Abstract
Biomass briquettes are increasingly used as renewable solid fuels, yet their durability under humid storage remains a key limitation. This study evaluated the mechanical performance and water resistance of briquettes made from fine (0–1 mm) and coarse (0–3 mm) charcoal fractions using molasses as a primary binder, polyvinyl alcohol (PVA, 3–7%) as a synthetic binder, and liquid soap (1–9%) as a surfactant additive. Compressive strength was measured in the dry state, after four days of water immersion, and after re-drying, while water absorption was monitored over immersion times from 15 min to 4 days. Fine-fraction briquettes showed higher strength and lower water uptake than coarse fractions, with optimal PVA contents of 6–7% providing maximum dry and post-drying strength. Moderate soap addition (2–3%) improved binder dispersion and early wet strength, whereas higher levels (>5%) reduced durability. Water absorption kinetics indicated that particle size controlled early swelling, while binder composition influenced the rate but not the final saturation. The best performance in humid storage was achieved by 0–1 mm + 4% PVA and 0–1 mm + 5% PVA + 3% soap formulations. These results support the formulation of eco-friendly binder systems that balance strength, moisture resistance, and cost for large-scale biomass briquette production.
1. Introduction
The global demand for sustainable solid biofuels continues to rise as an alternative to fossil-based energy [1,2]. Briquetting of biomass residues provides an avenue for improving energy density, transportability, and combustion efficiency while reducing waste and environmental [3,4]. However, briquettes are susceptible to mechanical degradation and moisture-induced disintegration during handling and storage, particularly in humid [5,6].
Binder selection plays a decisive role in determining the durability of briquettes. Among traditional binders, molasses, a by-product of sugar processing, is widely used because of its availability, low cost, and adhesive properties [7]. However, molasses-bonded briquettes often show high water solubility, which leads to reduced mechanical strength after moisture exposure [8]. To overcome this limitation, researchers have incorporated synthetic and semi-synthetic polymers, particularly polyvinyl alcohol (PVA), to improve wet strength and decrease binder solubility [9,10,11].
The effectiveness of PVA arises from its hydroxyl functionality, which enables strong hydrogen bonding with biomass carbohydrates, while its film-forming ability enhances particle adhesion and matrix cohesion [12,13]. Beyond briquetting, PVA has been extensively applied in polymer composites and functional gels due to these same properties. For instance, PVA has been explored as a biocarrier material in wastewater treatment, demonstrating high stability and tunable porosity [14]. It has also been incorporated into biopolymer–nanocomposite systems, such as PVA–chitosan–dandelion–CuNPs gels, which show enhanced antibacterial activity and sorption capacity [15]. Similarly, multifunctional PVA–chitosan–nanosilver composite gels have been developed, highlighting PVA’s adaptability to structural reinforcement and water-interaction control [16]. Taken together, these studies confirm PVA’s versatility and support its proven role in enhancing wet strength, durability, and functional performance in biomass briquettes and related composite systems.
Particle size also plays a decisive role in briquette performance. Finer particles provide higher specific surface area for binder adhesion and reduced macrovoids, leading to improved density and [17,18]. Conversely, coarse particles increase porosity and capillary pathways, accelerating water [19]. Binder modification through additives such as surfactants has been explored to improve binder dispersion and penetration; however, excessive surfactant can disrupt the binder matrix, increasing hydrophilicity and [20,21].
Previous studies have examined molasses–PVA systems in biomass and coal briquetting, identifying optimal PVA ranges between 5 and 8% for maximizing dry [9,10,22,23]. However, limited attention has been given to the interplay between PVA concentration, particle size, and surfactant additives on both mechanical and water-resistance properties, particularly under extended immersion conditions. This study addresses these gaps by systematically evaluating the compressive strength and water absorption behaviour of molasses–PVA briquettes across two particle size fractions and with varying surfactant additions. The aim is to determine optimal binder formulations that balance strength, water resistance, and cost-effectiveness for applications requiring long-term storage in humid environments.
Polyvinyl alcohol (PVA) is more expensive than traditional binders such as molasses, but only small additions (5–7 wt %) are required to achieve significant improvements in wet strength and durability. At current market prices (≈US $2–3 per kg), using 5–7% PVA in briquettes (charcoal density ~1 g/cm3) increases the material cost by about US $25–35 per tonne of briquettes. Although this represents a noticeable increase relative to molasses-only binders, the enhanced water resistance and improved mechanical durability can offset this in applications where storage stability, handling losses, or exposure to moisture are critical. In our study, the optimal formulation (≈6% PVA in the fine fraction) was chosen to minimize cost while still ensuring durability. Further techno-economic analysis would be beneficial to assess the viability of scaling these binder systems to commercial production, especially in comparison to existing studies on briquetting economics and durability [24,25,26].
2. Materials and Methods
2.1. Materials
Industrial hardwood charcoal fines produced at the LLC Perechin Timber (Perechyn, Ukraine) and Chemical Plant (Perechyn, Ukraine) were used as the base material. The PVA binder additive was tested. In all formulations, a baseline of 20 wt % cane molasses (a traditional binder) was added for initial cohesion, and the PVA binder was added on top of that in varying amounts (3%, 4%, 5%, 6%, or 7% by weight of dry charcoal) [27,28]. Thus, for example, a “5% PVA” formulation contained 20% molasses + 5% PVA (with the remainder charcoal). Charcoal particle size was controlled by sieving. “Fine” fraction refers to particles <0.5–1 mm (predominantly around 0.5 mm), and “Coarse” fraction refers to particles up to ~1.5 mm (with a distribution centered around that size) [29]. Some experiments also used blended fractions—e.g., mixtures of 0.5 mm and 1.5 mm material in specified ratios—to assess the influence of particle size [30]. All materials were used at room-temperature moisture content (char ~5% moisture, molasses ~20% water content). No chemical curing agents or cross-linkers were [31].
