Linear-Chain Nanostructured Carbon with a Silver Film Plated on Metal Components Has a Promising Effect for the Treatment of Periprosthetic Joint Infection
Abstract
:1. Introduction
2. Materials and Methods
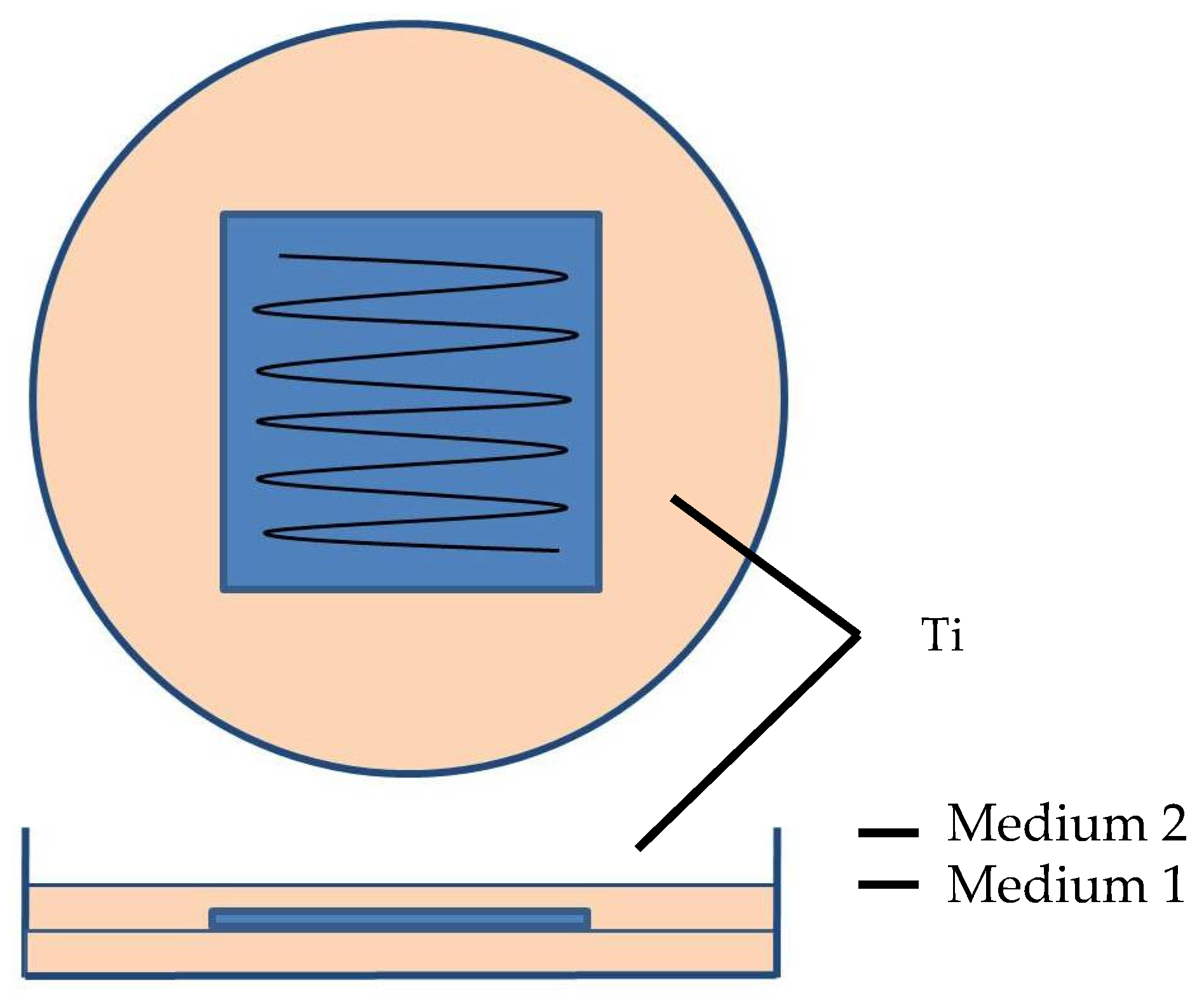
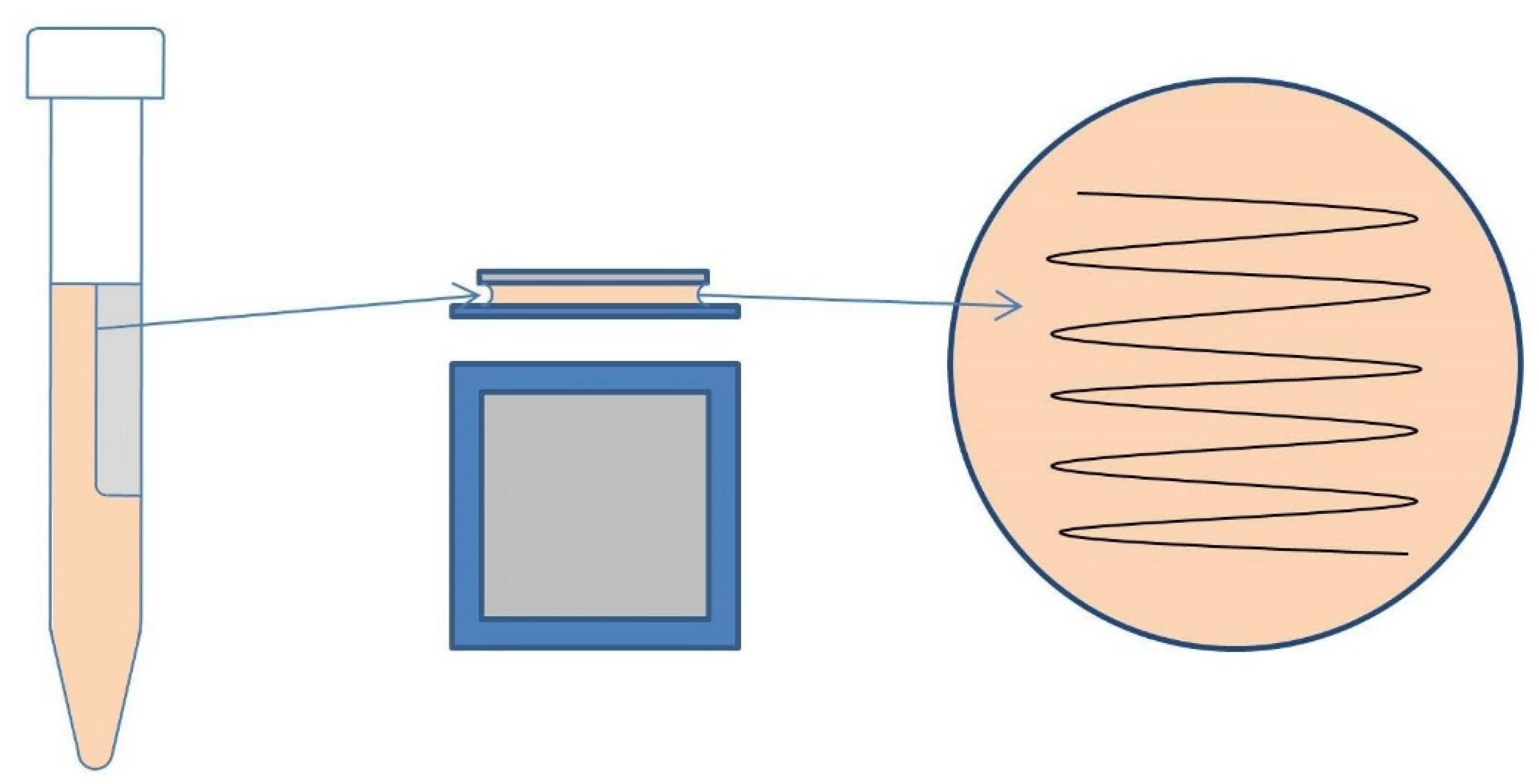
2.1. Fabrication of Silver-Doped Carbyne-like Carbon (S-CLC) Films
2.2. Evaluation of the Antibacterial Activity of Coatings
2.2.1. Assessment of the Release of Silver Ions from S-CLC Films
2.2.2. Qualifying the Direct Antibacterial Properties of S-CLC Films
2.2.3. Study of the Formation of Microbial Biofilm on the S-CLC Coating
3. Results
3.1. Antibacterial Activity of CLC or S-CLC Surface
3.1.1. S-CLC Film Had an Influence on P. aeruginosa in Solution Only at a Very Short Distance
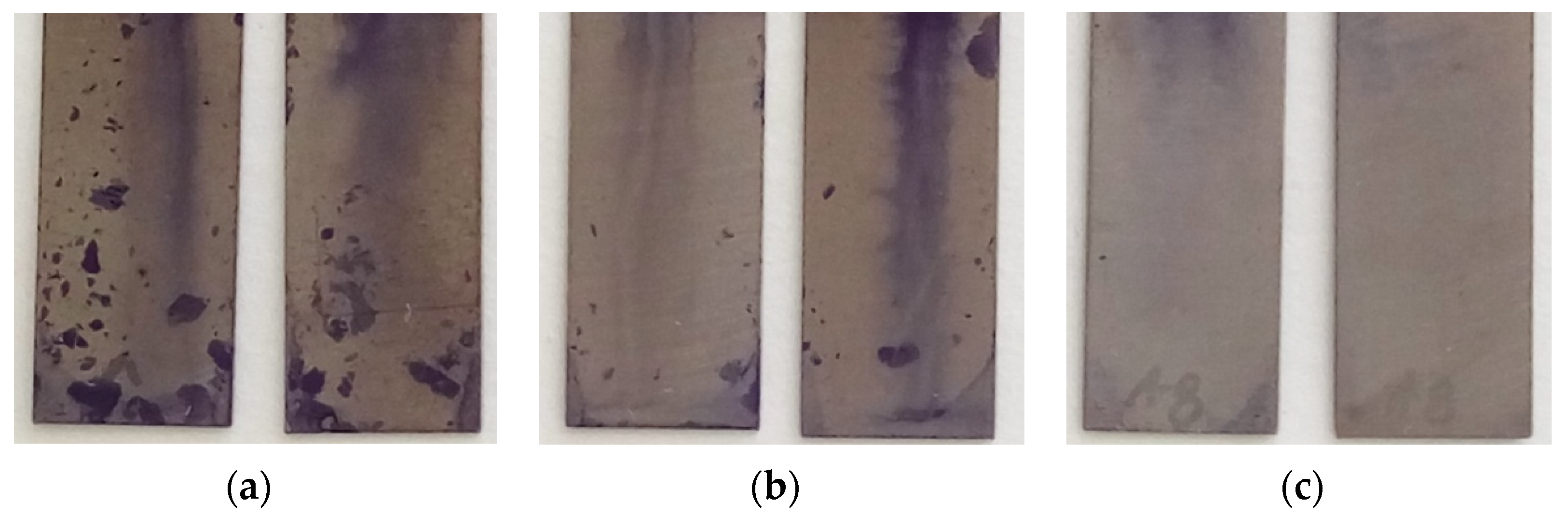
3.1.2. S-CLC Film Could Inhibit S. aureus, E. faecalis, and P. aeruginosa Surface Growth
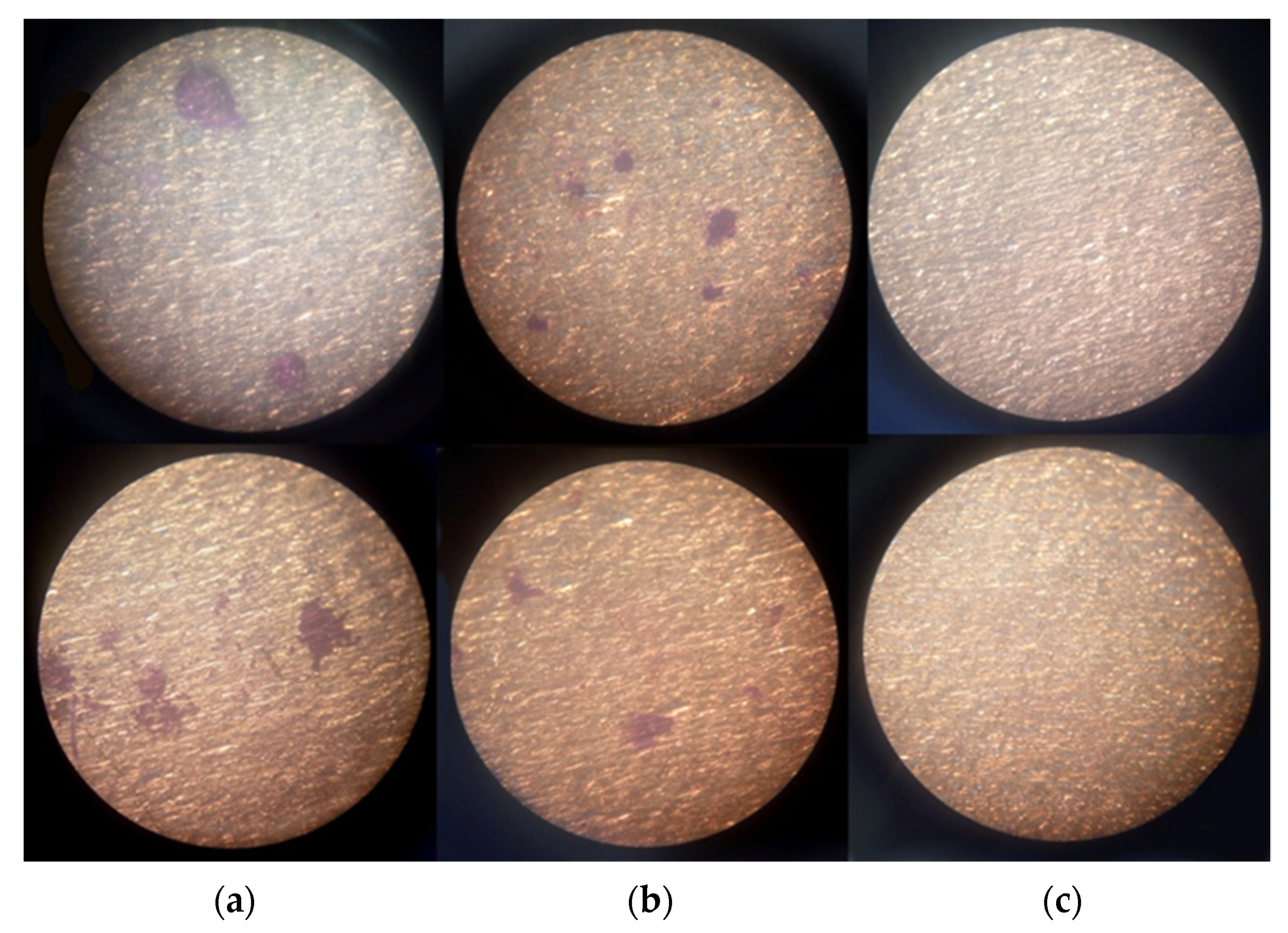
3.1.3. S-CLC Film Can Inhibit the P. aeruginosa Surface Biofilm Formation
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukeik, M.; Haddad, F.S. Periprosthetic joint infections after total hip replacement: An algorithmic approach. SICOT J. 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, A.M.; Farley, K.X.; Guild, G.N.; Bradbury, T.L., Jr. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2030. J. Arthroplast. 2020, 35, S79–S85. [Google Scholar] [CrossRef]
