On the Mechanism of the Synthesis of Nitrofunctionalised Δ2-Pyrazolines via [3+2] Cycloaddition Reactions between α-EWG-Activated Nitroethenes and Nitrylimine TAC Systems
Abstract
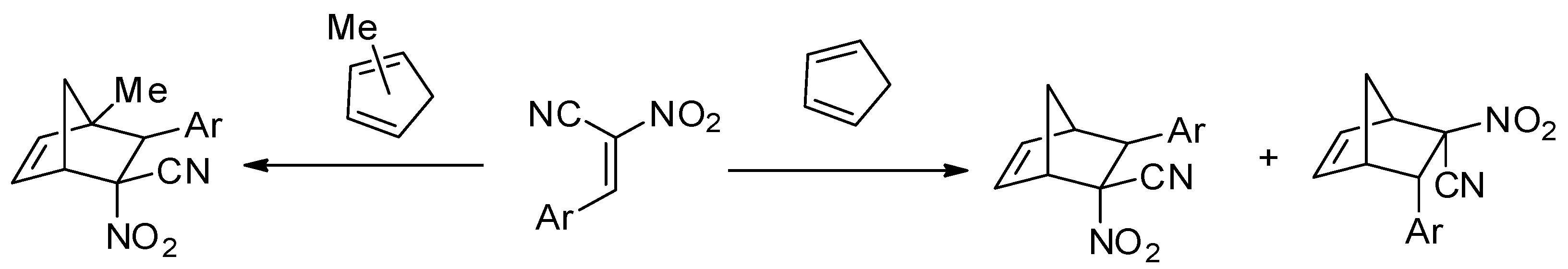
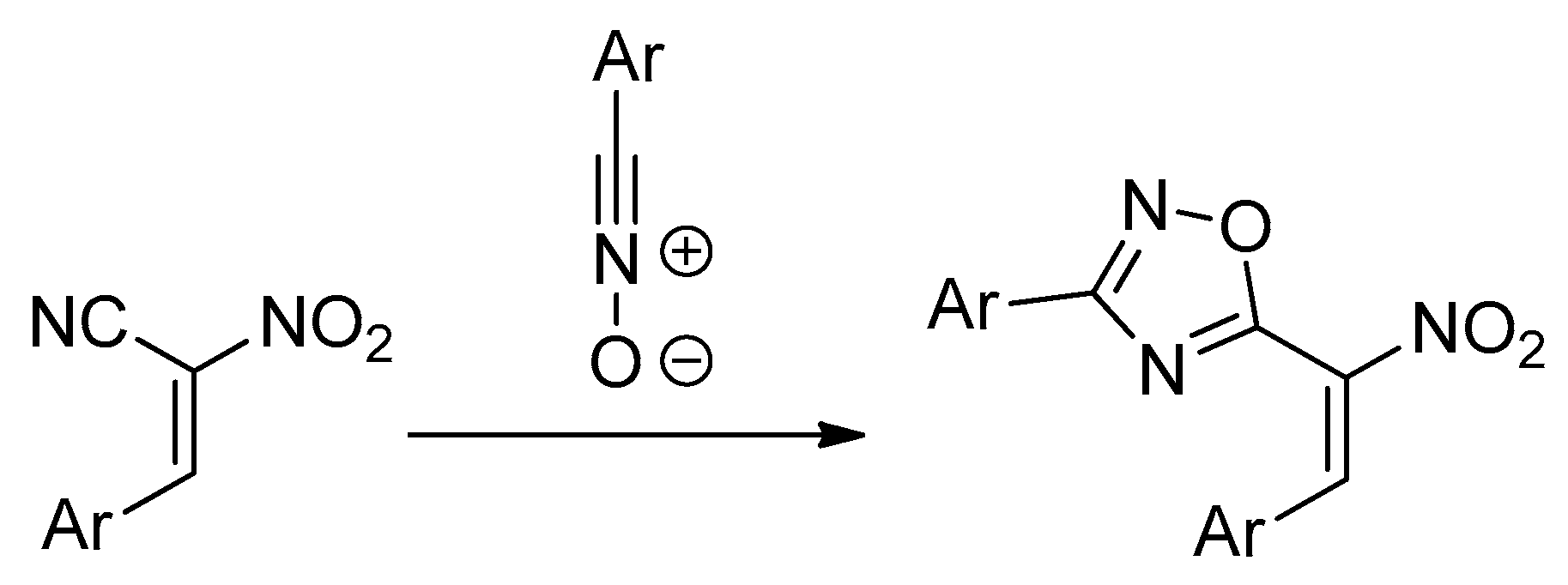
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Nature of Intermolecular Interactions of Addents According to the Analysis Based on the CDFT Reactivity Indices of Reagents
3.1.1. Global Reactivity
3.1.2. Local Reactivity
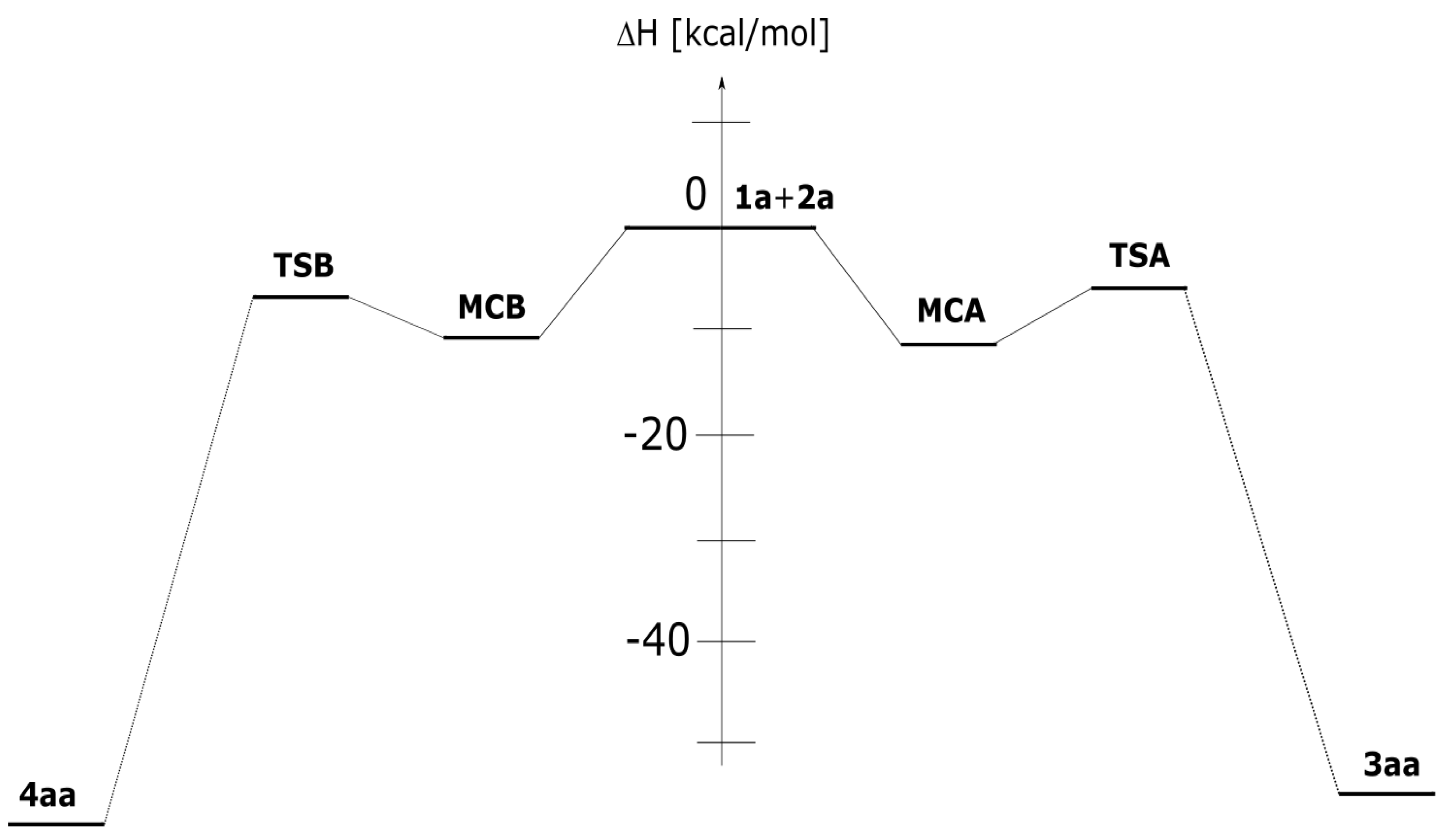
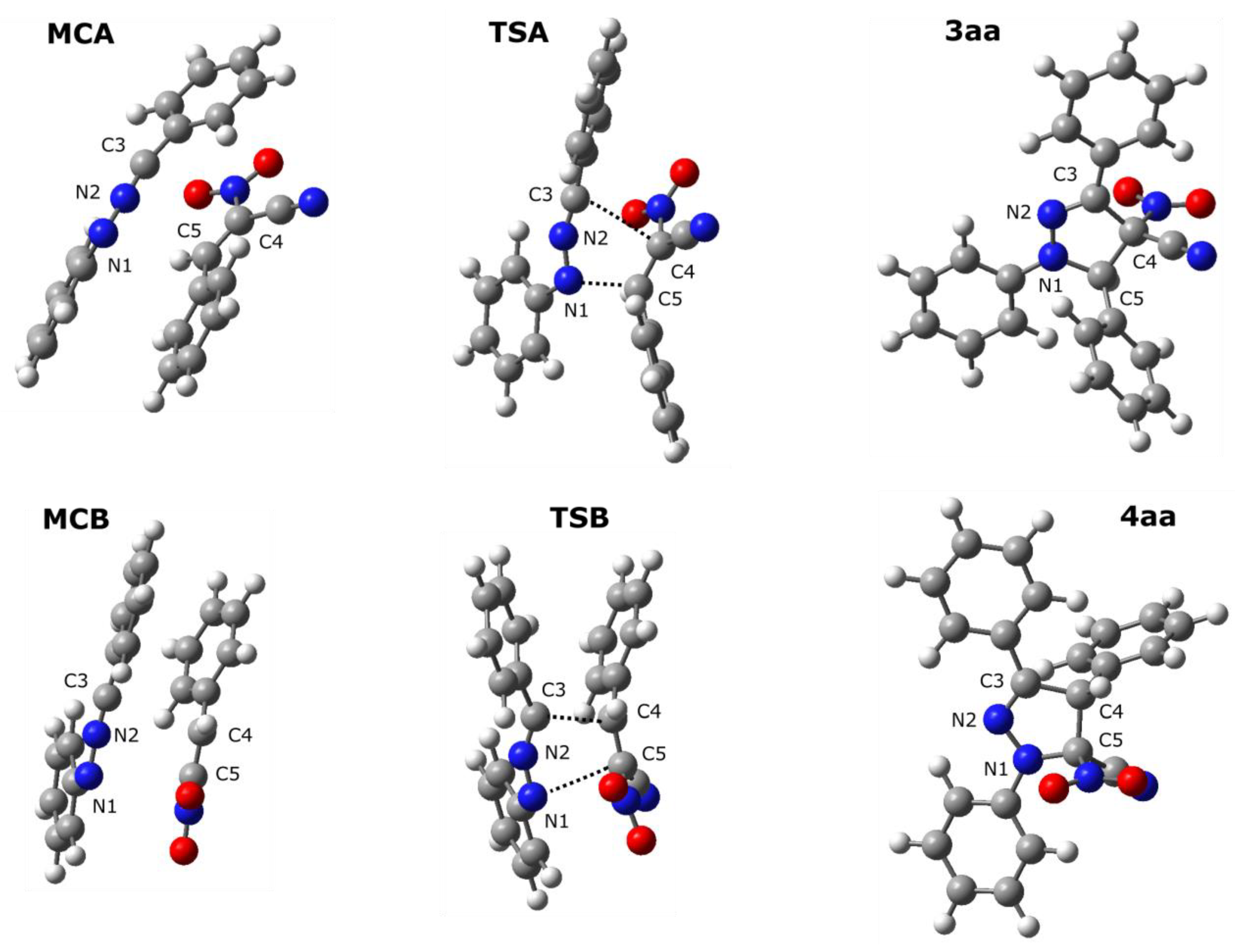
3.2. Reaction Profiles
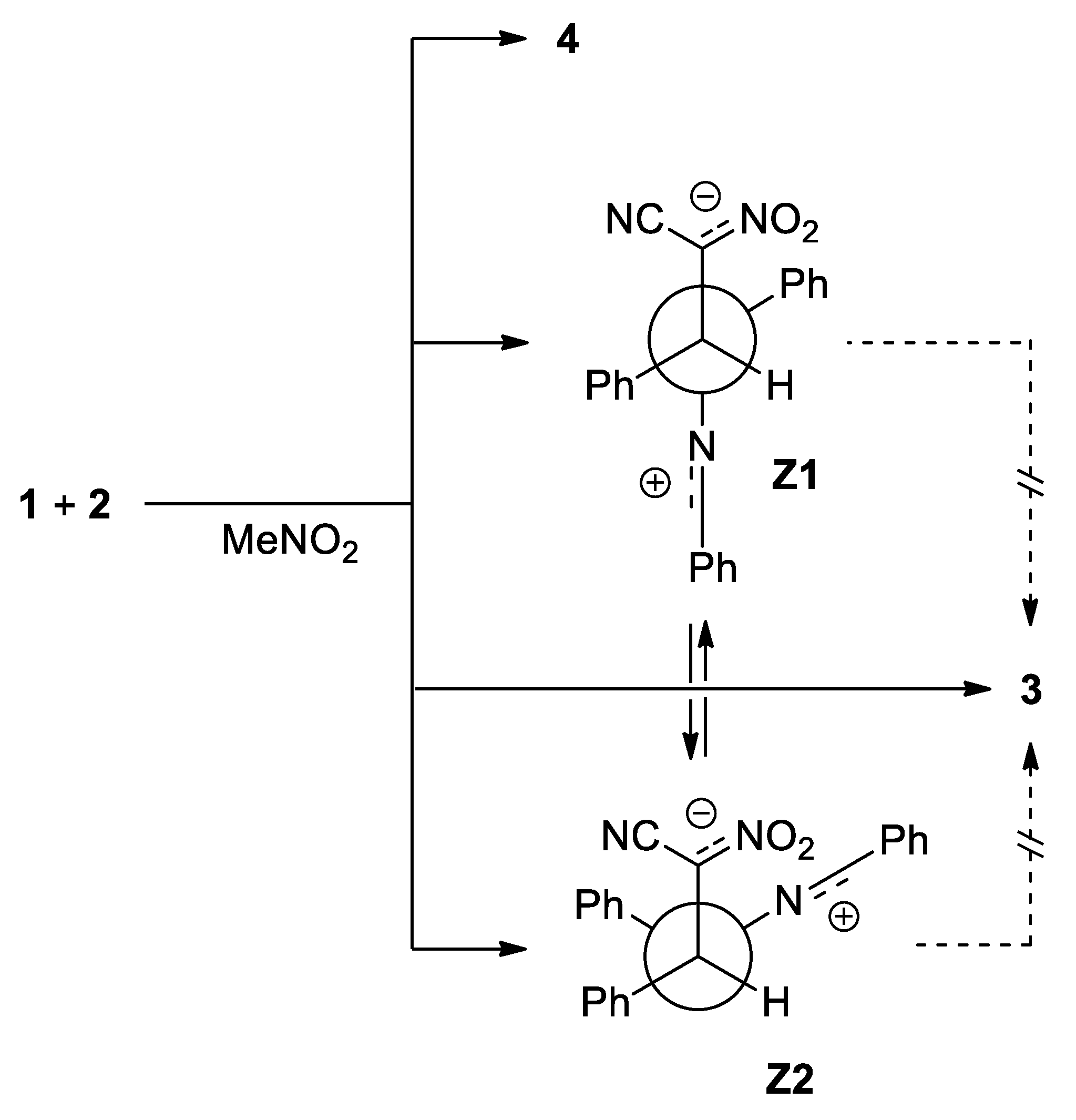
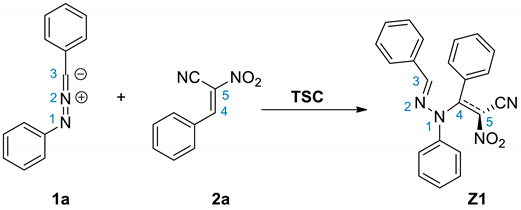
3.3. BET Analysis of the 32CA between C,N-diphenylnitrylimine 1a and (E)-2-phenyl-1-cyano-1-nitroethene 2a
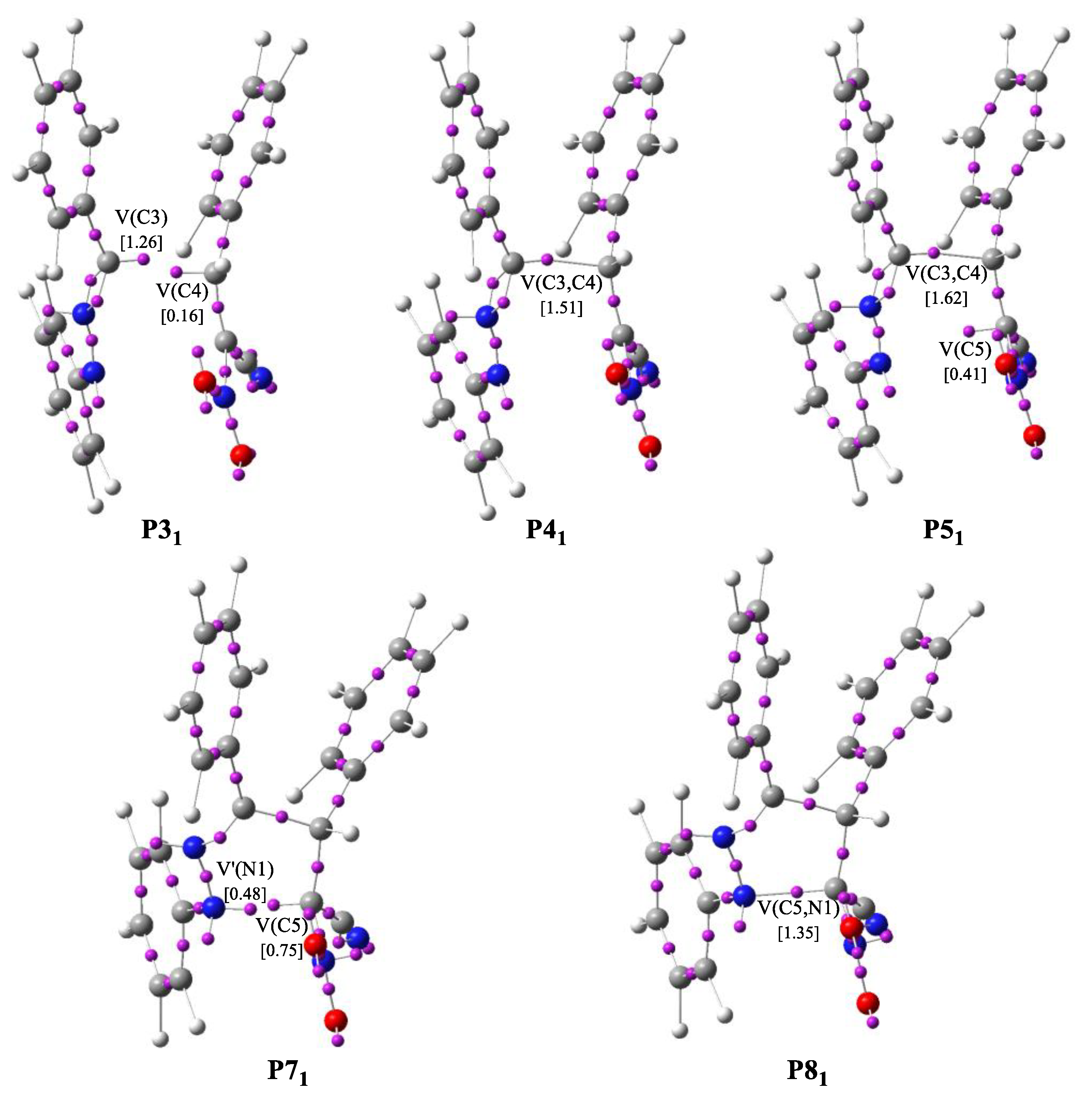
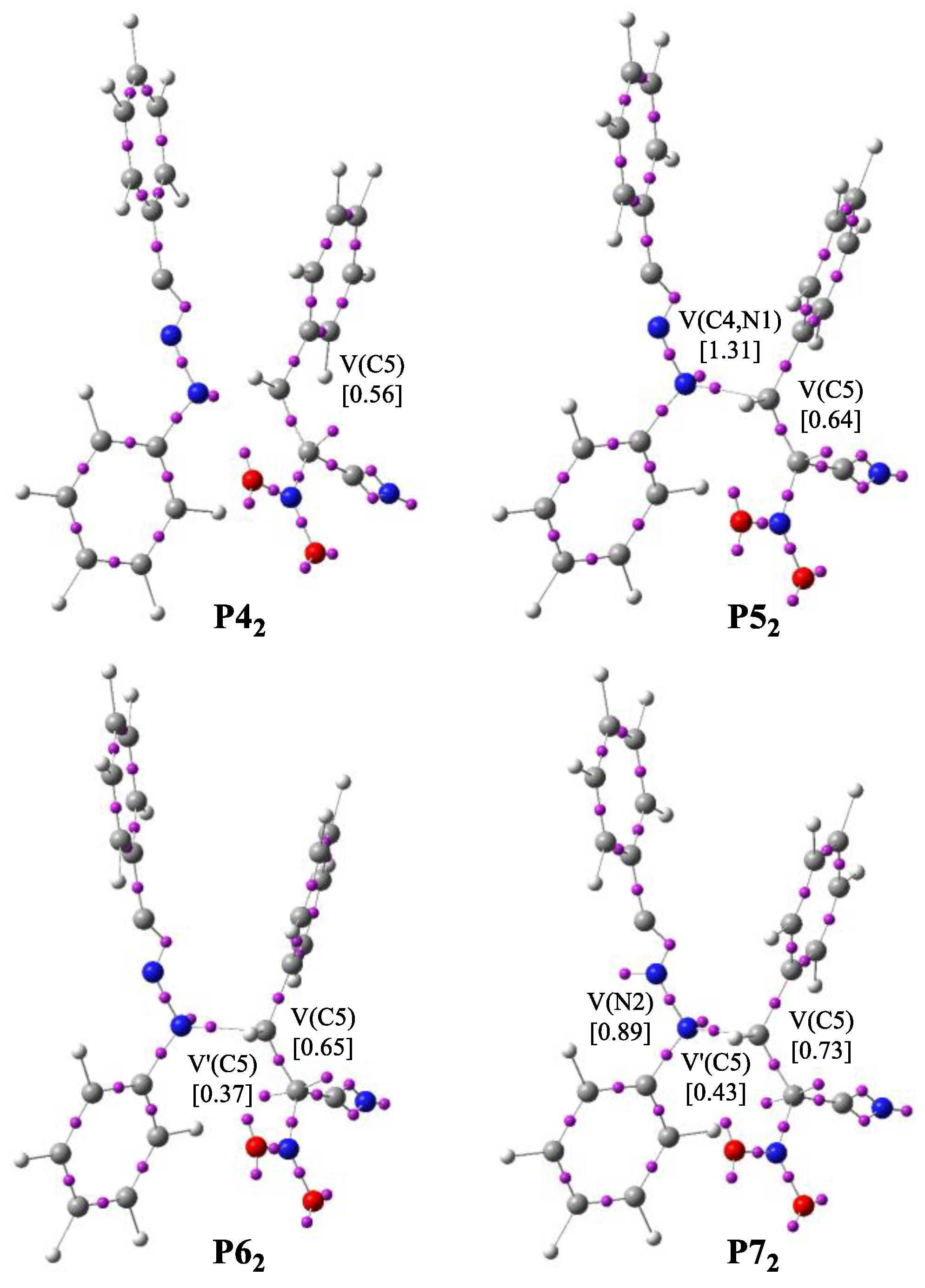
3.4. BET Study of the Creation of Acyclic Adduct Z1
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ried, W.; Köhler, E. Reaktionen mit Nitro-acetonitril. Lieb. Ann. Chem. 1956, 598, 145–158. [Google Scholar] [CrossRef]
- Kanishchev, M.I.; Korneeva, N.; Shevelev, S.A. Synthesis of 3-amino-5-benzylamino-4-nitropyrazole. Bull. Acad. Sci. USSR 1986, 35, 2145–2147. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Kula, K.; Wnorowski, A.; Biernasiuk, A.; Popiołek, Ł.; Miodowski, D.; Demchuk, O.M.; Jasiński, R. Novel functionalized β-nitrostyrenes: Promising candidates for new antibacterial drugs. Saudi Pharm. J. 2019, 27, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, R.; Mirosław, B.; Demchuk, O.M.; Babyuk, D.; Łapczuk-Krygier, A. In the search for experimental and quantumchemical evidence for zwitterionic nature of (2E)-3-[4-(dimethylamino)phenyl]-2-nitroprop-2-enenitrile—An extreme example of donor–π–acceptor push–pull molecule. J. Mol. Struct. 2016, 1108, 689–697. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Ponikiewski, Ł.; Jasiński, R. The crystal structure of (1RS,4RS,5RS,6SR)-5-cyano-5-nitro-6-phenyl-bicyclo[2.2.1]hept-2-ene. Crystallogr. Rep. 2014, 59, 961–963. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Jasiński, R. New Endo-Nitro-Substituted Norbornene Derivatives and Stereoselective Method for Producing New Endo-Nitro-Substituted Norbornene Derivatives. Patent No. PL 231749, 13 March 2017. [Google Scholar]
- Poos, G.I.; Kleis, J.; Wittekind, R.R.; Rosenau, J.D. Bicyclic Bases. III. Isomeric 2-Amino-3-phenylnorbornanes. J. Org. Chem. 1961, 26, 4898–4904. [Google Scholar] [CrossRef]