2.2. Briquette Formation
Approximately 100 g of charcoal powder was blended uniformly with molasses and binder solution. For formulations containing PVA, the required quantity of PVA powder was dissolved in hot water (~80 °C) to prepare a 10% w/v solution, which was then mixed with charcoal to ensure even dispersion [32]. The moisture content of the mixture was adjusted to ~30% to aid compaction [33].
Briquettes were fabricated using a laboratory hydraulic press (Enerpac H-Frame, maximum load 50 Ton, Figure 1) equipped with a cylindrical steel mold of 70 mm diameter and 25 mm height. A dwell time of 10 s was maintained at maximum pressure to achieve compaction. The resulting briquettes were carefully ejected from the mold and subjected to controlled air-drying at 120 °C and ~60% relative humidity for 2 h, ensuring stabilization of structure and removal of residual moisture.

Figure 1.
Photograph of a laboratory hydraulic press Enerpac H-Frame.
Typically, 10–12 briquettes were obtained per formulation (an example briquette is shown in Figure 2). All subsequent mechanical and combustion testing was conducted on fully dried briquettes unless otherwise specified [34].

Figure 2.
Photograph of a laboratory-produced charcoal briquette (70 mm × 25 mm) obtained using the Enerpac H-Frame hydraulic press. The image illustrates the typical shape and surface texture of the briquettes prior to testing.
2.3. Mechanical Strength Testing
The compressive strength of briquettes was measured using an axial compression [35]. Each briquette was placed between steel plates and loaded at a cross-head speed of 1 mm min−1 until fracture. The peak load at failure was recorded. Compressive strength (in MPa) was calculated by dividing the failure load by the briquette’s cross-sectional area. An average of three briquettes per formulation was taken. We report “dry compressive strength” for briquettes in their dry [36]. To assess water durability, “wet compressive strength” was measured for briquettes after water immersion (described below), and “re-dried compressive strength” was measured after those same briquettes were dried [37].
2.4. Water Immersion and Absorption Measurement
Briquettes were subjected to a static immersion test in deionized water at room temperature (~20 °C) to evaluate water uptake and its effect on [38]. For each formulation, a set of briquettes (typically 2–3, total dry mass ~250–320 g depending on briquette number and size) was weighed (dry mass noted) and then fully submerged in a container of [39]. No mechanical agitation was applied, but briquettes were kept separated to ensure water contact on all sides. At intervals of 15 min, 30 min, 45 min, 1 h, 2 h, 3 h, 5 h, and 24, 48, 72, 96 h (4 days), the briquettes were carefully removed, blotted of surface water, and [40]. They were then returned to the water for continued soaking (except at the final 4-day mark). From the mass gain, the water absorption (%) at each time point was calculated as:
where m0 is the initial dry mass and m(t) is the mass at time t [40]. After 4 days of immersion, briquettes were evaluated for wet strength by performing a gentle compression test (some briquettes were too fragile to test fully when wet; in those cases, a qualitative assessment “disintegrated” was noted) [41]. Subsequently, the soaked briquettes were dried in air for 24 h and then in an oven (60 °C, 24 h) to remove absorbed moisture. These re-dried briquettes were then tested for compressive strength to see how much of the original strength was [42]. Any visible physical changes (cracks, deformation) were recorded [43]. Representative soaked briquettes are shown in Figure 3.

Figure 3.
Photograph of charcoal briquettes after immersion in water for 180 min.
2.5. Data Analysis
The experimental data were analyzed to compare the effects of binder concentration and particle [44]. Plots of compressive strength vs. binder content were generated for each binder and particle size [45]. Water absorption curves (% vs. time) were plotted on semi-log axes to capture both the initial fast uptake and long-term [46]. A two-stage absorption model was considered, defining an “initial absorption rate” (within the first few hours) and a slower long-term [47]. Strength data before/after water were used to compute retention ratios (wet strength as % of original, and re-dried strength as % of original). Due to the consistent trends observed, we focus on representative [48]. All data processing and statistical analysis were performed using Microsoft Excel 2021 (Microsoft Corp., Redmond, WA, USA), while graphs were generated in Inkscape 1.3 (Inkscape Project, Free Software Foundation, Boston, MA, USA) [49].
3. Results
3.1. Concentration Effects of PVA Binder
The compressive strength data for the 0–1 mm fraction briquettes bound with molasses and varying PVA contents are shown in Figure 4 and summarised in Table 1. Before water immersion, briquettes with 3% PVA exhibited a mean compressive strength of 1.1 MPa, which progressively increased with higher PVA content, reaching 2.6 MPa at 7% PVA. This represents an overall increase of 136% relative to the lowest binder loading. The increase in strength is attributed to the enhanced formation of a continuous PVA–molasses matrix, which improves interparticle adhesion through hydrogen bonding between hydroxyl groups of PVA and carbohydrate components of molasses, as well as by creating a denser film over particle surfaces [12,13].
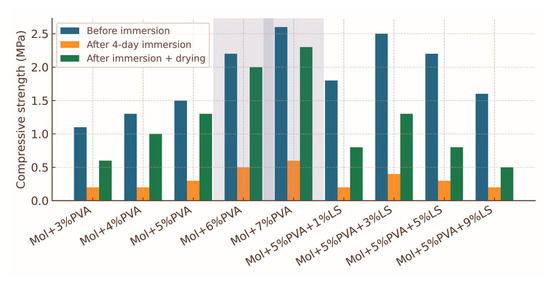
Figure 4.
Compressive strength of briquettes (0–1 mm fraction) with varying PVA binder content before water immersion, after 4 days of immersion, and after drying. Error bars indicate standard deviation (n = 3). The shaded area highlights the optimal range of PVA content (6–7%) for maximum dry and post-drying strength.

