Granular Activated Carbon and Organic Loading Interactions in Methane Fermentation: An Inverse Load-Dependent Relationship and Absolute Microbial Abundance Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Anaerobic Digestion Experiments
2.2.1. Effects of Different Addition Rates of GAC on Methanogenesis Under Normal and High Organic Loadings in Batch Fermentation System
2.2.2. Effects of GAC Addition on Methanogenesis at High Organic Loading in Continuous Fermentation System
2.3. Analytical Measurements
2.3.1. Measurement of pH and Methane Concentration
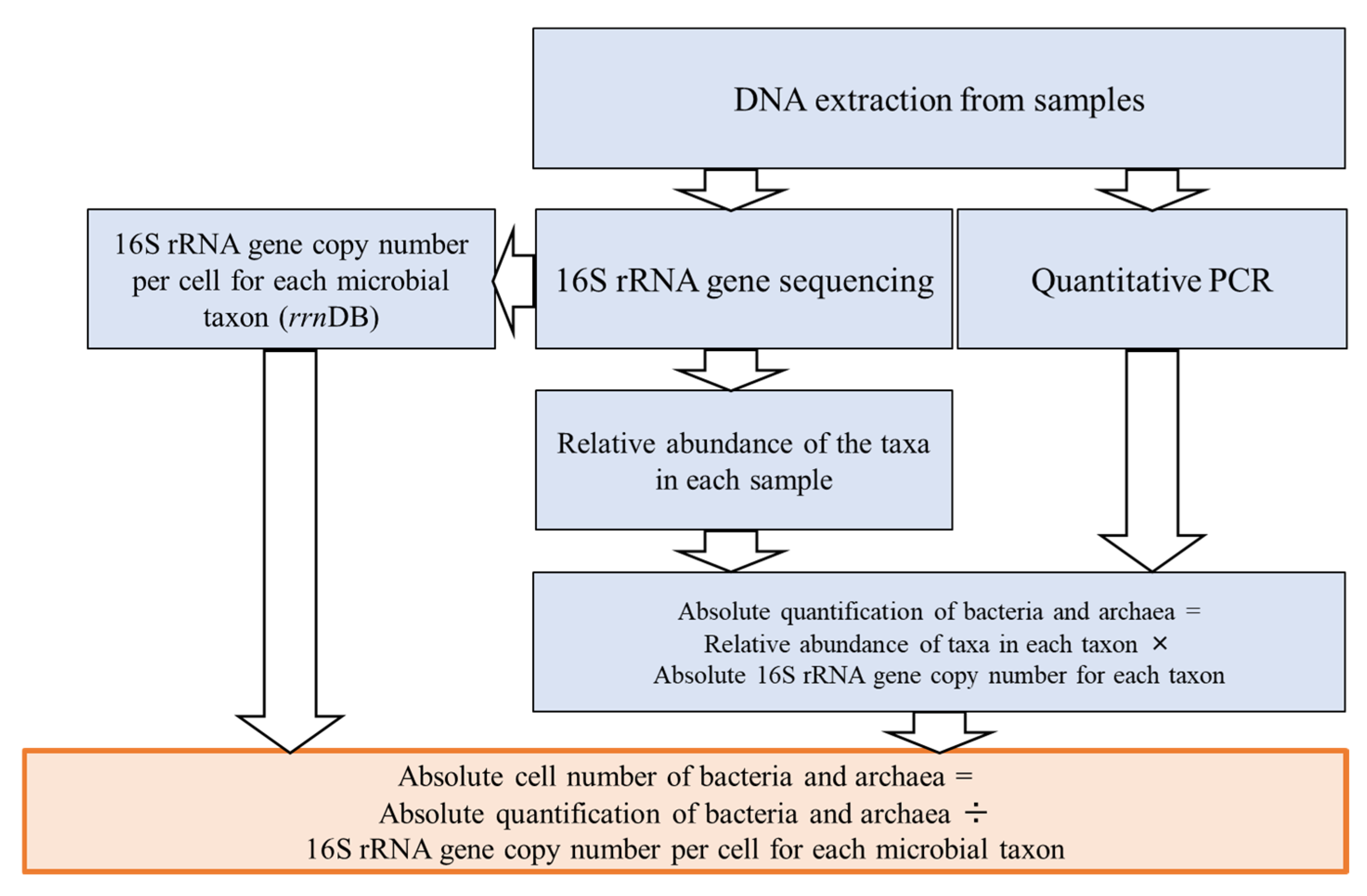
2.3.2. Microbial Analysis
3. Results
3.1. Batch Fermentation
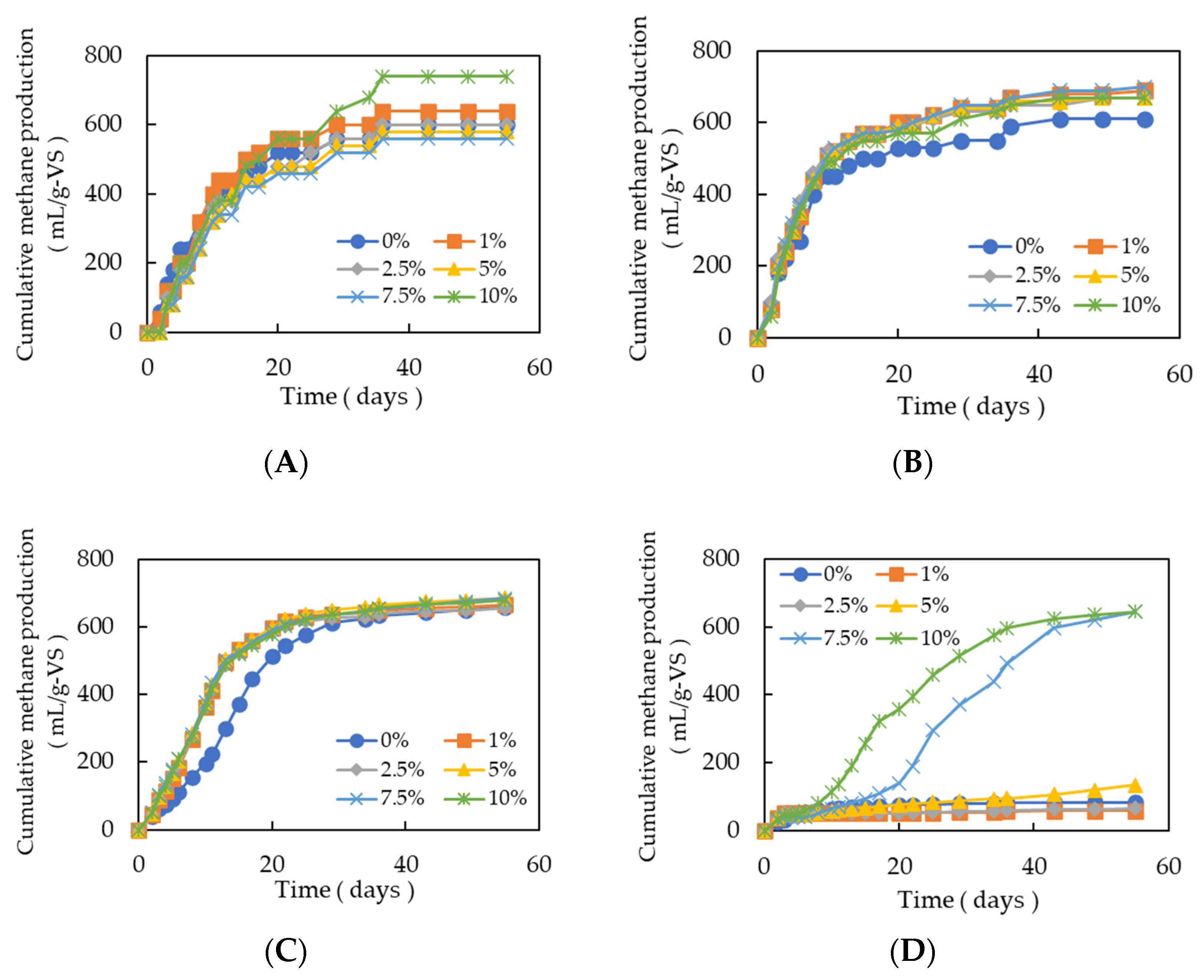
3.1.1. Effect of Different Levels of GAC on Methanogenesis Under Varying Organic Loading in Batch Fermentation
3.1.2. Effect of Different Percentages of GAC on Methanogenesis Under Varying Organic Loading in Batch Fermentation
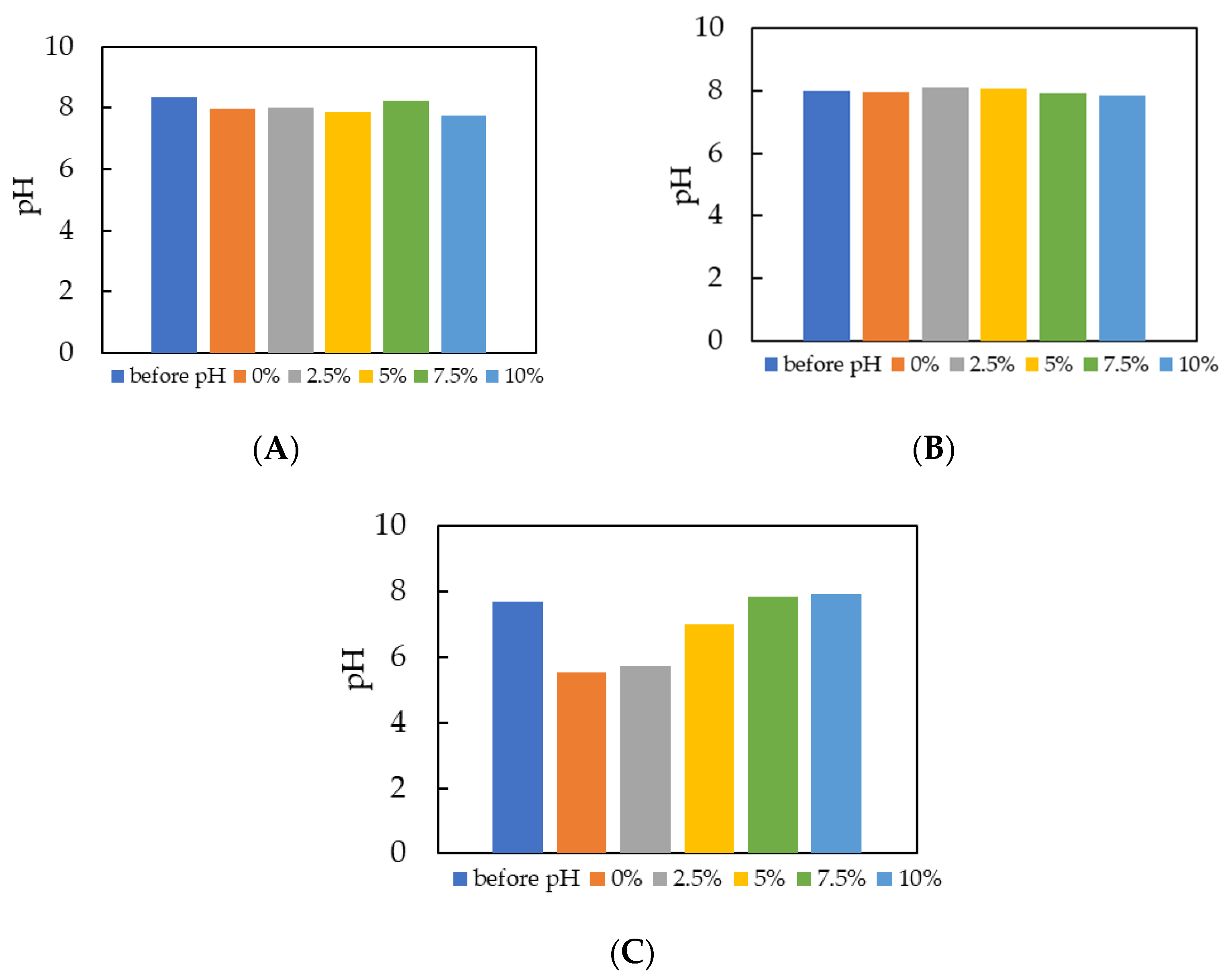
3.1.3. Change in pH Under High OLR and Different GAC Percentages for Batch Fermentation
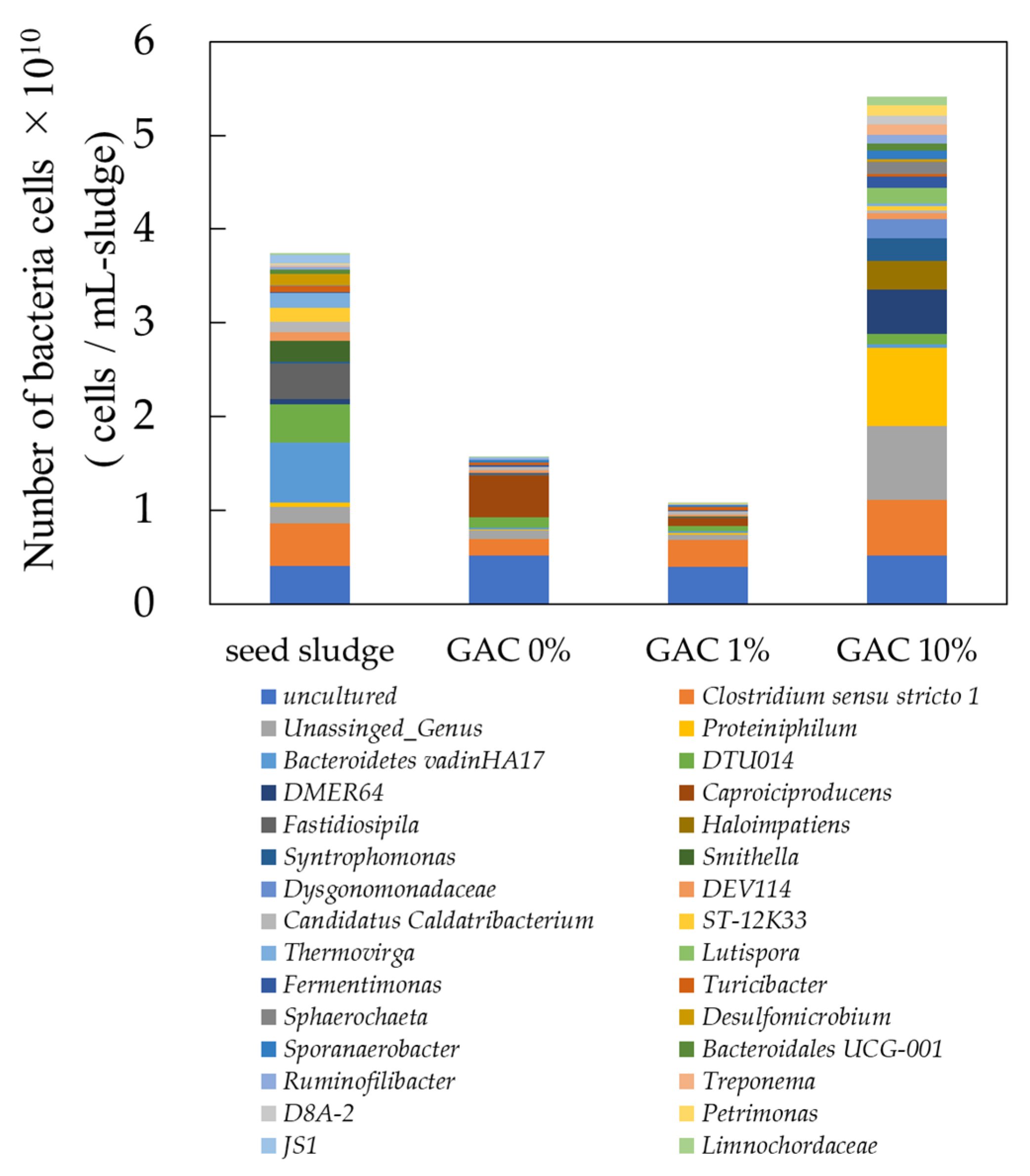
3.1.4. Microflora
Bacterial and Archaeal Abundance and Diversity at the Family and Kingdom
Bacterial Abundance and Diversity at the Genus Level
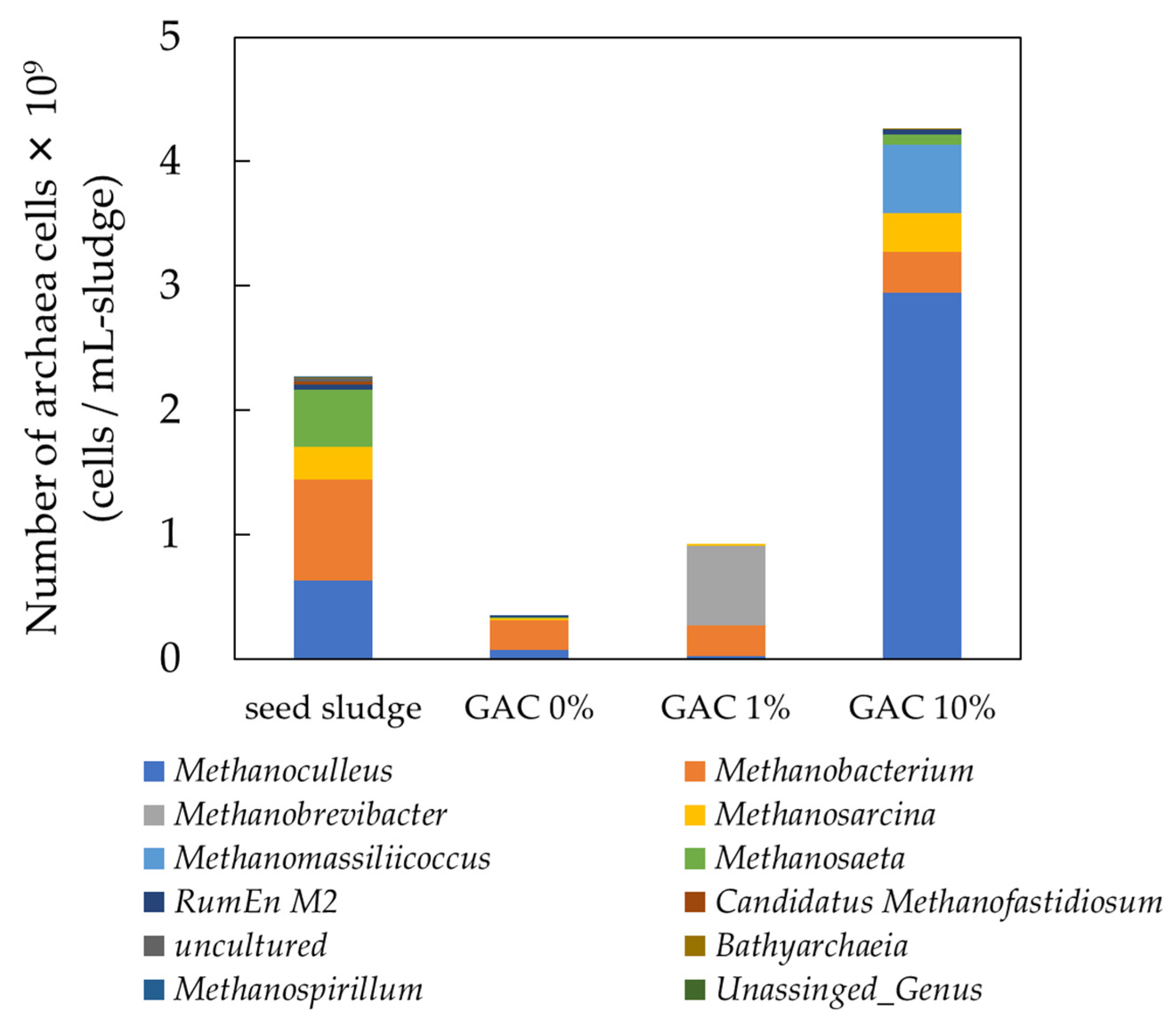
Archaeal Abundance and Diversity at the Genus Level
3.2. Continuous Fermentation
3.2.1. Effect of GAC Addition on Biogas and Methanogenesis at High Organic Loadings in Continuous Fermentation
3.2.2. pH Change
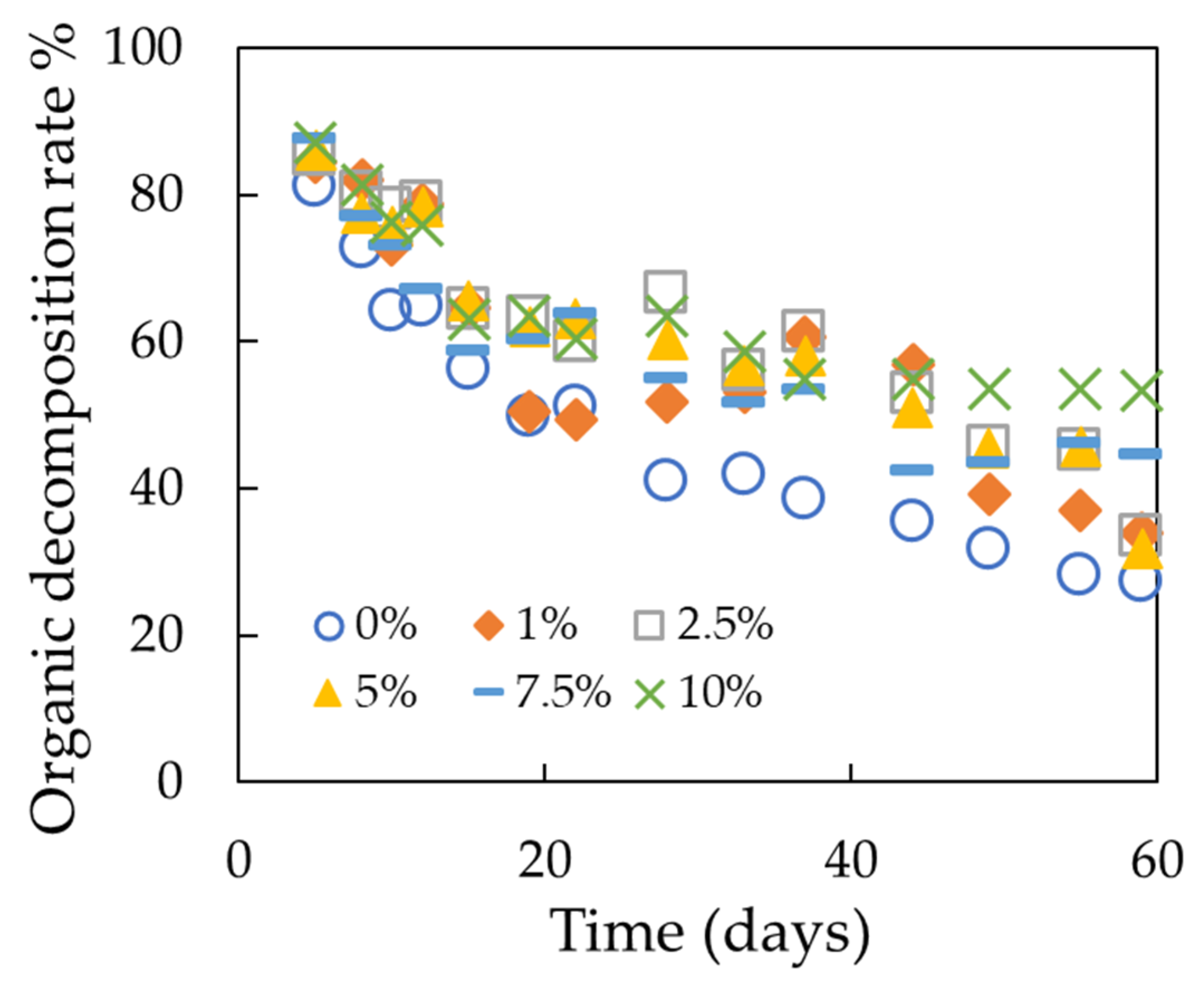
3.2.3. Organic Decomposition Rate
4. Discussion