- Żmigrodzka, M.; Dresler, E.; Hordyjewicz-Baran, Z.; Kulesza, R.; Jasiński, R. A unique example of noncatalyzed 32CAinvolving (2E)-3-aryl-2-nitroprop-2-enenitriles. Chem. Heterocycl. Compd. 2017, 53, 1161–1162. [Google Scholar] [CrossRef]
- Jasiński, R.; Kula, K.; Kącka, A.; Mirosław, B. Unexpected course of reaction between (E)-2-aryl-1-cyano-1-nitroethenes and diazafluorene: Why is there no 1,3-dipolar cycloaddition? Mon. Chem. 2017, 148, 909–915. [Google Scholar] [CrossRef] [Green Version]
- Woliński, P.; Kącka-Zych, A.; Demchuk, O.M.; Łapczuk-Krygier, A.; Mirosław, B.; Jasiński, R. Clean and molecularly programmable protocol for preparation of bis-heterobiarylic systems via a domino pseudocyclic reaction as a valuable alternative for TM-catalyzed cross-couplings. J. Clean. Prod. 2020, 275, 122086. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, P.; Domingo, L.R.; Duque-Noreña, M.; Chamorro, E. A condensed-to-atom nucleophilicity index. An application to the director effects on the electrophilic aromatic substitutions. J. Mol. Struct. 2009, 895, 86–91. [Google Scholar] [CrossRef]
- Greelings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef] [PubMed]
- Zawadzińska, K.; Kula, K. Application of ß-phosphorylated nitroethenes in [3+2] cycloaddition reactions involving benzonitrile N-oxide in the light of DFT computational study. Organics 2021, 2, 26–37. [Google Scholar] [CrossRef]
- Pérez, P.; Domingo, L.R.; Aurell, M.J.; Contreras, R. Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1.3-dipolar cycloaddition reactions. Tetrahedron 2003, 59, 3117–3125. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Saez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Fox, D.J.; et al. (Eds.) Gaussian 16; Revision, A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry Optimization of Molecular Structures in Solution by the Polarizable Continuum Model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef] [Green Version]
- Jasiński, R. A stepwise, zwitterionic mechanism for the 1,3-dipolar cycloaddition between (Z)-C-4-methoxyphenyl-N-phenylnitrone and gem-chloronitroethene catalysed by 1-butyl-3-methylimidazolium ionic liquid cations. Tetrahedron Lett. 2015, 56, 532–535. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Savin, A.; Silvi, B.; Colonna, F. Topological Analysis of the Electron Localization Function Applied to Delocalized Bonds. Can. J. Chem. 1996, 74, 1088–1096. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Krokidis, K.; Noury, S.; Silvi, B. Characterization of Elementary Chemical Processes by Catastrophe Theory. J. Phys. Chem. A 1997, 101, 7277–7281. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, K.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Mirosław, B.; Babyuk, D.; Łapczuk-Krygier, A.; Kącka-Zych, A.; Demchuk, O.M.; Jasiński, R. Regiospecific formation of the nitromethyl-substituted 3-phenyl-4,5-dihydroisoxazole via [3+2] cycloaddition. Chem. Mon. 2018, 149, 1877–1884. [Google Scholar] [CrossRef]