Table 1.
Compressive strength of briquettes with different PVA and liquid soap contents before and after 4 days of water immersion, and after subsequent drying, for two particle size fractions (0–1 mm and 0–3 mm).
These results align with earlier studies on PVA-containing biomass briquettes, where optimal binder contents in the range of 5–8% yielded significant gains in dry compressive strength and moderate improvements in wet performance [9,10,22,23]. Similar increases were reported for coal briquettes bonded with synthetic polymers, in which binder loadings above ~6% achieved diminishing returns in strength [8,11,50]. The present data confirm that for fine fractions (0–1 mm), PVA contents between 6 and 7% can be considered optimal for achieving a balance between mechanical performance and binder efficiency [9,13].
3.2. Strength vs. Binder Loading in Mixed Fractions
The 0–3 mm particle size fraction briquettes followed similar trends to the 0–1 mm fraction but with overall lower compressive strengths. At 3% PVA, pre-immersion strength was 0.9 MPa, increasing to 1.7 MPa at 7% PVA. The difference in strength compared to finer fractions is attributed to less efficient packing density, which reduces the number of effective particle–binder contacts [20,28]. Coarser particles also create larger voids within the briquette structure, which act as stress concentrators and reduce load-bearing capacity [21,27]. Post-immersion strength rose modestly from 0.2 MPa at 3% PVA to 0.5 MPa at 7% PVA, and post-drying recovery reached a maximum of 1.4 MPa. The relative wet strength recovery for coarse fractions (70–80% of pre-immersion strength) was lower than for fine fractions (85–90%), likely due to more extensive water ingress along larger pores and weaker particle–binder bonds [20,21,41,51].
3.3. Binder Combinations and Additive Effects
The influence of liquid soap as an additive to the 0–1 mm fraction briquettes containing 20% molasses and 5% PVA is illustrated in Figure 5. At 1% soap, pre-immersion strength increased from 1.5 MPa (no soap) to 1.8 MPa, indicating improved binder wetting and dispersion across particle surfaces [36]. The most pronounced enhancement occurred at 3% soap, where strength reached 2.5 MPa—approximately a 67% increase relative to the soap-free case [35,36]. After immersion, the 3% soap formulation retained 0.4 MPa, double the value observed for the 1% soap case. Post-drying strength also peaked at 1.3 MPa at this soap content. However, higher soap additions (≥5%) resulted in strength deterioration, dropping to 1.6 MPa at 9% soap. This can be attributed to excessive surfactant content interfering with binder cohesion, increasing brittleness and promoting microcrack formation during drying [21,42]. Comparable trends have been reported in biomass briquetting with surfactant-modified binders, where moderate levels improved matrix homogeneity and adhesion but excessive amounts disrupted the polymer network [21,34,42]. Thus, in combination binders containing molasses, PVA, and a surfactant, the optimal additive level appears to be in the 2–3% range.
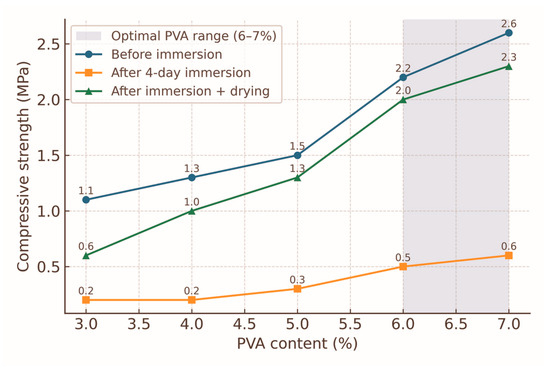
Figure 5.
Effect of liquid soap addition on compressive strength of briquettes (0–1 mm fraction, 20% molasses + 5% PVA) before water immersion, after 4 days of immersion, and after drying.
3.4. Influence of Particle Size
Direct comparison of 0–1 mm and 0–3 mm fractions in Table 1 reveals that fine fractions consistently outperform coarse fractions in all strength metrics. For example, at 5% PVA, pre-immersion strength is 1.5 MPa for 0–1 mm briquettes but only 1.3 MPa for 0–3 mm briquettes; post-drying recovery shows a similar pattern (1.3 MPa vs. 1.0 MPa). The superior performance of fine fractions is explained by their higher specific surface area, which increases binder coverage and enhances interparticle bonding [17,18,19]. Fine particles also fill voids more effectively, producing denser briquette structures with lower porosity and reduced water uptake. Coarse fractions, in contrast, form more open structures with larger pores, which facilitate water ingress and binder softening during immersion. These findings agree with prior studies on coal and biomass briquettes, where decreasing particle size improved both dry strength and durability [17,18,52]. However, extremely fine fractions may require higher compaction pressures to avoid excessive binder absorption into micropores [53], a factor to consider in scaling up production.
3.5. Water Uptake Measurement
Water absorption tests were conducted for all briquette formulations listed in Table 2, covering two particle size fractions (0–1 mm and 0–3 mm), five PVA binder contents (3–7% by dry mass) with constant 20% molasses, and four binder combinations containing 5% PVA plus 1–9% liquid soap as an additive [38]. Water uptake was quantified at 15, 30, 45, 60, 90, 120, 180, and 300 min, as well as at 1, 2, 3, and 4 days of immersion, both as absolute mass gain (g) and relative water absorption percentage [40]. The results reveal pronounced effects of particle size, binder content, and binder modification on both the rate and extent of water penetration [38,40]. Fine fractions generally exhibit slower early-stage absorption rates and marginally lower equilibrium uptake than coarse fractions at the same binder content. Increasing PVA content tends to reduce initial water ingress and delay saturation, although total long-term water uptake after 3–4 days converges for most formulations.

Table 2.