4.1. Enhanced Methane Production by GAC in Batch Fermentation
4.2. Effect of Different GAC Additions on Methanogenesis Under High Organic Loadings in Continuous Fermentation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study, Year | Additive | Substrate | Additive Dosage | Key Quantitative Outcome |
|---|---|---|---|---|
| Kalantzis et al., 2023 [24] | GAC | Agro-industrial wastewater | 5 g/L | Biogas production increased by 32% after the addition of GAC. |
| Ziganshina et al., 2020 [25] | GAC | Beet pulp and distillers grains with solubles | 1–10 g/L | The pH values in reactors remained stable, ranging from 6.91 to 7.57 throughout the experimental period. |
| Ziganshina et al., 2022 [26] | GAC | Chicken manure | 5–10 g/L | Under mesophilic conditions, methane production was highest with 5 g/L GAC, showing an approximately 4.1% increase compared to the control group. |
| Xu et al., 2018 [27] | GAC | VFA (acetate, propionate, butyrate) | 0.5–25 g/L | Methane generation rate increased significantly with GAC supplementation, reducing the lag phase from 4.2 days (0 g/L) to 0.9 days (25 g/L). |
| Zhang et al., 2024 [28] | GAC+ZVI | Organic fraction of municipal solid waste | 1–15% (w/w) | GAC alone (R-GAC) achieved 327.6 mL/gVS, and ZVI alone (R-ZVI) achieved 296.9 mL/gVS, both higher than the control group. |
| De Sousa e Silva et al., 2025 [29] | GAC | Swine manure | 10–30 g/L | GAC enhanced methane generation by promoting Direct Interspecies Electron Transfer (DIET) and adsorbing inhibitory compounds. |
