1. Introduction
The global demand for energy continues to rise, driven by the growing population and increasing energy needs. In the United States alone, energy consumption is projected to grow by up to 15% between 2022 and 2050 [
1]. While advancements in technology enable the integration of various renewable energy sources, such as wind and solar power, into electrical grids with load-following power plants [
2], biomass derived from animals and plants offers a renewable alternative to reduce reliance on finite non-renewable energy sources, such as coal and oil. Lignocellulosic biomass (LCB), can support base-load and peaking-power plants through conversion into biofuels (via conversion of polysaccharides into bioethanol [
3,
4]) and direct combustion (as seen in repurposed coal-fired boilers [
5]).
LCB consists of three major polymers: cellulose, a homopolysaccharide of β-D-glucopyranose; hemicelluloses, heteropolysaccharides composed of pentoses and hexoses; and lignin, an amorphous aromatic polymer, along with minor amounts of extractives, which are diverse low molecular weight organic compounds [
3,
6]. LCB can be classified as woody materials, including softwood and hardwood trees, [
7] and as agricultural materials, such as residues and herbaceous energy crops [
7]. Compared to coal, LCB has a lower energy content (typically listed as higher heating value (HHV)) [
8] and bulk density when chipped or baled, limiting its competitiveness as a fuel [
8]. For example, the HHV of coal can be in the range of 13.41–23.21 MJ/kg (lignite coal) [
9,
10,
11] and 17.33–29.19 MJ/kg (bituminous coal) [
9,
12] compared to LCB, such as hardwood (17.6–20.1 MJ/kg) [
13,
14], softwood (17.38–21.2 MJ/kg) [
13,
15,
16], wheat straw (12.3–17.5 MJ/kg) [
14,
15], miscanthus (17–20 MJ/kg) [
17], and willow (18.6–19 MJ/kg) [
18,
19]. Pelletization addresses this by densifying LCB into pellets, thereby increasing its energy and bulk density [
8]. Global pellet production increased from 18.05 million tonnes in 2012 to 47.59 million tonnes in 2022 [
20]—reflecting growing interest in LCB as a sustainable energy source.
Despite the increasing adoption of LCB fuel pellets, significant challenges remain. LCB pellets are prone to moisture absorption, which compromises mechanical integrity [
16,
21], reduces energy yield (if latent heat of vaporization is not recovered) [
22], and accelerates physical, chemical, and biological degradation [
23,
24]. Additionally, the high ash of LCB pellets can damage boilers utilized in steam production and hinder efficient combustion [
25,
26]. Another major issue is the accumulation of carbon monoxide (CO) during pellet storage. Densified LCB, such as pellets, emits significantly more CO than ground or chipped biomass [
27]. For instance, CO emissions from pellets made from softwood (pine and spruce) can range from 769 to 13,440 ppm [
23,
27,
28,
29,
30], far exceeding emissions from hardwood pellets ranging from 105 to 1200 ppm [
23,
30]. These emissions have been linked to injuries and fatalities among workers handling LCB pellets [
31,
32], underscoring the need for safer storage and improved pellet properties.
Hot water extraction (HWE) offers a potential solution to some of these challenges. HWE is a hydrothermal pretreatment in which LCB undergoes autohydrolysis under relatively mild conditions (typically at 160 °C for two hours [
33,
34,
35,
36]). Autohydrolysis, initiated by the deacetylation of acetylated hemicelluloses (such as xylans in angiosperms) and sustained by the release of other acidic compounds like aromatic acids and glucuronic acids, generates mildly acidic conditions that promote the cleavage of glycosidic bonds in the LCB [
37,
38]. As a result, hot water extracted LCB (HWE-LCB) typically has a lower hemicellulose content than untreated LCB. Prior studies [
34,
35,
39,
40] on shrub willow (
Salix spp.), miscanthus (
Miscanthus spp.), and wheat straw (
Triticum spp.) reported a loss of glucan and xylan after HWE—1.9% and 49.0% for willow, 9.8% and 57.1% for miscanthus, and 10.1% and 64.4% for wheat straw, respectively. HWE removed substantial amounts of ash, reducing ash by 44.6%, 21.6%, and 61.0% in willow, miscanthus, and wheat straw, respectively [
39,
40]. Although some lignin is lost in the hydrolysate [
8], the relative lignin content in HWE-LCB increases due to the more pronounced loss of hemicelluloses.
These compositional changes from HWE offer multiple benefits for fuel pellets. Hemicelluloses, which have a lower energy value (16.6 MJ/kg) [
41] compared to both cellulose (17.0 MJ/kg) [
14,
41], and especially, lignin (21.0–26.6 MJ/kg) [
14,
41], reduce the overall energy content of untreated LCB. Hemicelluloses are also more hydrophilic than both cellulose [
42] and lignin [
43], contributing to moisture absorption of untreated LCB. In contrast, lignin acts as a natural binder and assists particle adhesion. The combined effects of compression and heat [
44] during pelletization promote covalent bonding between lignin and polysaccharides, as well as solid bridge formation between particles [
45], both of which are necessary for producing long, durable pellets. Consequently, HWE-LCB pellets are anticipated to have higher energy content, reduced ash content, enhanced water resistance, and improved durability compared to untreated LCB pellets. The hypothesized effects of HWE on pellet properties have been supported by various studies throughout the literature.
Eisenbies et al. [
46] produced pellets by blending willow (shrub willow with bark), maple, HWE-willow, and HWE-maple in different ratios. For debarked maple/HWE-maple blends, the moisture content and ash content of the pellets decreased as the proportion of HWE-maple increased. Additionally, pure HWE-willow pellets had lower moisture and ash content than untreated willow pellets. The energy content also improved with increased incorporation of HWE-maple, and pure HWE-willow pellets showed superior energy content compared to untreated willow pellets. Furthermore, both HWE-maple and HWE-willow pellets produced fewer fines than their untreated counterparts, indicating improved durability.
Similar trends were observed by Sermyagina et al. [
36], Nguyen et al. [
21], and Melin et al. [
47] with pellets made from softwood sawdust [
36,
47], and sugar maple and birch particles (hammermilled and refined) [
21] that were hot water extracted. They reported increases in energy content, individual pellet density (determined via measurement of the mass and dimensions of a sample of pellets), and mechanical strength due to HWE. The changes in pellet density are supported in a study by Roman et al. [
48] in which the overall density of pellets made from HWE-miscanthus (ground to a particle size of 0.1 mm) increased after one HWE cycle compared to the control, due to improved compaction efficiency and a reduction in the energy required for compaction. All studies documented a decrease in ash in HWE-LCB pellets. Nguyen et al. [
21] also observed that HWE-LCB pellets absorbed less water than untreated LCB pellets, which disintegrated within five minutes after submersion in water, whereas HWE-LCB pellets completely disintegrated after one week. Graham et al. [
16] reported that pellets made from a steam exploded hardwood/softwood blend maintained a higher durability after exposure to elevated temperatures and relative humidities compared to torrefied pellets and white pellets (pellets made from untreated softwood).
Lignin’s role in pellet properties has been explored extensively. Studies have shown that adding lignin as a binding agent before pelletizing LCB tends to enhance pellet properties. Pellets produced from softwoods and hardwoods with additional kraft lignin [
49,
50,
51,
52], which inherently contains a low content of carbohydrates, consistently showed improved energy content. In contrast, the addition of lignosulfonates yielded inconsistent results, likely due to the higher content of low-energy-density carbohydrates present in this type of lignin. Durability of pellets with lignin as a binding agent was consistently enhanced across multiple studies [
36,
49,
51,
53,
54,
55]. However, inconsistent trends have been observed in ash [
36,
49,
50,
53], pellet length [
36,
49,
51], and bulk density [
49,
51,
54] in pellets with additional lignin.