Water absorption of briquettes with different binder formulations over time (fractions 0–1 mm and 0–3 mm).
3.6. Fine Fraction (0–1 mm)—Effect of PVA Content on Absorption Kinetics
For 0–1 mm briquettes with 3% PVA, water absorption reached 5.22% within 15 min and 16.18% at 60 min. After 120 min, uptake was 31.13%, rising sharply to 50.41% by 180 min [40]. By 1 day, briquettes had absorbed 54.53% of their dry mass, with no significant change thereafter (stabilising at ~51% after minor fluctuations). This profile indicates a two-phase absorption: (i) rapid infiltration in the first 3 h, and (ii) a slower approach to equilibrium over the next 21 h. At 4% PVA, early-stage uptake slowed: 3.59% at 15 min, 11.30% at 60 min, and 24.63% at 120 min. Saturation occurred around 46–47%, ~8% lower than the 3% PVA case. With 5% PVA, absorption at 15 min was 4.07%, only marginally above the 4% case, but the profile differed: a slow rise to 10.16% by 120 min, followed by a sharp jump to 33.24% at 180 min and a delayed but steep increase to 56.25% at 1 day. At 6% PVA, early absorption remained low (4.15% at 15 min, 6.69% at 60 min), and even at 180 min uptake was only 26.74%. Equilibrium was reached at ~53%. This steady resistance to penetration during the first 3 h supports literature reports [7,12,37], which indicate that high PVA content increases swelling resistance by forming dense, flexible networks. The 7% PVA samples showed the lowest initial uptake: 3.53% at 15 min, 5.50% at 60 min, and only 19.49% at 180 min. The final equilibrium absorption (~52.8%) was similar to other fine-fraction samples, confirming that binder content primarily affects the rate, not the total capacity, of water absorption [40].
3.7. Coarse Fraction (0–3 mm)—Elevated Uptake and Faster Saturation
The coarse fraction exhibited significantly faster and higher early absorption rates. At 3% PVA, 15 min uptake was already 25.42%, exceeding the fine fraction’s 180 min value. By 60 min, absorption reached 38.45%, and saturation (~56–57%) occurred by 1 day. At 4% PVA, early uptake was 22.23% (15 min) and 38.87% (60 min), with saturation at ~57.7%. Even with 5% PVA, 60 min absorption was 25.78%, considerably higher than the fine fraction’s 6–10% at this time. The 6% PVA coarse fraction displayed very high initial uptake (38.17% at 15 min, 45.56% at 60 min) and reached ~62% at 1 day—higher than any fine-fraction sample. Similarly, 7% PVA coarse briquettes had 26.05% uptake at 15 min and ~51.5% equilibrium [40] but still showed much faster initial wetting than fine equivalents. These data confirm the dominant role of particle size in controlling water ingress [17,18,53].
3.8. Binder Combinations—Liquid Soap as an Additive
Liquid soap additions to 5% PVA + 20% molasses briquettes modified water absorption behaviour in complex ways (Figure 6). At 1% soap, 15 min uptake was 6.12%, rising to 18.90% at 60 min, and stabilising around 54% after 1 day. At 3% soap, slightly lower early uptake (4.93% at 15 min) was observed, but similar saturation (~52.2%). At 5% soap, initial uptake was 4.53% at 15 min, but irregular behaviour occurred—14.42% at 45 min, dip at 60–90 min, then final equilibrium (~54%). At 9% soap, markedly higher absorption occurred—7.27% at 15 min, 30.13% at 60 min, final ~59.3% [40]. The non-linear effects arise from two competing mechanisms: (i) moderate surfactant improves binder wetting, film coverage, and reduces permeability [21,42]; (ii) excessive surfactant increases hydrophilicity, promoting water channeling [21,42].
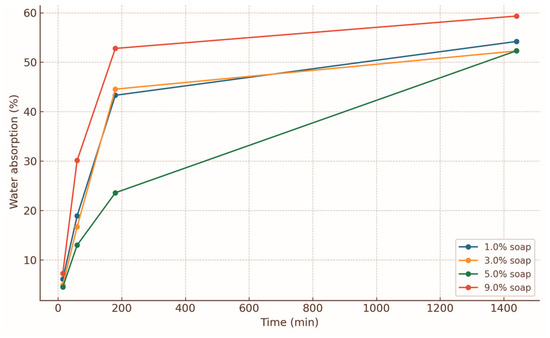
Figure 6.
Water absorption profiles for 0–1 mm briquettes with 5% PVA, 20% molasses, and varying liquid soap contents (1–9%).
3.9. Comparative Analysis Across All Formulations at Fixed Immersion Times
To enable a direct comparison of water absorption performance across the full range of tested binder compositions and particle sizes, the data from Table 2 were analysed at four representative immersion times: 15 min, 60 min, 300 min, and 4 days. These time points were selected to represent (i) early-stage wetting and capillary infiltration (15 min), (ii) intermediate swelling and binder hydration (60 min), (iii) near saturation of easily accessible pores (300 min), and (iv) final equilibrium moisture content (4 days). In the early stage (15 min), water absorption values ranged from a minimum of 3.53% for the fine fraction (0–1 mm) with 20% molasses + 7% PVA to a maximum of 38.17% for the coarse fraction (0–3 mm) with 20% molasses + 6% PVA. By 60 min, absorption levels increased significantly, with most fine-fraction samples stabilising in the 5–19% range, while coarse-fraction samples ranged from 25.78% (0–3 mm + 5% PVA) to 45.56% (0–3 mm + 6% PVA). The lowest intermediate absorption was recorded for 0–1 mm + 4% PVA (11.30%), suggesting that this composition effectively restricts capillary penetration without excessive binder brittleness [40]. At the 300 min mark, values converged into narrower ranges: fine-fraction formulations showed ~19–50% absorption, while coarse fractions exhibited ~43–55%. The addition of liquid soap notably altered these kinetics: the 0–1 mm + 5% PVA + 3% soap formulation demonstrated a controlled uptake profile, with 49.24% at 300 min—comparable to soap-free systems but with a smoother absorption curve, indicating reduced microcracking during wetting [12]. After 4 days, equilibrium water absorption was reached, with the lowest value recorded for 0–1 mm + 4% PVA (47.04%) and the highest for 0–3 mm + 6% PVA (61.29%) [40].