| Wu et al., 2022 [30] | AC/graphite | Food waste sludge | — | The cumulative biogas production with 100-mesh activated carbon was 468.2 mL/g VSS, which was 13.8% higher than the control group. |
| Chen et al., 2023 [31] | GAC | Garden waste | 50 g/L | The VFA/sCOD ratio in the GAC-amended group reached 70.01%, surpassing the control group’s 49.35%, indicating more efficient hydrolysis and acidogenesis. |
| Quintana-Najera et al., 2023 [32] | Biochar | Model carbohydrate | 0.03–8.0% (w/w) | he addition of biochar showed notable improvements in the digestion of complex substrates rich in lipids and proteins, as well as under stressful conditions. |
| Hu et al., 2023 [33] | Biochar | Dog food pellets | 10–25% (VS basis) | Under high substrate overloading (ISR 0.5), 25% biochar addition enhanced the removal rate of substrate volatile solids (VS). |

References
- Ibarra-Esparza, F.E.; González-López, M.E.; Ibarra-Esparza, J.; Lara-Topete, G.O.; Senés-Guerrero, C.; Cansdale, A.; Forrester, S.; Chong, J.P.J.; Gradilla-Hernández, M.S. Implementation of anaerobic digestion for valorizing the organic fraction of municipal solid waste in developing countries: Technical insights from a systematic review. J. Environ. Manag. 2023, 347, 118993. [Google Scholar] [CrossRef]
- Triviño-Pineda, J.S.; Sanchez-Rodriguez, A.; Peláez, N.P. Biogas production from organic solid waste through anaerobic digestion: A meta-analysis. Case Stud. Chem. Environ. Eng. 2024, 9, 100618. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, R.; Yamada, T.; Nishimura, M.; Kamahara, H.; Atsuta, Y.; Daimon, H. The Effect of a Small-scale, Distributed Methane Fermentation System on a Medium-sized Pig Farm. J. Jpn. Soc. Mater. Cycles Waste Manag. 2019, 30, 95–102. [Google Scholar] [CrossRef]