- Corro, S.; Vicente, M.; Rodriguez-Pardo, D.; Pigrau, C.; Lung, M.; Corona, P.S. Vancomycin-Gentamicin Prefabricated Spacers in 2-Stage Revision Arthroplasty for Chronic Hip and Knee Periprosthetic Joint Infection: Insights Into Reimplantation Microbiology and Outcomes. J. Arthroplast. 2020, 35, 247–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, C.L.; Tsuchiya, H.; Morelli, I.; Battaglia, A.G.; Drago, L. Antibacterial coating of implants: Are we missing something? Bone Jt. Res. 2019, 8, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; Ahrens, H.; Gebert, C.; Streitbuerger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Wedemeyer, C.; Saxler, G.; Winkelmann, W.; et al. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials 2007, 28, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldo, F. Polyynes: Synthesis, Properties, and Applications; Taylor & Francis: Boca Raton, FL, USA, 2005; p. xvii. 506p. [Google Scholar]
- Green, J.B.; Fulghum, T.; Nordhaus, M.A. Review of immobilized antimicrobial agents and methods for testing. Biointerphases 2011, 6, CL2-43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cendra, M.D.M.; Blanco-Cabra, N.; Pedraz, L.; Torrents, E. Optimal environmental and culture conditions allow the in vitro coexistence of Pseudomonas aeruginosa and Staphylococcus aureus in stable biofilms. Sci. Rep. 2019, 9, 16284. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, H.; Yoshida, K.; Nishida, Y.; Kikuchi, Y.; Sato, Y. Antibacterial Properties of Metallic Elements for Alloying Evaluated with Application of JIS Z 2801:2000. Isij Int. 2008, 48, 1299–1304. [Google Scholar] [CrossRef] [Green Version]
- Food, É.-U.; Administration, D.; Safety, C.F.F.; Nutrition, A. Bacteriological Analytical Manual (BAM); AOAC International: Gaithersburg, MD, USA, 2020. [Google Scholar]
- De Vries, T.A.; Hamilton, M.A. Estimating the Antimicrobial Log Reduction: Part 1. Quantitative Assays. Quant. Microbiol. 1999, 1, 29–45. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 30, 2437. [Google Scholar] [CrossRef] [PubMed]
- Gunputh, U.F.; Le, H.; Lawton, K.; Besinis, A.; Tredwin, C.; Handy, R.D. Antibacterial properties of silver nanoparticles grown in situ and anchored to titanium dioxide nanotubes on titanium implant against Staphylococcus aureus. Nanotoxicology 2020, 14, 97–110. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef] [PubMed]