From a performance perspective, the top performing formulations for minimising both initial and long-term water uptake were: (i) 0–1 mm + 4% PVA—lowest 4-day absorption (47.04%) and moderate early-stage wetting; and (ii) 0–1 mm + 5% PVA + 3% liquid soap—balanced performance, reduced early swelling (4.93% at 15 min) with controlled long-term absorption (52.24% after 4 days). These results have practical implications for briquette durability in humid storage. Compositions with fine particle size and moderate PVA content (4–5%), optionally with low levels of liquid soap, provide optimal resistance to prolonged moisture exposure. This is critical for preventing strength loss, microbial growth, and dimensional instability in biomass briquettes stored in non-climate-controlled environments [9,17,52].
4. Discussion
The collected data highlight clear trends in how binder composition and particle size influence the mechanical performance and moisture resistance of molasses-bound briquettes. Increasing the PVA content from 3% to 7% leads to substantial gains in compressive strength prior to immersion—particularly for the fine fraction (0–1 mm), where strength rises from 1.1 MPa at 3% PVA to 2.6 MPa at 7% PVA. This improvement reflects the formation of a continuous PVA–molasses matrix that enhances interparticle bonding through hydrogen bonding and film densification [12,13]. However, the marginal gains diminish above roughly 6% PVA, suggesting an optimal range of 6–7% PVA for balancing mechanical performance and binder cost [9,13,24,53,54,55]. For coarse fractions (0–3 mm), similar trends occur but at lower strength levels due to less efficient packing and larger voids [17,18]. The results therefore emphasise the importance of particle size optimisation in briquetting processes.
Water immersion tests reveal that higher PVA contents also improve water resistance: post-immersion strength increases from 0.2 MPa at 3% PVA to 0.6 MPa at 7% PVA for the fine fraction. Post-drying recovery underscores the reversibility of the swelling: at 7% PVA, briquettes regained 2.3 MPa after drying, nearly matching their original dry strength. This suggests that the PVA–molasses network swells but does not dissolve, preserving structural integrity [9,53,56]. In mixed fractions, recovery is more limited because larger pores allow greater water ingress and hinder bonding upon drying [17,18]. Coarse fractions also exhibit faster and higher water uptake, particularly during the first hour of immersion, confirming the dominant role of particle size on moisture transport [17,18,53].
Surfactant (liquid soap) additions exhibit a non-linear influence on both strength and water uptake. Moderate levels (2–3%) enhance binder wetting and distribution, leading to higher dry strength and moderate wet strength improvements. For example, the 0–1 mm + 5% PVA + 3% soap formulation achieved 2.5 MPa dry strength and showed controlled water absorption kinetics, with only 4.93% uptake after 15 min. However, at higher surfactant levels (≥5%), mechanical strength decreases and water uptake increases, likely due to the disruption of the polymer network and increased hydrophilicity [21,42]. Thus, there exists an optimal surfactant range (around 2–3%) that maximises mechanical benefits without compromising water durability.
Correlation analysis between PVA content, dry strength, and post-drying recovery yields strong positive relationships (r > 0.9), underscoring the dominant influence of binder content on mechanical integrity. The correlation between pre-immersion and post-immersion strengths is moderate (r ≈ 0.6), indicating that factors such as particle size distribution and surfactant content also play significant roles in water resistance [39,40,46,52]. From a process perspective, the findings suggest that for fine fractions, combining 6–7% PVA with 2–3% liquid soap offers the best balance of strength, water resistance, and cost [9,17,21]. For coarse fractions, additional strategies—such as higher compaction pressure, incorporation of secondary binders, or narrowing particle size distributions—may be required to achieve comparable performance [17,18,38].
Finally, the water absorption profiles emphasise the criticality of early-stage wetting (first 1–3 h) in determining long-term durability. Formulations that minimise rapid initial uptake, such as 0–1 mm + 4% PVA and 0–1 mm + 5% PVA + 3% soap, delay swelling and maintain structural integrity during the time when briquettes are most vulnerable. This insight is crucial for storage and handling: in humid environments, preventing rapid initial wetting can forestall mechanical degradation until drying conditions improve.
This work demonstrates that modest additions of PVA (6–7%) in combination with molasses significantly improve briquette durability and water resistance while remaining economically feasible. The systematic evaluation of particle size effects and surfactant additions provides clear, practical guidelines for optimizing briquette formulations. The results show high reproducibility and provide evidence for binder synergy that can be directly applied in real-world briquetting operations.
The main limitations include the small laboratory scale of the study, reliance on hydraulic press compaction rather than industrial-scale equipment, and immersion testing in deionized water, which does not fully represent field conditions. Coarse particle fractions remain less durable under wetting conditions, and the relatively higher cost of PVA compared to traditional binders like molasses could limit broader adoption without careful economic optimization.
Overall, the study indicates that binder optimization, particle size control, and judicious surfactant use are key strategies for producing durable, water-resistant briquettes. Future work should include pilot-scale production trials, compliance testing against EN ISO 17225 standards, and advanced characterization (SEM, FTIR) to better understand binder–substrate interactions. Techno-economic assessments and life cycle analyses will also be important to validate the industrial scalability and sustainability of these binder systems.