- Sun, M.; Yanagawa, K.; Prasitwuttisak, W.; Goel, R.; Watanabe, R.; Harada, H.; Liu, B.; Terashima, M.; Yasui, H. Kinetics for the Methanogen’s Death in the Acidic Environments. J. Water Environ. Technol. 2023, 21, 59–75. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Awasthi, M.K.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Mu, H.; Ding, X.; Zhu, X.; Wang, L.; Zhang, Y.; Zhao, C. Effects of different types of granular activated carbon on methanogenesis of carbohydrate-rich food waste: Performance microbial communities and optimization. Sci. Total Environ. 2023, 895, 165173. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, J.; Kumar, P.S.; Zhou, M.; Yu, J.; Lichtfouse, E. Enhanced methane production by granular activated carbon: A review. Fuel 2022, 320, 123903. [Google Scholar] [CrossRef]
- Zhao, Y.; Mu, H.; Su, Y.; Zhang, Y.; Qiao, X.; Zhao, C. Promotion of granular activated carbon on methanogenesis of readily acidogenic carbohydrate-rich waste at low inoculation ratio. Sci. Total Environ. 2022, 817, 152642. [Google Scholar] [CrossRef]
- Ozaki, Y.; Atsuta, Y.; Daimon, H. Addition of Granular Activated Carbon to Accelerate Decomposition of Propionic Acid in Anaerobic Digestion System. J. Jpn. Soc. Mater. Cycles Waste Manag. 2022, 33, 263–270. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015, 191, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Yuan, Y.; Mou, A.; Park, S.; Liu, Y. Impacts of Granular activated carbon addition on anaerobic granulation in blackwater treatment. Environ. Res. 2022, 206, 112406. [Google Scholar] [CrossRef]