- Kula, K.; Dobosz, J.; Jasiński, R.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Mirosław, B.; Demchuk, O.M. [3+2] Cycloaddition of diaryldiazomethanes with (E)-3,3,3-trichloro-1-nitroprop-1-ene: An experimental, theoretical and structural study. J. Mol. Struct. 2020, 1203, 127473. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Domingo, L.R.; Ríos-Gutiérrez, M.; Jasiński, R. Understanding the mechanism of the decomposition reaction of nitroethyl benzoate through the Molecular Electron Density Theory. Theor. Chem. Acc. 2017, 136, 129. [Google Scholar] [CrossRef] [Green Version]
- Kącka-Zych, A.; Jasiński, R. Unexpected molecular mechanism of trimethylsilyl bromide elimination from 2-(trimethylsilyloxy)-3-bromo-3-methyl-isoxazolidines. Theor. Chem. Acc. 2019, 138, 81. [Google Scholar] [CrossRef] [Green Version]
- Woliński, P.; Kącka-Zych, A.; Dziuk, B.; Ejsmont, K.; Łapczuk-Krygier, A.; Dresler, E. The structural aspects of the transformation of 3-nitroisoxazoline-2-oxide to 1-aza-2,8-dioxabicyclo[3.3.0]octane derivatives: Experimental and MEDT theoretical study. J. Mol. Struct. 2019, 1192, 27–34. [Google Scholar] [CrossRef]
- Kącka, A.; Jasiński, R. A dramatic change of kinetic conditions and molecular mechanism of decomposition processes of nitroalkyl carboxylates catalyzed by ethylammonium cations. Comput. Theor. Chem. 2017, 1104, 37–42. [Google Scholar] [CrossRef]
- Kącka-Zych, A. Participation of Phosphorylated Analogues of Nitroethene in Diels–Alder Reactions with Anthracene: A Molecular Electron Density Theory Study and Mechanistic Aspect. Organics 2020, 1, 36–48. [Google Scholar] [CrossRef]
- Kącka-Zych, A. The Molecular Mechanism of the Formation of Four-Membered Cyclic Nitronates and Their Retro (3+2) Cycloaddition: A DFT Mechanistic Study. Molecules 2021, 26, 4786. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R. Molecular Electron Density Theory: A modern view of reactivity in organic chemistry. Molecules 2016, 21, e1319. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Sarkar, U.; Roy, D.R. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Kula, K.; Zawadzinska, K. Local nucleophile-electrophile interactions in [3+2] cycloaddition reactions between benzonitrile N-oxide and selected conjugated nitroalkenes in the light of MEDT computational study. Curr. Chem. Lett. 2021, 10, 9–16. [Google Scholar] [CrossRef]