5. Conclusions
This study showed that the performance of molasses-bound biomass briquettes depends strongly on the interplay between particle size, PVA content, and surfactant addition. Increasing the PVA concentration from 3% to 7% substantially enhanced compressive strength, with fine fractions (0–1 mm) displaying up to a 136% improvement and an optimal range of 6–7% identified. Although coarse fractions (0–3 mm) followed the same trend, their absolute strengths were consistently lower due to reduced packing density and higher porosity. Water absorption experiments revealed that PVA primarily slowed the rate of wetting rather than lowering the final equilibrium moisture content; nevertheless, higher binder levels (≥6%) effectively delayed early swelling and improved strength recovery after drying. Particle size proved to be a decisive factor, with finer fractions outperforming coarser ones in both dry and wet conditions because of their denser structure and reduced macrovoid connectivity. The role of surfactant was more complex: moderate additions of liquid soap (2–3%) improved binder wetting and short-term water resistance, while excessive amounts (>5%) promoted hydrophilicity and weakened long-term durability. Taken together, the best formulations for use under humid storage conditions were 0–1 mm + 4% PVA and 0–1 mm + 5% PVA + 3% soap, as they combined minimal early swelling with controlled long-term water uptake. These findings provide practical guidance for designing eco-friendly binder systems, emphasising that fine particle size, moderate PVA loading, and carefully balanced surfactant levels yield the most durable and cost-effective briquettes. Future investigations should focus on scaling these formulations to industrial production, testing under higher compaction pressures, and evaluating their stability during extended storage under variable humidity.
Author Contributions
Conceptualization, N.K. and V.Y.; methodology, N.K.; validation, N.K. and V.Y.; formal analysis, N.K.; investigation, N.K.; resources, N.K.; data curation, N.K.; writing—original draft preparation, N.K.; writing—review & editing, V.Y.; visualization, N.K.; supervision, V.Y.; project administration, N.K.; funding acquisition, V.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon Europe Programme under the QUEEN Project (Grant Agreement No. 101178144). The APC was funded by the same source.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to institutional policy and confidentiality agreements with industrial partners.
Conflicts of Interest
Both authors (Nataliya Korol and Viktor Yankovych) are employees of LLC Perechin Timber and Chemical Plant (a commercial entity). The results presented in this study were generated in the context of the Horizon Europe QUEEN Project (Grant Agreement No. 101178144) and are relevant to product development at LLC Perechin Timber and Chemical Plant.
Abbreviations
The following abbreviations are used in this manuscript:
| PVA | Polyvinyl Alcohol |
| QUEEN | Quartz Enrichment Enabling Near-Zero Silicon |
References
- Obi, O.F.; Pecenka, R.; Clifford, M.J. A review of biomass briquette binders and quality parameters. Energies 2022, 15, 2426. [Google Scholar] [CrossRef]
- REN21. Renewables 2023 Global Status Report; REN21 Secretariat: Paris, France, 2023. [Google Scholar]
- Kaliyan, N.; Morey, R.V. Factors affecting strength and durability of densified biomass products. Biomass Bioenergy 2009, 33, 337–359. [Google Scholar] [CrossRef]
- Lubwama, M.; Yiga, V.A. Development of groundnut shells and bagasse briquettes as sustainable fuel sources for domestic cooking applications in Uganda. Renew. Energy 2017, 111, 532–542. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Z.; Cai, Z.; Fei, B.; Yu, Y.; Liu, X. Effects of carbonization conditions on properties of bamboo pellets. Renew. Energy 2013, 51, 1–6. [Google Scholar] [CrossRef]
- Grover, P.D.; Mishra, S.K. Biomass Briquetting: Technology and Practices; FAO Regional Wood Energy Development Programme in Asia: Bangkok, Thailand, 1996. [Google Scholar]
- Marreiro, H.M.P.; Peruchi, R.S.; Lopes, R.M.B.P.; Andersen, S.L.F.; Eliziário, S.A.; Junior, P.R. Empirical studies on biomass briquette production: A literature review. Energies 2021, 14, 8320. [Google Scholar] [CrossRef]
- Benk, A.; Coban, A. Molasses and air blown coal tar pitch binders for the production of metallurgical quality formed coke from anthracite fines or coke breeze. Fuel Process. Technol. 2011, 92, 1078–1086. [Google Scholar] [CrossRef]
- Makgobelele, N.W.; Mbaya, R.K.K.; Bunt, J.R.; Leokaoke, N.T.; Neomagus, H.W.J.P. Evaluation of the mechanical properties of wood-derived charcoal briquettes for use as a reductant. J. S. Afr. Inst. Min. Metall. 2021, 121, 187–192. [Google Scholar] [CrossRef]
- Rajput, S.P.; Thorat, B.N. Recovered polyvinyl alcohol as an alternative binder for the production of metallurgical quality coke breeze briquettes. Int. J. Coal Prep. Util. 2019, 42, 475–485. [Google Scholar] [CrossRef]
