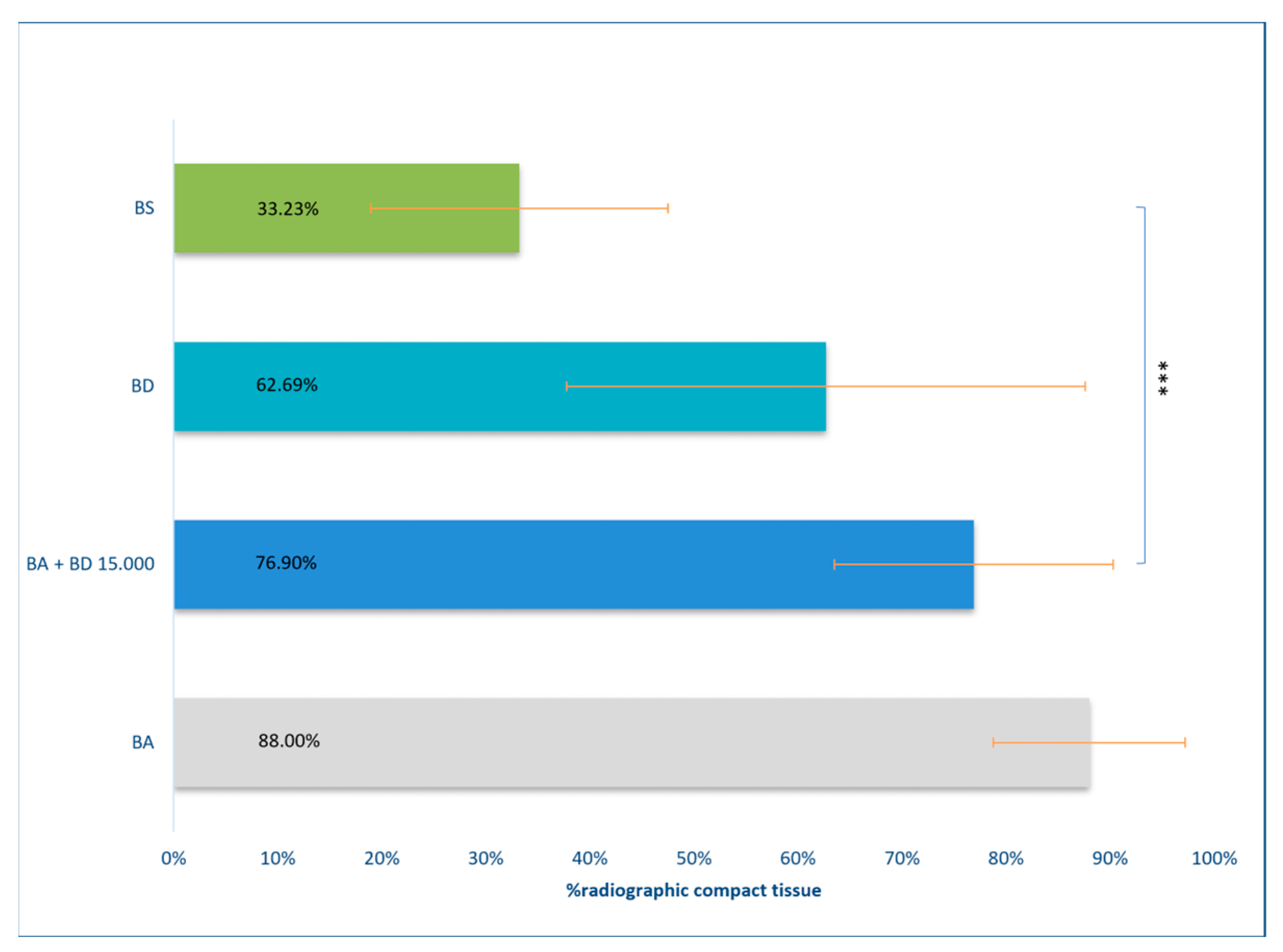
3.3. Results of DVT Measurement
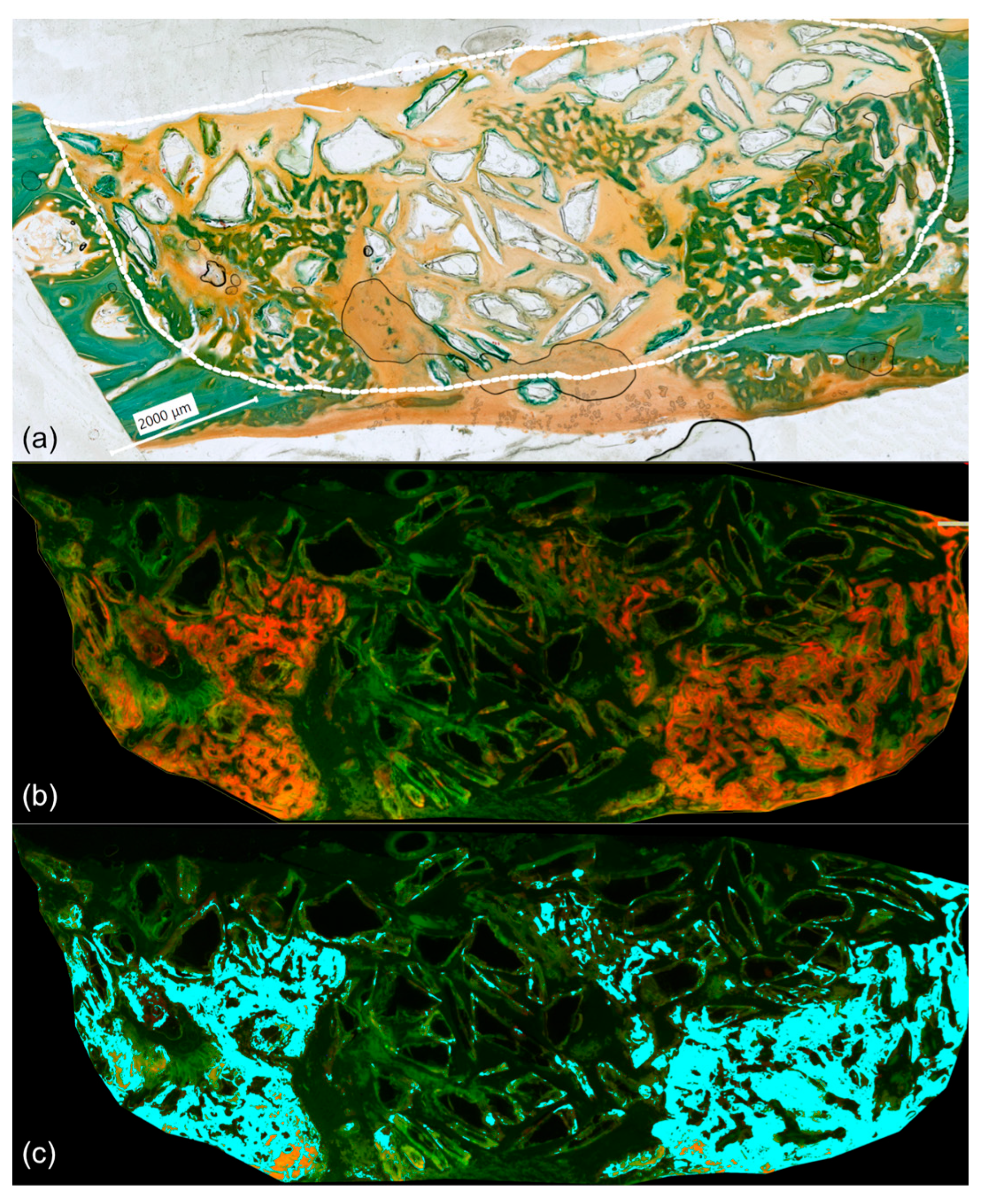
3.4. Results of Phase Colour-Coding System
4. Discussion
4.1. BD Harvesting Methods
4.2. Obliteration Techniques
4.3. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BA | bioactive glass S53P4 |
| BD | bone dust |
| BMD | bone mineral density |
| BS | blank sample |
| CI | confidence interval |
| DMEM | Dulbecco Modified Eagle Medium |
| DVT | digital volume tomography |
| FCS | fetal calf serum |
| HSD | Honestly Significant Difference |
| NCSD | non-critical size defects |
| PBS | phosphate-buffered saline |
| ROI | region of interest |
| Rpm | rotation per minute |
| pQCT | peripheral quantitative computed tomography |
| SD | standard deviation |
References
- Schimanski, G.; Schimanski, E. Obliteration von Mastoidhöhlen: 30 Jahre Erfahrung mit Empfehlungen zur Operationsstrategie. HNO 2015, 63, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Baron, M.; Busenlechner, D.; Kandler, B.; Fuerst, G.; Watzek, G. Proliferation and Osteogenic Differentiation of Cells from Cortical Bone Cylinders, Bone Particles from Mill, and Drilling Dust. J. Oral Maxillofac. Surg. 2005, 63, 238–243. [Google Scholar] [CrossRef]
- Urist, M.R. Introduction to Update on Osteochondral Allograft Surgery. In Bone Transplantation; Springer: Berlin/Heidelberg, Germany, 1989; pp. 1–6. ISBN 978-3-642-83573-5. [Google Scholar]
- Fassone, E.; Fabiano, B.; Caracciolo, A.; Sapino, S.; Ferrero, V. Use of Bonalive in Obliterative Mastoidectomy: Anatomical Results and Clinical Outcome. Eur. Arch. Otorhinolaryngol. 2023, 280, 3577–3583. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.; Cristofaro, G.; Berrettini, S.; Danti, S. Applications of Bioactive Glasses for Implants in the Ear. In Bioactive Glasses and Glass-Ceramics: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2022; pp. 459–477. ISBN 978-1-119-72419-3. [Google Scholar]