- Abalkhil, T.A.; Alharbi, S.A.; Salmen, S.H.; Wainwright, M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol. Biotechnol.Equip. 2017, 31, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Pisarcik, M.; Lukac, M.; Jampilek, J.; Bilka, F.; Bilkova, A.; Paskova, L.; Devinsky, F.; Horakova, R.; Brezina, M.; Opravil, T. Silver Nanoparticles Stabilized with Phosphorus-Containing Heterocyclic Surfactants: Synthesis, Physico-Chemical Properties, and Biological Activity Determination. Nanomaterials 2021, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Casolaro, I.; Akimoto, J.; Ueda, M.; Ueki, M.; Ito, Y. Antibacterial Properties of Silver Nanoparticles Embedded on Polyelectrolyte Hydrogels Based on alpha-Amino Acid Residues. Gels 2018, 4, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegand, C.; Abel, M.; Ruth, P.; Elsner, P.; Hipler, U.C. pH influence on antibacterial efficacy of common antiseptic substances. Skin Pharmacol. Physiol. 2015, 28, 147–158. [Google Scholar] [CrossRef]
- Liu, M.; Artyukhov, V.I.; Lee, H.; Xu, F.; Yakobson, B.I. Carbyne from First Principles: Chain of C Atoms, a Nanorod or a Nanorope. ACS Nano 2013, 7, 10075–10082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrasser, N.; Jussen, S.; Banke, I.J.; Kmeth, R.; von Eisenhart-Rothe, R.; Stritzker, B.; Gollwitzer, H.; Burgkart, R. Antibacterial efficacy