HWE and additional lignin may also address issues with CO emissions during the storage of LCB fuel pellets. CO generation has been linked to the autoxidation of lipophilic extractives, such as unsaturated fatty acids, and the formation of radicals that trigger a chain of reactions in hemicelluloses that ultimately produce CO [
28]. Therefore, decreasing the content of hemicelluloses while increasing the content of lignin, a natural radical quencher, may influence CO production. While the role of various lipophilic extractives in CO production is still under investigation [
56,
57], the involvement of radicals in CO generation appears to be significant. Therefore, the following two strategies may be proposed to mitigate CO emissions: the removal of hemicelluloses to limit their participation in radical reactions and the inhibition of radicals from reacting with hemicelluloses.
Several studies have examined the use of antioxidizing agents—such as 1-butanol [
28], ozone pretreatment [
58],
tert-butylhydroquinone (TBHQ) [
59], propyl gallate (PG) [
59], and acetylsalicylic acid (ASA) [
60]—to reduce CO emissions from LCB fuel pellets. Various lignins, including organosolv ethanol lignins [
61], kraft lignin [
8,
34], and HWE-recovered lignin [
8,
34], have also exhibited antioxidant activities exceeding that of BHT (3,5-di-tert-butyl-4-hydroxytoluene) as a synthetic antioxidant. In these analyses, 1,1-Diphenyl-2-picrylhydrazyl (DPPH) methods were utilized to examine the antioxidant activities of BHT [
61] and the various lignin samples [
8,
34,
61]. Notably, HWE-recovered lignin was 1.4 times more effective than the kraft lignin as an antioxidizing compound [
8], although less effective than natural antioxidants, vitamin E [
61], vitamin C (ascorbic acid) [
34], and ferulic acid [
8] (which were also examined using DPPH methods [
8,
34,
61]). The use of lignin as a radical scavenger to reduce CO emissions has demonstrated additional benefits, including enhancements in mechanical and energy properties, as well as reductions in moisture absorption and ash content of willow pellets [
8].
Based on the current proposed mechanisms of CO production and the ability of HWE to remove a significant portion of hemicelluloses while increasing the content of lignin as a radical scavenger, HWE emerges as a promising candidate for mitigating CO emissions while simultaneously improving the mechanical and energy properties of LCB fuel pellets. The aim of this work was to examine the effect of HWE on CO production, as well as mechanical and energy properties of pellets from three representative angiosperm lignocellulosics: shrub willow (Salix spp.), miscanthus (Miscanthus spp.), and wheat straw (Triticum spp.). Prior to this research, information on the mechanical and energy properties of HWE-miscanthus and-wheat straw was limited, and the potential of HWE to curb the generation of CO had not been explored to the best of our knowledge.
2. Materials and Methods
Procurement of Lignocellulosic Biomass (LCB) and Commercial Fuel Pellets. Shrub willow (Salix spp.) was procured from the SUNY-ESF Tully Field Station in Tully, NY, USA. The willow was chipped (2 cm long pieces) without bark removal. Miscanthus (Miscanthus spp.) and wheat straw (Triticum spp.) were obtained from Mesa Bioenergy Services, LLC (Cobleskill, NY, USA). Miscanthus and wheat straw were chopped to a particle size of <1.9 cm. All LCB was air-dried and stored in super sacks prior to hot water extraction and pelletizing. Lignetics Green Supreme (Broomfield, CO, USA) pellets of an unspecified “Northern” hardwood/softwood blend were obtained from a local distributor to represent commercial fuel pellets. Pellets were stored in a cold room (4 °C) until tested.
Hot Water Extraction (HWE) of LCB. HWE was conducted at 160 °C for 2 h at a liquid to LCB ratio of 8.0 for miscanthus and wheat straw, and an average of 4.4 for shrub willow in a 1.84 m3 Struthers-Well stainless lined batch digester (Sante Fe Springs, CA, USA) that used direct steam injection for miscanthus and wheat straw and indirect heating for willow. After HWE was completed, the hydrolysate was discharged from the digester, and the hot water extracted LCB (HWE-LCB) was rinsed twice with water at 80 °C for 15–20 min. The HWE-LCB was air-dried for several days prior to pelletizing.
Characterization of LCB and HWE-LCB. Organic solvent extraction of the LCB and HWE-LCB was performed in accordance with TAPPI standard T-204 [
62] in triplicate. A three-step sequential Soxhlet extraction of LCB and HWE-LCB was conducted using
n-hexane, ethanol:toluene (1:2), and ethanol (Pharmco/Greenfield Global, Brookfield, CT, USA) to cover a range of polarity indices (0.1, 3.33, and 5.2, respectively) [
63,
64]. The chemical composition of the extractive-free LCB and HWE-LCB was determined by the USDA-FS—Forest Products Lab (FPL) following the standard two-step acid hydrolysis and the high-performance anion exchange chromatographic method with pulsed amperometric detection (HPAEC/PAD) [
65] analysis of the hydrolysate for monosaccharides. The content of monosaccharides was converted to the corresponding content of polysaccharides using anhydro-correction factors of 0.88 (132/150) and 0.90 (162/180) for pentoses (C
5 monosaccharides—xylose and arabinose) and hexoses (C
6 monosaccharides—glucose, galactose, and mannose), respectively. The acid-insoluble lignin was determined gravimetrically, and the acid-soluble lignin was determined by measuring the absorbance at 205 nm (spectrophotometer Genesys 180 UV-vis from ThermoFisher Scientific (Waltham, MA, USA)), using 110 L (g cm)
−1 absorption coefficient [
66]. Each sample was analyzed in duplicate.
Hammermilling and Pelletizing of LCB. A pilot-scale hammermill and pelletizer from Lawson Mills Biomass Solutions (now Kovo Novak, division of Kesir Industrial, Mount Herbert, PE, Canada) were utilized to produce fuel pellets from each LCB and HWE-LCB. The hammermill with a 6 mm perforation screen was used to reduce the particle size of the feedstock, which was then stored under dry, ambient conditions. Pelletizing was carried out using a flat die pelletizer equipped with a hopper, screw augers for transporting biomass, an in-line water addition system, and a die with 6 mm diameter holes. Pellets were cooled at ambient conditions and stored in sealed buckets at 4 °C until testing occurred.
Characterization of Fuel Pellets. The moisture content of pellets was determined using a modified version of ISO 18134-2 [
67], adhering to the maximum loading specifications to test 10–20 g of pellets per replicate. Moisture content results were subsequently used in calculations for ash content, energy content, and moisture absorption properties of the fuel pellets. Ash content was examined using ISO 18122 at 550 °C [
68]. Energy content measurements were conducted using a ISO 18125 [
69] procedure in a Parr 6200 isoperibol oxygen bomb calorimeter (Moline, IL, USA). Pellets were conditioned to constant mass under laboratory conditions consistent with the calorimeter environment and then directly placed into the oxygen bomb for testing.
The bulk density of pellets was examined according to ISO 17828 [
70] using the “small container” apparatus specifications and an ISO certified 5 L steel cylinder tester (Bioenergy Institute, Vienna, Austria). Pellet durability was measured according to ISO 17831 [
71], using an ISO certified TUMBLER 100 R Durability Tester (Bioenergy Institute, Vienna, Austria). Pellets were screened before and after tumbling using a 3.35 mm screen. The durability index was calculated as the ratio of the mass of pellets remaining after tumbling to their initial mass. Pellet length was measured in compliance with ISO 17829 [
72], and any pellets shorter than 3.15 mm were discarded as per the definition of pellets in ISO 17225-1 [
73]. The individual pellet density was determined via the measurement of the average diameter, length, and mass of the pellets examined in ISO 17829 [
72].
