Participation of Phosphorylated Analogues of Nitroethene in Diels–Alder Reactions with Anthracene: A Molecular Electron Density Theory Study and Mechanistic Aspect
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Analysis of the CDFT Reactivity Indices and ELF Characterization of the Reactants
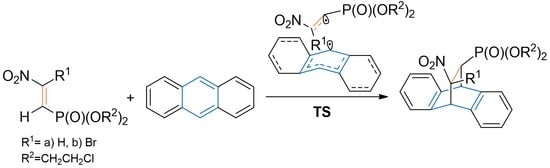
3.2. DA Reaction Profiles of the bis(2-chloroethyl) 2-nitro- 1a and bis(2-chloroethyl) 2-bromo-2-Nitroethenylphosphonates 1b with Anthracene 2
3.3. BET Study of the DA Reaction between bis(2-chloroethyl) 2-nitro-ethenylphosphonate 1a and Anthracene 2
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Coarey, E.J. Catalytic enantioselective Diels--Alder reactions: Methods, mechanistic fundamentals, pathways, and applications. Angew. Chem. Int. Ed. Engl. 2002, 17, 1650–1667. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels–Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. Engl. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Heravi, M.M.; Ahmadi, T.; Ghavidel, M.; Heidari, B.; Himidi, H. Recent applications of the hetero Diels–Alder reaction in the total synthesis of natural products. RSC Adv. 2015, 5, 101999–102075. [Google Scholar] [CrossRef]
- Feuer, H. Nitrile Oxides, Nitrones and Nitronates in Organic Synthesis: Novel Strategies in Synthesis, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kącka-Zych, A.; Jasiński, R. Molecular mechanism of Hetero Diels-Alder reactions between (E)-1,1,1-trifluoro-3-nitrobut-2-enes and enamine systems in the light of Molecular Electron Density Theory. J. Mol. Graph. Model. 2020, 101, 107714. [Google Scholar] [CrossRef] [PubMed]
- Korotaev, V.Y.; Barkov, A.Y.; Slepukihin, P.A.; Kodess, M.I.; Sosnovskikh, V.Y. Diastereoselective reactions of 1,1,1-trichloro(trifluoro)-3-nitrobut-2-enes with 2-morpholinoalk-1-enes. Mendeleev Commun. 2011, 21, 112–114. [Google Scholar] [CrossRef]
- Korotaev, V.Y.; Barkov, A.Y.; Slepukihin, P.A.; Kodess, M.I.; Sosnovskikh, V.Y. Uncatalyzed reactions of α-(trihaloethylidene)nitroalkanes with push–pull enamines: A new type of ring–ring tautomerism in cyclobutane derivatives and the dramatic effect of the trihalomethyl group on the reaction pathway. Tetrahedron Lett. 2011, 52, 5764–5768. [Google Scholar] [CrossRef]
- Lee, C.K.; Hahn, C.S. Diels-Alder Reaction of Pyrrole with Dimethyl Acetylenedicarboxylate. J. Org. Chem. 1978, 43, 3727–3729. [Google Scholar] [CrossRef]
- Domingo, L.R.; Picher, M.T.; Zaragoza, R.J. Toward an Understanding of the Molecular Mechanism of the Reaction between 1-Methylpyrrole and Dimethyl Acetylenedicarboxylate. An ab Initio Study. J. Org. Chem. 1998, 63, 9183–9189. [Google Scholar] [CrossRef]