- Patra, S.K.; Ghorai, S.; Sahu, N.; Kapure, G.U.; Tripathy, S.K. Synthesis of bio-polymer based composite binder for utilization of industrial coke dust waste. Fuel 2022, 311, 122502. [Google Scholar] [CrossRef]
- Nganko, J.M.; Koffi, E.P.M.; Gbaha, P.; Toure, A.O.; Kane, M.; Ndiaye, B.; Faye, M.; Nkounga, W.M.; Tekounegning, C.T.; Bile, E.E.J.; et al. Modeling and Optimization of Compaction Pressure, Binder Percentage and Retention Time in the Production Process of Carbonized Sawdust-Based Biofuel Briquettes Using Response Surface Methodology (RSM). Heliyon 2024, 10, e25376. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, T.; Wei, S.; Zhao, C.; Zhang, X.; Li, X.; Tang, X.; Liu, Y.; Yang, Z.; Chen, L. Molecular Docking, Molecular Dynamics Simulations, and Free Energy Calculation Insights into the Binding Mechanism between VS-4718 and Focal Adhesion Kinase. ACS Omega 2022, 7, 32442–32456. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.A.; Saharuddin, S.N.D.M.; Muhamad, M.H. Unlocking the Potential of Polyvinyl Alcohol (PVA) as a Biocarrier for Enhanced Wastewater Treatment: A Comprehensive Review. J. Water Process Eng. 2025, 74, 107780. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Huang, M.; Zhang, M.; He, Y.-C. Preparation of Dandelion Flower Extract-Based Polyvinyl Alcohol-Chitosan-Dandelion-CuNPs Composite Gel for Efficient Bacteriostatic and Dye Adsorption. Int. J. Biol. Macromol. 2024, 281, 136512. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; He, Y. Preparation and Application of Multifunctional Chitosan-Polyvinyl Alcohol-Nanosilver-Chrysanthemum Extract Composite Gel. Processes 2025, 13, 517. [Google Scholar] [CrossRef]
- Chin, O.C.; Siddiqui, K.M. Characteristics of Some Biomass Briquettes Prepared under Modest Die Pressures. Biomass Bioenergy 2000, 18, 223–228. [Google Scholar] [CrossRef]
- Mitchual, S.J.; Frimpong-Mensah, K.; Darkwa, N.A. Effect of Species, Particle Size and Compacting Pressure on Relaxed Density and Compressive Strength of Fuel Briquettes. Int. J. Energy Environ. Eng. 2013, 4, 30. [Google Scholar] [CrossRef]
- Okot, D.K.; Bilsborrow, P.E.; Phan, A.N. Effects of Operating Parameters on Maize Cob Briquette Quality. Biomass Bioenergy 2018, 112, 61–72. [Google Scholar] [CrossRef]
- Major, B.J.; Radu, G. Briquette Binder Composition. U.S. Patent 6,013,116, 11 January 2000. [Google Scholar]
- Yang, X.; Wang, W.; Long, M.; Xiang, L.; Cao, Y.; Hao, J. Relationship between Surfactant Content and the Properties of Microfibrillated Cellulose/High Density Polyethylene Composites. Polym. Compos. 2025, 46, 4947–4957. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Wright, C.T. A Review on Biomass Densification Technologies for Energy Application; Idaho National Laboratory (INL): Idaho Falls, ID, USA, 2010. [CrossRef]
- Olugbade, T.; Ojo, O.; Mohammed, T. Influence of Binders on Combustion Properties of Biomass Briquettes: A Recent Review. BioEnergy Res. 2019, 12, 241–259. [Google Scholar] [CrossRef]
- Kpalo, S.Y.; Zainuddin, M.F.; Manaf, L.A.; Roslan, A.M. A Review of Technical and Economic Aspects of Biomass Briquetting. Sustainability 2020, 12, 4609. [Google Scholar] [CrossRef]
- Gilvari, H.; de Jong, W.; Schott, D.L. Quality Parameters Relevant for Densification of Bio-Materials: Measuring Methods and Affecting Factors—A Review. Biomass Bioenergy 2019, 120, 117–134. [Google Scholar] [CrossRef]
- Awwal, O.A.; Israel, O.K.; Yashim, Z. Physico-Chemical, Calorific, and Emission Performance of Briquettes Produced from Maize Cob, Sugarcane Bagasse, and Polythene Composites. Avicenna J. Environ. Health Eng. 2019, 6, 49–54. [Google Scholar] [CrossRef]
- Omoniyi, T.E.; Igbo, P.K. Physico-Mechanical Characteristics of Rice Husk Briquettes Using Different Binders. Agric. Eng. Int. CIGR J. 2016, 18, 70–81. [Google Scholar]
- Davies, R.M.; Davies, I.E.E.; Davies, G.O. Studies on the Effect of Particle Size, Binder Ratio and Pressure on Compaction Energy of Water Hyacinth Briquettes. Asian Sci. Bull. 2024, 2, 148–155. [Google Scholar] [CrossRef]
- Filimon, A.; Dobos, A.M.; Onofrei, M.D.; Serbezeanu, D. Polyvinyl Alcohol-Based Membranes: A Review of Research Progress on Design and Predictive Modeling of Properties for Targeted Application. Polymers 2025, 17, 1016. [Google Scholar] [CrossRef]
- Davies, R.M.; Davies, O.A. Physical and Combustion Characteristics of Briquettes Made from Water Hyacinth and Phytoplankton Scum as Binder. J. Combust. 2013, 2013, 549894. [Google Scholar] [CrossRef]
- Song, B.; Cooke-Willis, M.; van Leeuwen, R.; Fahmy, M.; Hall, P. Insights into the Swelling Behaviours of Biomass and Biomass/Thermoplastic Briquettes under Water Penetration and Moisture Adsorption. Biomass Bioenergy 2023, 168, 106673. [Google Scholar] [CrossRef]
- Borowski, G.; Stępniewski, W.; Wójcik-Oliveira, K. Effect of Starch Binder on Charcoal Briquette Properties. Int. Agrophys. 2017, 31, 571–574. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Odusote, J.K.; Ikubanni, P.P.; Adebisi, J.A.; Adesina, O.S.; Babatunde, D.E. Briquetting of Subbituminous Coal and Torrefied Biomass Using Bentonite as Inorganic Binder. Sci. Rep. 2022, 12, 8716. [Google Scholar] [CrossRef]
