Harnessing Mechanical Force for Greenhouse Gas Conversion: A Mini-Review on Mechanochemistry in the Dry Reforming of Methane
Abstract
1. Introduction
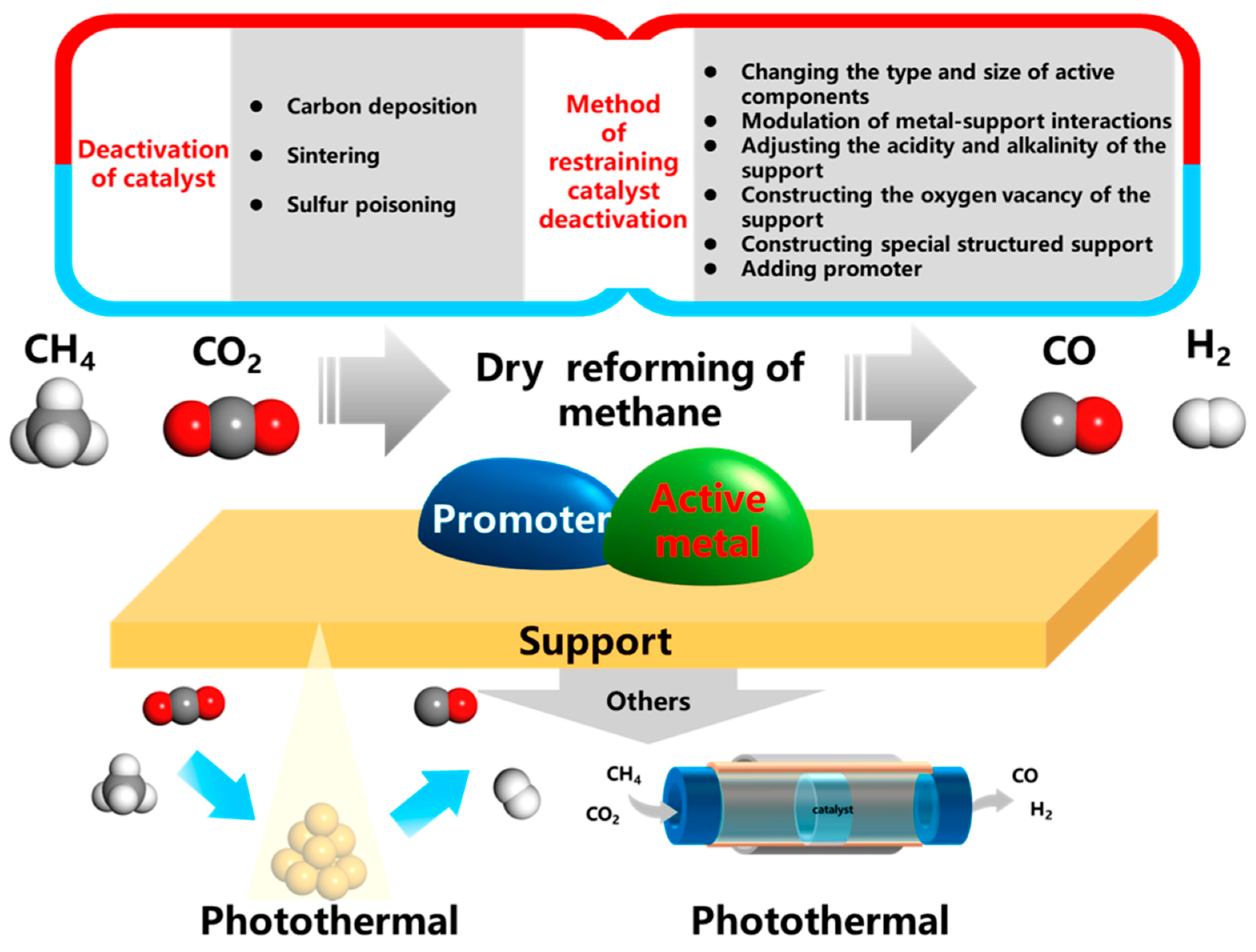
2. Mechanochemical Strategies for Advanced DRM Catalyst Synthesis
2.1. Principle of Mechanochemical Catalyst Synthesis
Core Advantages of Mechanochemical Catalyst Synthesis
2.2. Enhancing Catalyst Stability I: Mitigating Sintering via Strong Metal-Support Interactions (SMSI)
2.3. Enhancing Catalyst Stability II: Designing Coke-Resistant Bimetallic Alloys
2.4. Improving Catalyst Activity: Synthesis of Highly Dispersed Nanocatalysts
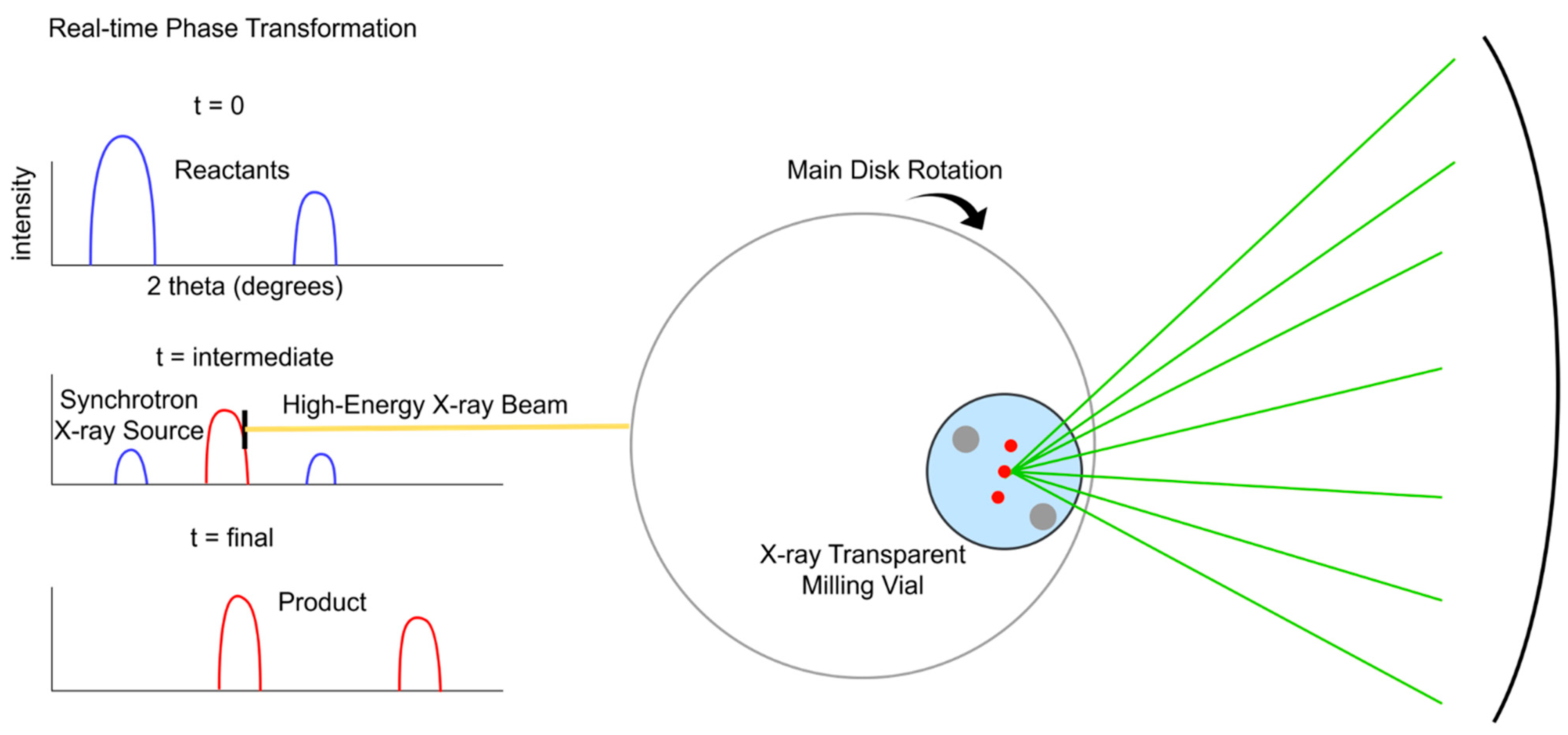
3. Direct Mechanochemical Dry Reforming of Methane
3.1. Proof of Concept and Related Studies
3.1.1. Mechanistic Insights into Force-Induced CH4 and CO2 Activation
| Application | Catalyst | Synthesis Details | Reaction Conditions | Performance Metrics | Advantage | Ref. |
|---|---|---|---|---|---|---|
| CO2 Methanation | Ni-Co bimetallic | Mechanochemical: Co + Ni precursors, 500 rpm, 2 h | 360 °C, 0.1 MPa, GHSV 9600 mL/(h·gcat) | 84.5% CO2 conversion, 99.8% CH4 selectivity, STY 1325.6 g/(kg·h) | Electron transfer from Ni to Co; enhanced CO2 adsorption; superior to literature reports | [91] |
| Biogas Reforming | 0.2% Ru/MgO-0.2CTAB | Soft template mechanochemical: CTAB-assisted, 400 rpm, 1.5 h | CH4:CO2 = 1.5:1, 750 °C, GHSV 30,000 h−1 | 94% CH4 conversion, 61% CO2 conversion, stable 120 h | Excellent Ru dispersion; MgO alkalinity for CO2 activation; coke suppression | [92] |
| Methanol Steam Reforming | 5% Pd/porous ZnO | Ball-milling salt-templating: ZnO + NaCl, 500 rpm, 3 h | 300 °C, H2O:CH3OH = 1.3:1, WHSV 1.2 h−1 | 98% methanol conversion, 72% H2 selectivity | High oxygen vacancy concentration; enhanced Pd-support interaction | [93] |
| Methane Oxidation | 2% Pd/CeO2 | Dry ball milling: metallic Pd + CeO2, 900 rpm, 10 min | Lean: 0.5% CH4, 2% O2, He balance, GHSV 200,000 h−1 | T50 = 350 °C, 100% conversion at 450 °C, stable in H2O (10%) | Pd-Ce-O amorphous interface; superior water tolerance; low-temperature activity | [78] |
| Dry Reforming of Methane | Ni-Al2O3 (mechanochemical) | One-step milling: Ni + Al precursors, 300 rpm, 20 min | 800 °C, CH4:CO2 = 1:1, GHSV 15,000 mL/(g·h) | CH4 conversion 85%, CO2 conversion 88%, H2/CO = 0.95, stable 100 h | Reduced carbon deposition; strong metal-support interaction; coke resistance | [94] |
| Hydrodesulfurization | V2O5 with oxygen vacancies | Ball milling: V2O5 + oxalic acid, 400 rpm, 3 h | 350 °C, H2 pressure 3 MPa, LHSV 1 h−1 | 94% sulfur removal from diesel (500 ppm to <30 ppm) | Abundant oxygen vacancies; enhanced adsorption; room temperature synthesis | [95] |
| CO-PROX | CuO-CeO2 nanocomposite | Ball milling: Cu + CeO2, 400 rpm, 90 min, air atmosphere | 120 °C, 1% CO, 1% O2, 50% H2, He balance | 97% CO conversion, >98% CO2 selectivity, stable 100 h | Four active oxygen sites; strong Cu-Ce interaction; H2-tolerant | [96] |
| Nitrobenzene Hydrogenation | Pt/meso-Al2O3 | Solvent-free milling: H2PtCl6 + Al2O3, 50 Hz, 30 min, 400 °C calcination | 80 °C, 1 MPa H2, ethanol solvent, substrate:catalyst = 100:1 | >95% conversion, 98% aniline selectivity, TOF 285 h−1 | High Pt dispersion (2–4 nm); 465 m2/g support area; excellent recyclability (5 cycles) | [97] |
| Ammonia Synthesis | Fe powder catalyst | Mechanochemical: N2 + H2 flow, Ti/Fe milling media, ball mill | 45 °C, 1 bar, continuous N2+H2 flow | 82.5 vol% NH3 concentration | Avoids Haber-Bosch harsh conditions; dynamic surface regeneration; energy-efficient | [98] |
| Ethanol Steam Reforming | Ni-CeO2 (ball-milled) | 600 °C, H2O:C2H5OH = 3:1, GHSV 10,000 h−1 | 600 °C, H2O:C2H5OH = 3:1, GHSV 10,000 h−1 | 98% ethanol conversion, 65% H2 yield, low carbon deposition | High Ni dispersion; strong Ni-CeO2 interaction; stable 50 h | [99] |
| Catalytic Oxidation | MnO_x (defect-rich) | One-step milling: MnO2, 400 rpm, 2 h, air | 200 °C, gaseous POPs removal, GHSV 30,000 h−1 | >95% removal efficiency for hexachlorobenzene | Mechanochemically induced oxygen vacancies; reactive oxygen species; low-temperature activity | [100] |