- Almegbl, A.M.; Rahmani, A.M.; Kamal, K.; Munshi, F.M.A.; Khursheed, A.; Ali, M.; Khursheed, A. Retrospective of DIET process for enhanced biogas production during anaerobic digestion of thermal/chemically pretreated waste activated sludge. Front. Environ. Eng. 2025, 4, 1597684. [Google Scholar] [CrossRef]
- Mou, A.; Yu, N.; Yang, X.; Liu, Y. Enhancing methane production and organic loading capacity from high solid-content wastewater in modified granular activated carbon (GAC)-amended up-flow anaerobic sludge blanket (UASB). Sci. Totla Environ. 2024, 906, 167609. [Google Scholar] [CrossRef]
- Hasani, Z.A.; Nayak, J.K.; Balushi, N.J.A.; Al-Mamun, A.; Samal, K. Prospect of Conductive Materials in the Anaerobic Digester Matrix for Methane Production: Electron Transfer and Microbial Communication. Water 2025, 17, 1321. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Xu, L.; Zhou, P.; Graham, N.; Yu, W. Carboxyl Group on Granular Activated Carbon Enhances the Metabolism of Nom in Surface Water by Modulating Microbial Colonization. SSRN eLibr. 2025, 29, 5202511. [Google Scholar]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Tao, A.; Zhao, L.; Wang, P.; Wang, A.; Huang, X.; Chen, M. Roles of modified biochar in the performance, sludge characteristics, and microbial community features of anaerobic reactor for treatment food waste. Sci. Total Environ. 2021, 770, 144668. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zhao, Y.; Zhang, H.; Chen, S.; Zhu, W.; Yuan, X.; Cui, Z. A comparison and evaluation of the effects of biochar on the anaerobic digestion of excess and anaerobic sludge. Sci. Total Environ. 2020, 736, 139159. [Google Scholar] [CrossRef]
