Biochar Surface Chemistry Modification by Blending Hardwood, Softwood, and Refuse-Derived Fuel: Insights from XPS, FTIR, and Zeta Potential Analysis
Abstract
1. Introduction
2. Materials and Methodology
2.1. Biochar Preparation
2.2. Surface and Electrochemical Characterization
2.2.1. X-Ray Photoelectron Spectroscopy (XPS)
2.2.2. FTIR Spectroscopy
2.2.3. pH Measurement
2.2.4. Zeta Potential and Electrophoretic Mobility
3. Results and Discussion
3.1. Surface Elemental Composition and Chemical State Analysis Using XPS
3.2. Functional Group Characterization Using FTIR Spectroscopy
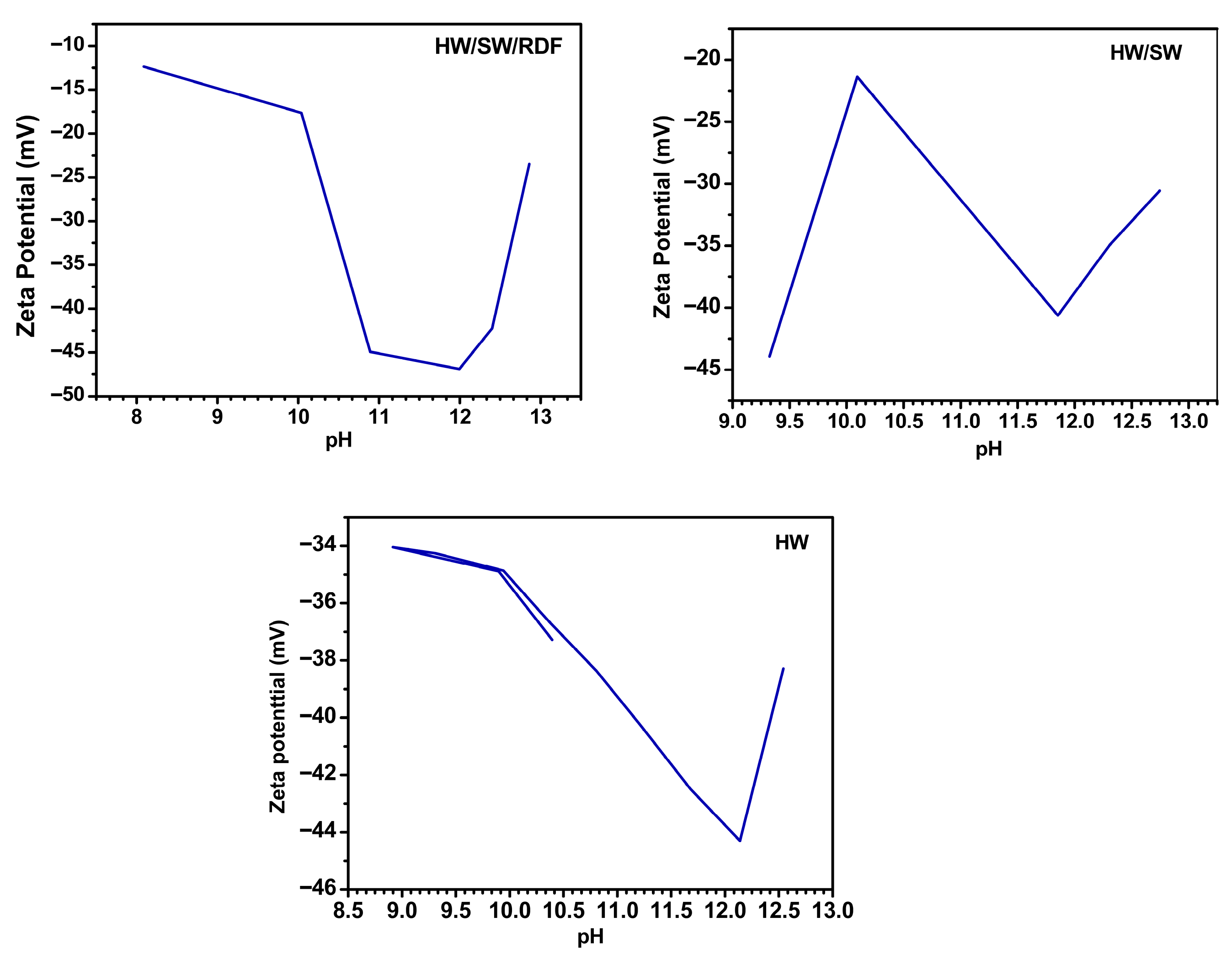
3.3. Electrostatic Surface Behavior and Colloidal Stability Assessments Using Zeta Potential and Electrophoretic Mobility
3.4. Influence of RDF Inclusion on Biochar Alkalinity and Surface pH Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Rajput, V.; Saini, I.; Parmar, S.; Pundir, V.; Kumar, V.; Kumar, V.; Naik, B.; Rustagi, S. Biochar production methods and their transformative potential for environmental remediation. Discov. Appl. Sci. 2024, 6, 408. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-facilitated soil remediation: Mechanisms and efficacy variations. Front. Environ. Sci. 2020, 8, 521512. [Google Scholar] [CrossRef]
- Al-Rubaye, H.A.; Yu, J.; Smith, J.D.; Al-Abedi, H.J. Experimental investigation of tar recycling in pilot-scale down-draft biomass gasifiers: Prospects, operating procedures, process variations, and controls. Biofuels 2023, 14, 201–210. [Google Scholar] [CrossRef]
- Alfè, M.; Gargiulo, V.; Porto, M.; Migliaccio, R.; Le Pera, A.; Sellaro, M.; Pellegrino, C.; Abe, A.A.; Urciuolo, M.; Caputo, P.; et al. Pyrolysis and gasification of a real refuse-derived fuel (RDF): The potential use of the products under a circular economy vision. Molecules 2022, 27, 8114. [Google Scholar] [CrossRef] [PubMed]
- Golpour, H.; Boravelli, T.; Smith, J.D.; Safarpour, H.R. Production of syngas from biomass using a downdraft gasifier. Int. J. Eng. Res. Appl. 2017, 7, 61–71. [Google Scholar] [CrossRef]
- Sher, F.; Hameed, S.; Omerbegović, N.S.; Chupin, A.; Hai, I.U.; Wang, B.; Teoh, Y.H.; Yildiz, M.J. Cutting-edge biomass gasification technologies for renewable energy generation and achieving net zero emissions. Energy Convers. Manag. 2025, 323, 119213. [Google Scholar] [CrossRef]
- Bhavanam, A.; Sastry, R. Biomass gasification processes in downdraft fixed bed reactors: A review. Int. J. Chem. Eng. Appl. 2011, 2, 425. [Google Scholar]