| Photo-Fenton Catalysis | TiO2/Magnetite (10%) | Ball milling: TiO2 (P25) + natural magnetite, 250 rpm, 20 min | UV light (365 nm), H2O2 5 mM, pollutant 20 mg/L | 92% degradation of methylene blue in 60 min | Enhanced H2O2 decomposition; OH· generation; Fe(III)/Fe(II) cycle acceleration | [101] |
C–H Bond Activation Mechanisms
CO2 Activation Mechanisms
Mechanochemical Force-Induced Reactivity
Compression, Shear, and Amorphization Pathways
3.2. Key Parameters Influencing Mechanochemical Dry Reforming of Methane
4. Summary, Challenges, and Future Outlook
4.1. Overarching Challenges
4.2. Future Research Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DRM | Dry Reforming of Methane |
| SMSI | Strong Metal-Support Interactions |
| GHG | Greenhouse Gas |
| RWGS | Reverse Water-Gas Shift |
| SRM | Steam Reforming of Methane |
| POX | Partial Oxidation |
| NTP | Non-Thermal Plasma |
| HEA | High-Entropy Alloys |
| BPR | Ball-to-Powder Ratio |
| PCA | Process Control Agents |
| TSE | Twin-Screw Extrusion |
| DFT | Density Functional Theory |
| TOS | Time-On-Stream |
| TPO | Temperature-Programmed Oxidation |
| FESEM | Field Emission Scanning Electron Microscopy |
| NAP-XPS | Near-Ambient Pressure X-ray Photoelectron Spectroscopy |
References
- Kanna, V.; Roseline, S.; Balamurugan, K.; Jeeva, S.; Santhiyagu, I.A. The effects of greenhouse gas emissions on global warming. Encycl. Renew. Energy Sustain. Environ. 2024, 1, 143–154. [Google Scholar]
- Hake, J.-F.; Kuckshinrichs, W. Greenhouse Gas Mitigation Strategies in Germany and the European Community. In Strategies and Technologies for Greenhouse Gas Mitigation; Routledge: Oxfordshire, UK, 2019; pp. 11–32. [Google Scholar]
- Balogun, B.; Adigu, I.; Adeleke, O.; Bosini, O.; Wordu, E.; Usiagu, G.; Onyeme, C.; Ewuzie, V.O. Crude Oil Bulk Flow Strategy to Manage Emission Abatement and Gas Flaring—An Adafill Eastern Asset Success Story. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeri, 5–7 August 2024; p. D032S029R001. [Google Scholar]
- von Rosing, M.; Wackernagel, M. CO- compensation. In The Sustainability Handbook, Volume 1; Elsevier: Amsterdam, The Netherlands, 2025; pp. 151–169. [Google Scholar]
- Gan, K.E.; Taikan, O.; Gan, T.Y.; Weis, T.; Yamazaki, D.; Schüttrumpf, H. Enhancing renewable energy systems, contributing to Sustainable Development Goals of United Nation and building resilience against climate change impacts. Energy Technol. 2023, 11, 2300275. [Google Scholar] [CrossRef]
- Olsen, K.H.; Arens, C.; Mersmann, F. Learning from CDM SD tool experience for Article 6.4 of the Paris Agreement. Clim. Policy 2018, 18, 383–395. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Zhang, S.E.; Nwaila, G.T.; Bourdeau, J.E.; Rose, D.H. Embracing a diverse approach to a globally inclusive green energy transition: Moving beyond decarbonisation and recognising realistic carbon reduction strategies. J. Clean. Prod. 2024, 434, 140414. [Google Scholar] [CrossRef]
- Duranti, L.; Laverdura, U.P.; Di Bartolomeo, E.; Grilli, M.L.; Chierchia, R.; Larosa, C.; Varotto, A.; Tuti, S.; Licoccia, S.; Luisetto, I. Coking resistant Ru supported on Sm-substituted CaZrO3 catalyst for dry reforming of methane: The effect of Ru loading on catalytic activity. Int. J. Hydrogen Energy 2025, 106, 1403–1416. [Google Scholar] [CrossRef]
- Durán, I.; Dietrich, B.; Hofberger, C.; Stoppel, L.; Uhlenbruck, N.; Wetzel, T. CO2 Impact on Methane Pyrolysis as a Key Issue of Using Biogas as an Educt: A Theoretical Study. Int. J. Energy Res. 2023, 2023, 3684046. [Google Scholar] [CrossRef]
- Albano, M.; Madeira, L.M.; Miguel, C. V Use of Pd-Ag membrane reactors for low-temperature dry reforming of biogas—A simulation study. Membranes 2023, 13, 630. [Google Scholar] [CrossRef]
- Tao, Z.; Shen, D.; Liu, Y.; Zhang, X.; Zhang, G. Enhanced Stability and Activity of Nitrogen-Doped Carbon Nanotube-Supported Ni Catalysts for Methane Dry Reforming. Catalysts 2025, 15, 559. [Google Scholar] [CrossRef]