- Bao, R.; Wei, Y.; Guan, R.; Li, X.; Lu, X.; Rong, S.; Zuo, X.; Yuan, H. High-solids anaerobic co-digestion performances and microbial community dynamics in co-digestion of different mixing ratios with food waste and highland barley straw. Energy 2023, 262, 125529. [Google Scholar] [CrossRef]
- Chen, X.; Yan, W.; Sheng, K.; Sanati, M. Comparison of high-solids to liquid anaerobic co-digestion of food waste and green waste. Bioresour. Technol. 2014, 154, 215–221. [Google Scholar] [CrossRef]
- Yan, M.; Treu, L.; Campanaro, S.; Tian, H.; Zhu, X.; Khoshnevisan, B.; Tsapekos, P.; Angelidaki, I.; Fotidis, I.A. Effect of ammonia on anaerobic digestion of municipal solid waste: Inhibitory performance, bioaugmentation and microbiome functional reconstruction. Chem. Eng. J. 2020, 401, 126159. [Google Scholar] [CrossRef]
- Kapantzis, D.; Daskaloudis, I.; Lacoere, T.; Stasinakis, A.S.; Lekkas, D.F.; Vrieze, J.D.; Fountoulakis, M.S. Granular activated carbon stimulates biogas production in pilot-scale anaerobic digester treating agro-industrial wastewater. Bioresour. Tech. 2023, 376, 128908. [Google Scholar]
- Ziganshina, E.E.; Belostotskliy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of Granular Activated Carbon on Anaerobic Co-Digestion of Sugar Beet Pulp and Distillers Grains with Solubles. Processes 2020, 8, 1226. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of Granular Activated Carbon on Anaerobic Process and Microbial Community Structure during Mesophilic and Thermophilic Anaerobic Digestion of Chicken Manure. Sustainability 2022, 14, 447. [Google Scholar] [CrossRef]
