Abstract
Triazolo[4,3-a]pyrimidine is one of the promising structural fragments for the development of drugs, including anticancer drugs. This work is devoted to the synthesis of a number of new 2-arylhydrazone derivatives of thiazolo[3,2-a]pyrimidine, which are synthetic precursors for triazolo[4,3-a]pyrimidines. The crystal structure of 6-acetyl-7-methyl-5-phenyl-2-(2-phenylhydrazineylidene)-5H-thiazolo[3,2-a]pyrimidin-3(2H)-one was established by SCXRD. In the reduction reaction of the compound, the following system was used: vanadium(V) oxide, and sodium borohydride in ethanol at room temperature, which led to the formation of only one pair of diastereomers (1R*)-1-((5S*,6R*,7R*)-(1-(hydroxymethyl)-7-methyl-1,5-diphenyl-1,5,6,7-tetrahydro[1,2,4]triazolo[4,3-a]pyrimidin-6-yl)ethan-1-ol.
1. Introduction
Triazolopyrimidines, which are analogues of purines, are the subject of research for chemists and biologists due to their wide range of pharmacological activities, antimicrobial, antimalarial, cardiac stimulant, antifungal, anti-HBV, anticancer, analgesic, antipyretic, anti-inflammatory, namely antihypertensive, leishmanicidal and potential herbicidal action [1,2,3,4,5,6,7,8]. Thus, triazolopyrimidines are one of the promising structural fragments for new methods of new potential drug synthesis.
Several synthetic methods for the preparation of triazolo[4,3-a]pyrimidine derivatives are described in the literature, consisting of interaction with subsequent cyclization of 3-ethoxycarbonyl-2-hydrazinylpyrimidines [9] (Scheme 1) or 2-hydrazinylpyrimidines [10,11] with various reagents (carbon disulfide, ethyl chloroformate, triethylorthoformate, acetic anhydride) (Scheme 2).
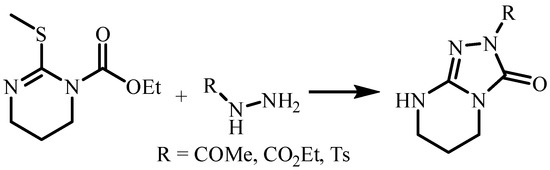
Scheme 1.
Cyclization of 3-ethoxycarbonyl-2-hydrazinylpyrimidines.
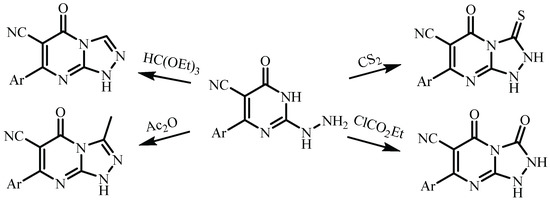
Scheme 2.
Cyclization2-hydrazinylpyrimidines with various reagents (carbon disulfide, ethyl chloroformate, triethylorthoformate, acetic anhydride).
Another way to prepare of triazolo[4,3-a]pyrimidines is the dipolar 1,3-addition of nitrile imide (formed in situ from hydrazonoyl halide and triethylamine) to 1,2,3,4-tetrahydropyrimidin-2-thione at the C=S bond and Smiles rearrangement with loss of hydrogen sulfide (Scheme 3) [12,13].
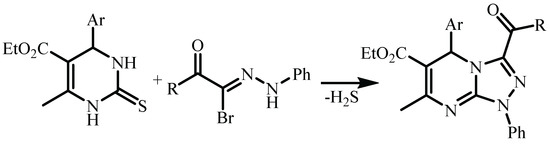
Scheme 3.
Dipolar 1,3-addition of nitrile imide to 1,2,3,4-tetrahydropyrimidin-2-thione at the C=S bond and Smiles rearrangement with loss of hydrogen sulfide.
Recently, our scientific group has shown that 2-arylhydrazone derivatives of thiazolo[3,2-a]pyrimidine can be transformed into 1,5-dihydrotriazolo[4,3-a]pyrimidines (Scheme 4) in the presence of a new reducing system—vanadium(V) oxide and a fourfold excess of sodium borohydride at room temperature [14,15]. This method includes the hydrogenation of a hydrozone moiety at the first stage and subsequent rearrangement with hydrogen sulfide elimination. It is a promising method for triazolo[4,3-a]pyrimidine derivative synthesis containing a hydroxymethylene substituent.