- Fryda, L.; Visser, R. Biochar for soil improvement: Evaluation of biochar from gasification and slow pyrolysis. Agriculture 2015, 5, 1076–1115. [Google Scholar] [CrossRef]
- Ulusal, A.; Varol, E.A.; Bruckman, V.J.; Uzun, B.B. Opportunity for sustainable biomass valorization to produce biochar for improving soil characteristics. Biomass Convers. Biorefin. 2021, 11, 1041–1051. [Google Scholar] [CrossRef]
- Al-Abedi, H.J.; Smith, J.D.; Al-Rubaye, H.; Shakor, Z.M.; Erdem, A.; Ani, P.C. Synergistic co-pyrolysis of corn stover and refuse-derived fuel with microplastics: Kinetic and thermodynamic study. Biofuels 2024, 15, 1197–1213. [Google Scholar] [CrossRef]
- Al-Abedi, H.J.; Smith, J.D.; Al-Rubaye, H.; Ani, P.C.; Moellenhoff, C.; McLeland, T.; Zagorac, K. Experimental and Aspen Simulation Study of the Co-Pyrolysis of Refuse-Derived Fuel and Oil Shale: Product Yields and Char Characterization. Fuels 2025, 6, 38. [Google Scholar] [CrossRef]
- Rezaei, H.; Panah, F.Y.; Lim, C.J.; Sokhansanj, S. Pelletization of refuse-derived fuel with varying compositions of plastic, paper, organic, and wood. Sustainability 2020, 12, 4645. [Google Scholar] [CrossRef]
- Zhao, L.; Giannis, A.; Lam, W.-Y.; Lin, S.-X.; Yin, K.; Yuan, G.-A.; Wang, J.-Y. Characterization of Singapore RDF resources and analysis of their heating value. Sustain. Environ. Res. 2016, 26, 51–54. [Google Scholar] [CrossRef]
- Robinson, T.; Bronson, B.; Gogolek, P.; Mehrani, P. Air-blown bubbling fluidized bed co-gasification of woody biomass and refuse-derived fuel. Can. J. Chem. Eng. 2017, 95, 55–61. [Google Scholar] [CrossRef]
- Abbas, S.; Abbas, A.; Zahra, T.; Kazmi, J.; Ahmad, W.; Ahmed, N.; Lim, T.M.; Cong, H. Green and gram-scale synthesis of uniform graphene quantum dots from biomass waste: A highly selective probe for nanomolar Hg2+ sensing. Mater. Today Chem. 2025, 47, 102830. [Google Scholar] [CrossRef]
- Pattnaik, B.K.; Behera, R.; Santra, S.C.; Choudhury, S.; Biswas, J.K.; Hossain, A.; Moulick, D. Potentials of urban waste-derived biochar in minimizing heavy metal bioavailability: A techno-economic review. Iscience 2025, 28, 111915. [Google Scholar] [CrossRef]
- Kumar, A.; Joseph, S.; Tsechansky, L.; Privat, K.; Schreiter, I.J.; Schüth, C.; Graber, E.R. Biochar aging in contaminated soil promotes Zn immobilization due to changes in biochar surface structural and chemical properties. Sci. Total Environ. 2018, 626, 953–961. [Google Scholar] [CrossRef]