- Luyben, W.L. Control of parallel dry methane and steam methane reforming processes for Fischer–Tropsch syngas. J. Process Control 2016, 39, 77–87. [Google Scholar] [CrossRef]
- Quatrevalet, M.; Ai, X.; Pérez-Serrano, A.; Adamiec, P.; Barbero, J.; Fix, A.; Rarity, J.G.; Ehret, G.; Esquivias, I. Random-modulation differential absorption lidar based on semiconductor lasers and single photon counting for atmospheric CO2 sensing. In Proceedings of the International Conference on Space Optics—ICSO 2016, Biarritz, France, 18–21 October 2016; Volume 10562, pp. 1561–1569. [Google Scholar]
- Polychronopoulou, K.; AlKhoori, S.; AlBedwawi, S.; Alareeqi, S.; Hussien, A.G.S.; Vasiliades, M.A.; Efstathiou, A.M.; Petallidou, K.C.; Singh, N.; Anjum, D.H. Decoupling the Chemical and Mechanical Strain Effect on Steering the CO2 Activation over CeO2-Based Oxides: An Experimental and DFT Approach. ACS Appl. Mater. Interfaces 2022, 14, 33094–33119. [Google Scholar] [CrossRef]
- Burange, A.S.; Alothman, Z.A.; Luque, R. Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus. Nanotechnol. Rev. 2023, 12, 20230172. [Google Scholar] [CrossRef]
- Li, M.; Zhang, T.; Yang, S.-Z.; Sun, Y.; Zhang, J.; Polo-Garzon, F.; Siniard, K.M.; Yu, X.; Wu, Z.; Driscoll, D.M. Mechanochemistry-induced strong metal–support interactions construction toward enhanced hydrogenation. ACS Catal. 2023, 13, 6114–6125. [Google Scholar] [CrossRef]
- Ahasan, M.R.; Hossain, M.M.; Barlow, Z.; Ding, X.; Wang, R. Low-temperature plasma-assisted catalytic dry reforming of methane over CeO2 nanorod-supported NiO catalysts in a dielectric barrier discharge reactor. ACS Appl. Mater. Interfaces 2023, 15, 44984–44995. [Google Scholar] [CrossRef]
- Tu, Z.; Mu, C.; Yao, Y.; Wu, L.; Zou, Y.; Tong, Z.; Huang, K. Recent advances in unconventional heating and external field-assisted enhancement for dry reforming of methane. Chem. Eng. J. 2024, 481, 148899. [Google Scholar] [CrossRef]
- Yuan, B.; Zhu, T.; Han, Y.; Zhang, X.; Wang, M.; Li, C. Deactivation mechanism and anti-deactivation measures of metal catalyst in the dry reforming of methane: A review. Atmosphere 2023, 14, 770. [Google Scholar] [CrossRef]
- de Oliveira, R.P.P.; Fuziki, M.E.K.; Costa, P.M.L.Z.; Tusset, A.M.; Lenzi, G.G. Syngas Generation Process Simulation: A Comparative Study. Int. J. Robot. Control Syst. 2022, 2, 187–200. [Google Scholar] [CrossRef]
- Moravvej, Z.; Rahimpour, M.R. Comparative study of modified Ni catalysts over hexagonal & cubic-ordered mesoporous Al2O3 and cubic-ordered mesoporous SBA-16 support for effective hydrogen production through CO2/CH4 reforming. Int. J. Hydrogen Energy 2025, 142, 1168–1183. [Google Scholar]
- Fantozzi, N.; Volle, J.-N.; Porcheddu, A.; Virieux, D.; García, F.; Colacino, E. Green metrics in mechanochemistry. Chem. Soc. Rev. 2023, 52, 6680–6714. [Google Scholar] [CrossRef]
- Reynes, J.F.; Isoni, V.; García, F. Tinkering with mechanochemical tools for scale up. Angew. Chemie Int. Ed. 2023, 62, e202300819. [Google Scholar] [CrossRef]
- Hergesell, A.H.; Baarslag, R.J.; Seitzinger, C.L.; Meena, R.; Schara, P.; Tomović, Z.; Li, G.; Weckhuysen, B.M.; Vollmer, I. Surface-Activated Mechano-Catalysis for Ambient Conversion of Plastic Waste. J. Am. Chem. Soc. 2024, 146, 26139–26147. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Xue, L.; Wang, Q.; You, F.; Dai, L.; Wu, J.; Kramer, S.; Lian, Z. Mechanochemical synthesis of aryl fluorides by using ball milling and a piezoelectric material as the redox catalyst. Angew. Chemie 2023, 135, e202307054. [Google Scholar] [CrossRef]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical Synthesis of Catalytic Materials. Chem.-A Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef]
- Su, D.S. Ball-Milling as an Effective Method for Preparation and Activation of Catalysts. Available online: https://pure.mpg.de/rest/items/item_740540/component/file_740539/content (accessed on 8 September 2025).