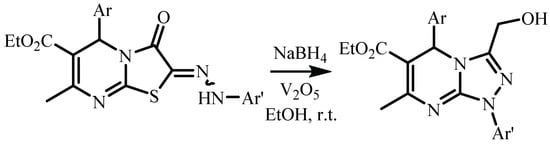
Scheme 4.
Method for triazolo[4,3-a]pyrimidine derivatives synthesis containing a hydroxy methylene substituent from 2-arylhydrazone derivatives of thiazolo[3,2-a]pyrimidine.
In the present work, the synthesis of new 2-arylhydrazone thiazolo[3,2-a]pyrimidine derivatives containing an acetyl group at C6, the crystal structure of the 2-phenylhydrazone derivative, and unique and diastereoselective reduction of the 2-phenylhydrazonethiazolo[3,2-a]pyrimidine molecule under the action of the reducing system—NaBH4/V2O5 were discussed.
2. Materials and Methods
NMR experiments were performed on Bruker Avance 500 (Saarbrucken, Germany). Chemical shifts were determined relative to the signals of residual protons of the DMSO-d6. Electrospray ionization (ESI) mass spectra were obtained using a Bruker AmaZon X ion trap mass spectrometer. IR spectra in KBr tablets were recorded on a Bruker Vector-22.
The method of halogens determination is based on the combustion at 1200 °C of organic compound in oxygen in the presence of a platinum catalyst; the combustion products are adsorbed by the alkali and the halides formed were determined by mercurimetric titration with diphenylcarbazone as an indicator.
CHNS elemental analysis was carried out using a high-temperature one-/two-reactor analyzer (oxidation tube and reduction tube) EuroEA3028-HT-OM “Eurovector SpA. Synthesis of 6-acetyl-5-(4-bromophenyl)-7-methyl-5H-thiazolo[3,2-a]pyrimidin-3(2H)-one hydrochloride 2b.
1,2,3,4-Tetrahydripyrimidine-2-thion 1b (0.3 g, 1 mmol) was mixed with ethyl chloroacetate (5.4 mL, 5 mmol) without solvent. The mixture was stirred at a temperature of 120 °C for 1 h, then cooled to room temperature; ethyl acetate (20 mL) was added and precipitate was filtered out followed by washing with ethyl acetate and recrystallization from ethyl alcohol. Yield 87%, yellow powder, mp 238–240 °C. 1H NMR (400 MHz, DMSO-d6, 25 °C) δH ppm: 2.23 (s, 3H, CH3), 2.35 (s, 3H, CH3), 4.12–4.13 (m, 2H, SCH2), 5.99 (s, 1H, CH-Ar), 7.20–7.22(m, 2H, CH (Ar)), 7.53–7.55 (m, 2H, CH (Ar)). 13C NMR (100 MHz, DMSO-d6, 25 °C) δC ppm: 23.93, 31.36, 33.11, 54.24, 116.63, 122.16, 130.29, 132.10, 139.96, 171.52, 196.71. IR (KBr, cm−1): 2965 (CH2); 1744 (C=O); 1650 (C=O). Anal. Calcd. for C15H13BrN2O2S, %: C 49.33, H 3.59, Br 21.88; N 7.67; O 8.76, S 8.78. Found C 49.31; H 3.54; Br 21.91; N 7.69; S 8.80.
General Method for the Preparation of Compounds 3a–c.
Sodium nitrite cold solution (1 mmol) in water (3 mL) was added drop by drop to an aromatic amine hydrochloride (1 mmol) suspension in water (5 mL) with stirring at 0–5 °C for 1 h. The resultant solution of aryldiazonium chloride (1 mmol) was added drop by drop with stirring at 0–5 °C to a cold solution of the corresponding thiazolo[3,2-a]pyrimidine 2a,b (1 mmol) and sodium acetate (1.1 mmol) in ethyl alcohol (10 mL). The mixture was stirred at room temperature for 2 h. Next, the reaction mixture was diluted with water, and the crude precipitate was collected by filtration, washed with water, and crystallized from ethyl alcohol.