- Sioshansi, P.C.; Alyono, J.C.; Blevins, N.H. Mastoid Obliteration Using Autologous Bone Dust Following Canal Wall Down Mastoidectomy. Otol. Neurotol. 2021, 42, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kluge, A.; Neudert, M.; Kunert-Keil, C.; Lailach, S.; Zahnert, T.; Kemper, M. The Obliteration of Noncritical Size Bone Defects With Bone Dust or Bone Replacement Material (Bioactive Glass S53P4). Otol. Neurotol. 2019, 40, e415–e423. [Google Scholar] [CrossRef]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Virolainen, P.; Heikkilä, J.; Yli-Urpo, A.; Vuorio, E.; Aro, H.T. Histomorphometric and Molecular Biologic Comparison of Bioactive Glass Granules and Autogenous Bone Grafts in Augmentation of Bone Defect Healing. J. Biomed. Mater. Res. 1997, 35, 9–17. [Google Scholar] [CrossRef]
- Pernaa, K.; Koski, I.; Mattila, K.; Gullichsen, E.; Heikkila, J.; Aho, A.J.; Lindfors, N. Bioactive Glass S53P4 and Autograft Bone in Treatment of Depressed Tibial Plateau Fractures—A Prospective Randomized 11-Year Follow-Up. JLT 2011, 21, 139–148. [Google Scholar] [CrossRef]
- Eder, C.; Chavanne, A.; Meissner, J.; Bretschneider, W.; Tuschel, A.; Becker, P.; Ogon, M. Autografts for Spinal Fusion: Osteogenic Potential of Laminectomy Bone Chips and Bone Shavings Collected via High Speed Drill. Eur. Spine J. 2011, 20, 1791–1795. [Google Scholar] [CrossRef]
- Gao, R.; Street, M.; Tay, M.L.; Callon, K.E.; Naot, D.; Lock, A.; Munro, J.T.; Cornish, J.; Ferguson, J.; Musson, D. Human Spinal Bone Dust as a Potential Local Autograft: In Vitro Potent Anabolic Effect on Human Osteoblasts. Spine 2018, 43, E193–E199. [Google Scholar] [CrossRef]