- Anisimova, N.A.; Kuzhaeva, A.A.; Berkova, G.A.; Deiko, L.I.; Berestovitskaya, V.M. Reactions of 2-Nitro- and 2-Bromo-2-nitroethenylphosphonates with Anthracene. Russ. J. Gen. Chem. 2005, 75, 689–693. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Krokidis, X.; Noury, S.; Silvi, B. Characterization of elementary chemical processes by catastrophe theory. J. Phys. Chem. A 1997, 101, 7277–7283. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.J.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 16 Rev A.1; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Stephens, P.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Dresler, E.; Kącka-Zych, A.; Kwiatkowska, M.; Jasiński, R. Regioselectivity, stereoselectivity, and molecular mechanism of [3 + 2] cycloaddition reactions between 2-methyl-1-nitroprop-1-ene and (Z)-C-aryl-N-phenylnitrones: A DFT computational study. J. Mol. Model. 2018, 24, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kącka-Zych, A. Understanding the molecular mechanism of the rearrangement of internal nitronic ester into nitronorbornene in light of the MEDT study. Molecules 2019, 24, 462. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Jasiński, R. Unexpected molecular mechanism of trimethylsilyl bromide elimination from 2-(trimethylsilyloxy)-3-bromo-3-methyl-isoxazolidines. Theor. Chem. Acc. 2019, 138, 81–86. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Ríos-Gutiérrez, M.; Domingo, L. A molecular electron density theory study of the Lewis acid–catalyzed decomposition reaction of nitroethyl benzoate using aluminum derivatives. J. Phys. Org. Chem. 2019, 32, e3938. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Unveiling the Lewis Acid Catalyzed Diels–Alder Reactions through the Molecular Electron Density Theory. Molecules 2020, 25, 2535. [Google Scholar] [CrossRef]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- Tapia, O. Solvent effect theories: Quantum and classical formalism and their applications in chemistry and biochemistry. J. Math. Chem. 1992, 10, 131–181. [Google Scholar] [CrossRef]
- Tomasi, J.; Perisco, M. Molecular interactions in solution: An overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2017–2094. [Google Scholar] [CrossRef]
- Simkin, Y.; Sheikhet, I. Quantum Chemical and Statistical Theory of Solutions: A Computational Approach; Ellis Horwood: London, UK, 1995. [Google Scholar]
- Cances, E. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Chem. 1996, 225, 327–335. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Phys. Chem. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Greelings, P.; De Proft, F.; Langenaeker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView Version 6.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Ahrens, J.; Geveci, B.; Law, C. ParaView: An End-User Tool for Large Data Visualization. In Visualization Handbook; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Ayachit, U. The ParaView Guide: A Parallel Visualization Application; Kitware: New York, NY, USA, 2015. [Google Scholar]
- Kącka-Zych, A. Push-pull nitronates in the [3+2] cycloaddition with nitroethylene: Molecular Electron Density Theory study. J. Mol. Graph. Model. 2020, 97, 107549. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels–Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struct. THEOCHEM 2008, 865, 68–72. [Google Scholar] [CrossRef]












| µ | η | ω | N | |
|---|---|---|---|---|
| 1a | −5.59 | 5.19 | 3.01 | 0.94 |
| 1b | −5.62 | 4.74 | 3.33 | 1.13 |