- Domingo, L.R.; Kula, K.; Ríos-Gutiérrez, M. Unveiling the reactivity of cyclic azomethine ylides in [3+2] cycloaddition reactions within the Molecular Electron Density Theory. Eur. J. Org. Chem. 2020, 5938–5948. [Google Scholar] [CrossRef]
- Mlostoń, G.; Kula, K.; Jasiński, R. A DFT Study on the Molecular Mechanism of Additions of Electrophilic and Nucleophilic Carbenes to Non-Enolizable Cycloaliphatic Thioketones. Molecules 2021, 26, 5562. [Google Scholar] [CrossRef]
- Domingo, L.R.; Kula, K.; Ríos-Gutiérrez, M.; Jasiński, R. Understanding the Participation of Fluorinated Azomethine Ylides in Carbenoid-Type [3+2] Cycloaddition Reactions with Ynal Systems: A Molecular Electron Density Theory Study. J. Org. Chem. 2021, 86, 12644–12653. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A molecular electron density theory study of the participation of tetrazines in aza-Diels–Alder reactions. RSC Adv. 2020, 10, 15394–15405. [Google Scholar] [CrossRef] [Green Version]
- Zawadzińska, K.; Ríos-Gutiérrez, M.; Kula, K.; Woliński, P.; Mirosław, B.; Krawczyk, T.; Jasiński, R. The Participation of 3,3,3-Trichloro-1-nitroprop-1-ene in the [3+2] Cycloaddition Reaction with Selected Nitrile N-Oxides in the Light of the Experimental and MEDT Quantum Chemical Study. Molecules 2021, 26, 6774. [Google Scholar] [CrossRef]
- Mlostoń, G.; Jasiński, R.; Kula, K.; Heimgartner, H. A DFT Study on the Barton-Kellogg Reaction—The molecular mechanism of the formation of thiiranes in the reaction between diphenyldiazomethane and diaryl thioketones. Eur. J. Org. Chem 2020, 176–182. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. Analysis of the possibility and molecular mechanism of carbon dioxide consumption in the Diels-Alder processes. Pure Appl. Chem. 2021, 93, 427–446. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos Gutiérrez, M.; Castellanos Soriano, J. Understanding the Origin of the Regioselectivity in Non-Polar [3+2] Cycloaddition Reactions through the Molecular Electron Density Theory. Organics 2020, 1, 19–35. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the High Reactivity of the Azomethine Ylides in [3+2] Cycloaddition Reactions. Lett. Org. Chem. 2010, 7, 432–439. [Google Scholar] [CrossRef]




| μ | η | ω | N | |
|---|---|---|---|---|
| 1a | –3.37 | 3.78 | 1.50 | 3.86 |
| 1b | –2.99 | 3.47 | 1.29 | 4.40 |
| 1c | –3.95 | 3.40 | 2.29 | 3.47 |
| 1d | –3.02 | 3.97 | 1.15 | 4.11 |
| 1e | –3.92 | 3.25 | 2.36 | 3.58 |
| 2a | –5.28 | 4.07 | 3.42 | 1.81 |