- Xu, S.; Han, R.; Zhang, Y.; He, C.; Liu, H. Differentiated stimulating effects of activated carbon on methanogenic degradation of acetate, propionate and butyrate. Waste Manag. 2018, 76, 394–403. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, H.; Zuo, X.; Zhang, L.; Li, X. Adding Granular Activated Carbon and Zerovalent Iron to the High-Solid Anaerobic Digestion System of the Organic Fraction of Municipal Solid Waste: Anaerobic Digestion Performance and Microbial Community Analysis. ACS Omega 2024, 9, 3401–3411. [Google Scholar] [CrossRef]
- De Sousa e Silva, A.; Moraes dos Santos, A.L.; Cavalcante Malveira, I.C.; Holanda Albano Girao, B.; Bezerra dos Santos, A. Influence of Granular Activated Carbon Addition on Methane Production in Dry and Semidry Anaerobic Digestion of Swine Manure. ACS Omega 2025, 10, 29386–29399. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xie, J.; Xin, X.; He, J. Effect of activated carbon/graphite on enhancing anaerobic digestion of waste activated sludge. Front. Microbiol. 2022, 13, 999647. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zeng, Y.; Liu, H.; Sun, D.; Liu, X.; Xu, H.; Wu, H.; Qiu, B.; Dang, Y. Granular activated carbon enhances volatile fatty acid production in the anaerobic fermentation of garden wastes. Front. Bioeng. Biotechnol. 2023, 11, 1330293. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. Understanding the Influence of Biochar Augmentation in Anaerobic Digestion by Principal Component Analysis. Energies 2023, 16, 2523. [Google Scholar] [CrossRef]