- El-Eskandarany, M.S.; Al-Hazza, A.; Al-Hajji, L.A.; Ali, N.; Al-Duweesh, A.A.; Banyan, M.; Al-Ajmi, F. Mechanical milling: A superior nanotechnological tool for fabrication of nanocrystalline and nanocomposite materials. Nanomaterials 2021, 11, 2484. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.; Lei, Y.; Sala, X.; García-Antón, J. A review on photocatalytic methane conversion systems: From fundamental mechanisms to the emerging role of ferroelectric materials. J. Mater. Chem. A 2025, 13, 12712–12745. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, S. High-entropy materials for catalysis: A new frontier. Sci. Adv. 2021, 7, eabg1600. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Sun, X.; Li, Z.; Hao, X.; Fan, M.; Ning, P.; Li, K. Plasma-assisted reforming of methane. Adv. Sci. 2022, 9, 2203221. [Google Scholar] [CrossRef]
- Geng, F.; Haribal, V.P.; Hicks, J.C. Non-thermal plasma-assisted steam methane reforming for electrically-driven hydrogen production. Appl. Catal. A Gen. 2022, 647, 118903. [Google Scholar] [CrossRef]
- Daurio, D.; Jacobsen, C.S.; Nagapudi, K.; Saw, R.; Elipe, M.V.S.; Thiel, O.; Balgley, R.; Chamarthy, S.P.; Alvarez-Nunez, F. Application of mechanochemistry to green, scalable, and continuous manufacturing of pharmaceutically relevant peptides by twin-screw extrusion. J. Pharm. Sci. 2025, 103941. [Google Scholar] [CrossRef]
- Katsenis, A.D.; Puškarić, A.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Užarević, K.; Pham, M.-H.; Do, T.-O.; Kimber, S.A.J.; Lazić, P. In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework. Nat. Commun. 2015, 6, 6662. [Google Scholar] [CrossRef]
- Ghayour, H.; Abdellahi, M.; Bahmanpour, M. Optimization of the high energy ball-milling: Modeling and parametric study. Powder Technol. 2016, 291, 7–13. [Google Scholar] [CrossRef]
- Fecht, H.J.; Hellstern, E.; Fu, Z.; Johnson, W.L. Nanocrystalline metals prepared by high-energy ball milling. Metall. Trans. A 1990, 21, 2333–2337. [Google Scholar] [CrossRef]
- Leroy, C.; Métro, T.-X.; Hung, I.; Gan, Z.; Gervais, C.; Laurencin, D. From Operando Raman Mechanochemistry to “NMR Crystallography”: Understanding the Structures and Interconversion of Zn-Terephthalate Networks Using Selective 17O-Labeling. Chem. Mater. 2022, 34, 2292–2312. [Google Scholar] [CrossRef]
- Lukin, S.; Užarević, K.; Halasz, I. Raman spectroscopy for real-time and in situ monitoring of mechanochemical milling reactions. Nat. Protoc. 2021, 16, 3492–3521. [Google Scholar] [CrossRef]
- Xu, M.; Peng, M.; Tang, H.; Zhou, W.; Qiao, B.; Ma, D. Renaissance of strong metal–support interactions. J. Am. Chem. Soc. 2024, 146, 2290–2307. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, J.; Liu, T.; Xu, M.; Dong, Y.; Wang, C.-A. Constructing morphologically stable supported noble metal catalysts in heterogeneous catalysis: Mechanisms and strategies. Nano Energy 2024, 129, 109975. [Google Scholar] [CrossRef]
- Wang, T.; Hu, J.; Ouyang, R.; Wang, Y.; Huang, Y.; Hu, S.; Li, W.-X. Nature of metal-support interaction for metal catalysts on oxide supports. Science 2024, 386, 915–920. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Li, Q.; Ye, Z.; Wang, L.; Gong, Y.; Li, S.; Wu, J.; Lu, Z.; Wu, S.; Zhang, J. Regulating Atomically-Precise Pt Sites for Boosting Light-Driven Dry Reforming of Methane. Angew. Chemie Int. Ed. 2024, 63, e202412308. [Google Scholar] [CrossRef]
- Jiménez, J.D.; Betancourt, L.E.; Danielis, M.; Zhang, H.; Zhang, F.; Orozco, I.; Xu, W.; Llorca, J.; Liu, P.; Trovarelli, A. Identification of highly selective surface pathways for methane dry reforming using mechanochemical synthesis of Pd–CeO2. ACS Catal. 2022, 12, 12809–12822. [Google Scholar] [CrossRef]
- Choi, H.S.; Ahn, K.J.; Nam, J.-D.; Chun, H.J. Hygroscopic aspects of epoxy/carbon fiber composite laminates in aircraft environments. Compos. Part A Appl. Sci. Manuf. 2001, 32, 709–720. [Google Scholar] [CrossRef]
- Dang, C.-Y.; Liu, K.; Fan, M.-X.; Zhu, S.-Q.; Zhao, S.-H.; Shen, X.-J. Investigation on cryogenic interlaminar shear properties of carbon fabric/epoxy composites improved by graphene oxide-coated glass fibers. Compos. Commun. 2020, 22, 100510. [Google Scholar] [CrossRef]
- Wu, P.; Tan, S.; Moon, J.; Yan, Z.; Fung, V.; Li, N.; Yang, S.-Z.; Cheng, Y.; Abney, C.W.; Wu, Z. Harnessing strong metal–support interactions via a reverse route. Nat. Commun. 2020, 11, 3042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Mohamedali, M.; Ren, Y.; Lu, Q.; Mahinpey, N. Facile synthesis of multi-layered nanostructured Ni/CeO2 catalyst plus in-situ pre-treatment for efficient dry reforming of methane. Appl. Catal. B Environ. 2022, 316, 121696. [Google Scholar] [CrossRef]
- Han, S.; Doi, R.; Stoltz, B.M. Nickel-Catalyzed Intramolecular C− O Bond Formation: Synthesis of Cyclic Enol Ethers. Angew. Chemie 2016, 128, 7563–7566. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Y.; Huang, L.; Liu, Y.; Cui, S.; Liu, H.; Xu, J.; Wang, L. Three-Dimensional mesoporous Ni-CeO2 catalyst for dry reforming of methane. Catalysts 2024, 14, 291. [Google Scholar] [CrossRef]