- Pandey, R.K.; Panda, S.S. Drilling of Bone: A Comprehensive Review. J. Clin. Orthop. Trauma 2013, 4, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Pautke, C.; Vogt, S.; Tischer, T.; Wexel, G.; Deppe, H.; Milz, S.; Schieker, M.; Kolk, A. Polychrome Labeling of Bone with Seven Different Fluorochromes: Enhancing Fluorochrome Discrimination by Spectral Image Analysis. Bone 2005, 37, 441–445. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Martin, K.L. (Eds.) Handbook of Histology Methods for Bone and Cartilage; Humana Press: Totowa, NJ, USA, 2003; ISBN 978-0-89603-960-5. [Google Scholar]
- Donath, K.; Breuner, G. A Method for the Study of Undecalcified Bones and Teeth with Attached Soft Tissues. The Säge-Schliff (Sawing and Grinding) Technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef]
- Bucher, O.; Wartenberg, H. Cytologie, Histologie und Mikroskopische Anatomie des Menschen, 12th ed.; Hans Huber: Berching, Germany, 1997; ISBN 978-3-456-82785-8. [Google Scholar]
- Kunert-Keil, C.; Gredes, T.; Heinemann, F.; Dominiak, M.; Botzenhart, U.; Gedrange, T. Socket Augmentation Using a Commercial Collagen-Based Product—An Animal Study in Pigs. Mater. Sci. Eng. C 2015, 46, 177–183. [Google Scholar] [CrossRef]
- Trinkaus, K.; Wenisch, S.; Siemers, C.; Hose, D.; Schnettler, R. Bohrmehl: Eine Quelle vitaler Zellen!: Erste Ergebnisse von humanen Proben. Der Unfallchirurg 2005, 108, 650–656. [Google Scholar] [CrossRef]
- Street, M.; Gao, R.; Martis, W.; Munro, J.; Musson, D.; Cornish, J.; Ferguson, J. The Efficacy of Local Autologous Bone Dust: A Systematic Review. Spine Deform 2017, 5, 231–237. [Google Scholar] [CrossRef]
- Ye, S.; Seo, K.-B.; Park, B.-H.; Song, K.-J.; Kim, J.-R.; Jang, K.-Y.; Chae, Y.J.; Lee, K.-B. Comparison of the Osteogenic Potential of Bone Dust and Iliac Bone Chip. Spine J. 2013, 13, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.V.; Estes, S.M.; Naar, E.M.; Lindley, E.M.; Burger, E. Histologic Evaluation of High Speed Burr Shavings Collected During Spinal Decompression Surgery. Orthopedics 2009, 32, 1–5. [Google Scholar] [CrossRef]