| 2 | −3.43 | 3.59 | 1.64 | 3.89 |
| Transition | ΔH353 | ΔG353 | ΔS353 |
|---|---|---|---|
| 1+2→MC1 | −2.1 | 7.9 | −33.7 |
| 1+2→TS1 | 21.8 | 36.8 | −50.4 |
| 1+2→3a | −13.6 | 1.5 | −50.7 |
| 1+2→MC2 | −3.6 | 4.8 | −28.0 |
| 1+2→TS2 | 18.7 | 35.2 | −55.3 |
| 1+2→3b | −15.0 | 3.8 | −63.0 |
| Structure | C2-C3 | C1-C6 | Δl | GEDT [e] | Imaginary Frequency [cm−1] | ||
|---|---|---|---|---|---|---|---|
| r [Å] | l | r [Å] | l | ||||
| MC1 | 6.816 | 3.925 | |||||
| TS1 | 2.106 | 0.664 | 2.430 | 0.448 | 0.22 | 0.30 | −399.48 |
| 3a | 1.576 | 1.566 | |||||
| MC2 | 7.256 | 5.187 | |||||
| TS2 | 1.958 | 0.762 | 2.805 | 0.203 | 0.56 | 0.36 | −332.75 |
| 3b | 1.581 | 1.561 | |||||
| Points | 1a | 2 | MC1 | P1 | P2 | P3 | P4 | P5 | P6 | P7 | 3a | TS1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phases | I | II | III | IV | V | VI | VII | VIII | ||||
| d(C2-C3) | 3.489 | 2.659 | 2.502 | 2.449 | 2.412 | 2.356 | 2.316 | 2.184 | 1.566 | 2.430 | ||
| d(C1-C6) | 3.643 | 2.429 | 2.208 | 2.132 | 2.081 | 2.005 | 1.956 | 1.821 | 1.576 | 2.106 | ||
| ΔE | 0.0 | 8.5 | 17.1 | 22.0 | 21.8 | 15.8 | 5.5 | −2.7 | −11.5 | 24.0 | ||
| V(C1,C2) | 1.74 | 1.75 | 3.32 | 3.19 | 2.95 | 2.69 | 2.55 | 2.45 | 2.22 | 1.99 | 2.87 | |
| V’(C1,C2) | 1.74 | 1.69 | ||||||||||
| V(C3,C4) | 2.71 | 2.81 | 2.65 | 2.58 | 2.55 | 2.53 | 2.49 | 2.45 | 2.33 | 2.05 | 2.54 | |
| V(C4,C5) | 2.47 | 2.45 | 2.59 | 2.60 | 2.61 | 2.62 | 2.65 | 2.66 | 2.70 | 2.75 | 2.61 | |
| V(C5,C6) | 2.71 | 2.79 | 2.65 | 2.50 | 2.40 | 2.35 | 2.29 | 2.23 | 2.16 | 2.05 | 2.37 | |
| V(C6,C7) | 2.93 | 2.83 | 2.69 | 2.53 | 2.46 | 2.41 | 2.34 | 2.31 | 2.22 | 2.07 | 2.44 | |
| V(C7,C8) | 2.47 | 2.48 | 2.54 | 2.60 | 2.61 | 2.62 | 2.64 | 2.65 | 2.69 | 2.77 | 2.61 | |
| V(C8,C3) | 2.93 | 2.81 | 2.76 | 2.69 | 2.64 | 2.61 | 2.61 | 2.47 | 2.33 | 2.06 | 2.61 | |
| V(C3) | 0.13 | 0.23 | 0.32 | 0.28 | ||||||||
| V(C2) | 0.34 | 0.45 | 0.40 | |||||||||
| V(C1) | 0.38 | 0.50 | 0.57 | |||||||||
| V(C2,C3) | 0.96 | 1.07 | 1.38 | 1.76 | ||||||||
| V(C6) | 0.15 | |||||||||||
| V(C1,C6) | 1.02 | 1.79 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kącka-Zych, A. Participation of Phosphorylated Analogues of Nitroethene in Diels–Alder Reactions with Anthracene: A Molecular Electron Density Theory Study and Mechanistic Aspect. Organics 2020, 1, 36-48. https://doi.org/10.3390/org1010004
Kącka-Zych A. Participation of Phosphorylated Analogues of Nitroethene in Diels–Alder Reactions with Anthracene: A Molecular Electron Density Theory Study and Mechanistic Aspect. Organics. 2020; 1(1):36-48. https://doi.org/10.3390/org1010004
Chicago/Turabian StyleKącka-Zych, Agnieszka. 2020. "Participation of Phosphorylated Analogues of Nitroethene in Diels–Alder Reactions with Anthracene: A Molecular Electron Density Theory Study and Mechanistic Aspect" Organics 1, no. 1: 36-48. https://doi.org/10.3390/org1010004
APA StyleKącka-Zych, A. (2020). Participation of Phosphorylated Analogues of Nitroethene in Diels–Alder Reactions with Anthracene: A Molecular Electron Density Theory Study and Mechanistic Aspect. Organics, 1(1), 36-48. https://doi.org/10.3390/org1010004