| 2b | –4.51 | 3.48 | 2.92 | 2.87 |
| 2c | –5.98 | 4.00 | 4.47 | 1.14 |
| Reaction | Path | Transition | ΔH | ΔG | ΔS |
|---|---|---|---|---|---|
| 1a+2a | A | 1a+2a→MCA | −12.1 | 1.4 | −45.2 |
| 1a+2a→TSA | −6.1 | 8.4 | −48.6 | ||
| 1a+2a→3aa | −56.5 | −40.0 | −55.4 | ||
| B | 1a+2a→MCB | −11.2 | 2.2 | −45.0 | |
| 1a+2a→TSB | −7.2 | 8.3 | −52.1 | ||
| 1a+2a→4aa | −58.5 | −42.3 | −54.3 | ||
| 1b+2a | A | 1b+2a→MCA | −13.1 | 0.4 | −45.3 |
| 1b+2a→TSA | −6.9 | 7.8 | −49.5 | ||
| 1b+2a→3ba | −56.6 | −41.5 | −50.7 | ||
| B | 1b+2a→MCB | −13.1 | 0.4 | −45.3 | |
| 1b+2a→TSB | −9.6 | 6.1 | −52.8 | ||
| 1b+2a→4ba | −59.0 | −42.9 | −54.0 | ||
| 1c+2a | A | 1c+2a→MCA | −7.8 | 4.8 | −42.5 |
| 1c+2a→TSA | −2.5 | 12.8 | −51.2 | ||
| 1c+2a→3ca | −53.8 | −37.2 | −55.7 | ||
| B | 1c+2a→MCB | −7.8 | 4.8 | −42.5 | |
| 1c+2a→TSB | −2.6 | 13.1 | −52.5 | ||
| 1c+2a→4ca | −55.0 | −38.3 | −55.9 | ||
| 1d+2a | A | 1d+2a→MCA | −10.2 | 4.8 | −50.2 |
| 1d+2a→TSA | −8.2 | 7.5 | −52.9 | ||
| 1d+2a→3da | −54.7 | −36.7 | −60.6 | ||
| B | 1d+2a→MCB | −10.2 | 4.8 | −50.2 | |
| 1d+2a→TSB | −6.7 | 9.9 | −55.5 | ||
| 1d+2a→4da | −56.9 | −39.1 | −59.9 | ||
| 1e+2a | A | 1e+2a→MCA | −11.7 | 3.5 | −51.1 |
| 1e+2a→TSA | −3.0 | 13.0 | −53.8 | ||
| 1e+2a→3ea | −57.1 | −40.2 | −56.7 | ||
| B | 1e+2a→MCB | −11.7 | 3.5 | −51.1 | |
| 1e+2a→TSB | −5.9 | 10.5 | −55.2 | ||
| 1e+2a→4ea | −58.6 | −41.1 | −58.7 | ||
| 1a+2b | A | 1a+2b→MCA | −12.9 | 0.4 | −44.4 |
| 1a+2b→TSA | −2.3 | 13.0 | −51.5 | ||
| 1a+2b→3ab | −52.8 | −36.3 | −55.5 | ||
| B | 1a+2b→MCB | −15.4 | −0.6 | −49.5 | |
| 1a+2b→TSB | −3.9 | 11.6 | −52.2 | ||
| 1a+2b→4ab | −54.3 | −37.3 | −57.1 | ||
| 1a+2c | A | 1a+2c→MCA | −17.0 | −1.6 | −51.5 |
| 1a+2c→TSA | −8.8 | 5.8 | −49.0 | ||
| 1a+2c→3ac | −58.7 | −42.9 | −53.0 | ||
| B | 1a+2c→MCB | −17.0 | −1.6 | −51.5 | |
| 1a+2c→TSB | −9.7 | 5.7 | −51.4 | ||
| 1a+2c→4ac | −61.4 | −42.6 | −63.2 |
| Reaction | Path | Transition | ΔH | ΔG | ΔS |
|---|---|---|---|---|---|
| 1a+2a | A | 1a+2a→MCA | −10.9 | 1.6 | −42.1 |
| 1a+2a→TSA | −5.1 | 7.5 | −42.4 | ||
| 1a+2a→3aa | −53.3 | −38.4 | −50.0 | ||
| B | 1a+2a→MCB | −10.9 | 1.6 | −42.1 | |
| 1a+2a→TSB | −5.6 | 9.0 | −48.9 | ||
| 1a+2a→4aa | −55.3 | −40.1 | −50.9 | ||
| C | 1a+2a→MCC | −12.0 | −0.5 | −38.4 | |
| 1a+2a→TSC | −3.9 | 9.1 | −43.5 | ||
| 1a+2a→Z1 | −11.0 | 1.3 | −41.4 | ||
| Z1→TSrot | −11.1 | 3.5 | −49.0 | ||
| D | 1a+2a→MCD | −12.0 | −0.5 | −38.4 | |
| 1a+2a→TSD | −2.3 | 10.9 | −44.2 | ||
| 1a+2a→Z2 | −10.5 | 2.4 | −43.3 |
| Reaction | Structure | Interatomic Distances [Å] | IC3-C4 | IC5-N1 | ΔI | GEDT [e] | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N1-N2 | N2-C3 | C3-C4 | C4-C5 | C5-N1 | ||||||