6-Acetyl-7-methyl-5-phenyl-2-(2-phenylhydrazineylidene)-5H-thiazolo[3,2-a]pyrimidin-3(2H)-one 3a. Yield 76%, orange powder, mp 245–247 °C. 1H NMR (400 MHz, DMSO-d6, 25 °C) δH ppm: 2.26 (s, 3H, CH3), 2.38 (s, 3H, CH3), 6.17 (s, 1H, CH-Ph), 6.98–7.00 (m, 1H, CH (Ph)), 7.22–7.23 (m, 2H, CH (Ph)), 7.30–7.37 (m, 7H, CH (Ph)), 10.92 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6, 25 °C) δC ppm: 23.73, 31.26, 54.75, 114.69, 118.52, 120.71, 123.04, 128.08, 129.06, 129.27, 129.83, 140.39, 143.46, 149.76, 153.73, 160.89, 197.31. IR (KBr, cm−1): 3222, 3189 (NH); 1731 (C=O); 1621 (C=O); 1514 (C-C(Ph)). MS (ESI), m/z, [M+H]+: calcd. for C16H16BrN2O3S+: 391,47; found: 391,23. Anal. Calcd. for C21H19N4O2S, %: C 64.43; H 4.89; N 14.31; O 8.17, S 8.19. Found C 64.45; H 4.86; N 14.34; S 8.19.
6-Acetyl-5-(4-bromophenyl)-7-methyl-2-(2-phenylhydrazineylidene)-5H-thiazolo[3,2-a]pyrimidin-3(2H)-one 3b. Yield 69%, orange powder, mp 236–238 °C. 1H NMR (400 MHz, DMSO-d6, 25 °C) δH ppm: 2.27 (s, 3H, CH3), 2.38 (s, 3H, CH3), 6.13 (s, 1H, CH-Ar), 6.98–7.01 (m, 1H, CH (Ar)), 7.19–7.33 (m, 6H, CH (Ar)), 7.49–7.51 (m, 1H, CH (Ar)), 7.55–7.56 (m, 1H, CH (Ar)), 10.24, 10.29, 10.53, 10.61, 10.96 (five s, 1H, NH). 13C NMR (100 MHz, DMSO-d6, 25 °C) δC ppm: 23.86, 31.39, 54.19, 114.71, 118.16, 120.57, 122.29, 123.07, 129.83, 130.38, 132.17, 139.70, 143.44, 150.24, 160.89, 197.14. IR (KBr, cm−1): 3235 (NH); 1706 (C=O); 1642 (C=O); 1544 (C-C(Ph)). MS (ESI), m/z, [M+H]+: calcd. for C21H18BrN4O2S+: 470,36; found: 471,16. Anal. Calcd. for C11H17BrN4O2S, %: C 53.74; H 3.65; Br 17.02; N 11.94; O 6.82; S 6.83. Found C 53.71; H 3.62; Br 17.06; N 11.95; S 6.81.
6-Acetyl-2-(2-(2-methoxyphenyl)hydrazineylidene)-7-methyl-5-phenyl-5H-thiazolo[3,2-a]pyrimidin-3(2H)-one 3c. Yield 75%, orange powder, mp 210–212 °C. 1H NMR (400 MHz, DMSO-d6, 25 °C) δH ppm: 2.25 (s, 3H, CH3), 2.36, 2.37 (two s, 3H, CH3), 3.86, 3.88 (two s, 3H, CH3), 6.16 (s, 1H, CH-Ph), 6.91–6.95 (m, 1H, CH (Ar)), 6.99–7.08 (m, 2H, CH (Ar)), 7.27–7.40 (m, 6H, CH (Ar)), 10.24, 12.18 (two s, 1H, NH). 13C NMR (100 MHz, DMSO-d6, 25 °C) δC ppm: 24.26, 31.74, 55.23, 56.75, 112.46, 112.75, 113.17, 116.71, 118.98, 122.12, 124.33, 124.61, 128.60, 129.58, 129.66, 129.80, 129.90, 132.95, 140.97, 148.63, 150.34, 154.78, 161.63, 197.80. IR (KBr, cm−1): 3269 (NH); 1729 (C=O); 1633 (C=O); 1525 (C-C(Ph)). MS (ESI), m/z, [M+H]+: calcd. for C22H21N4O3S+: 421,50; found: 421,26. Anal. Calcd. for C22H20N4O3S, %: C 62.84; H 4.79; N 13.32; O 11.41; S 7.62. Found C 62.86; H 4.77; N 13.35; S 7.56.