Moisture absorption of pellets was analyzed using a modified version of TAPPI standard T-550 [
74]. Initially, the pellets were dried following ISO 18134-2 [
67] and then stored in a sealed desiccator placed in a temperature and humidity-controlled room (23 °C and 50 ± 2% relative humidity) overnight to equilibrate to the room temperature. The initial mass of the dry pellets was recorded before exposing them to the conditioned atmosphere of the room. The mass of the pellets was subsequently monitored at regular intervals until constant mass was achieved.
Carbon Monoxide (CO) Emissions of Fuel Pellets. CO emissions of the pellets were measured in two experiments. For the first set of experiments, pellets were tested under dark, ambient conditions. Pellets were placed in glass jars with an approximate volume of 3.84 L, ensuring 50% headspace (
Figure 1) following previous study on CO measurements in sealed pellet containers [
23,
28]. EasyLog EL-USB-CO and EasyLog EL-SIE-1 temperature sensors obtained from LASCAR Electronics (Erie, PA, USA) were utilized to measure CO emissions in individual jars and the temperature of the overall storage area, respectively. Only CO was monitored due to its significant health and safety risks in pellet-related working environments [
31]. The EL-USB-CO sensors were positioned in the center of the headspace within each jar. The jars were then sealed and stored in a darkened laboratory hood under ambient conditions. CO measurements were taken continuously over a 95-day period.
In the second experiment, pellets were tested under dark, temperature-controlled conditions as described in prior work [
8]. Glass jars with an approximate volume of 0.47 L were filled halfway with pellets to yield a 50% headspace, with each jar containing approximately 150 g of pellets. The same LASCO CO and temperature sensors used in the ambient experiments were employed in these temperature-controlled experiments. Once sealed, the jars were placed in a dark Fisher Scientific Isotemp Low Temperature Incubator (Model Number 13-987-626, Waltham, MA, USA) set at 22 ± 1 °C, consistent with studies of CO emissions of fuel pellets conducted at or near room temperature [
23,
27,
28,
29].
Statistical Analysis. This experiment utilized a two-factorial design of experiment in a 2 × 3 arrangement. LCB species (willow, miscanthus, wheat straw) and pretreatment (control, HWE) were the factors in the design. Statistical analysis of the data was performed using analysis of variance (ANOVA) tools in MiniTab (Version 22.3.1 (64-bit), State College, PA, USA). ANOVA is a statistical method used to analyze differences between treatment means (µ
i) by comparing variation within each treatment group and between them. The null hypothesis assumed equivalence of the means (µ
1 = µ
2 … = µ
i), while the alternative hypothesis stated the means were different (µ
1 ≠ µ
2 … ≠ µ
i). Main effects and interaction effects were analyzed, and full ANOVA tables are included in the
Supplementary Materials.
The main effects of treatment and species—as well as interaction effects—were examined with two-way ANOVA. One-way ANOVA followed by Tukey’s HSD was utilized for within-species comparisons between control and HWE treatments. In addition, one-way ANOVA with Tukey’s HSD was conducted across seven levels—all six species and treatment combinations and the commercial pellets—to evaluate overall differences in pellet properties and to position the commercial reference relative to the LCB and HWE-LCB samples. These complementary Tukey’s HSD approaches were used to control familywise error and provide consistent, conservative adjustment of pairwise p-values. Statistical analysis was conducted using triplicate samples unless otherwise noted in the method. All tests were performed with an alpha (α) value of 0.05.
Pearson product-moment correlation analysis was utilized to examine the relationship between extractives content and CO production using MiniTab (Version 22.3.1 (64-bit)). Extractives (hexane, ethanol:toluene, ethanol, and total) were treated as the predictor variable, and carbon monoxide was assessed under ambient and isothermal conditions. Correlations were conducted with correlation coefficients (r), 95% confidence intervals, and two-tailed significance values reported. Linearity was assessed visually via scatterplots prior to analysis.
3. Results
Characterization of LCB and HWE-LCB. The results of the sequential extraction of LCB and HWE-LCB with
n-hexane, ethanol:toluene (1:2), and ethanol are summarized in
Table 1. HWE-LCB exhibited a higher total extractive removal compared to untreated LCB. However, the increase in extractive content due to HWE was more pronounced for willow and wheat straw (~2-fold) compared to miscanthus (~1.5-fold). Among the solvent fractions, ethanol:toluene extracted the highest percentage of extractives for HWE-LCB, while ethanol extracted the least. This trend is attributed to the ability of the ethanol:toluene to solubilize low-molecular-weight lignin fractions from hot-water extracted LCB, as reported in earlier studies [
75]. Although ethanol alone can also dissolve these fractions, it likely contained fewer of them because the ethanol:toluene extraction preceded the ethanol extraction in the sequence. For untreated LCB, the overall extractive content was highest in miscanthus and lowest in wheat straw, which contrasts with previous studies where wheat straw was reported to have the highest content of extractives. However, both previous studies and our findings confirm that HWE-willow yielded the highest amount of extractives in this three-step sequential extraction.
The chemical composition results obtained for LCB and HWE-LCB are summarized in
Table 2. The decrease in acid-soluble lignin is consistent with the HWE process [
34,
35], which creates mildly acidic conditions through autohydrolysis. However, the total lignin content in HWE-LCB was higher than in its LCB counterpart. As expected, willow had the highest total lignin content of all the LCB samples (26.88%), while miscanthus and wheat straw displayed comparable levels (20.35% and 20.38%, respectively). The largest increase in lignin content due to HWE was observed for wheat straw, which rose by almost 30% (from 20.38% to 26.24%, a relative increase of 28.75%). In contrast, HWE-willow exhibited the overall highest lignin content at 31.00%, reflecting an increase of approximately 15% due to HWE (from 26.88% to 31.00%, a relative increase of 15.33%). HWE-miscanthus showed both the lowest lignin content and the smallest increase in lignin content due to HWE (from 20.35% to 22.85%, a relative increase of 12.28%). This could be attributed to various extents of lignin condensation occurring during hydrothermal pretreatment of LCB [
76]; however, detailed analysis of lignin modifications is needed to confirm relationships between these phenomena.
In comparison, the carbohydrate profiles show a distinct trend between the LCB and HWE-LCB. Overall, HWE-miscanthus had the highest total carbohydrate content (69.71%), followed by HWE-wheat straw (68.18%) and HWE-willow (66.47%). This trend is like that observed in the control LCB, where miscanthus also had the highest carbohydrate content (66.27%) and willow the lowest (61.21%). Similar trends were observed in xylan content, in which willow and HWE-willow had the lowest xylan content, as expected. Miscanthus and wheat straw had comparable xylan contents (20.94% and 20.87%), and this is reflected in the xylan content of the HWE-miscanthus (10.08%) and HWE-wheat straw (10.86%) as well. The loss of xylans ranged from 50.47 to 67.77%—with willow at 50.47%, miscanthus at 67.77%, and wheat straw at 67.46%—based on the xylan content of HWE-LCB and LCB, and the OD yields of HWE-LCB from pilot scale HWE (Yield, % OD starting LCB: 76.47% for willow, 66.95% for miscanthus, 62.54% for wheat straw).
Willow and miscanthus exhibited comparable glucan contents, whereas wheat straw had the lowest. Among HWE-LCB samples, glucan content was highest in HWE-miscanthus and lowest in HWE-willow. Regarding minor polysaccharides, willow and HWE-willow had the highest mannan content, consistent with the presence of glucomannan hemicelluloses typically found in hardwoods (~2–5%) [
77]. In contrast, herbaceous species such as agricultural residues and grasses are richer in arabino-containing hemicelluloses—glucuronoarabinoxylans [15–30%] and arabinoglucuronoxylans [5–10%] [
77]—as reflected by the higher arabinan content in miscanthus and wheat straw compared to that in willow in this study.