- Liu, Z.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.; Zhou, Y.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, D.J.; Zhou, J. Dry reforming of methane on a highly-active Ni-CeO2 catalyst: Effects of metal-support interactions on C− H bond breaking. Angew. Chemie Int. Ed. 2016, 55, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, J.; Felderhoff, M.; Schuth, F. Mechanochemical synthesis of supported bimetallic catalysts. Chem. Mater. 2021, 33, 2037–2045. [Google Scholar] [CrossRef]
- Gunnarson, A.; De Bellis, J.; Imhof, T.; Pfänder, N.; Ledendecker, M.; Schüth, F. Facile solid-state synthesis of supported PtNi and PtCo bimetallic nanoparticles for the oxygen reduction reaction. Chem. Mater. 2023, 35, 2006–2015. [Google Scholar] [CrossRef]
- Bitters, J.S.; He, T.; Nestler, E.; Senanayake, S.D.; Chen, J.G.; Zhang, C. Utilizing bimetallic catalysts to mitigate coke formation in dry reforming of methane. J. Energy Chem. 2022, 68, 124–142. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, C.; Tian, H.; Liu, T.; Zhang, X.; Chen, S.; Xiao, Q.; Wang, X.; Gong, J. Role of Fe species of Ni-based catalysts for efficient low-temperature ethanol steam reforming. JACS Au 2021, 1, 1459–1470. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Zhou, N.; Zhang, P.; Liu, Z.; Vovk, E.I.; Zhu, Y.-A.; Yang, Y.; Zhu, K. Realization of high-pressure dry methane reforming by suppressing coke deposition with Co-Rh intermetallic clusters. Appl. Catal. B Environ. 2023, 339, 123102. [Google Scholar] [CrossRef]
- Wang, C.; Sun, N.; Zhao, N.; Wei, W.; Zhao, Y. Template-free preparation of bimetallic mesoporous Ni-Co-CaO-ZrO2 catalysts and their synergetic effect in dry reforming of methane. Catal. Today 2017, 281, 268–275. [Google Scholar] [CrossRef]
- Xu, Z.; Park, E.D. Recent advances in coke management for dry reforming of methane over Ni-based catalysts. Catalysts 2024, 14, 176. [Google Scholar] [CrossRef]
- Zhang, Q.; Akri, M.; Yang, Y.; Qiao, B. Atomically dispersed metals as potential coke-resistant catalysts for dry reforming of methane. Cell Reports Phys. Sci. 2023, 4, 101310. [Google Scholar] [CrossRef]
- Liu, J.; Peng, H.; Liu, W.; Xu, X.; Wang, X.; Li, C.; Zhou, W.; Yuan, P.; Chen, X.; Zhang, W. Tin modification on Ni/Al2O3: Designing potent coke-resistant catalysts for the dry reforming of methane. ChemCatChem 2014, 6, 2095–2104. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, L.; Zheng, X.; Liu, W.; Cao, Z.; Peng, H. Coke-resistance over Rh–Ni bimetallic catalyst for low temperature dry reforming of methane. Int. J. Hydrogen Energy 2023, 48, 13890–13901. [Google Scholar] [CrossRef]
- Liu, K.; Xing, F.; Xiao, Y.; Yan, N.; Shimizu, K.; Furukawa, S. Development of a highly stable ternary alloy catalyst for dry reforming of methane. ACS Catal. 2023, 13, 3541–3548. [Google Scholar] [CrossRef]
- Ma, W.; Morales-Vidal, J.; Tian, J.; Liu, M.-T.; Jin, S.; Ren, W.; Taubmann, J.; Chatzichristodoulou, C.; Luterbacher, J.; Chen, H.M. Encapsulated Co–Ni alloy boosts high-temperature CO2 electroreduction. Nature 2025, 641, 1156–1161. [Google Scholar] [CrossRef]
- Martín, N.; Cirujano, F.G. Supported single atom catalysts for C− H activation: Selective C− H oxidations, dehydrogenations and oxidative C− H/C− H couplings. ChemCatChem 2021, 13, 2751–2765. [Google Scholar] [CrossRef]
- Lucas, J.; Padmanabha Naveen, N.S.; Janik, M.J.; Alexopoulos, K.; Noh, G.; Aireddy, D.; Ding, K.; Dorman, J.A.; Dooley, K.M. Improved Selectivity and Stability in Methane Dry Reforming by Atomic Layer Deposition on Ni-CeO2–ZrO2/Al2O3 Catalysts. ACS Catal. 2024, 14, 9115–9133. [Google Scholar] [CrossRef]
- Pan, Y.; Zhen, S.; Liu, X.; Ge, M.; Zhao, J.; Gu, L.; Su, D. Looping metal-support interaction in heterogeneous catalysts during redox reactions. Nat. Commun. 2025, 16, 8627. [Google Scholar] [CrossRef]
- Nosbi, N.; Akil, H.M. Controlling the number of walls in multi walled carbon nanotubes/alumina hybrid compound via ball milling of precipitate catalyst. Appl. Surf. Sci. 2015, 340, 78–88. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhan, L.; Guo, P.; Dai, Y.; Shen, L.; Zhang, Y.; Wang, G.; Wang, Z.; Zhao, L. Recent advances in atomically dispersed MNC coupling Pt-based oxygen reduction catalysts. Sustain. Energy Fuels 2025, 9, 10–27. [Google Scholar] [CrossRef]
- Chen, Z.; Han, G.-F.; Mahmood, A.; Hou, J.; Wei, W.; Shon, H.K.; Wang, G.; Waite, T.D.; Baek, J.-B.; Ni, B.-J. Mechanosynthesized electroactive materials for sustainable energy and environmental applications: A critical review. Prog. Mater. Sci. 2024, 145, 101299. [Google Scholar] [CrossRef]
- Bandurist, P.S.; Pichugina, D.A. Quantum-Chemical Study of C–H Bond Activation in Methane on Ni–Cu Oxide and Sulfide Clusters. Kinet. Catal. 2023, 64, 362–370. [Google Scholar] [CrossRef]