Synthesis of (1R*)-1-((5S*,6R*,7R*)-1-(Hydroxymethyl)-7-methyl-1,5-diphenyl-1,5,6,7-tetrahydro[1,2,4]triazolo[4,3-a]pyrimidin-6-yl)ethan-1-ol 4a. Vanadium(V) oxide (0.2 g, 1 mmol) and sodium borohydride (0.3 g, 7 mmol) were added to thiazolo[3,2-a]pyrimidine 2-phenylhydrazone derivative 3a (0.4 g, 1 mmol) dissolved in ethanol (5 mL). Next, the reaction mixture was stirred at room temperature for 72 h. The mixture was filtered, and the filtrate was diluted with water. The precipitate that formed was filtered off and recrystallized from ethanol. Yield 44%, white powder, mp 165–166 °C. 1H NMR (400 MHz, CDCl3, 25 °C) δH ppm: 0.75 (d, J = 6.9 Hz, 3H, CH(OH)CH3), 1.17 (d, J = 6.0 Hz, 3H, CH3), 1.99–2.05 (m, 1H, CH-6), 3.60–3.65 (m, 1H, CH-N), 4.31–4.36 (m, 1H, CH-OH), 4.39 (d, J = 5.9 Hz, 2H, CH2OH), 5.15 (d, J = 4.6 Hz, 1H, CHOH), 5.63 (t, J = 6.0 Hz, 1H, CH2OH), 7.01–7.07 (m, 1H, CH (Ph)), 7.12–7.16 (m, 1H, CH (Ph)), 7.21–7.25 (m, 2H, CH (Ph)), 7.43–7.45 (m, 2H, CH (Ph)), 8.17–8.19 (m, 2H, CH (Ph)). 13C NMR (100 MHz, CDCl3, 25 °C) δC ppm: 16.0, 22.7, 47.1, 48.6, 54.7, 55.7, 64.3, 117.7, 123.5, 126.7, 128.1, 129.0, 129.2, 139.9, 143.0, 147.6, 148.2. IR (KBr, cm−1): 3348 (OH); 1631 (C=N); 1593 (C-C(Ph)). Anal. Calcd. for C21H24N4O2, %: C 69.21; H 6.64; N 15.37; O 8.78. Found C 69.27; H 6.53; N 15.23.
Crystals of 3a suitable for X-ray diffraction study were obtained by slow evaporation of ethanol solution (20 mL) containing 0.02 mol of the dissolved compound after 5 days.
X-ray diffraction analysis of 3a was performed on a Bruker D8 QUEST automatic three-circle diffractometer with a PHOTON III two-dimensional detector and an IμS DIAMOND microfocus X-ray tube (λ[Mo Kα] = 0.71073 Å) at 100 (2) K. Data collection and processing of diffraction data were performed using the APEX3 software package.
Structure 3a was solved by the direct method using the SHELXT program [16] and refined by the full-matrix least-squares method over F2 using the SHELXL program [17]. All calculations were performed in the WinGX software package [18]. The calculation of the geometry of molecules and intermolecular interactions in crystals was carried out using the PLATON program [19], and the drawings of molecules were done using the ORTEP-3 [18] and MERCURY [20] programs.
Non-hydrogen atoms were refined in the anisotropic approximation. The positions of the hydrogen atoms H(O) were determined using difference Fourier maps, and these at-oms were refined isotropically. The remaining hydrogen atoms were placed in geometrically calculated positions and included in the refinement in the “riding” model. The crystallographic data of structure 7 were deposited at the Cambridge Crystallographic Data Center, and the registration numbers and the most important characteristics are given in Table 1.

Table 1.
Crystallographic data for compound 3a.
3. Results and Discussion
2-Arylhydrazinylidenethiazolo[3,2-a]pyrimidin-3-one 3a–c were synthesized according to Scheme 5. The first step was a three-component Biginelli condensation between acetylacetone, thiourea and an aromatic aldehyde (benzaldehyde or 4-bromobenzaldehyde) carried out in boiling acetonitrile in the presence of catalytic amounts of molecular iodine [21]. The obtained 1,2,3,4-tetrahydropyrimidine-2-thiones 1a,b were involved in the reaction of sulfur atom alkylation by ethyl chloroacetate followed by cyclization with the formation of thiazolo[3,2-a]pyrimidine-3-one 2a,b [22,23]. Finally, the interaction of CH-active derivatives 2a,b with aryldiazonium salts upon cooling to 0–5 °C gave the target derivatives 3a–c in good yields (69–76%).

Scheme 5.
Synthesis of 2-arylhydrazone derivatives of thiazolo[3,2-a]pyrimidine 3a–c. Reagents and conditions: (a) I2, CH3CN, reflux, 8 h; (b) ClCH2CO2Et, 120 °C, no solvent; (c) R’C6H4N2+Cl− (R’=H or 2-OMe), AcONa, EtOH, 2 h, 0–5 °C. *—asymmetric carbon atom.