Fuel Pellet Properties. The ash content, energy content, bulk density, durability, pellet length, moisture absorption, and pellet density of LCB and HWE-LCB pellets are presented in
Table 3. The
p-values for the ANOVA analysis of main effects and interaction effects for each property are shown in
Table 4. Overall, the properties of HWE-LCB pellets were generally consistent with expected trends based on findings in the literature.
The ANOVA analysis showed significant main effects of species and pretreatment on the ash content of LCB and HWE-LCB pellets, indicating differences among both species and pretreatment (
Table 4). However, no interaction effect was observed, showing that the two factors (species and treatment) influenced ash content independently. Tukey’s HSD across treatment groups showed that willow pellets (both control and HWE) formed a distinct group compared with miscanthus and wheat straw, indicating that the species main effect in ash was primarily driven by willow. Within-species analysis revealed a significant reduction in ash content due to HWE. Ash of HWE-willow and HWE-wheat straw pellets was significantly lower than that of their control counterparts (Tukey’s HSD,
p < 0.05), with reductions ranging from 17.9 to 27.4% (average decrease of 22.7%). Although the ash of HWE-miscanthus was also lower than that of the control (reduction of 7.4%), the difference was not statistically significant. The observed reduction in ash may be attributed to the removal of water-soluble inorganics during the HWE of LCB, as documented in previous HWE studies [
34,
35], representing the pretreatment main effect observed. These findings are consistent with significant main effects of species and pretreatment, but no interaction effect. HWE reduced ash content overall, but the differences among willow, wheat straw, and miscanthus were not large enough to indicate a true interaction effect between species and pretreatment.
When all groups were analyzed together, Tukey’s HSD grouped commercial pellets into a distinct group, indicating the commercial pellets contained significantly less ash than both LCB and HWE-LCB pellets. A key challenge in using LCB like shrub willow, wheat straw, and miscanthus for fuel pellets—compared to softwoods and hardwoods—is their inherently higher inorganic content (ash) and a high oxygen content. Wheat straw and miscanthus as herbaceous species are naturally rich in ash, and shrub willow used in this study was not debarked. The typical ash of shrub willow (1.47%) [
39], miscanthus (4.24%) [
39], and wheat straw (4.41%) [
40] contrasts sharply with that of commonly used hardwoods (e.g., 0.5% on average for maple, poplar, and white oak) [
78] and softwoods (e.g., 0.35–0.60% for spruce [
36,
79]). This difference is reflected in the ash content of the fuel pellets produced in this study relative to the commercial hardwood/softwood blend pellets. While HWE significantly reduced the ash in willow and wheat straw, the ash content of all HWE-LCB pellets remained significantly higher, approximately 3.6 to 7.5 times, than that of the commercial pellets.
The ANOVA analysis of moisture absorption revealed main effects of species and pretreatment, as well as an interaction effect (
Table 4). Tukey’s HSD comparison (
Table 3) confirmed significant differences in moisture absorption between species (different groupings) and within species due to HWE (asterisks). While all species showed reduced moisture absorption after HWE, the magnitude of the reduction varied significantly among species. HWE significantly reduced moisture absorption, with reductions ranging from 16.05% to 23.84% (average decrease of 20.69%). Similarly, the ANOVA analysis of energy content revealed main effects of species and pretreatment, as well as an interaction effect (
Table 4), despite similar values of energy content for willow and HWE-wheat straw (19.8 and 19.6 MJ/kg, respectively) (
Table 3). The pattern of differences between miscanthus and the other groups—as indicated by different groupings (
Table 3)—was strong enough to support a significant interaction effect. HWE-LCB pellets showed a statistically significant increase in energy content due to HWE, with an average increase of 2.94% (ranging from 2.62% for wheat straw to 3.54% for willow). These improvements in energy content and moisture absorption are attributed to the partial removal of hemicelluloses and ash, along with a simultaneous increase in lignin content in LCB following HWE, as discussed earlier.
When compared to commercial pellets, only HWE-willow pellets were competitive in terms of energy content, exhibiting significantly higher energy than untreated willow pellets. This aligns with their relatively higher lignin, lower ash, and lower xylan contents (
Table 2 and
Table 3). However, all HWE-LCB pellets outperformed commercial pellets in terms of lower moisture absorption—a key advantage for energy efficiency, especially when the latent heat of vaporization cannot be recovered during combustion in pellet stoves or burners. This is supported by the distinct grouping between the various LCB and HWE-LCB pellets and the commercial pellets, indicating this performance was significant. Wheat straw and willow pellets absorbed significantly more moisture than the commercial reference, and only after HWE did their moisture absorption decrease to more favorable levels. Miscanthus pellets, in contrast, initially absorbed less moisture than commercial pellets, but still exhibited a significant further reduction in moisture absorption after HWE (Tukey’s HSD,
p < 0.05). Notably, wheat straw showed the greatest benefit from HWE, with moisture absorption decreasing by approximately 24%.
Two-way ANOVA analysis indicated significant main effects of species and pretreatment, as well as an interaction effect for the bulk density of LCB and HWE-LCB pellets (
Table 4). Tukey’s HSD showed that HWE resulted in a significant increase in bulk density for miscanthus (19.5%) and wheat straw (19.7%), whereas willow remained unchanged after HWE (
Table 3). These results reveal that miscanthus and wheat straw drive the pretreatment main effect observed, while willow contributes to the overall species main effect. Although the magnitude of change between species after HWE was sufficient to produce an interaction effect, the actual effect of HWE varied among species.
Similarly, significant main effects of species and pretreatment, as well as an interaction effect, were observed for the durability index (
Table 4). While some similarities existed between species in the Tukey’s HSD comparison (
Table 3)—such as willow (96.6) and wheat straw (96.9), the overall variance between species was significant enough to demonstrate a species main effect. Within-species comparisons also confirmed a significant main effect of HWE on each species (
Table 3). However, the significant interaction effect is due to variations in the direction and magnitude of change among species. Durability of HWE-miscanthus pellets increased relative to the control (93.9 vs. 92.4, a 1.62% increase) whereas durability of HWE-willow and HWE-wheat straw pellets decreased (6.11% and 5.37% decrease, respectively). While the interaction effect was statistically significant, the overall trend for the effect of HWE on durability varied by species.
Two-way ANOVA of pellet length revealed a significant main effect of treatment and an interaction effect (
Table 4). Overall, HWE changed pellet length, but only miscanthus pellets had a significant reduction in pellet length (21.76% decrease) after HWE (
Table 3). There were no significant changes for willow and wheat straw pellets after HWE, and the direction of the variations differed between species (pellet length increased for HWE-willow pellets and decreased for HWE-miscanthus pellets). Like bulk density and durability, the variation in pellet length between species after HWE was significant enough to show an interaction effect, reflecting that the response to HWE is species-dependent, but the effect of HWE on pellet length is variable.
In principle, an increase in lignin content resulting from HWE should enhance the bulk density and durability of pellets due to lignin’s proposed binding or bridging effect on LCB particles [
44]. Supporting this, a study on HWE-miscanthus [
48] reported an increase in the density of compacted HWE-miscanthus after a single HWE cycle. Similarly, pellets from HWE-miscanthus and HWE-wheat straw exhibited significantly higher bulk density, approximately 18% greater than their respective control pellets, confirming the positive impact of HWE on these herbaceous biomass sources.