- Santos, D.C.B.D.; Evaristo, R.B.W.; Dutra, R.C.; Suarez, P.A.Z.; Silveira, E.A.; Ghesti, G.F. Advancing biochar applications: A review of production processes, analytical methods, decision criteria, and pathways for scalability and certification. Sustainability 2025, 17, 2685. [Google Scholar] [CrossRef]
- Nworie, F.S.; Mgbemena, N.; Ike-Amadi, A.C.; Ebunoha, J. Functionalized biochars for enhanced removal of heavy metals from aqueous solutions: Mechanism and future industrial prospects. J. Hum. Earth Future 2022, 3, 377–395. [Google Scholar] [CrossRef]
- Bachmann, H.J.; Bucheli, T.D.; Dieguez-Alonso, A.; Fabbri, D.; Knicker, H.; Schmidt, H.-P.; Ulbricht, A.; Becker, R.; Buscaroli, A.; Buerge, D.; et al. Toward the standardization of biochar analysis: The cost action TD1107 interlaboratory comparison. J. Agric. Food Chem. 2016, 64, 513–527. [Google Scholar] [CrossRef]
- Li, S.; Tasnady, D. Biochar for soil carbon sequestration: Current knowledge, mechanisms, and future perspectives. J. Carbon Res. 2023, 9, 67. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, G.; Kumar, A.; Dhiman, P.; Stadler, F.J. Recent Advancements in Biochar and its Composite for the Remediation of Hazardous Pollutants. Curr. Anal. Chem. 2025, 21, 15–56. [Google Scholar] [CrossRef]
- Vijayaraghavan, K. The importance of mineral ingredients in biochar production, properties and applications. Crit. Rev. Environ. Sci. Technol. 2021, 51, 113–139. [Google Scholar] [CrossRef]
- Syguła, E.; Świechowski, K.; Stępień, P.; Koziel, J.A.; Białowiec, A. The prediction of calorific value of carbonized solid fuel produced from refuse-derived fuel in the low-temperature pyrolysis in CO2. Materials 2020, 14, 49. [Google Scholar] [CrossRef]
- Ani, P.C.; Alhameedi, H.; Al-Abedi, H.J.; Al-Rubaye, H.; Zeitoun, Z.; Ewuzie, U.; Smith, J.D. The Comprehensive Quantification and Characterization of Oak Biochar Produced via a Gasification Process Using a Downdraft Reactor. Fuels 2025, 6, 51. [Google Scholar] [CrossRef]
- ISO 18115-1; Surface Chemical Analysis—Vocabulary—Part 1: General Terms and Terms Used in Spectroscopy. International Organization for Standardization: Geneva, Switzerland, 2013.
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Ohshima, H. Electrophoretic mobility of a colloidal particle with constant surface charge density. Langmuir 2010, 26, 18016–18019. [Google Scholar] [CrossRef] [PubMed]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Elemental composition of biochars is affected by methods used for its determination. J. Anal. Appl. Pyrolysis 2021, 156, 105174. [Google Scholar] [CrossRef]