The structure of compounds 2a and 3a–c was established by 1H and 13C NMR IR-, and mass-spectra (see Figures S1–S15). The structure of derivative 3a was additionally confirmed by SCXRD (Table 1). According to SCXRD data, the bicyclic tiazolo[3,2-a]pyrimidine fragment was almost flat (Figure 1a). The six-membered cycle assumed a sofa conformation. The sp3-hybridized C5 carbon atom deviates slightly from the plane formed by the other five atoms of the pyrimidine ring. The acetyl group was located in the plane of the bicyclic thiazolopyrimidine fragment. The formation of hydrogen bonds between the oxygen of the acetyl group and the N-H hydrazone fragment (dO…N = 2.953(1) Å, φ = 172.07(4)°) was observed in the crystal (Figure 1b). It is interesting to note that the established hydrogen interaction leads to the formation of zigzag heterochiral chains in the crystalline phase (Figure 1c). Heterochiral chains consisting of alternating R- and S-isomers were arranged parallel to each other due to π-stacking (Figure 1c). Thus, it was found that hydrogen bonding determines the crystal packing of 3a. It should be noted that, as was shown in our previous work [24], the formation of the Z-isomer was observed both in solution and in the crystalline phase.
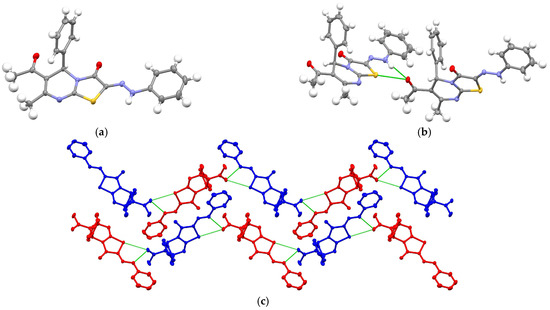
Figure 1.
ORTEP view of molecule 3a in the crystalline phase (a) (C, O, N, S, and H-atoms are presented as grey, red, light-violet, yellow, and light grey ellipsoids with 50% probability, respectively); hydrogen bond between the oxygen of the acetyl group of R-isomer of the molecule of compound 3a and the NH hydrazone fragment of the S-isomer (b); part of crystal packing 3a showing the formation of parallel zigzag heterochiral chains consisting of alternating R- and S-isomers (colored in blue and red, respectively) (c). The hydrogen bond is colored green.
The obtained 2-arylhidrazone derivatives 3a–c were involved in the reaction with reducing system—vanadium(V) oxide and a fourfold excess of sodium borohydride at room temperature [14]. However, the complicated mixture of hard-to-separate substances which we obtained instead yielded 1,5-dihydrotriazolo[4,3-a]pyrimidines (Scheme 4). The temperature decrease up to 0–5 °C did not affect the reaction. The individual product was isolated in the case of the increase in sodium borohydride in excess of seven equivalents. In these experimental conditions, compound 4—1-(1-(hydroxymethyl)-7-methyl-1,5-diphenyl- 1,5,6,7-tetrahydro[1,2,4]triazolo[4,3-a]pyrimidin-6-yl)ethan-1-ol was isolated in 44% yield (Scheme 6). Thus, it was found that the hydroxymethylene derivative of triazolo[4,3-a]pyrimidine was formed in agreement with [14]. However, the reaction was complicated by the hydrogenation of the conjugated C=C-C=O system due to a large excess of sodium borohydride.
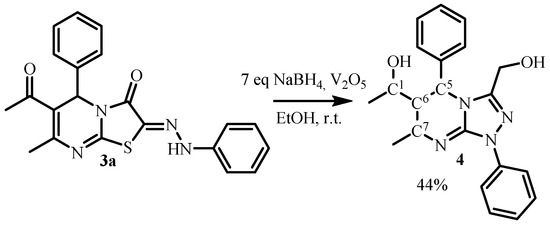
Scheme 6.
Reduction of 2-arylhydrazonothiazolopyrimidine derivative 3a.
The signals of the C6 and C7 atoms of triazolo[4,3-a]pyrimidine 4 in the 13C NMR spectrum shift were upfield and appeared at 47.1 and 48.6 ppm, respectively. In the 1H NMR spectrum, the signals of hydrogen atoms at C6 and C7 resonated at 1.99–2.05 ppm and 3.60–3.65 ppm as multiplets. Additionally, in the carbon spectrum, there was no signal of the carbonyl group carbon in the region of 197.3 ppm, but a signal of the methine carbon atom was found in the region of 64.3 ppm, which in the proton spectrum corresponds to a proton signal in the form of a multiplet in the region of 4.31–4.36 ppm (see Figures S16–S18).