- Tang, L.; Huang, X.; Ran, J.; Guo, F.; Niu, J.; Qiu, H.; Ou, Z.; Yan, Y.; Yang, Z.; Qin, C. Density functional theory studies on direct and oxygen assisted activation of C–H bond for dry reforming of methane over Rh–Ni catalyst. Int. J. Hydrogen Energy 2022, 47, 30391–30403. [Google Scholar] [CrossRef]
- Akri, M.; El Doukkali, M. Enhanced coking resistance in methane dry reforming using anti-sintering Ni nanoparticles and atomically re-dispersed oxygen vacancy-rich CeOx on MgAl2O4 support. Mater. Today Chem. 2025, 45, 102673. [Google Scholar] [CrossRef]
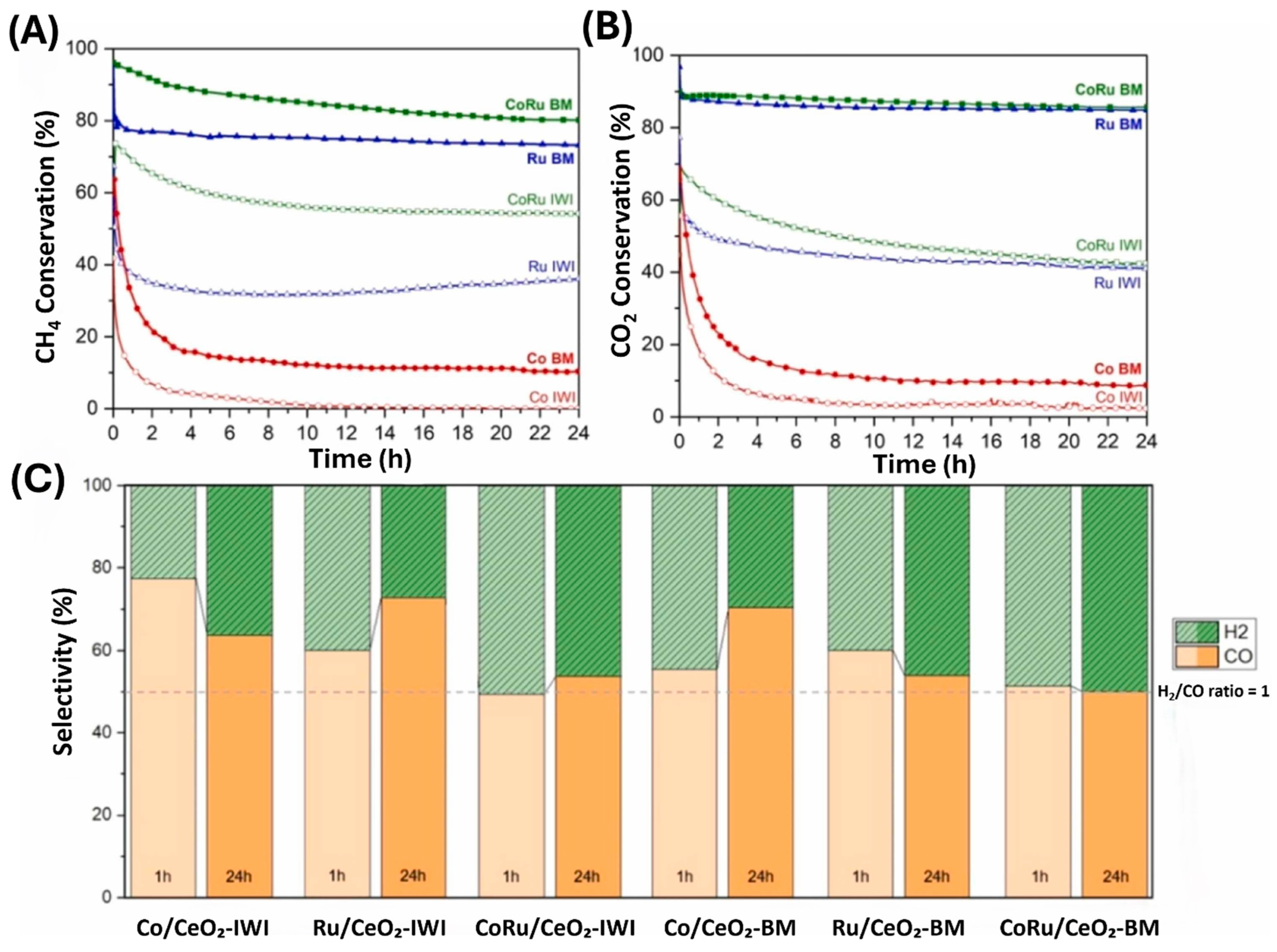
- Armengol-Profitós, M.; Braga, A.; Pascua-Solé, L.; Lucentini, I.; Garcia, X.; Soler, L.; Vendrell, X.; Serrano, I.; Villar-Garcia, I.J.; Pérez-Dieste, V. Enhancing the performance of a novel CoRu/CeO2 bimetallic catalyst for the dry reforming of methane via a mechanochemical process. Appl. Catal. B Environ. Energy 2024, 345, 123624. [Google Scholar] [CrossRef]
- Giordano, F.; Trovarelli, A.; de Leitenburg, C.; Giona, M. A model for the temperature-programmed reduction of low and high surface area ceria. J. Catal. 2000, 193, 273–282. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Zhang, S.; Akter, N.; Palomino, R.M.; Vovchok, D.; Orozco, I.; Salazar, D.; Rodriguez, J.A.; Llorca, J. In situ elucidation of the active state of Co–CeOx catalysts in the dry reforming of methane: The important role of the reducible oxide support and interactions with cobalt. ACS Catal. 2018, 8, 3550–3560. [Google Scholar] [CrossRef]
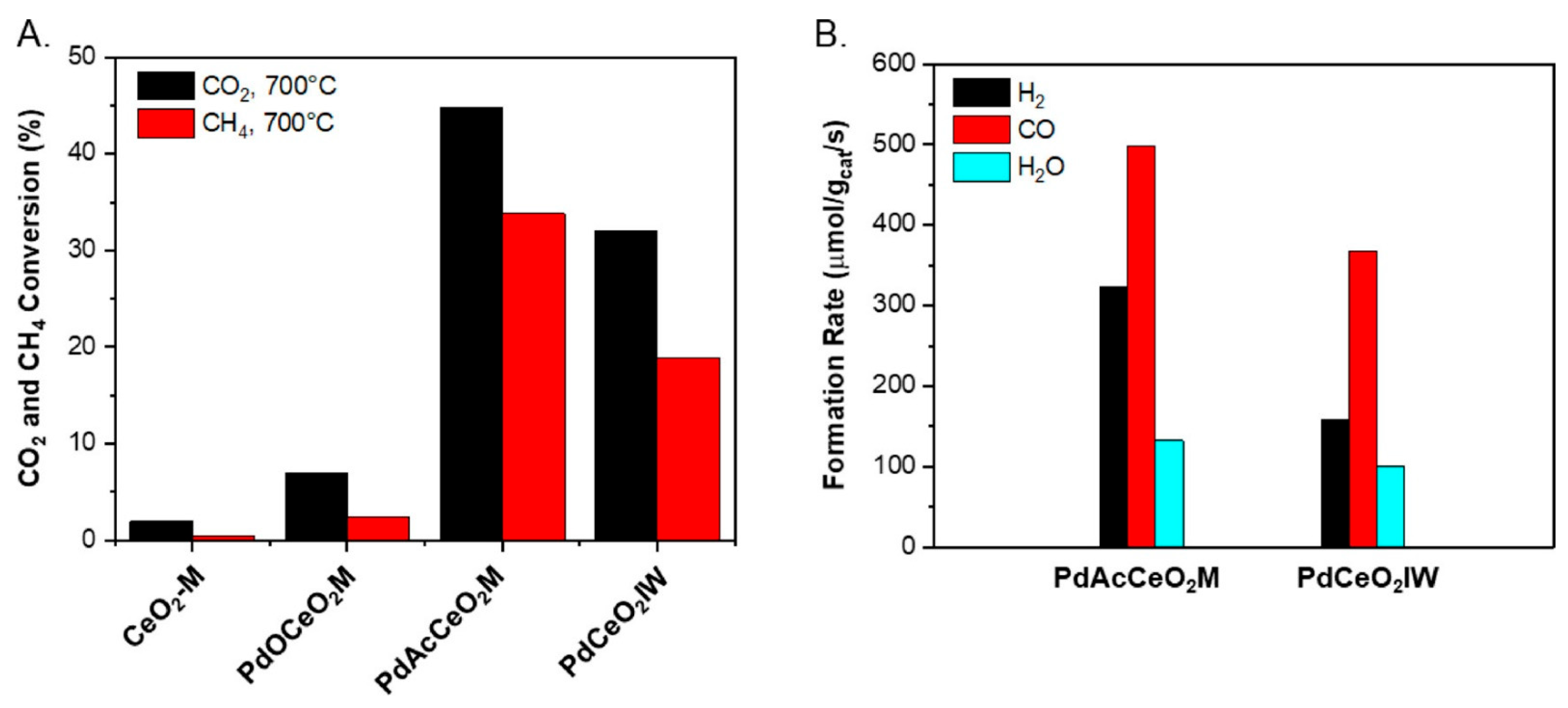
- Danielis, M.; Betancourt, L.E.; Orozco, I.; Divins, N.J.; Llorca, J.; Rodríguez, J.A.; Senanayake, S.D.; Colussi, S.; Trovarelli, A. Methane oxidation activity and nanoscale characterization of Pd/CeO2 catalysts prepared by dry milling Pd acetate and ceria. Appl. Catal. B Environ. 2021, 282, 119567. [Google Scholar] [CrossRef]
- Li, L.; Vozniuk, O.; Cao, Z.; Losch, P.; Felderhoff, M.; Schüth, F. Hydrogenation of different carbon substrates into light hydrocarbons by ball milling. Nat. Commun. 2023, 14, 5257. [Google Scholar] [CrossRef]
- Carta, M.; Sanna, A.L.; Porcheddu, A.; Garroni, S.; Delogu, F. Mechanochemical effects underlying the mechanically activated catalytic hydrogenation of carbon monoxide. Sci. Rep. 2023, 13, 2470. [Google Scholar] [CrossRef]
- Park, G.-J.; Kim, S.-J.; Ko, C.H. Pore-size Controlled Ni/Al2O3 Catalyst for Methane Reforming via Mechanochemical One-pot Synthesis. Appl. Catal. A Gen. 2025, 706, 120500. [Google Scholar] [CrossRef]
- AlKetbi, M.; Polychronopoulou, K.; Abi Jaoude, M.; Vasiliades, M.A.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Zedan, A.F.; Efstathiou, A.M. Cu-Ce-La-Ox as efficient CO oxidation catalysts: Effect of Cu content. Appl. Surf. Sci. 2020, 505, 144474. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Patel, J.; Bordoloi, A. A study of the synergy between support surface properties and catalyst deactivation for CO2 reforming over supported Ni nanoparticles. Appl. Catal. A Gen. 2017, 545, 113–126. [Google Scholar] [CrossRef]
- Kasatkin, I.; Kurr, P.; Kniep, B.; Trunschke, A.; Schlögl, R. Role of lattice strain and defects in copper particles on the activity of Cu/ZnO/Al2O3 catalysts for methanol synthesis. Angew. Chemie 2007, 119, 7465–7468. [Google Scholar] [CrossRef]
- Danielis, M.; Colussi, S.; de Leitenburg, C.; Soler, L.; Llorca, J.; Trovarelli, A. Outstanding Methane Oxidation Performance of Palladium-Embedded Ceria Catalysts Prepared by a One-Step Dry Ball-Milling Method. Angew. Chemie 2018, 130, 10369–10373. [Google Scholar] [CrossRef]
- Danielis, M.; Colussi, S.; de Leitenburg, C.; Soler, L.; Llorca, J.; Trovarelli, A. The effect of milling parameters on the mechanochemical synthesis of Pd–CeO2 methane oxidation catalysts. Catal. Sci. Technol. 2019, 9, 4232–4238. [Google Scholar] [CrossRef]
- Grigoryan, R.R.; Aloyan, S.G.; Harutyunyan, V.R.; Arsentev, S.D.; Tavadyan, L.A. Dry Reforming of Methane over Nanosized Tungsten Carbide Powders Obtained by Mechanochemical and Plasma-Mechanochemical Methods. Pet. Chem. 2019, 59, 1256–1263. [Google Scholar] [CrossRef]
- Babakouhi, R.; Alavi, S.M.; Rezaei, M.; Jokar, F.; Varbar, M.; Akbari, E. Hydrogen production through combined dry reforming and partial oxidation of methane over the Ni/Al2O3–CeO2 catalysts. Int. J. Hydrogen Energy 2024, 60, 503–514. [Google Scholar] [CrossRef]
- Leroy, C.; Mittelette, S.; Félix, G.; Fabregue, N.; Špačková, J.; Gaveau, P.; Métro, T.-X.; Laurencin, D. Operando acoustic analysis: A valuable method for investigating reaction mechanisms in mechanochemistry. Chem. Sci. 2022, 13, 6328–6334. [Google Scholar] [CrossRef]
- Kamran, K.; Tahir, M. Photothermal Dry Reforming of Methane: A Comprehensive Review of Synergistic Interactions among Thermal, Photonic, Catalytic, and Reactor Engineering with Their Techno-Economic Aspects. Ind. Eng. Chem. Res. 2025, 64, 15171–15204. [Google Scholar] [CrossRef]