- Lefebvre, J.; Galli, F.; Bianchi, C.L.; Patience, G.S.; Boffito, D.C. Experimental methods in chemical engineering: X-ray photoelectron spectroscopy-XPS. Can. J. Chem. Eng. 2019, 97, 2588–2593. [Google Scholar] [CrossRef]
- Gale, M.; Nguyen, T.; Moreno, M.; Gilliard-AbdulAziz, K.L. Physiochemical properties of biochar and activated carbon from biomass residue: Influence of process conditions to adsorbent properties. ACS Omega 2021, 6, 10224–10233. [Google Scholar] [CrossRef]
- Kabir, E.; Kim, K.-H.; Kwon, E.E. Biochar as a tool for the improvement of soil and environment. Front. Environ. Sci. 2023, 11, 1324533. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Zhao, W.; Li, Z.; Zang, L. Facile fabrication of calcium-doped carbon for efficient phosphorus adsorption. ACS Omega 2020, 6, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Jiang, Y.; An, J.; Huang, X.; Elgarhy, A.H.; Cao, H.; Liu, G. Novel Fe/Ca oxide co-embedded coconut shell biochar for phosphorus recovery from agricultural return flows. RSC Adv. 2024, 14, 27204–27214. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, L.; Huang, R.; Lin, H.; Wang, D. Immobilization of heavy metals in biochar derived from co-pyrolysis of sewage sludge and calcium sulfate. J. Hazard. Mater. 2021, 403, 123648. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Islam, S.; Li, Q.; Fu, Q.; Zhu, J.; Hu, H. Combined Application of Biochar and Calcium Superphosphate Can Effectively Immobilize Cadmium and Reduce Its Uptake by Cabbage. Agronomy 2024, 14, 2538. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256, 1–9. [Google Scholar] [CrossRef]
- Bahcivanji, L.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. The effect of post-pyrolysis treatment on waste biomass-derived hydrochar. Waste Manag. 2020, 106, 55–61. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Gao, P.; Hu, Z.; Sheng, Y.; Pan, W.; Ding, L.; Tang, L.; Chen, X.; Wang, F. Pyrolysis of municipal plastic waste: Chlorine distribution and formation of organic chlorinated compounds. Sci. Total Environ. 2024, 912, 169572. [Google Scholar] [CrossRef]
- Strezov, V.; Zhou, X.; Evans, T.; Kan, T.; Taylor, M.P. Investigation of the effect of chlorine in different additives on dioxin formation during high temperature processing of iron ore. Ecotoxicol. Environ. Saf. 2024, 288, 117406. [Google Scholar] [CrossRef]
- Yang, P.; Jia, D.; Lin, B.; Zhuang, X.; Bi, X. Microwave-assisted catalytic pyrolysis of refuse-derived fuel (RDF) to improve pyrolysis performance and biochar properties. Fuel Process. Technol. 2022, 227, 107129. [Google Scholar] [CrossRef]