Four carbon atoms are asymmetric in compound 4; therefore, the formation of a mixture of diastereomers is possible. The only set of signals in the 1H and 13C NMR spectra indicated the formation of one diastereomer. Obviously, 2D NMRs and SCXRD are the best ways to establish the compound configuration. Unfortunately, our attempts to prepare a single crystal of compound 4 failed. The low solubility of 4 in most organic solvents did not allow recording the 2D NOESY spectrum. For this reason, molecular mechanics calculations using the MMFF94s force field were performed to estimate the thermodynamic stability of all possible stereoisomers. The calculated data are presented in Table 2.

Table 2.
Relative energies of diastereomer 4.
According to these data, (1R*)-1-((5S*, 6R*,7R*)-(1-(hydroxymethyl)-7-methyl-1,5-diphenyl-1,5,6,7-tetrahydro[1,2,4]triazolo[4,3-a]pyrimidin-6-yl)ethan-1-ol (Figure 2) was relatively more stable other diastereomers. So, at the thermodynamic reaction control, the formation of this stereoisomer is preferable. On the other hand, the phenyl substituent located in a pseudo-axial position (Figure 1a) blocks the approach of the reagent from one side of the pyrimidine ring and leads to cis-orientation of substituents at C5 and C6 carbon atoms. The orientation of substituents at C7 and C6 carbon atoms can be assigned to the hydrogen trans-addition to carbon–carbon double bonds in the case of reduction by sodium borohydride. So, the formation of (1R*)-1-((5S*, 6R*,7R*)-(1-(hydroxymethyl)-7-methyl-1,5-diphenyl-1,5,6,7-tetrahydro[1,2,4]triazolo[4,3-a]pyrimidin-6-yl)ethan-1-ol can be explained from a kinetic reaction control point of view as well.
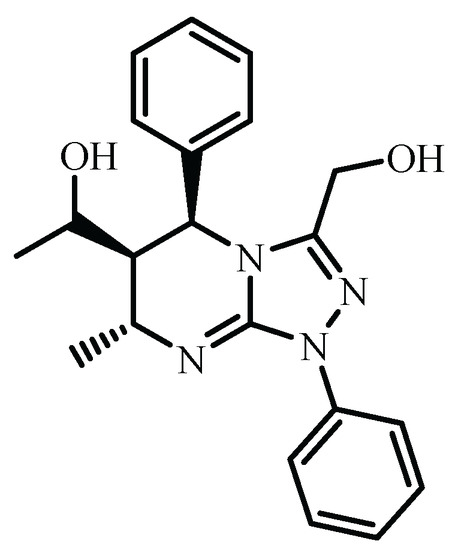
Figure 2.
Most stable diastereomer of compound 4 according to molecular mechanics calculations.
4. Conclusions
In this study, new 6-acetyl-2-arylhydrazone derivatives of thiazolo[3,2-a]pyrimidine were obtained. The structure of 6-acetyl-7-methyl-5-phenyl-2-(2-phenylhydrazineylidene)-5H-thiazolo[3,2-a]pyrimidine-3(2H)-one was confirmed by X-ray diffraction. It was shown that a zigzag heterochiral chain of hydrogen-bonded molecules was formed in the crystalline phase. The reaction of the 6-acetyl-2-phenylhydrazone derivative of thiazolo[3,2-a]pyrimidine with a seven-fold excess of sodium borohydride in the presence of vanadium(V) oxide led to the diastereoselective formation of 1-(hydroxymethyl)-7-methyl-1,5-diphenyl-1,5,6,7-tetrahydro[1,2,4]triazolo[4,3-a]pyrimidin-6-yl)ethan-1-ol.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org4030031/s1, Figure S1: 1H NMR spectrum of compound 2b (DMSO-d6, 400 MHz, 25 °C); Figure S2: 13C NMR spectrum of compound 2b (DMSO-d6, 100 MHz, 25 °C); Figure S3: IR spectrum of compound 2b (KBr tablet); Figure S4: 1H NMR spectrum of compound 3a (DMSO-d6, 400 MHz, 25 °C); Figure S5: 13C NMR spectrum of compound 3a (DMSO-d6, 100 MHz, 25 °C); Figure S6: IR spectrum of compound 3a (KBr tablet); Figure S7: ESI MS spectrum of compound 3a (Ion Polarity: Positive); Figure S8: 1H NMR spectrum of compound 3b (DMSO-d6, 400 MHz, 25 °C); Figure S9: 13C NMR spectrum of compound 3b (DMSO-d6, 100 MHz, 25 °C); Figure S10: IR spectrum of compound 3b (KBr tablet); Figure S11: ESI MS spectrum of compound 3b (Ion Polarity: Positive); Figure S12: 1H NMR spectrum of compound 3c (DMSO-d6, 400 MHz, 25 °C); Figure S13: 13C NMR spectrum of compound 3c (DMSO-d6, 100 MHz, 25 °C); Figure S14: IR spectrum of compound 3c (KBr tablet); Figure S15: ESI MS spectrum of compound 3c (Ion Polarity: Positive); Figure S16: 1H NMR spectrum of compound 4a (DMSO-d6, 400 MHz, 25 °C); Figure S18: 13C NMR spectrum of compound 4a (DMSO-d6, 100 MHz, 25 °C); Figure S18: DEPT spectrum of compound 4a (DMSO-d6, 100 MHz, 25 °C).