In contrast, no significant difference in the bulk density of HWE-willow vs. untreated willow pellets was observed (Tukey’s HSD,
p > 0.05). Interestingly, while HWE resulted in shorter pellets made from wheat straw and miscanthus, it led to longer pellets in the case of willow. This increase in length may partly explain the lack of improvement in bulk density for HWE-willow pellets. Although not statistically significant, this increase in average pellet length could have influenced the bulk density measurements performed according to ISO 17828 [
70] using the “small container” protocol. However, the bulk density of all HWE-LCB pellets was significantly higher than that of the commercial reference pellets. This improvement is likely due, at least in part, to the significantly shorter average length of HWE-LCB pellets compared to commercial pellets.
Analysis of individual pellet density provides further insight into the effects of HWE on pellet density. Similarly to pellet length, two-way ANOVA only revealed a significant pretreatment main effect and an interaction effect. HWE-miscanthus (1058 ± 68 kg/m
3) and HWE-wheat straw (981 ± 84 kg/m
3) pellets showed significantly higher densities than their respective controls, miscanthus (872 ± 108 kg/m
3) and wheat straw (891 ± 97 kg/m
3) pellets (Tukey’s HSD
p < 0.05). Additionally, the individual pellet density of HWE-miscanthus and HWE-wheat straw pellets was significantly higher than that of commercial pellets (904 ± 69 kg/m
3), as indicated by the unique groupings from Tukey’s HSD (
Table 3). In contrast, HWE-willow pellets had a lower average density than control willow pellets (936 ± 97 kg/m
3 vs. 972 ± 49 kg/m
3), diverging from bulk density trends and indicating minimal effect from HWE. A single-pellet press (SPP), where variables such as mass of hammermilled material, compression force, and temperature can be precisely controlled, could provide clearer insights into the specific impact of HWE on pellet density, though discrepancies in pellet properties may exist when trying to compare and project to pilot-scale pellets [
80].
Variability was also observed in the effects of HWE on pellet durability. HWE-miscanthus pellets presented a significantly higher durability index compared to the control and exhibited the greatest overall improvement. The durability decreased in the following order: HWE-miscanthus > HWE-wheat straw > HWE-willow, with both HWE-willow and HWE-wheat straw pellets displaying significant reductions in durability relative to the control counterparts. This is in contrast to expectations based on lignin’s known contribution to pellet durability and previous studies reporting reduced fines, which are typically associated with an improved durability index for HWE-LCB pellets [
46]. These results demonstrate the need for further analysis of the effects of HWE on the distribution, structure and properties of lignin remaining in HWE-LCB, particularly its glass transition temperature. It could be concluded that lignin distribution and properties rather than content alone govern its role as an in situ binder during pelletizing.
Unique groupings of commercial pellets via Tukey’s HSD demonstrated significantly greater durability and length than both LCB and HWE-LCB pellets, likely due to differences in equipment capabilities between pilot-scale and commercial-scale pelletizers. Commercial-scale pelletizers apply higher and more consistent pressure, enhancing particle interlocking [
81] and increasing operating temperature. Elevated temperatures allow polymeric constituents within LCB, particularly lignin and hemicelluloses, to reach their glass transition temperatures, enabling them to soften and flow in a manner that—upon cooling and resolidifying—creates a bridging effect, contributing to stronger pellet structure [
44,
82,
83,
84,
85,
86]. Additionally, the energy required for pelletization is influenced by hemicellulose composition and degree of branching. Xylans, which are typically more branched and prevalent in angiosperms (such as hardwoods, shrub willow, miscanthus, and wheat straw), require more energy and pressure [
83] than the less-branched glucomannans, the dominant hemicellulose in softwoods.
A reduction in pellet density and compression strength was observed when wheat straw pellets were produced below the lignin glass transition temperature [
87], whereas this difference in quality was not present when operating temperatures exceeded it [
87]. A similar effect was observed in wheat straw pellets produced by a single pellet press (SPP) compared to a pilot-scale pellet press (PSPP), where the PSPP produced stronger and denser pellets than the SPP [
80]. That study also noted a temperature effect on pellet quality; pellets produced at lower temperatures showed higher moisture absorption than those produced at higher temperatures [
80]. Collectively, these findings demonstrated that lower operating temperatures can negatively affect pellet quality. The greater consistency and higher operating pressure and temperature achievable with commercial pelletizers may help resolve the discrepancies observed in pellet samples from this study.
Additionally, rapid spikes in operating temperatures can also have a negative effect on pellet formation and quality. Issues with pressure and temperature stabilization can occur with herbaceous materials like miscanthus during pilot-scale pelletizing, as seen in one study [
88] in which herbaceous material mixtures (miscanthus, oats) caused temperature spikes and jamming within the pelletizer die. Herbaceous materials like miscanthus [
89] and corn stover [
90], and grain-like materials such as wheat-based distiller’s dried grains [
91], typically require and benefit from a higher compression die (greater ratio of length of the die to the diameter of the die (L/D)). However, smaller pelletizers can struggle with pushing this material through such dies [
88], leading to operating issues that could potentially be circumvented with more stable pressure and temperature controls. Modeling of pelletizer pressure generated experimentally with an SPP predicted higher obtainable pressures with an industrial-scale pellet press at higher L/D ratios compared to the SPP [
92]. As such, it may be beneficial to control the pressure and temperature occurring during the pelletizing process to examine the phenomenon occurring with the materials in this study, which may require the use of greater-scale pelletizers. Future studies should explore the impact of pelletizer scale by using commercial-scale pressure for LCB and HWE-LCB pelletizing or testing a comparable hardwood/softwood blend on the pilot-scale pelletizer used in this study. This would help clarify whether the observed differences in mechanical properties stem from inherent material properties or machine capabilities.
Another potential avenue of investigation concerns the consistency of feedstock flowrate, which should be closely monitored in future experiments. HWE results in a more porous, lower-density material [
33] prior to pelletization. This reduction in density can create challenges for material flow in gravity-fed systems, such as hoppers and augers. Adapa [
93] studied four types of straw—barley straw, canary straw, oat straw, and wheat straw—pretreated with steam explosion prior to pelletizing, and concluded that increasing the fraction of steam-exploded straw in blends with untreated straws led to clogging and reduced flowability, ultimately hindering pellet formation. This was attributed, in part, to the lower density of the steam-exploded straw compared to untreated straw. Inconsistent feed rates can disrupt pellet formation and contribute to variability in the mechanical properties of HWE-LCB pellets. Future research should focus on strategies to ensure a consistent flow of HWE-LCB to the pelletizer, thereby reducing this source of variability in pellet production.