- Hu, J.; Stenchly, K.; Gwenzi, W.; Wachendorf, M.; Kaetzl, K. Critical evaluation of biochar effects on methane production and process stability in anaerobic digestion. Front. Energy Res. 2023, 11, 1205818. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneko, H.; Ozaki, Y.; Takezaki, J.; Daimon, H. Granular Activated Carbon and Organic Loading Interactions in Methane Fermentation: An Inverse Load-Dependent Relationship and Absolute Microbial Abundance Analysis. Fuels 2025, 6, 72. https://doi.org/10.3390/fuels6030072
Kaneko H, Ozaki Y, Takezaki J, Daimon H. Granular Activated Carbon and Organic Loading Interactions in Methane Fermentation: An Inverse Load-Dependent Relationship and Absolute Microbial Abundance Analysis. Fuels. 2025; 6(3):72. https://doi.org/10.3390/fuels6030072
Chicago/Turabian StyleKaneko, Hikaru, Yusuke Ozaki, Jun Takezaki, and Hiroyuki Daimon. 2025. "Granular Activated Carbon and Organic Loading Interactions in Methane Fermentation: An Inverse Load-Dependent Relationship and Absolute Microbial Abundance Analysis" Fuels 6, no. 3: 72. https://doi.org/10.3390/fuels6030072
APA StyleKaneko, H., Ozaki, Y., Takezaki, J., & Daimon, H. (2025). Granular Activated Carbon and Organic Loading Interactions in Methane Fermentation: An Inverse Load-Dependent Relationship and Absolute Microbial Abundance Analysis. Fuels, 6(3), 72. https://doi.org/10.3390/fuels6030072