Author Contributions
Conceptualization, A.S.A., S.E.S. and I.S.A.; methodology, A.S.O. and I.A.L.; validation, A.S.A. and I.A.L.; formal analysis, I.A.L. and A.K.S.; investigation, A.A.N., D.O.M., A.S.A. and I.A.L.; resources A.K.S. and I.A.L.; data curation, A.S.A., I.S.A., S.E.S. and A.K.S.; writing—original draft preparation, A.S.A. and S.E.S.; writing—review and editing, A.S.A. and S.E.S.; visualization A.S.A., A.A.N. and D.O.M.; supervision, A.S.A., I.S.A. and S.E.S.; project administration, A.S.A., I.S.A. and S.E.S.; funding acquisition, S.E.S. and I.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by financial support from a government assignment for the Arbuzov Institute of Organic and Physical Chemistry, FRC Kazan Scientific Center, Russian Academy of Sciences (122011800132-5).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article or in Supplementary Materials, or are available on request from the corresponding author Artem Agarkov.
Acknowledgments
The authors are grateful to the Assigned Spectral-Analytical Center of Shared Facilities for Study of Structure, Composition and Properties of Substances and Materials of the Federal Research Center of Kazan Scientific Center of Russian Academy of Sciences (CSF-SAC FRC KSC RAS) for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fischer, G. Recent progress in 1,2,4-triazolo [1,5-a] pyrimidine chemistry. Adv. Heterocycl. Chem. 2007, 95, 143–219. [Google Scholar]
- Gujjar, R.; Marwaha, A.; El Mazouni, E.; White, J.; White, K.L.; Creason, S.; Shackleford, D.M.; Baldwin, J.; Charman, W.N.; Buckner, F.S.; et al. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J. Med. Chem. 2009, 52, 1864–1872. [Google Scholar] [PubMed]
- Yu, W.; Goddard, C.; Clearfield, E.; Mills, C.; Xiao, T.; Guo, H.; Morrey, J.D.; Motter, N.E.; Zhao, K.; Block, T.M.; et al. Design, synthesis, and biological evaluation of triazolo-pyrimidine derivatives as novel inhibitors of hepatitis B virus surface antigen (HBsAg) secretion. J. Med. Chem. 2011, 54, 5660–5670. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Shi, D.Q. Synthesis and herbicidal activity of O,O-dialkyl N-[2-(5,7-dimethyl-[1,2,4] triazolo [1,5-a] pyrimidin-2-yloxy) benzoxyl]-1-amino-1-substitutedbenzyl phosphonates. J. Heterocycl. Chem. 2010, 47, 162–166. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.M.; Chen, C.N.; Jiang, L.L.; Yang, G.F. Synthesis and Fungicidal Activities of New 1,2,4-Triazolo [1,5-a] pyrimidines. Chem. Biodivers. 2009, 6, 1254–1265. [Google Scholar] [CrossRef]
- Łakomska, I.; Wojtczak, A.; Sitkowski, J.; Kozerski, L.; Szłyk, E. Platinum (IV) complexes with purine analogs. Studies of molecular structure and antiproliferative activity in vitro. Polyhedron 2008, 27, 2765–2770. [Google Scholar] [CrossRef]