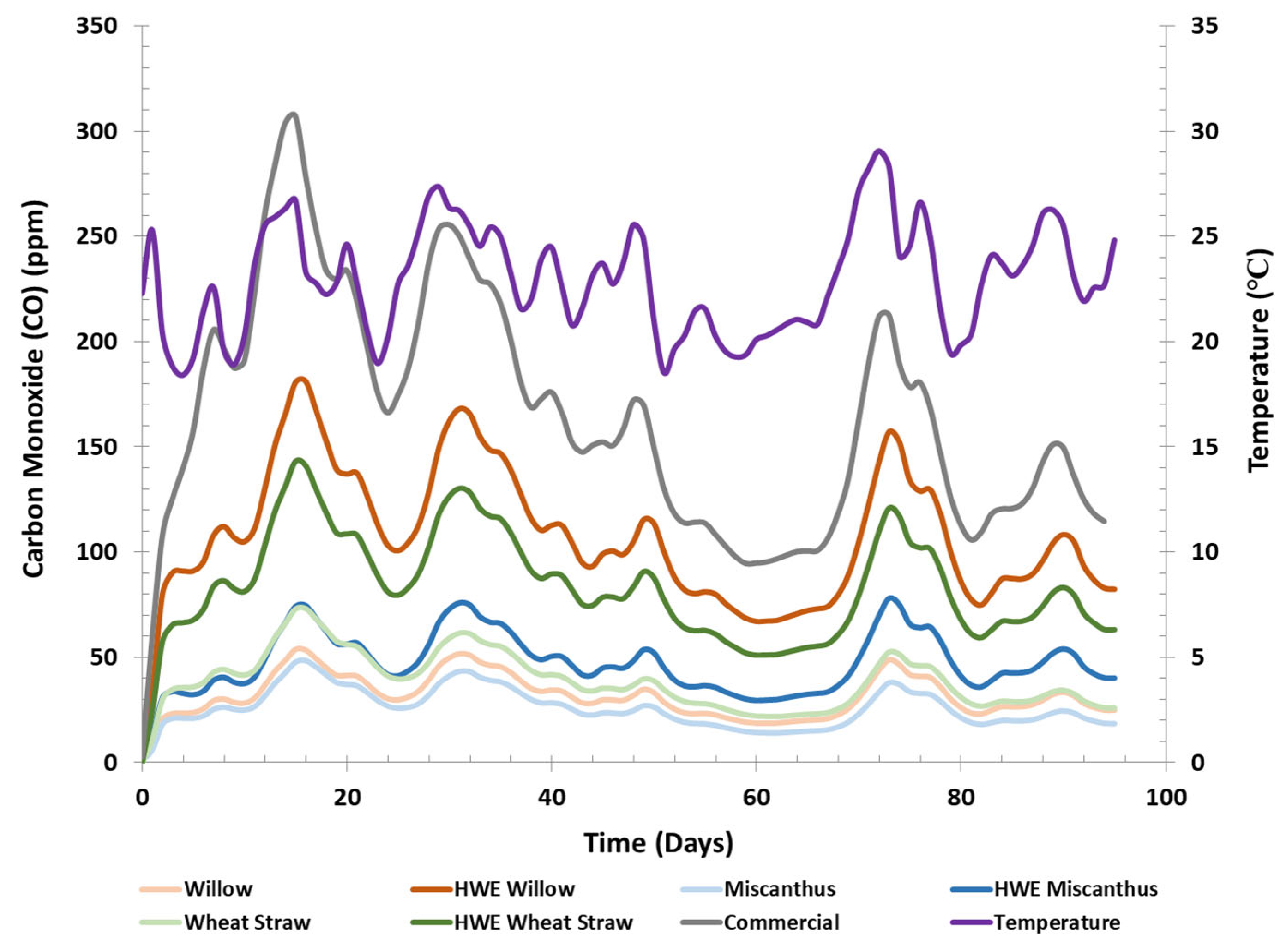
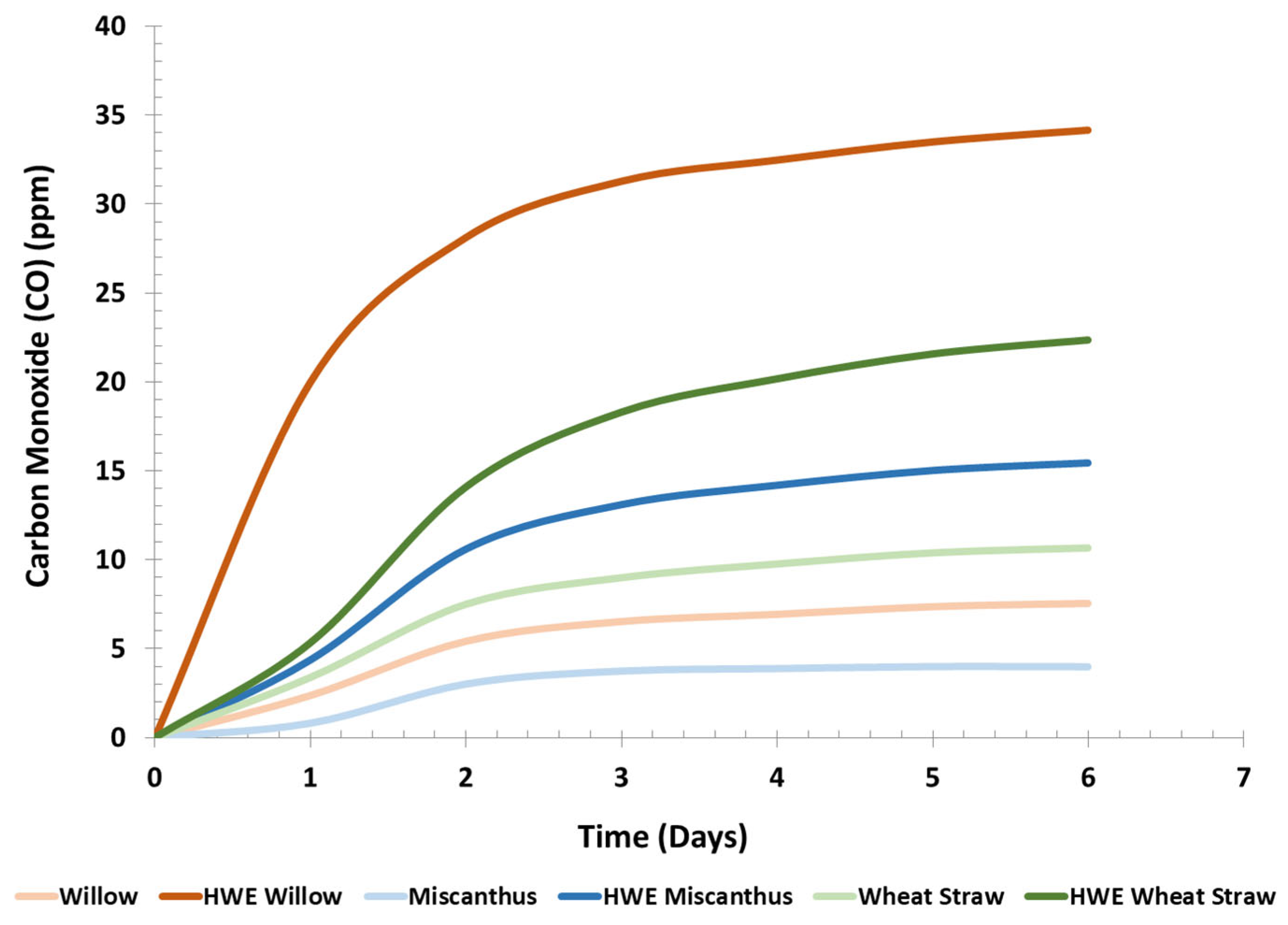
Carbon Monoxide Emission Measurements of Fuel Pellets. Carbon monoxide (CO) emissions were measured using two experimental setups: one under ambient temperature conditions (
Figure 2) and the other in an isothermal environment (
Figure 3). Additional data for CO emissions from commercial pellets under isothermal conditions are provided in
Figure S1 of the Supplementary Information, as including these results in the main presentation would have skewed the display of the emission data. In both experiments, commercial pellets produced significantly higher CO emissions than any of the LCB and HWE-LCB pellets. This is strongly supported by Tukey’s HSD analysis (
Table 5), where commercial pellets were consistently placed in unique groupings compared to LCB and HWE-LCB samples. Under isothermal testing, the difference in peak CO emissions between commercial pellets (152 ppm) and the next highest CO concentration (HWE-willow pellets, 34 ppm) was large enough that Tukey’s HSD grouped all LCB and HWE-LCB pellets as statistically similar. This large difference in CO production by commercial pellets is attributed to their softwood content, as softwoods typically generate more CO than hardwoods [
23,
27,
28,
29,
30]. Rahman and Hopke [
28] suggest that this difference arises from the types of extractives characteristically present in softwoods compared to those present in hardwoods.