- Barman, R.; Gupta, A.; Kandpal, G. Combined Application of Biochar with Fertilizers Influence available Nitrogen, Phosphorus and Potassium Quantity in Soil. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1218–1224. [Google Scholar] [CrossRef]
- Zou, X.; Debiagi, P.; Amjed, M.A.; Zhai, M.; Faravelli, T. Impact of high-temperature biomass pyrolysis on biochar formation and composition. J. Anal. Appl. Pyrolysis 2024, 179, 106463. [Google Scholar] [CrossRef]
- Zeitoun, Z.; El-Shazly, A.H.; Nosier, S.; Elmarghany, M.R.; Salem, M.S.; Taha, M.M. Performance evaluation and kinetic analysis of photocatalytic membrane reactor in wastewater treatment. Membranes 2020, 10, 276. [Google Scholar] [CrossRef]
- Michalak, I.; Baśladyńska, S.; Mokrzycki, J.; Rutkowski, P. Biochar from a freshwater macroalga as a potential biosorbent for wastewater treatment. Water 2019, 11, 1390. [Google Scholar] [CrossRef]
- Moradi-Choghamarani, F.; Moosavi, A.A.; Baghernejad, M. Determining organo-chemical composition of sugarcane bagasse-derived biochar as a function of pyrolysis temperature using proximate and Fourier transform infrared analyses. J. Therm. Anal. Calorim. 2019, 138, 331–342. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Uchimiya, M. Comparison of biochar formation from various agricultural by-products using FTIR spectroscopy. Mod. Appl. Sci. 2015, 9, 246. [Google Scholar] [CrossRef]
- Li, B.; Huang, Y.; Wang, Z.; Li, J.; Liu, Z.; Fan, S. Enhanced adsorption capacity of tetracycline on tea waste biochar with KHCO3 activation from aqueous solution. Environ. Sci. Pollut. Res. 2021, 28, 44140–44151. [Google Scholar] [CrossRef]
- Ganesan, K.; Guin, B.; Wilbanks, E.; Sternberg, J. Synthesis and Characterization of Soy Hull Biochar-Based Flexible Polyurethane Foam Composites. Materials 2025, 18, 2006. [Google Scholar] [CrossRef]
- Ben Salem, I.; El Gamal, M.; Sharma, M.; Hameedi, S.; Howari, F.M. Utilization of the UAE date palm leaf biochar in carbon dioxide capture and sequestration processes. J. Environ. Manag. 2021, 299, 113644. [Google Scholar] [CrossRef]
- Hou, J.; Yu, J.; Li, W.; He, X.; Li, X. The effects of chemical oxidation and high-temperature reduction on surface functional groups and the adsorption performance of biochar for sulfamethoxazole adsorption. Agronomy 2022, 12, 510. [Google Scholar] [CrossRef]
- Fan, Q.; Cui, L.; Quan, G.; Wang, S.; Sun, J.; Han, X.; Wang, J.; Yan, J. Effects of wet oxidation process on biochar surface in acid and alkaline soil environments. Materials 2018, 11, 2362. [Google Scholar] [CrossRef] [PubMed]