- Ashour, H.M.; Shaaban, O.G.; Rizk, O.H.; El-Ashmawy, I.M. Synthesis and biological evaluation of thieno [2′,3′:4,5] pyrimido [1,2-b][1,2,4] triazines and thieno [2,3-d][1,2,4] triazolo [1,5-a] pyrimidines as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2013, 62, 341–351. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, S.; Liu, Z.J.; Chen, W.; Fu, J.; Zeng, Q.F.; Zhu, H.L. Synthesis and antimicrobical evaluation of a novel class of 1,3,4-thiadiazole: Derivatives bearing 1,2,4-triazolo [1,5-a] pyrimidine moiety. Eur. J. Med. Chem. 2013, 64, 54–61. [Google Scholar] [CrossRef]
- Krezel, I. s-Triazolo[4,3-a] [1,3]diazacycloalkans. III: A novel synthesis of 2-aryl-3-oxo-2,3,5,6,7,8-hexahydro-s-triazolo[4,3-a] pyrimidines. Heterocycles 1986, 24, 93–100. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Khalil, A.K.; Abbass, E.M.; El-Naggar, A.M. Design, synthesis of new pyrimidine derivatives as anticancer and antimicrobial agents. Synth. Commun. 2017, 47, 1441–1457. [Google Scholar] [CrossRef]
- El-Zahar, M.I.; Abd El-Karim, S.S.; Haiba, M.E.; Khedr, M.A. Synthesis, antitumor activity and molecular docking study of novel benzofuran-2-yl pyrazole pyrimidine derivatives. Acta Pol. Pharm. Drug Res. 2011, 68, 357–373. [Google Scholar]
- Abdelhamid, A.O.; Sayed, A.R.; Zaki, Y.H. Reaction of Hydrazonoyl Halides 511: A Facile Synthesis of 5-Arylthiazoles and Triazolino[4,3-a]pyrimidines as Antimicrobial Agents. Phosphorus Sulfur Silicon 2007, 182, 1447–1457. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Edrees, M.M. Ethyl 7-Methyl-1-(4-nitrophenyl)-5-phenyl-3-(thiophen-2-yl)-1,5-dihydro-[1,2,4]triazolo [4,3-a]pyrimidine-6-carboxylate. Molbank 2017, 2, M942. [Google Scholar] [CrossRef]
- Lashmanova, E.A.; Agarkov, A.S.; Rybakov, V.B.; Shiryaev, A.K. Rearrangement of thiazolo[3,2-a]pyrimidines into triazolo[4,3-a]pyrimidines induced by C=N bond reduction. Chem. Heterocycl. Compd. 2019, 55, 1217–1221. [Google Scholar] [CrossRef]
- Agarkov, A.S.; Konorov, G.V.; Nefedova, A.A.; Gabitova, E.R.; Islamov, D.R.; Ovsyannikov, A.S.; Shiryaev, A.K.; Solovieva, S.E.; Antipin, I.S. Synthesis and structure of new derivatives of triazolo[4,3-a]pyrimidine and 2-phenylhydrazones of thiazolo[3,2-a]pyrimidine. Butlerov Commun. 2021, 68, 122–128. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J.V.D. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Foroughifar, N.; Jirandehi, H.F. Investigation of the effects of some heat sinks in microwave-assisted synthesis of some biginelli compounds. Phosphorus Sulfur Silicon 2004, 179, 2259–2263. [Google Scholar] [CrossRef]
- Zhang, G.P.; Tian, D.Y.; Shi, W.M. Efficient Catalytic Synthesis of 3,4-Dihydropyrimidin-2-ones/thiones via Little Acidic Ionic Liquid Combined with Rapid Heating Ways. J. Heterocycl. Chem. 2018, 55, 2522–2531. [Google Scholar] [CrossRef]
- Amrollahi, M.; Mobinikhaledi, A.; Forughifar, N. Synthesis of some new thiazolo pyrimidines using cyclocondensation reactions. Asian J. Chem. 2005, 17, 902–906. [Google Scholar]
- Agarkov, A.S.; Gabitova, E.R.; Galieva, F.B.; Ovsyannikov, A.S.; Voloshina, A.D.; Shiryaev, A.K.; Litvinov, I.A.; Solovieva, S.E.; Antipin, I.S. Structure and Biological Properties of 2-Phenylhydrazone Derivatives of Thiazolopyrimidines. Dokl. Chem. 2022, 503, 45–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).