- Hassanein, A.H.; Arany, P.R.; Couto, R.A.; Clune, J.E.; Glowacki, J.; Rogers, G.F.; Mulliken, J.B.; Greene, A.K. Cranial Particulate Bone Graft Ossifies Calvarial Defects by Osteogenesis. Plast. Reconstr. Surg. 2012, 129, 796e–802e. [Google Scholar] [CrossRef]
- Karamese, M.; Toksoz, M.R.; Selimoglu, M.N.; Akdağ, O.; Toy, H.; Tosun, Z. Comparison of Bone Dust With Other Types of Bone Grafts for Cranioplasty. J. Craniofacial Surg. 2014, 25, 1155–1158. [Google Scholar] [CrossRef]
- Turner, A.S. The Sheep as a Model for Osteoporosis in Humans. Vet. J. 2002, 163, 232–239. [Google Scholar] [CrossRef]
- de Pollak, C.; Arnaud, E.; Renier, D.; Marie, P.J. Age-Related Changes in Bone Formation, Osteoblastic Cell Proliferation, and Differentiation during Postnatal Osteogenesis in Human Calvaria. J. Cell. Biochem. 1997, 64, 128–139. [Google Scholar] [CrossRef]
- Nagle, S.K.; Hebbar, C.R.; Kumar, R. An Effective Method to Collect Bone Dust for Mastoid Cavity Obliteration. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 4450–4454. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Lobocki, C.; Malhotra, G.; Jackson, I.T. Comparison of Osteogenic Potential of Calvarial Bone Dust, Bone Fragments, and Periosteum. J. Craniofacial Surg. 2009, 20, 1995–1999. [Google Scholar] [CrossRef]
- Lindeboom, J.J.; Van Kempen, P.M.W.; Buwalda, J.; Westerlaken, B.O.; Van Zuijlen, D.A.; Bom, S.J.H.; Van Der Beek, F.B. Mastoid Obliteration with Hydroxyapatite vs. Bone Pâté in Mastoidectomy Surgery Performed on Patients with Cholesteatoma and Chronic Suppurative Otitis Media: A Retrospective Analysis. Eur. Arch. Otorhinolaryngol. 2023, 280, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Beutner, D.; Stumpf, R.; Zahnert, T.; Hüttenbrink, K.-B. Long-Term Results following Mastoid Obliteration in Canal Wall Down Tympanomastoidectomy. Laryngo-Rhino-Otol. 2007, 86, 861–866. [Google Scholar] [CrossRef]