- Beć, K.B.; Grabska, J.; Badzoka, J.; Huck, C.W. Spectra-structure correlations in NIR region of polymers from quantum chemical calculations. The cases of aromatic ring, C=O, C≡ N, and C-Cl functionalities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120085. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Zhang, P.; Li, H.; Yang, Q.; Oleszczuk, P.; Pan, B. Generation mechanism of persistent free radicals in lignocellulose-derived biochar: Roles of reducible carbonyls. Environ. Sci. Technol. 2022, 56, 10638–10645. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.X.; Zhu, Q.; Dong, W.P.; Fan, Y.Q.; Wang, W.L. Preparation and characterization of phosphoric acid-modified biochar nanomaterials with highly efficient adsorption and photodegradation ability. Langmuir 2021, 37, 9253–9263. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Zhang, G.; Zhi, Y.; Yang, D.; Lai, X.; Ren, T. Characterization of acid-aged biochar and its ammonium adsorption in an aqueous solution. Materials 2020, 13, 2270. [Google Scholar] [CrossRef]
- Liu, A.; Feng, L.-J.; Ou, Y.; Zhang, X.; Zhang, J.; Chen, H. Competitive adsorption of polycyclic aromatic hydrocarbons on phosphorus tailing-modified sludge biochar provides mechanistic insights. Environ. Geochem. Health 2024, 46, 497. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Krushnamurty, K.; Mahammadunnisa, S.K.; Dayamani, A.; Subrahmanyam, C. Preparation of activated carbons from bio-waste: Effect of surface functional groups on methylene blue adsorption. Int. J. Environ. Sci. Technol. 2015, 12, 1363–1372. [Google Scholar] [CrossRef]
- Ramezanzadeh, H.; Zarehaghi, D.; Baybordi, A.; Bouket, A.C.; Oszako, T.; Alenezi, F.N.; Belbahri, L. The impacts of biochar-assisted factors on the hydrophysical characteristics of amended soils: A review. Sustainability 2023, 15, 8700. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for wastewater treatment—Conversion technologies and applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Yu, P.; Baker, M.C.; Crump, A.R.; Vogler, M.; Strawn, D.G.; Möller, G. Biochar integrated reactive filtration of wastewater for P removal and recovery, micropollutant catalytic oxidation, and negative CO2e: Process operation and mechanism. Water Environ. Res. 2023, 95, e10926. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, P.; Kots, P.A.; Cohen, M.; Chen, Y.; Quinn, C.M.; de Mello, M.D.; Boscoboinik, J.A.; Shaw, W.J.; Caratzoulas, S.; et al. Tuning the reactivity of carbon surfaces with oxygen-containing functional groups. Nat. Commun. 