- Bhuiyan, H.; Li, Y.-S.; Kim, S.H.; Martini, A. Shear-activation of mechanochemical reactions through molecular deformation. Sci. Rep. 2024, 14, 2992. [Google Scholar] [CrossRef]
- Wensink, F.J.; Roos, N.; Bakker, J.M.; Armentrout, P.B. C–H Bond Activation and C–C Coupling of Methane on a Single Cationic Platinum Center: A Spectroscopic and Theoretical Study. Inorg. Chem. 2022, 61, 11252–11260. [Google Scholar] [CrossRef]
- Ninkovic, D.B.; Moncho, S.; Petrovic, P.; Hall, M.B.; Zaric, S.D.; Brothers, E.N. Improving a Methane C–H Activation Complex by Metal and Ligand Alterations from Computational Results. Inorg. Chem. 2023, 62, 5058–5066. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Zhang, C.; Liu, H.; Jin, Y.; Zhang, R.; Ran, J. Unraveling the effects of Ni particle size and facet on CH4 activation: From cluster to nanoparticle. Int. J. Hydrogen Energy 2023, 48, 19486–19493. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, S.; Zhang, T.; Wu, Z.; Li, J.; Wang, W.; Wu, D. Unveiling the mechanistic synergy in Mn-doped NiO catalysts with atomic-burry structure: Enhanced CO oxidation via Ni-OH and Mn bifunctionality. Sep. Purif. Technol. 2025, 354, 129330. [Google Scholar] [CrossRef]
- Liu, W.; Feng, H.; Yang, Y.; Niu, Y.; Wang, L.; Yin, P.; Hong, S.; Zhang, B.; Zhang, X.; Wei, M. Highly-efficient RuNi single-atom alloy catalysts toward chemoselective hydrogenation of nitroarenes. Nat. Commun. 2022, 13, 3188. [Google Scholar] [CrossRef]
- Gobindlal, K.; Zujovic, Z.; Yadav, P.; Sperry, J.; Weber, C.C. The mechanism of surface-radical generation and amorphization of crystalline quartz sand upon mechanochemical grinding. J. Phys. Chem. C 2021, 125, 20877–20886. [Google Scholar] [CrossRef]
- O’Neill, R.T.; Boulatov, R. The many flavours of mechanochemistry and its plausible conceptual underpinnings. Nat. Rev. Chem. 2021, 5, 148–167. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A force of synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar]
- Kerrache, A.; Mousseau, N.; Lewis, L.J. Crystallization of amorphous silicon induced by mechanical shear deformations. Phys. Rev. B—Condensed Matter Mater. Phys. 2011, 84, 14110. [Google Scholar] [CrossRef]
- Chetry, A.B. Mechanochemistry: A new frontier in chemical synthesis. J. Chem. Res. 2025, 49, 17475198251339300. [Google Scholar] [CrossRef]
- Cleary, P.W. A multiscale method for including fine particle effects in DEM models of grinding mills. Miner. Eng. 2015, 84, 88–99. [Google Scholar] [CrossRef]
- Yeh, J.; Chen, S.; Lin, S.; Gan, J.; Chin, T.; Shun, T.; Tsau, C.; Chang, S. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar]
- Löffler, T.; Meyer, H.; Savan, A.; Wilde, P.; Garzón Manjón, A.; Chen, Y.; Ventosa, E.; Scheu, C.; Ludwig, A.; Schuhmann, W. Discovery of a multinary noble metal–free oxygen reduction catalyst. Adv. Energy Mater. 2018, 8, 1802269. [Google Scholar] [CrossRef]
- Kumar, K.S.; Van Swygenhoven, H.; Suresh, S. Mechanical behavior of nanocrystalline metals and alloys. Acta Mater. 2003, 51, 5743–5774. [Google Scholar] [CrossRef]
- Carta, M.; Delogu, F.; Porcheddu, A. A phenomenological kinetic equation for mechanochemical reactions involving highly deformable molecular solids. Phys. Chem. Chem. Phys. 2021, 23, 14178–14194. [Google Scholar] [CrossRef]
- Jafter, O.F.; Lee, S.; Park, J.; Cabanetos, C.; Lungerich, D. Navigating Ball Mill Specifications for Theory-to-Practice Reproducibility in Mechanochemistry. Angew. Chemie Int. Ed. 2024, 63, e202409731. [Google Scholar]
- Urakaev, F.K.; Shevchenko, V.S. Phenomenology, kinetics and application of abrasive-reactive wear during comminution (Overview). KONA Powder Part. J. 2007, 25, 162–179. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Qi, F.; Peng, J.; Liang, Z.; Guo, J.; Liu, J.; Fang, T.; Mao, H. Strong metal-support interaction (SMSI) in environmental catalysis: Mechanisms, application, regulation strategies, and breakthroughs. Environ. Sci. Ecotechnol. 2024, 22, 100443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-C.; Xu, H.-M.; Huang, C.-J.; Zhu, H.-R.; Li, G.-R. Recent Progress in the Design and Application of Strong Metal–Support Interactions in Electrocatalysis. Inorg. Chem. 2025, 64, 4713–4748. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.M.; Liang, Y.X.; Fan, Y.; Wang, P.P.; Du, J.L.; Zhao, Y.B.; Fu, E.G. The ball to powder ratio (BPR) dependent morphology and microstructure of tungsten powder refined by ball milling. Powder Technol. 2018, 339, 256–263. [Google Scholar] [CrossRef]
- Shadab, M.; Miryala, M. Enhancing bulk MgB2 performance through optimized ball milling variables using Taguchi design approach. Ceram. Int. 2025, 51, 9647–9659. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Shao, X.-Y.; Wang, K.; Wang, Q.; Xing, Y.-Z. Effect of ball-to-powder ratio on morphology, structure, and flowability of ball-milled gray cast iron powder. J. Therm. Spray Technol. 2021, 30, 1679–1691. [Google Scholar] [CrossRef]