Regardless of the temperature conditions, a steady trend in CO emissions was observed: two-way ANOVA analysis (
Table 4) revealed a significant main effect of pretreatment present in all cases (ambient and isothermal), with Tukey’s HSD confirming that HWE-LCB pellets consistently produced significantly more CO than control pellets. A significant main effect of species was also observed in all cases (
p < 0.05), though the effect was weaker under isothermal conditions (
p = 0.031). This effect was less evident in Tukey’s HSD groupings when commercial pellets were included (
Table 5), due to their disproportionally high emissions.
A separate Tukey’s HSD analysis of the CO production restricted to LCB and HWE-LCB pellets (see
Tables S24–S27 in Supplementary Materials) showed similar grouping patterns under ambient conditions, but different groupings under isothermal conditions: CO production from HWE-willow was significantly higher than from all other species except HWE-wheat straw (
p = 0.302). The other groupings generally overlapped, with only miscanthus having a distinct group, significantly different from both HWE-willow (
p = 0.001) and HWE-wheat straw (
p = 0.033). This supports the main effect of species revealed by two-way ANOVA analysis (
Table 4). A significant interaction effect in CO production was observed under ambient conditions but not under isothermal conditions, indicating that the effect of HWE on CO production was species-dependent under ambient conditions, but not under isothermal conditions. Future work should examine the CO emissions from a larger mass and volume of LCB and HWE-LCB pellets under isothermal conditions to better assess the potential for an interaction effect.
In the ambient storage experiment (
Figure 2), CO generation occurred independently of temperature fluctuations during the first 3 days of storage, as indicated by a sharp increase in CO levels despite a temperature drop. This value, referred to as the “Day 3 Peak,” is shown in
Figure 2 as the measurements around the initial inflection point or plateau of CO production. During this period, wheat straw pellets produced more CO than the other two control species (36 ± 7 ppm for wheat straw versus 23 ± 5 ppm for willow and 21 ± 3 ppm for miscanthus). When reanalyzing Day 3 Peak measurements for the LCB and HWE-LCB pellets with Tukey’s HSD and the commercial pellet sample removed, the grouping presented in
Table 5 remains the same (
Supplementary Table S24).
Among the HWE-LCB pellets, HWE-willow pellets emitted the highest level of CO (92 ± 6 ppm), significantly exceeding emissions from HWE-wheat straw (66 ± 2 ppm) and HWE-miscanthus pellets (34 ± 6 ppm). HWE resulted in a fourfold increase in CO from willow pellets, and a 1.6- and 1.8-fold increase in CO from miscanthus and wheat straw pellets, respectively, with HWE-miscanthus pellets still producing lower CO emissions than the wheat straw control pellets. However, commercial pellets generated the highest overall CO emissions, reaching 133 ± 6 ppm, surpassing even HWE-willow pellets by 44%.
The CO emissions from HWE-LCB pellets remained significantly higher than those from the control when examining the maximum peak (highest recorded CO value during the testing period) and the average CO measurement throughout the testing period under ambient conditions, as shown in the Tukey’s HSD within-species comparisons in
Table 5. The groupings from Tukey’s HSD across the seven levels of pellet samples show a significant difference in the peak CO produced from HWE-willow and HWE-wheat straw samples in ambient conditions but do not differentiate the miscanthus and HWE-miscanthus treatments. When examining average CO production in ambient conditions, a similar trend is observed. Upon excluding the commercial pellet sample to better resolve the LCB and HWE-LCB samples in the Tukey’s HSD analysis, the same peak and average CO production groupings in ambient conditions are observed (
p-values presented in
Tables S25 and S26 of the Supplementary Information). CO emissions from control species were not significantly different in both Tukey’s HSD analyses (including commercial pellets [
Table 5] and excluding commercial samples [
Tables S24–S26 in Supplementary Information]). There was some significance between the three-day peak CO production within miscanthus and wheat straw pellets, but this trend was not upheld in the other ambient condition measurements (average and peak).
After the three-day period, the reported CO values fluctuated in tandem with temperature variations. This is not an uncommon occurrence. Soto-Garcia [
31] reported similar fluctuations in CO concentration and temperature in a pellet bin with a storage capacity of 30 tons over the course of a month. These variations were found to coincide with temperature changes in the system, although they appeared to be unrelated to ventilation. A comparable trend was observed by Svedberg et al. [
94] in a 5-ton capacity pellet storage bin over a three-month period.
The reason for the observed CO–temperature relationship in a sealed, unventilated system remains unclear. While prior studies [
23,
27,
95,
96] have demonstrated that increasing temperature in pellet storage systems can elevate CO emissions, it is perplexing that a decrease in temperature would lead to a reduction in CO values in such a sealed system. One potential explanation is the sensitivity of the CO sensor to temperature variations, even though ambient conditions in this study remained within the device’s listed operating temperature range. Alternatively, fluctuations in temperature could influence the desorption and re-adsorption of CO from the fuel pellets, with CO being released at higher temperatures and re-adsorbed as temperatures decrease. A visual inspection of CO changes across different temperature peaks and valleys (such as between Day 15 to Day 25, Day 60 to Day 73, and Day 73 to Day 81), as shown in
Figure 2, reveals a larger swing in CO levels for commercial and HWE-LCB pellets. However, when comparing the percent changes in CO concentrations across these periods, it becomes evident that the greatest fluctuations occurred with the control pellets, while HWE-LCB pellets displayed more stable values.
Additionally, Yazdanpanah et al. [
97] examined the gas adsorption properties of wood pellets (control, torrefied pellets, and steam exploded pellets) and reported that the desorption energy for CO was significantly higher (97.8 kJ/mol) compared to other gases, such as CO
2 (7.24 kJ/mol). This suggests that CO may be chemically adsorbed onto the surface of the pellets rather than physically adsorbed, with desorption requiring relatively high temperatures (over 100 °C). The temperature range in this study (18.40–29.05 °C), however, was much lower, raising the question of whether these findings are applicable to CO behavior at these temperatures. Future work could examine CO sensor performance across different temperature ranges using a sealed container with standard gases, as opposed to a porous medium such as lignocellulosic biomass, to better understand temperature effects and calibrate sensor recordings.
This trend of CO production (LCB < HWE-LCB < commercial pellets) in ambient conditions was consistent with findings from isothermal conditions (
Figure 3) as well. When excluding the commercial pellets from the Tukey’s HSD analysis to better resolve the other samples, new distinct groupings emerge (
p-values in
Table S27). In particular, the CO production from HWE-willow pellets is significantly greater than from the control pellets, as confirmed by the Tukey’s HSD within-species comparison (
Table 5). The other levels (HWE-wheat straw, HWE-miscanthus, and the three control species) show slightly different groupings than the Tukey’s HSD that includes the commercial sample, but not in a significant manner. Notably, the CO concentrations in the isothermal experiment showed much less fluctuation than under ambient conditions over time, likely due to the more stable temperature in the isothermal setup (22.37 ± 0.4 °C). The CO production curve in this study mirrors trends reported in the literature involving CO generation under isothermal conditions.
Tumuluru et al. [
27] examined CO emissions from various LCB samples (ground switchgrass, softwood chips, torrefied woodchips, and softwood pellets) at two temperatures (20 °C and 40 °C). They reported a plateau effect in CO emissions from wood pellets after 12 days of storage at 40 °C. Although CO accumulation was observed in wood chips and pellets at these temperatures, production did not cease during the storage period. It is possible that extending the incubation time would result in an emission curve similar to that observed in this study. In another study, Tumuluru et al. [
96] investigated CO production from wood pellets stored at 5 °C, 10 °C, and 20 °C, over 28 days. In this case, CO production tapered off more quickly at lower temperatures, reaching a plateau sooner. Early work by Tumuluru et al. [
98] on wood pellets stored with controlled temperature increases (from room temperature to 30 °C, 40 °C, and 50 °C, over 70 days) also demonstrated a gradual decline in CO production over time. These findings align with other studies by Soto-Garcia et al. [
23] and Rahman and Hopke [
28].