2023, 14, 2293. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, L.; Shao, L.; Wu, Z.; Zhan, P.; Zhou, X.; Chen, J. Versatile strategy for the preparation of woody biochar with oxygen-rich groups and enhanced porosity for highly efficient Cr (VI) removal. ACS Omega 2021, 7, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Zhang, L.; Tan, Z.; Huang, Q. Effect mechanism of biochar’s zeta potential on farmland soil’s cadmium immobilization. Environ. Sci. Pollut. Res. 2019, 26, 19738–19748. [Google Scholar] [CrossRef] [PubMed]
- Bayar, J.; Ali, N.; Dong, Y.; Ahmad, U.; Anjum, M.M.; Khan, G.R.; Zaib, M.; Jalal, A.; Ali, R.; Ali, L. Biochar-based adsorption for heavy metal removal in water: A sustainable and cost-effective approach. Environ. Geochem. Health 2024, 46, 428. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and water quality. J. Environ. Qual. 2019, 48, 2–15. [Google Scholar] [CrossRef]
- Boçe, M.T.; Hoxha, J. Blockchain technology as a catalyst for sustainable development: Exploring economic, social, and environmental synergies. Acad. J. Interdiscip. Stud. 2024, 13, 151. [Google Scholar] [CrossRef]
- Chaves Fernandes, B.C.; Ferreira Mendes, K.; Dias Júnior, A.F.; da Silva Caldeira, V.P.; da Silva Teófilo, T.M.; Severo Silva, T.; Mendonça, V.; de Freitas Souza, M.; Valadão Silva, D. Impact of pyrolysis temperature on the properties of eucalyptus wood-derived biochar. Materials 2020, 13, 5841. [Google Scholar] [CrossRef]
- Long, L.; Xue, Y.; Hu, X.; Zhu, Y. Study on the influence of surface potential on the nitrate adsorption capacity of metal modified biochar. Environ. Sci. Pollut. Res. 2019, 26, 3065–3074. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Valentim, B.; Białecka, B.; Gonçalves, P.A.; Guedes, A.; Guimarães, R.; Cruceru, M.; Całus-Moszko, J.; Popescu, L.G.; Predeanu, G.; Santos, A.C. Undifferentiated inorganics in coal fly ash and bottom ash: Calcispheres, magnesiacalcispheres, and magnesiaspheres. Minerals 2018, 8, 140. [Google Scholar] [CrossRef]
- Santos, A.C.; Cruz, C.; Font, E.; French, D.; Guedes, A.; Moreira, K.; Sant’ovaia, H.; Vieira, B.J.C.; Waerenborgh, J.C.; Valentim, B. Physicochemical properties of Fe-bearing phases from commercial Colombian coal ash. Minerals 2023, 13, 1055. [Google Scholar] [CrossRef]
- Araujo, R.O.; Anjos, O.C.D.; Souza, L.K.D.; Bataglion, G.A. Biochar from Lignocellulosic Biomass: A Sustainable Circular Economy Approach For Removing Organic And Inorganic Contaminants. Química Nova 2024, 47, e-20240079. [Google Scholar] [CrossRef]