| 1a+2a | MCA | 1.262 | 1.161 | 3.629 | 1.345 | 4.034 | 0.00 | |||
| TSA | 1.280 | 1.163 | 2.867 | 1.393 | 2.032 | 0.120 | 0.608 | 0.49 | 0.67 | |
| 3aa | 1.335 | 1.284 | 1.525 | 1.566 | 1.460 | |||||
| MCB | 1.260 | 1.160 | 3.044 | 1.343 | 3.047 | 0.00 | ||||
| TSB | 1.244 | 1.200 | 2.176 | 1.380 | 2.646 | 0.566 | 0.119 | 0.45 | 0.39 | |
| 4aa | 1.379 | 1.278 | 1.517 | 1.561 | 1.406 | |||||
| 1b+2a | MCA | 1.253 | 1.167 | 3.354 | 1.345 | 4.727 | 0.00 | |||
| TSA | 1.277 | 1.162 | 2.904 | 1.391 | 2.065 | 0.094 | 0.584 | 0.49 | 0.62 | |
| 3ba | 1.328 | 1.286 | 1.524 | 1.567 | 1.458 | |||||
| MCB | 1.253 | 1.167 | 3.354 | 1.345 | 4.727 | 0.00 | ||||
| TSB | 1.240 | 1.200 | 2.267 | 1.374 | 2.714 | 0.507 | 0.062 | 0.45 | 0.34 | |
| 4ba | 1.376 | 1.280 | 1.518 | 1.557 | 1.400 | |||||
| 1c+2a | MCA | 1.253 | 1.162 | 4.200 | 1.344 | 3.658 | 0.00 | |||
| TSA | 1.289 | 1.164 | 2.793 | 1.400 | 1.972 | 0.172 | 0.651 | 0.48 | 0.72 | |
| 3ca | 1.343 | 1.281 | 1.528 | 1.564 | 1.462 | |||||
| MCB | 1.253 | 1.162 | 4.200 | 1.344 | 3.658 | 0.00 | ||||
| TSB | 1.251 | 1.203 | 2.131 | 1.382 | 2.569 | 0.595 | 0.182 | 0.41 | 0.31 | |
| 4ca | 1.383 | 1.278 | 1.517 | 1.562 | 1.413 | |||||
| 1d+2a | MCA | 1.267 | 1.160 | 3.040 | 1.343 | 2.965 | 0.00 | |||
| TSA | 1.284 | 1.165 | 2.937 | 1.386 | 2.109 | 0.077 | 0.554 | 0.48 | 0.58 | |
| 3da | 1.344 | 1.282 | 1.528 | 1.564 | 1.459 | |||||
| MCB | 1.267 | 1.160 | 3.040 | 1.343 | 2.965 | 0.00 | ||||
| TSB | 1.246 | 1.205 | 2.213 | 1.377 | 2.605 | 0.542 | 0.144 | 0.40 | 0.30 | |
| 4da | 1.386 | 1.280 | 1.518 | 1.559 | 1.404 | |||||
| 1e+2a | MCA | 1.246 | 1.168 | 3.355 | 1.342 | 3.187 | 0.00 | |||
| TSA | 1.278 | 1.165 | 2.745 | 1.399 | 1.990 | 0.198 | 0.639 | 0.44 | 0.67 | |
| 3ea | 1.325 | 1.287 | 1.524 | 1.564 | 1.462 | |||||
| MCB | 1.246 | 1.168 | 3.355 | 1.342 | 3.187 | 0.00 | ||||
| TSB | 1.239 | 1.201 | 2.158 | 1.381 | 2.683 | 0.577 | 0.098 | 0.48 | 0.43 | |
| 4ea | 1.370 | 1.278 | 1.516 | 1.563 | 1.411 | |||||
| 1a+2b | MCA | 1.264 | 1.159 | 3.705 | 1.358 | 4.033 | 0.00 | |||
| TSA | 1.283 | 1.161 | 2.871 | 1.405 | 1.982 | 0.116 | 0.644 | 0.53 | 0.69 | |
| 3ab | 1.334 | 1.284 | 1.523 | 1.568 | 1.461 | |||||
| MCB | 1.260 | 1.159 | 4.983 | 1.354 | 4.679 | 0.00 | ||||
| TSB | 1.243 | 1.202 | 2.143 | 1.387 | 2.650 | 0.587 | 0.115 | 0.47 | 0.38 | |
| 4ab | 1.380 | 1.279 | 1.517 | 1.562 | 1.406 | |||||
| 1a+2c | MCA | 1.266 | 1.157 | 4.178 | 1.342 | 3.512 | 0.00 | |||
| TSA | 1.281 | 1.162 | 2.874 | 1.389 | 2.045 | 0.118 | 0.596 | 0.48 | 0.67 | |
| 3ac | 1.337 | 1.283 | 1.527 | 1.564 | 1.456 | |||||