Overall, HWE-miscanthus generated the least CO under both temperature conditions among the HWE-LCB, while HWE-willow generated the most. In examining the xylan and total lignin content of the HWE-LCB (
Table 2), HWE-willow had a lower xylan content but a higher lignin content compared to HWE-miscanthus, which had the lowest lignin and xylan content. The xylan-to-lignin ratio of these samples decreased in the following order: HWE-miscanthus (0.441) > HWE-wheat straw (0.414) > HWE-willow (0.312). Theoretically, HWE-willow pellets should have produced less CO, as HWE-willow contained more lignin and less xylan. However, the opposite occurred, and HWE-willow pellets had the highest CO emissions.
One potential reason for the higher CO production from HWE-LCB pellets could be the difference in particle surface area between HWE-LCB and untreated LCB. As reported by Duarte et al. [
33], the porosity of the cell wall and average pore size of LCB increase following HWE, likely increasing the available surface area for reactions with remaining hemicelluloses in HWE-LCB. Another contributing factor may be related to extractives. Sequential extraction of LCB and HWE-LCB demonstrated that more total extractives were removed from HWE-LCB, likely due to the enhanced penetration of extraction solvents into the more porous HWE-LCB. This increased exposure of extractives could facilitate the oxidation of unsaturated fatty acids, further contributing to increased CO emissions from HWE-LCB pellets compared to the control.
Of note is the hexane extractives content of HWE-LCB and LCB (
Table 1), which includes fatty acids of interest for CO production. Willow and HWE-willow contained the highest amount of hexane extractives (3.50% and 4.43%, respectively) compared to the other LCB and HWE-LCB, whereas wheat straw (1.83%) and HWE-miscanthus (2.75%) had the lowest hexane extractives. The CO emissions under both isothermal and ambient conditions (
Table 5) for HWE-LCB pellets followed a similar trend: HWE-miscanthus < HWE-wheat straw < HWE-willow. However, this pattern was reversed for control pellets, where wheat straw produced the most CO. Pearson correlation analysis (
Table 6) was performed to evaluate relationships between extractives content and CO production. Hexane and ethanol:toluene fractions showed moderate positive correlations (r = 0.701–0.736 and 0.711–0.781, respectively), though these were not statistically significant (
p > 0.05). Ethanol extractives showed no correlation, potentially due to differences in both concentration and composition of ethanol extractives compared to hexane extractives. However, total extractives exhibited strong and significant correlations with CO production under most conditions (r = 0.825–0.855,
p < 0.05 for significant correlations), indicating a likely role in the production of CO. Future studies should focus on quantifying the types of extractives present in LCB and HWE-LCB to better understand their role in CO production. However, it is apparent that a combination of factors contributes to CO emissions, rather than a single parameter.
4. Discussion
This study provides valuable insights into the utilization of hot water extracted (HWE) lignocellulosic biomass (LCB) for fuel pellet production, highlighting both its advantages and challenges. The HWE process consistently improved key pellet properties, including significant reductions in ash and moisture absorption, along with an increase in energy content. However, further optimization is necessary to enhance the mechanical properties and address CO emissions, ensuring HWE-LCB pellets can compete with commercial alternatives.
Regarding ash, one of the limitations identified in this study is the potential constraint on ash removal despite additional washing cycles. Previous reports [
48] have suggested that increasing HWE cycles enhances water absorption but does not proportionally reduce ash, indicating an inherent limit to ash removal. This could be related to the change in porosity of HWE-LCB [
33], in which water retention values increased with HWE severity on hardwood fibers. Further studies should investigate whether alternative washing techniques or additives can enhance ash extraction without compromising pellet integrity.
One potential solution to improving energy content is the addition of lignin to hammermilled HWE-LCB prior to pelletization. Previous work [
8] has demonstrated a significant increase in energy content when lignin additives were used, with similar findings reported in other studies as well [
49,
50,
51,
52], depending on the lignin source. Additional lignin may also improve other properties of HWE-LCB pellets. Utilization of lignin as a binding agent across several studies [
8,
36,
49,
51,
53,
54,
55] consistently led to increases in the durability of lignin-treated pellets compared to the controls. While some research has shown improvements in bulk density [
8], others have found no effect [
51,
54] or even a decrease [
49]. Lignin can potentially reduce the moisture absorbed by pellets, as documented with the addition of lignin recovered from the HWE of shrub willow to pellets produced from untreated shrub willow [
8]. However, two studies [
8,
36] showed an increase in moisture absorption when kraft lignin was utilized as an additive.
Most importantly, lignin may play a key role in reducing CO emissions when incorporated into HWE-LCB before pelletization. One study [
8] examined the effect of adding two types of lignin—commercial kraft lignin and lignin recovered from the HWE of shrub willow—to hammermilled shrub willow that was then pelletized. Pellets containing the recovered lignin were reported to have significantly lower CO emissions compared to the control. This was correlated to the radical quenching capabilities of the recovered lignin based on the half maximal inhibitory concentration (IC
50 50.0 ± 0.0 µg/mL) compared to the commercial kraft lignin (IC
50 72.0 ± 6.1 µg/mL) [
8], indicating the importance of examining more than just the amount of lignin in the LCB or HWE-LCB sample (whether inherently in the biomass or added as a binding agent). Characteristics of lignin such as the type of lignin (e.g., the content of
p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) structural units, determined with techniques such as Fourier-transform infrared (FTIR) spectroscopy and Fourier-transform Raman spectroscopy) [
99], particle size and morphology (e.g., X-ray scattering [
100], atomic force microscopy (AFM) [
8]), molecular weight distribution (e.g., high-performance liquid chromatography (HPLC) in combination with light scattering (LS), ultraviolet (UV) absorbance, or refractive index (dRI) to measure elution) [
99], glass transition temperature (e.g., differential scanning calorimetry (DCS)) [
8,
99], and free phenolic hydroxyl group content (e.g., phosphorous-31 nuclear magnetic resonance (
31P-NMR) [
34]) could influence the various properties of fuel pellets. As such, future work should explore the effect of both the characteristics of lignin and how adding lignin to HWE-LCB prior to pelletizing can affect CO production.
Other potential treatments, such as saturation with ozone [
58], may be particularly effective at mitigating oxidation within the pores formed from HWE. However, unlike lignin additives, ozone treatment has not shown additional benefits such as improved energy content or durability. Another approach is the removal of fatty acids, which could theoretically lower CO emissions. Notably, HWE-LCB contained higher amounts of extractives compared to untreated LCB (
Table 1), suggesting that an extraction treatment may be more effective post-HWE. However, this approach has drawbacks, including solvent costs, processing complexity, environmental concerns, and the potential loss of lignin fractions, which could negatively impact pellet properties.
The viability of these and other processes regarding the intersection of CO emissions, pellet properties, and HWE-LCB require further research. Future work should aim to identify strategies that effectively reduce CO emissions from HWE-LCB pellets while maintaining the reported beneficial effects of HWE on pellet properties. This study clearly shows that CO production in LCB-based fuel pellets is a complex, multifaceted phenomenon. Both morphological attributes (porosity and surface area) and chemical composition (xylan content, lignin content and characteristics, and fatty acids content) collectively influence CO emissions. A deeper understanding of the interplay among these factors is essential for optimizing fuel pellet performance and emissions.