- Pandian, K.; Vijayakumar, S.; Mustaffa, M.R.A.F.; Subramanian, P.; Chitraputhirapillai, S. Biochar—A sustainable soil conditioner for improving soil health, crop production and environment under changing climate: A review. Front. Soil Sci. 2024, 4, 1376159. [Google Scholar] [CrossRef]


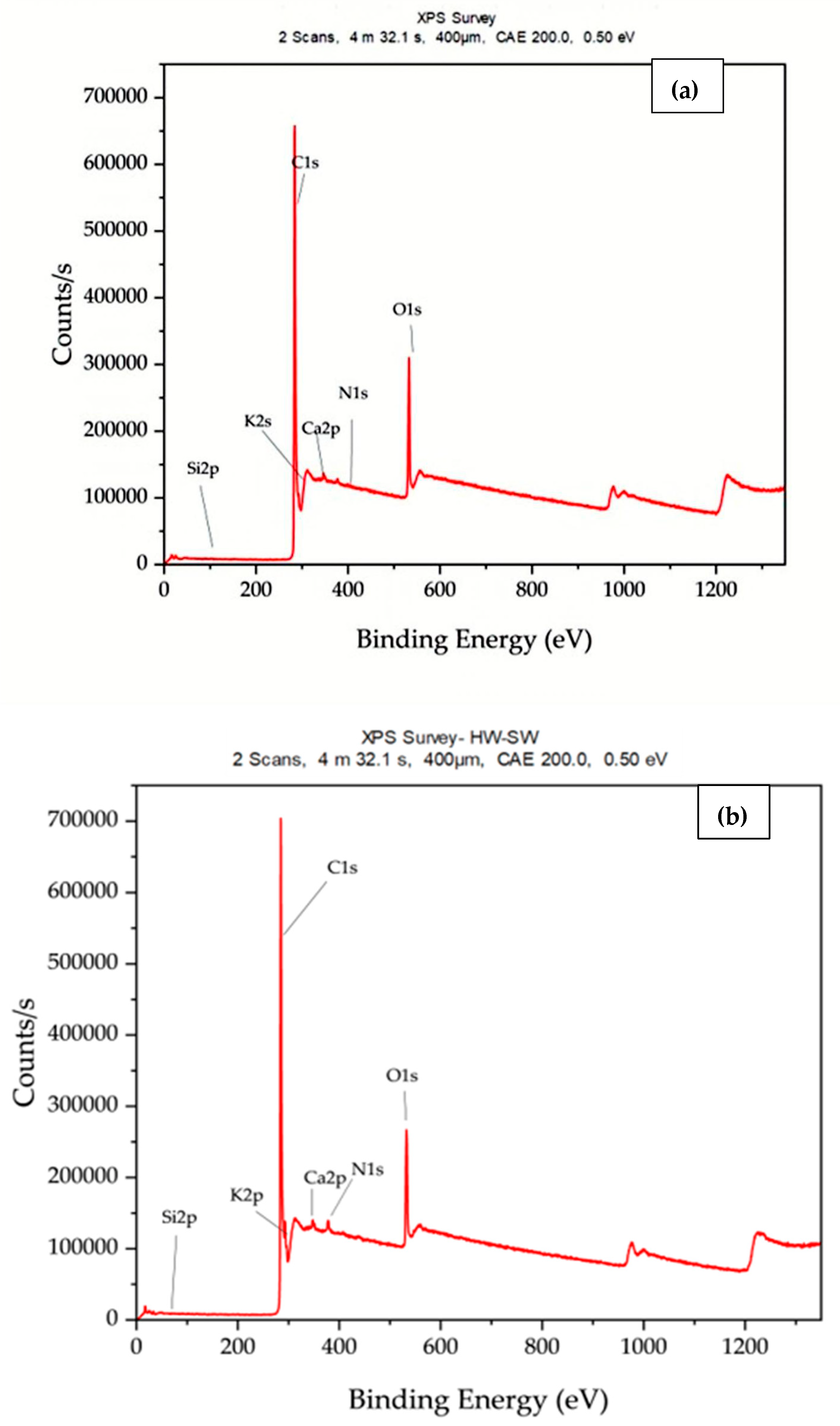
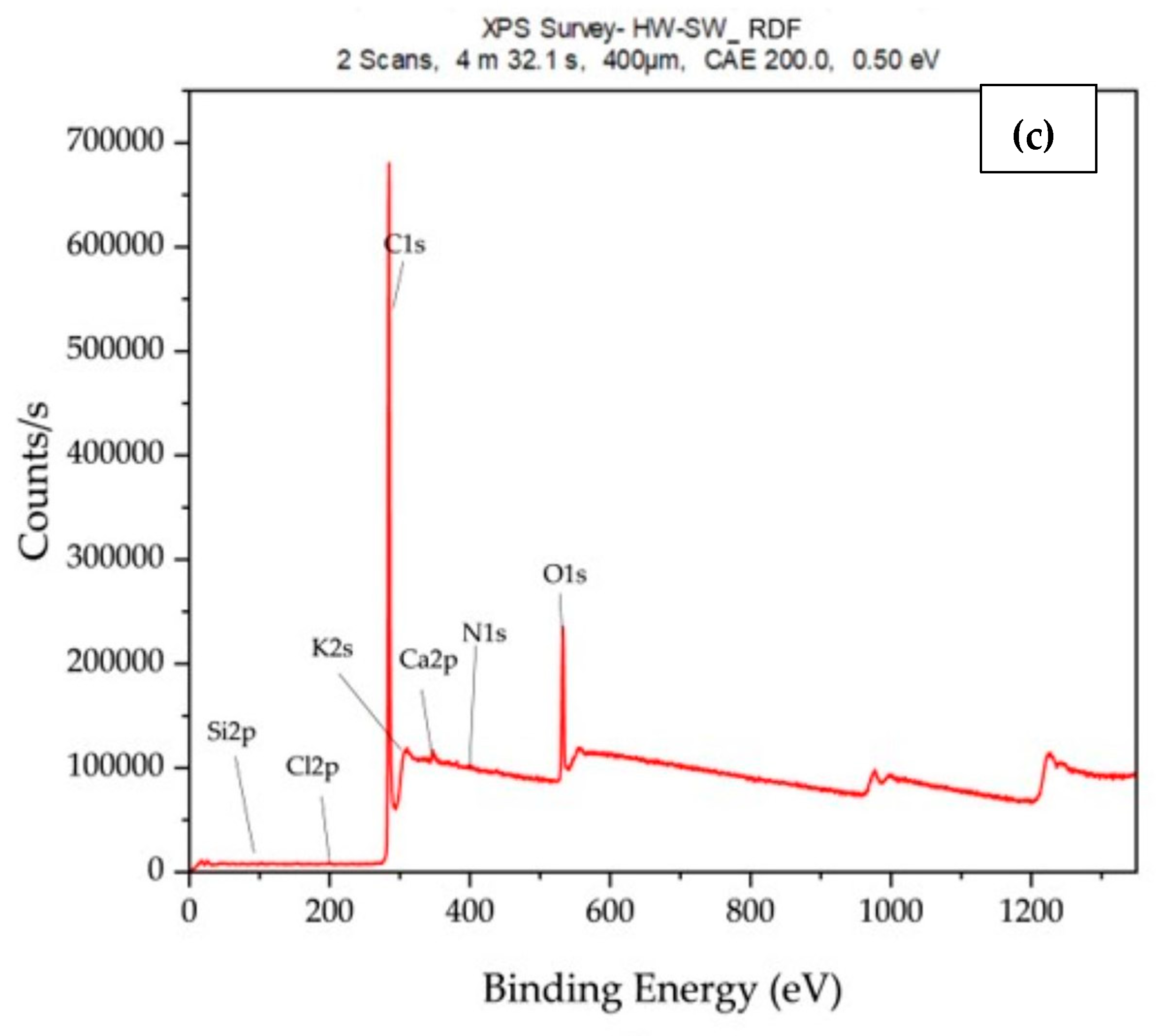
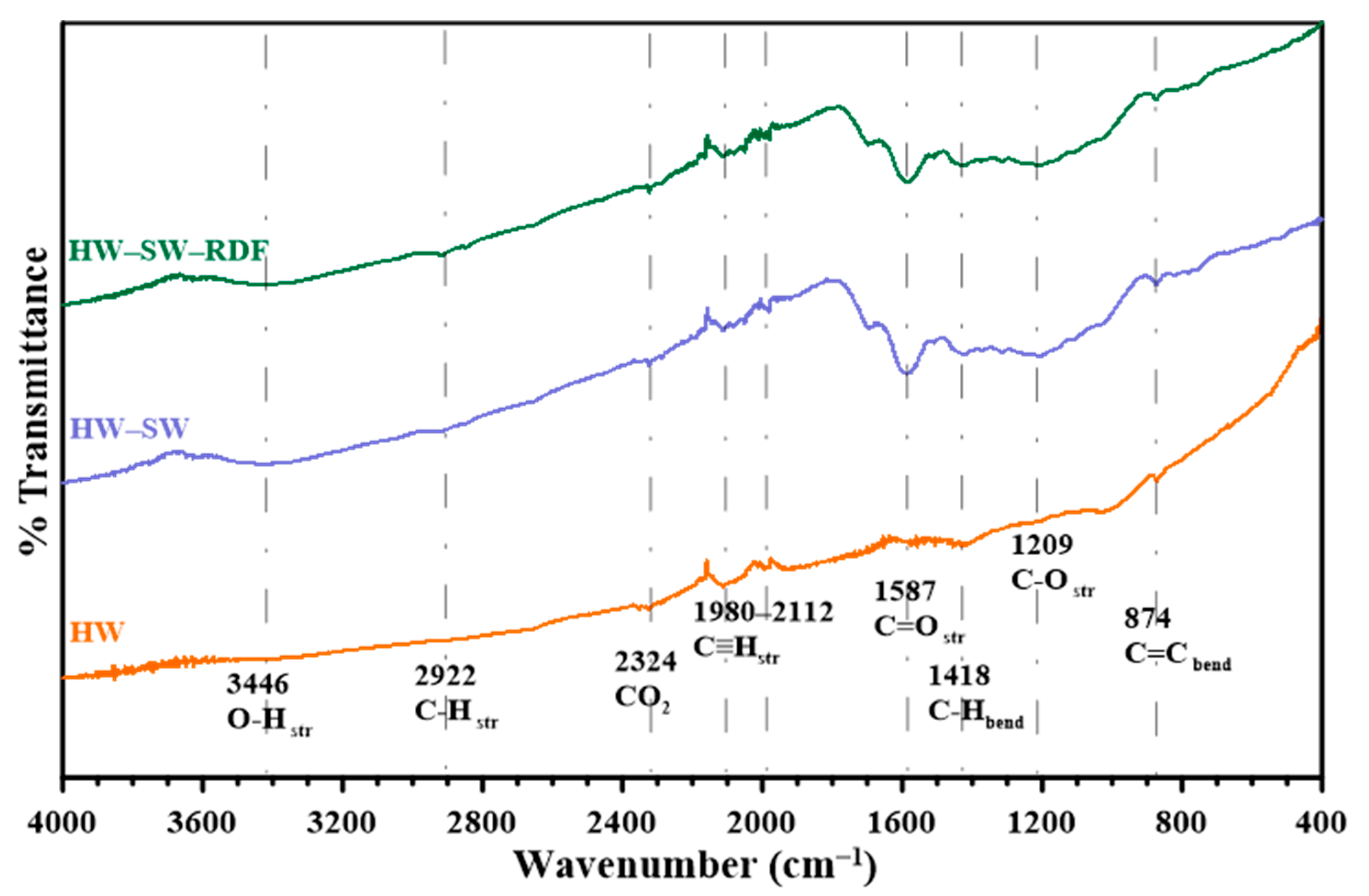
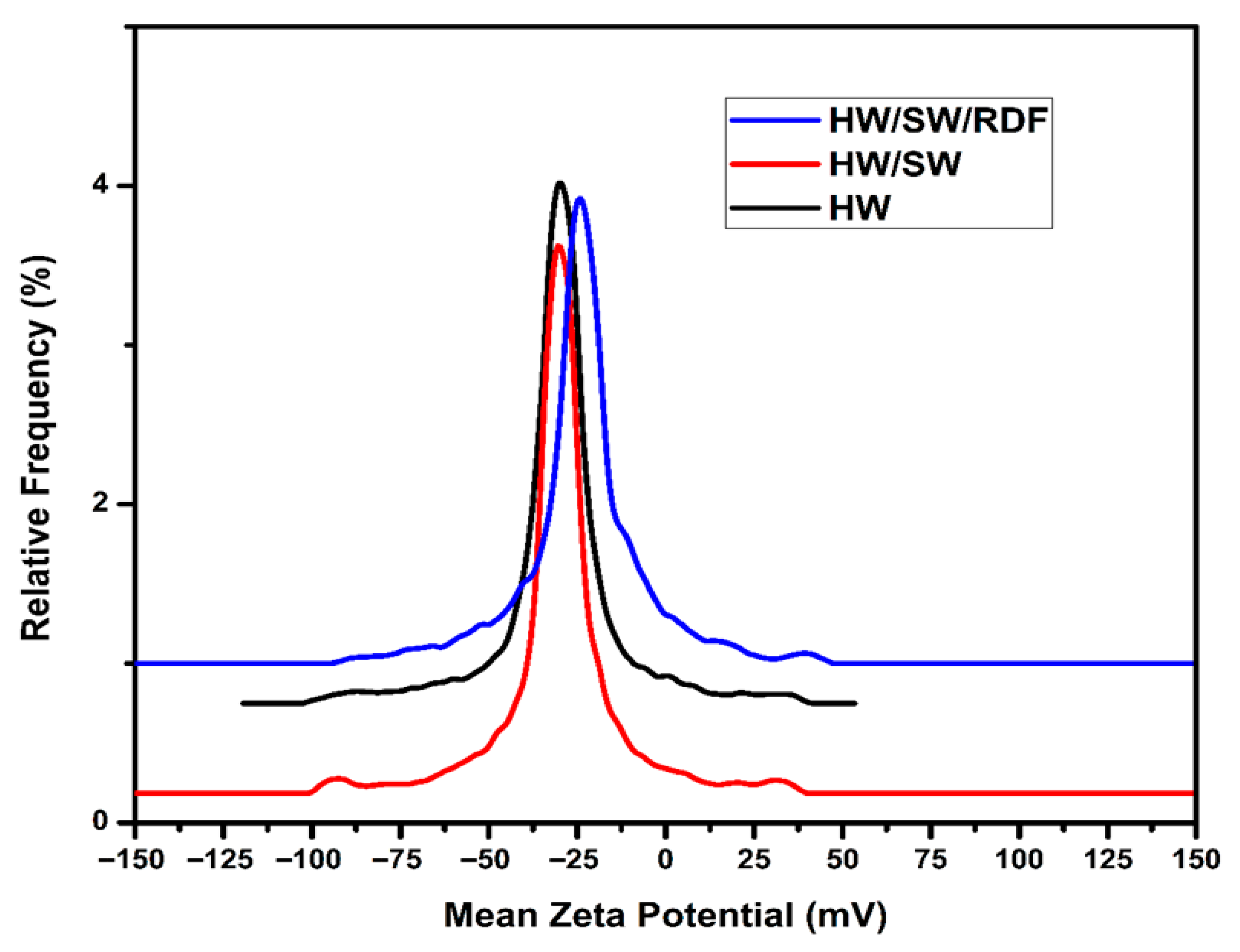
| Element | HW Weight % | HW/SW Weight % | HW/SW/RDF Weight % |
|---|---|---|---|
| N1s | 0.164481 | 0.240593 | 0.903234 |
| C1s | 81.07511 | 82.45155 | 82.57513 |
| O1s | 15.7526 | 13.02327 | 13.36913 |
| Ca2p | 1.224915 | 2.067382 | 2.271619 |
| Si2p | 0.374155 | 0.109751 | 0.685372 |
| K2p | 1.408742 | 2.107454 | - |
| Cl2p | - | - | 0.195517 |
| Sample | Mean Zeta Potential (mV) | Electrophoretic Mobility (μm.cm/V·s) | pH | Electrochemical Conductivity (μs/cm) |
|---|---|---|---|---|
| HW | −31.5 ± 0.53 | −2.4567 | 10.10 | 113 ± 0.123 |
| HW/SW | −30.0 ± 0.64 | −2.3385 | 10.25 | 170 ± 0.115 |
| HW/SW/RDF | −24.2 ± 0.92 | −1.8875 | 7.76 | 53 ± 0.172 |
| Biochar Sample | pH Range | Initial Zeta Potential (mV) | Final Zeta Potential (mV) |
|---|---|---|---|
| HW | 9–13 | −34 | −38 |
| HW/SW | 9–13 | −44 | −30 |
| HW/SW/RDF | 8–13 | −12.5 | −23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ani, P.C.; Al-Abedi, H.J.; Smith, J.D.; Zeitoun, Z. Biochar Surface Chemistry Modification by Blending Hardwood, Softwood, and Refuse-Derived Fuel: Insights from XPS, FTIR, and Zeta Potential Analysis. Fuels 2025, 6, 71. https://doi.org/10.3390/fuels6030071
Ani PC, Al-Abedi HJ, Smith JD, Zeitoun Z. Biochar Surface Chemistry Modification by Blending Hardwood, Softwood, and Refuse-Derived Fuel: Insights from XPS, FTIR, and Zeta Potential Analysis. Fuels. 2025; 6(3):71. https://doi.org/10.3390/fuels6030071
Chicago/Turabian StyleAni, Paul C., Hasan J. Al-Abedi, Joseph D. Smith, and Zeyad Zeitoun. 2025. "Biochar Surface Chemistry Modification by Blending Hardwood, Softwood, and Refuse-Derived Fuel: Insights from XPS, FTIR, and Zeta Potential Analysis" Fuels 6, no. 3: 71. https://doi.org/10.3390/fuels6030071
APA StyleAni, P. C., Al-Abedi, H. J., Smith, J. D., & Zeitoun, Z. (2025). Biochar Surface Chemistry Modification by Blending Hardwood, Softwood, and Refuse-Derived Fuel: Insights from XPS, FTIR, and Zeta Potential Analysis. Fuels, 6(3), 71. https://doi.org/10.3390/fuels6030071