- Kim, K.-C.; Jiang, T.; Kim, N.-I.; Kwon, C. Effects of ball-to-powder diameter ratio and powder particle shape on EDEM simulation in a planetary ball mill. J. Indian Chem. Soc. 2022, 99, 100300. [Google Scholar] [CrossRef]
- Tommasi, M.; Degerli, S.N.; Ramis, G.; Rossetti, I. Advancements in CO2 methanation: A comprehensive review of catalysis, reactor design and process optimization. Chem. Eng. Res. Des. 2024, 201, 457–482. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.E.; Miskimmin, C.K.G.; Albadarin, A.B.; Walker, G.; James, S.L. Organic synthesis by Twin Screw Extrusion (TSE): Continuous, scalable and solvent-free. Green Chem. 2017, 19, 1507–1518. [Google Scholar] [CrossRef]
| Features | Mechanochemical Synthesis | Conventional Wet Methods (Impregnation, Sol–Gel) |
|---|---|---|
| Operating conditions | Near room temperature; reactions driven by impact/shear/friction | Multiple solvent steps; high-temperature calcination |
| Defect generation | High defect density (vacancies, dislocations) and fresh surfaces | Limited defect creation; defects typically induced thermally or via doping |
| Metal dispersion | Uniform nanoscale dispersion; intimate metal–support contact | Often broad size distribution; agglomeration during calcination/reduction |
| Alloy/solid solution formation | Achievable at low temperature via repeated cold welding and fracture | Generally requires high T; risk of phase segregation |
| Environmental footprint | Solvent-free, energy-lean; aligns with green chemistry | Solvent-intensive; wastewater and drying energy burdens |
| Scalability | Scalable with modern high-energy mills | Scalable but often costlier due to multi-step wet chemistry |
| Catalytic impact in DRM | Higher activity and coke resistance via dispersion, SMSI, and alloying | Moderate activity; deactivation by sintering/coking more prevalent |
| Parameter | Optimal Range/Condition | Effect on DRM | Quantitative Impact |
|---|---|---|---|
| Active Metal | Ni-based (cost-effective), Noble metals (Pd, Ru) | Provides C-H and C-O bond cleavage sites | Ni: ~15–25 wt% optimal loading; Ru: active at <5 wt% [92] |
| Support Material | Redox-active oxides (e.g., CeO2, ZrO2) or high surface area oxides (e.g., Al2O3). | Stabilizes metal, prevents sintering, activates CO2 | CeO2: promotes 30–40% higher CO2 conversion vs. Al2O3 [65,109] |
| Metal-Support Interaction | Strong interaction (mechanochemically enhanced) | Improves stability and activity | SMSI catalysts: <5 wt% coke vs. >35 wt% for weak interaction [39,41] |
| Milling Time | System-dependent (typically 4–24 h) | Increases conversion via energy input | Optimal at ~8–12 h for most Ni-based systems [110,111] |
| Milling Speed | High, below critical centrifugation speed | Higher energy transfer increases defect density | 300–600 RPM typical range [65] |
| Ball-to-Powder Ratio | High (10:1 to 40:1) | Enhances energy transfer and particle refinement | Optimal 20:1 for most oxide-supported catalysts [112,113] |
| Temperature | Moderate external heating (200–400 °C) | Overcomes endothermic barrier | Each 100 °C increase: ~15–20% conversion improvement [90] |
| Gas Pressure | Slightly above atmospheric (1–5 bar) | Influences surface concentration | Higher pressure (>3 bar) increases conversion but may enhance coking [110] |
| Feature | Laboratory-Scale Mills (e.g., Planetary, Shaker) | Industrial-Scale Mills (e.g., Attritor, Twin-Screw Extruder) |
|---|---|---|
| Typical Capacity | Milligrams to ~100 g | Kilograms to multiple tons per hour |
| Primary Force | High-energy impact, shear | Attrition, shear, compression |
| Operation Mode | Batch | Continuous or semi-continuous |
| Throughput | Very low | High |
| Key Challenge | Low throughput, batch-to-batch variation | Translating lab-scale impact conditions to continuous attrition/shear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, A.; Alao, K.T.; Bello, I.T.; Olarinoye, F.O.; Hamzat, A.K. Harnessing Mechanical Force for Greenhouse Gas Conversion: A Mini-Review on Mechanochemistry in the Dry Reforming of Methane. Fuels 2025, 6, 86. https://doi.org/10.3390/fuels6040086
Saad A, Alao KT, Bello IT, Olarinoye FO, Hamzat AK. Harnessing Mechanical Force for Greenhouse Gas Conversion: A Mini-Review on Mechanochemistry in the Dry Reforming of Methane. Fuels. 2025; 6(4):86. https://doi.org/10.3390/fuels6040086
Chicago/Turabian StyleSaad, Abdulwahab, Kehinde Temitope Alao, Idris Temitope Bello, Fawziyah Oyefunke Olarinoye, and Abdulhammed K. Hamzat. 2025. "Harnessing Mechanical Force for Greenhouse Gas Conversion: A Mini-Review on Mechanochemistry in the Dry Reforming of Methane" Fuels 6, no. 4: 86. https://doi.org/10.3390/fuels6040086
APA StyleSaad, A., Alao, K. T., Bello, I. T., Olarinoye, F. O., & Hamzat, A. K. (2025). Harnessing Mechanical Force for Greenhouse Gas Conversion: A Mini-Review on Mechanochemistry in the Dry Reforming of Methane. Fuels, 6(4), 86. https://doi.org/10.3390/fuels6040086