| MCB | 1.266 | 1.157 | 4.178 | 1.342 | 3.512 | 0.00 | ||||
| TSB | 1.244 | 1.200 | 2.216 | 1.375 | 2.619 | 0.540 | 0.138 | 0.40 | 0.38 | |
| 4ac | 1.378 | 1.278 | 1.517 | 1.560 | 1.406 | |||||
| Reaction | Structure | Interatomic Distances [Å] | IC3-C4 | IC5-N1 | ΔI | GEDT [e] | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N1-N2 | N2-C3 | C3-C4 | C4-C5 | C5-N1 | ||||||
| 1a+2a | MCA | 1.269 | 1.158 | 3.787 | 1.346 | 3.576 | 0.00 | |||
| TSA | 1.280 | 1.160 | 2.938 | 1.389 | 2.096 | 0.072 | 0.566 | 0.49 | 0.59 | |
| 3aa | 1.333 | 1.284 | 1.524 | 1.566 | 1.461 | |||||
| MCB | 1.269 | 1.158 | 3.205 | 1.346 | 3.221 | 0.00 | ||||
| TSB | 1.242 | 1.198 | 2.179 | 1.383 | 2.704 | 0.564 | 0.075 | 0.49 | 0.43 | |
| 4aa | 1.381 | 1.278 | 1.517 | 1.561 | 1.404 | |||||
| MCC | 1.270 | 1.157 | 5.700 | 1.347 | 4.125 | 0.00 | ||||
| TSC | 1.290 | 1.155 | 4.699 | 1.393 | 2.062 | 0.65 | 0.60 | |||
| Z1 | 1.308 | 1.151 | 4.628 | 1.482 | 1.529 | 1.05 | ||||
| MCD | 1.270 | 1.157 | 5.700 | 1.347 | 4.125 | 0.00 | ||||
| TSD | 1.291 | 1.152 | 4.290 | 1.399 | 2.042 | 0.65 | 0.48 | |||
| Z2 | 1.296 | 1.150 | 4.410 | 1.487 | 1.515 | 1.12 | ||||
| TS rot | 1.294 | 1.151 | 4.613 | 1.483 | 1.511 | 1.21 | ||||
| Points | 1a | 2a | MCB | P11 | P21 | P31 | P41 | P51 | P61 | P71 | P81 | 4aa | TSB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phases | I | I–II | II–III | III–IV | IV–V | V–VI | VI–VII | VII–VIII | VIII–IX | IX | |||
| d(C3−C4) | 2.803 | 2.634 | 2.240 | 2.079 | 2.014 | 1.884 | 1.605 | 1.548 | 1.541 | 1.521 | 2.176 | ||
| d(C5−N1) | 2.871 | 2.811 | 2.664 | 2.619 | 2.600 | 2.562 | 2.335 | 1.841 | 1.750 | 1.473 | 2.646 | ||
| ΔE | 0.0 | 1.9 | 3.6 | 3.3 | 2.6 | 0.2 | −10.0 | −27.7 | −39.4 | −47.3 | 4.0 | ||
| V(N1,N2) | 2.26 | 2.19 | 2.25 | 2.09 | 2.08 | 2.09 | 2.06 | 1.94 | 1.69 | 1.61 | 1.55 | 2.09 | |
| V(N1) | 3.45 | 3.43 | 3.32 | 3.10 | 2.96 | 2.93 | 2.83 | 2.76 | 2.44 | 2.34 | 2.09 | 3.02 | |
| V(N2,C3) | 2.72 | 2.76 | 2.67 | 2.27 | 2.14 | 2.10 | 2.00 | 3.17 | 3.23 | 3.18 | 3.17 | 2.20 | |
| V’(N2,C3) | 2.17 | 2.39 | 2.35 | 1.81 | 1.53 | 1.51 | 1.50 | 1.69 | |||||
| V(C3) | 0.99 | 0.70 | 0.85 | 1.16 | 1.26 | 1.20 | |||||||
| V(N2) | 0.94 | 1.42 | 1.54 | 1.74 | 2.37 | 2.68 | 2.74 | 2.78 | 1.17 | ||||
| V(C4,C5) | 1.79 | 1.93 | 3.45 | 3.42 | 3.28 | 3.25 | 2.84 | 2.35 | 2.13 | 2.06 | 2.00 | 3.53 | |
| V’(C4,C5) | 1.74 | 1.58 | |||||||||||
| V(C4) | 0.16 | ||||||||||||
| V(C3,C4) | 1.41 | 1.62 | 1.90 | 1.94 | 1.95 | 1.98 | |||||||
| V(C5) | 0.41 | 0.60 | 0.75 | ||||||||||
| V’(N1) | 0.48 | ||||||||||||
| V(C5,N1) | 1.35 | 1.71 | |||||||||||