- Kodji, E.; Tize, K.J.; Awono, A.; Djoulde, D.R. Accessibility and Effects of Binder Types on the Physical and Energetic Properties of Ecological Coal. Heliyon 2022, 8, e11410. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, S.; Wang, C.; Mu, C.; Huang, X. High-Strength Charcoal Briquette Preparation from Hydrothermal Pretreated Biomass Wastes. Fuel Process. Technol. 2018, 171, 293–300. [Google Scholar] [CrossRef]
- Song, L.; Li, Y.; Lei, T.; Yang, Y.; Shen, Y.; Yang, M.; Wang, Y.; Zheng, H. Research on the Water Absorption Diffusion Model and Kinetics of Pretreated Straw. Sci. Rep. 2025, 15, 9927. [Google Scholar] [CrossRef]
- Akbar, A.; Aslam, U.; Asghar, A.; Aslam, Z. Effect of Binding Materials on Physical and Fuel Characteristics of Bagasse-Based Pellets. Biomass Bioenergy 2021, 150, 106118. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Peterson, T.; Sharma, N.; Shojaeiarani, J.; Bajwa, S.G. A Review of Densified Solid Biomass for Energy Production. Renew. Sustain. Energy Rev. 2018, 96, 296–305. [Google Scholar] [CrossRef]
- Craven, J.M.; Swithenbank, J.; Sharifi, V.N.; Peralta-Solorio, D.; Kelsall, G.; Sage, P. Hydrophobic Coatings for Moisture Stable Wood Pellets. Biomass Bioenergy 2015, 80, 278–285. [Google Scholar] [CrossRef]
- Ajith Kumar, J.; Vinoth Kumar, K.; Petchimuthu, M.; Iyahraja, S.; Vignesh Kumar, D. Comparative Analysis of Briquettes Obtained from Biomass and Charcoal. Mater. Today Proc. 2021, 45, 857–861. [Google Scholar] [CrossRef]
- Dai, X.; Theppitak, S.; Yoshikawa, K. Pelletization of Carbonized Wood Using Organic Binders with Biomass Gasification Residue as Additive. Energy Procedia 2019, 158, 509–515. [Google Scholar] [CrossRef]
- Butler, J.W.; Skrivan, W.; Lotfi, S. Identification of Optimal Binders for Torrefied Biomass Pellets. Energies 2023, 16, 3390. [Google Scholar] [CrossRef]
- Kabas, O.; Ercan, U.; Dinca, M.N. Prediction of Briquette Deformation Energy via Ensemble Learning Algorithms Using Physico-Mechanical Parameters. Appl. Sci. 2024, 14, 652. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Guan, F.; Liu, Y.; Yang, Q.; Zhang, X.; Xu, Y. Preparation of Electrospun Polyvinyl Alcohol/Nanocellulose Composite Film and Evaluation of Its Biomedical Performance. Gels 2021, 7, 223. [Google Scholar] [CrossRef]
- Ichsan, A.C.; Ningsih, R.V.; Rini, D.S.; Webliana, K. Combustion Performance and Physicochemical Characteristics of Sawdust-Based Bio-Charcoal Briquettes Using Molasses Adhesive. J. Sylva Lestari 2025, 13, 578–600. [Google Scholar] [CrossRef]
- Tambuwal, A.A.; Salihu, A.; Boyi, Y.M.; Garba, S.; Muhammad, A.S.; Sahabi, Y.; Umar, H.; Muhammad, M. Comparative Analysis of Gum Arabic and Molasses (Binders) in Briquette Produced from Millet Husk. J. Phys. Sci. Innov. 2022, 14, 13–27. [Google Scholar] [CrossRef]
- Alsaqoor, S.; Borowski, G.; Alahmer, A.; Beithou, N. Using of Adhesives and Binders for Agglomeration of Particle Waste Resources. Adv. Sci. Technol. Res. J. 2022, 16, 124–135. [Google Scholar] [CrossRef]
- Pacorel, B.; Hampson, C.A. Molasses Binder. European Patent EP2462169A2, 13 June 2012. [Google Scholar]
- Inkscape Project. Inkscape 1.3: Free and Open Source Vector Graphics Editor. 2023. Available online: https://inkscape.org (accessed on 18 September 2025).
- Clarke, D.E.; Marsh, H. Influence of coal/binder interactions on mechanical strength of briquettes. Fuel 1989, 68, 1023–1030. [Google Scholar] [CrossRef]
- Granado, M.P.P.; Suhogusoff, Y.V.M.; Santos, L.R.O.; Yamaji, F.M.; De Conti, A.C. Effects of pressure densification on strength and properties of cassava waste briquettes. Renew. Energy 2021, 167, 306–312. [Google Scholar] [CrossRef]
- Zepeda-Cepeda, C.O.; Goche-Tilles, J.R.; Palacios-Mendoza, C.; Moreno-Anguiano, O.; Niez-Retana, V.D.; Heya, M.N.; Carrillo-Parra, A. Effect of Sawdust Particle Size on Physical, Mechanical, and Energetic Properties of Pinus durangensis Briquettes. Appl. Sci. 2021, 11, 3805. [Google Scholar] [CrossRef]
- Kudo, S.; Otsuka, E.; Suzuki, A. Swelling behavior of chemically crosslinked PVA gels in mixed solvents. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1978–1986. [Google Scholar] [CrossRef]
- Ahn, B.J.; Chang, H.-S.; Lee, S.M.; Choi, D.H.; Cho, S.T.; Han, G.-S.; Yang, I. Effect of binders on the durability of wood pellets fabricated from Larix kaemferi C. and Liriodendron tulipifera L. sawdust. Renew. Energy 2014, 62, 18–23. [Google Scholar] [CrossRef]
- Somerville, M.A. The strength and density of green and reduced briquettes made with iron ore and charcoal. J. Sustain. Metall. 2016, 2, 228–238. [Google Scholar] [CrossRef]
- Kocer, A.; Kabas, O.; Zabava, B.S. Estimation of Compressive Resistance of Briquettes Obtained from Groundnut Shells with Different Machine Learning Algorithms. Appl. Sci. 2023, 13, 9826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).