of titanium-containing alloy with silver-nanoparticles enriched diamond-like carbon coatings. AMB Express 2015, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, M.; Alkubaisi, N.; Gopinath, K.; Karthika, V.; Arumugam, A.; Govindarajan, M. Facile and Cost-Effective Ag Nanoparticles Fabricated by Lilium lancifolium Leaf Extract: Antibacterial and Antibiofilm Potential. J. Clust. Sci. 2019, 30, 1081–1089. [Google Scholar] [CrossRef]
| Thickness of Growth Media Layer above the Plate | No Cover | CLC | S-CLC |
|---|---|---|---|
| 1 mm | Heavy growth | Heavy growth | No growth |
| 2 mm | Heavy growth | Heavy growth | Heavy growth |
| 4 mm | Heavy growth | Heavy growth | Heavy growth |
| No Cover | CLC | S-CLC | ||||
|---|---|---|---|---|---|---|
| R | I% | R | I% | R | I% | |
| S. aureus | 0.0 | 0.0 | 0.4 | 61.8 | 1.9 | 98.7 |
| E. faecalis | 0.0 | 0.0 | 0.4 | 51.7 | 2.0 | 99.9 |
| P. aeruginosa | 0.0 | 0.0 | 0.0 | 0.0 | 1.9 | 98.7 |
| No Covering | CLC | S-CLC | |
|---|---|---|---|
| Dye mass absorbed by biofilm, μg | 17.2 | 3.9 | 0.4 |
| Dye mass absorbed by biofilm, μg | 2.8 | 0.6 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliuchenko, L.I.; Nikolaev, N.S.; Pchelova, N.N.; Nikolaevich Efimov, D.; Preobrazhenskaia, E.V.; Emelianov, V.U. Linear-Chain Nanostructured