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Points | 1a | 2a | MCC | P12 | P22 | P32 | P42 | P52 | P62 | P72 | Z1 | TSC |
| Phases | I | I–II | II–III | III–IV | IV–V | V–VI | VI–VII | VII–VIII | VIII | |||
| d(C4−N1) | 3.074 | 2.775 | 2.381 | 2.242 | 1.937 | 1.875 | 1.844 | 1.753 | 1.544 | 2.062 | ||
| ΔE | 0.0 | 2.3 | 5.4 | 7.6 | 6.8 | 5.1 | 3.7 | 2.2 | 1.0 | 8.1 | ||
| V(N1,N2) | 2.26 | 2.15 | 2.09 | 2.10 | 2.06 | 1.97 | 1.93 | 1.91 | 1.87 | 1.75 | 2.00 | |
| V(N1) | 3.45 | 3.49 | 3.51 | 3.41 | 3.47 | 3.48 | 2.26 | 2.20 | 2.09 | 1.92 | 3.40 | |
| V(N2,C3) | 2.72 | 2.97 | 3.20 | 3.81 | 5.87 | 5.83 | 5.85 | 5.86 | 4.98 | 4.30 | 5.84 | |
| V’(N2,C3) | 2.17 | 2.36 | 2.13 | 2.00 | ||||||||
| V(C3) | 0.99 | 0.45 | 0.27 | |||||||||
| V(C4,C5) | 1.79 | 1.89 | 3.49 | 3.51 | 3.53 | 2.95 | 2.54 | 2.31 | 2.28 | 2.04 | 3.52 | |
| V’(C4,C5) | 1.74 | 1.66 | ||||||||||
| V(C5) | 0.56 | 0.64 | 0.65 | 0.73 | 0.74 | |||||||
| V(C4,N1) | 1.31 | 1.41 | 1.52 | 1.77 | ||||||||
| V’(C5) | 0.37 | 0.43 | 0.57 | |||||||||
| V(N2) | 0.89 | 1.64 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fryźlewicz, A.; Olszewska, A.; Zawadzińska, K.; Woliński, P.; Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. On the Mechanism of the Synthesis of Nitrofunctionalised Δ2-Pyrazolines via [3+2] Cycloaddition Reactions between α-EWG-Activated Nitroethenes and Nitrylimine TAC Systems. Organics 2022, 3, 59-76. https://doi.org/10.3390/org3010004
Fryźlewicz A, Olszewska A, Zawadzińska K, Woliński P, Kula K, Kącka-Zych A, Łapczuk-Krygier A, Jasiński R. On the Mechanism of the Synthesis of Nitrofunctionalised Δ2-Pyrazolines via [3+2] Cycloaddition Reactions between α-EWG-Activated Nitroethenes and Nitrylimine TAC Systems. Organics. 2022; 3(1):59-76. https://doi.org/10.3390/org3010004
Chicago/Turabian StyleFryźlewicz, Agnieszka, Aleksandra Olszewska, Karolina Zawadzińska, Przemysław Woliński, Karolina Kula, Agnieszka Kącka-Zych, Agnieszka Łapczuk-Krygier, and Radomir Jasiński. 2022. "On the Mechanism of the Synthesis of Nitrofunctionalised Δ2-Pyrazolines via [3+2] Cycloaddition Reactions between α-EWG-Activated Nitroethenes and Nitrylimine TAC Systems" Organics 3, no. 1: 59-76. https://doi.org/10.3390/org3010004
APA StyleFryźlewicz, A., Olszewska, A., Zawadzińska, K., Woliński, P., Kula, K., Kącka-Zych, A., Łapczuk-Krygier, A., & Jasiński, R. (2022). On the Mechanism of the Synthesis of Nitrofunctionalised Δ2-Pyrazolines via [3+2] Cycloaddition Reactions between α-EWG-Activated Nitroethenes and Nitrylimine TAC Systems. Organics, 3(1), 59-76. https://doi.org/10.3390/org3010004