- Schimanski, G.; Schimanski, E. Mastoid Cavity Obliteration with Bioactive Glass Granules. In Recent Advances in Otolaryngology Head & Neck Surgery; JP Medical Ltd.: New York, NY, USA, 2016; pp. 249–281. ISBN 978-93-5152-940-8. [Google Scholar]
- Fernandes, J.S.; Gentile, P.; Pires, R.A.; Reis, R.L.; Hatton, P.V. Multifunctional Bioactive Glass and Glass-Ceramic Biomaterials with Antibacterial Properties for Repair and Regeneration of Bone Tissue. Acta Biomater. 2017, 59, 2–11. [Google Scholar] [CrossRef]
- Kemper, M.; Kluge, A.; Ney, M.; Beleites, T.; Zeidler-Rentzsch, I.; Keil, C.; Zahnert, T.; Neudert, M. Visualization of Bone Formation in Sheep’s Middle Ear by Using Fluorochrome Sequential Labelling (FSL). Sci. Rep. 2024, 14, 7046. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemper, M.; Kluge, A.; Zeidler-Rentzsch, I.; Günther, S.I.; Neudert, M. Improvement of the Obliteration of Non-Critical Size Defects by Using a Mixture of Bone Dust and Bone Replacement Material (Bioactive Glass S53P4). Osteology 2025, 5, 15. https://doi.org/10.3390/osteology5020015
Kemper M, Kluge A, Zeidler-Rentzsch I, Günther SI, Neudert M. Improvement of the Obliteration of Non-Critical Size Defects by Using a Mixture of Bone Dust and Bone Replacement Material (Bioactive Glass S53P4). Osteology. 2025; 5(2):15. https://doi.org/10.3390/osteology5020015
Chicago/Turabian StyleKemper, Max, Anne Kluge, Ines Zeidler-Rentzsch, Susanne Isabella Günther, and Marcus Neudert. 2025. "Improvement of the Obliteration of Non-Critical Size Defects by Using a Mixture of Bone Dust and Bone Replacement Material (Bioactive Glass S53P4)" Osteology 5, no. 2: 15. https://doi.org/10.3390/osteology5020015
APA StyleKemper, M., Kluge, A., Zeidler-Rentzsch, I., Günther, S. I., & Neudert, M. (2025). Improvement of the Obliteration of Non-Critical Size Defects by Using a Mixture of Bone Dust and Bone Replacement Material (Bioactive Glass S53P4). Osteology, 5(2), 15. https://doi.org/10.3390/osteology5020015