Carbon with a Silver Film Plated on Metal Components Has a Promising Effect for the Treatment of Periprosthetic Joint Infection. Osteology 2021, 1, 238-246. https://doi.org/10.3390/osteology1040022
Maliuchenko LI, Nikolaev NS, Pchelova NN, Nikolaevich Efimov D, Preobrazhenskaia EV, Emelianov VU. Linear-Chain Nanostructured Carbon with a Silver Film Plated on Metal Components Has a Promising Effect for the Treatment of Periprosthetic Joint Infection. Osteology. 2021; 1(4):238-246. https://doi.org/10.3390/osteology1040022
Chicago/Turabian StyleMaliuchenko, Leonid I., Nikolay S. Nikolaev, Nadezhda N. Pchelova, Dmitry Nikolaevich Efimov, Elena V. Preobrazhenskaia, and Vladimir U. Emelianov. 2021. "Linear-Chain Nanostructured Carbon with a Silver Film Plated on Metal Components Has a Promising Effect for the Treatment of Periprosthetic Joint Infection" Osteology 1, no. 4: 238-246. https://doi.org/10.3390/osteology1040022
APA StyleMaliuchenko, L. I., Nikolaev, N. S., Pchelova, N. N., Nikolaevich Efimov, D., Preobrazhenskaia, E. V., & Emelianov, V. U. (2021). Linear-Chain Nanostructured Carbon with a Silver Film Plated on Metal Components Has a Promising Effect for the Treatment of Periprosthetic Joint Infection. Osteology, 1(4), 238-246. https://doi.org/10.3390/osteology1040022






