Physicochemical Properties of Coconut and Waste Cooking Oils for Biofuel Production and Lubrication
Abstract
1. Introduction
2. Materials and Methods
2.1. Biofuel Synthesis and Characterization
2.2. Lubrication Applications
3. Results and Discussion
3.1. Physicochemical Properties of Transesterified Oils
3.2. Infrared and Gas Chromatography Analysis of Transesterified Coconut Oil (CO)
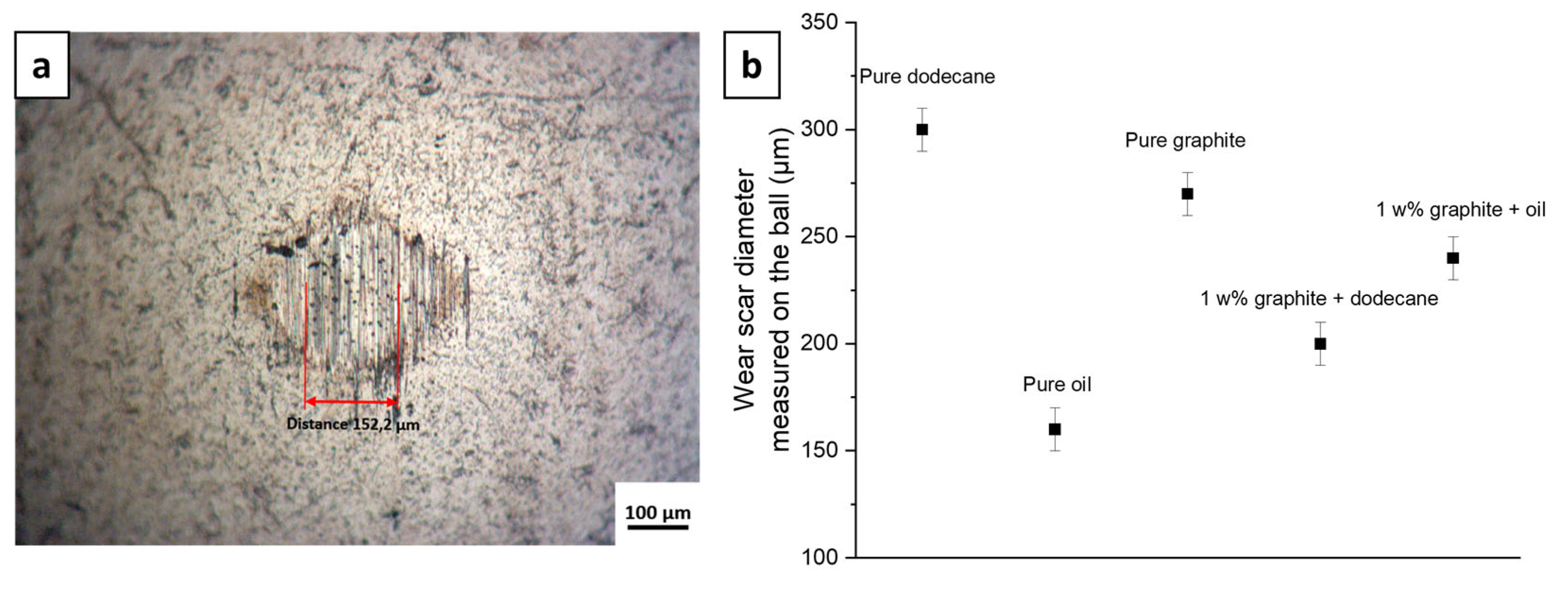
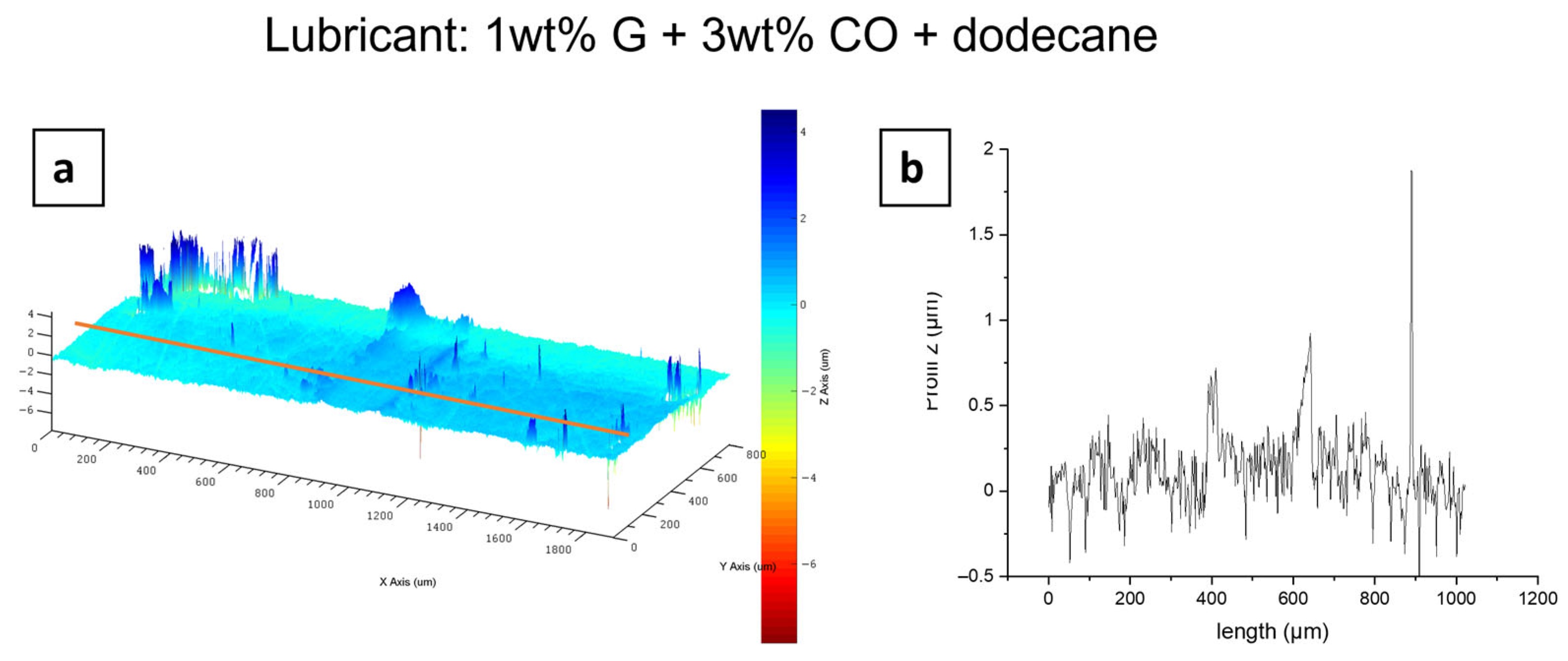
3.3. Tribological Performances
3.3.1. Oils as Base Oil Lubricant
3.3.2. Oils as Liquid Additive Lubricant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NIST | National Institute of Standards and Technology |
| CO | Coconut oil |
| COME | Coconut oil methyl ester |
| COEE | Coconut oil ethyl ester |
| WCOs | Waste cooking oils |
| WCOME | Waste cooking oils methyl ester |
| WCOEE | Waste cooking oils ethyl ester |
| µ | Friction coefficient |
| FN | Normal load |
| FT | Tangential load |
References
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a sustainable future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Dágosto, M.D.A.; Da Silva, M.A.V.; De Oliveira, C.M.; Franca, L.S.; Da Costa Marques, L.G.; Murta, A.L.S.; De Freitas, M.A.V. Evaluating the potential of the use of biodiesel for power generation in Brazil. Renew. Sustain. Energy Rev. 2015, 43, 807–817. [Google Scholar] [CrossRef]
- Crichton, R.; Farhidi, F.; Patel, A.; Ellegate, N. Clearing up the benefits of a fossil fuel sector diversified board: A climate change mitigation strategy. Bus. Soc. Rev. 2021, 126, 433–453. [Google Scholar] [CrossRef]
- Farhidi, F.; Farhidi, F. Impact of fossil fuel transition and population expansion on economic growth. Environ. Dev. Sustain. 2023, 25, 2571–2609. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Sugiawan, Y.; Managi, S. New evidence of energy-growth nexus from inclusive wealth. Renew. Sustain. Energy Rev. 2019, 103, 40–48. [Google Scholar] [CrossRef]
- Pillot, B.; Muselli, M.; Poggi, P.; Dias, J.B. Historical trends in global energy policy and renewable power system issues in Sub-Saharan Africa: The case of solar PV. Energy Policy 2019, 127, 113–124. [Google Scholar] [CrossRef]
- Chavarria-Hernandez, J.C.; Pacheco-Catalán, D.E. Predicting the kinematic viscosity of FAMEs and biodiesel: Empirical models. Fuel 2014, 124, 212–220. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Umeh, S.I.; Okonkwo, P.A.; Umeh, S.I.; Okonkwo, P.A. The Essential Properties of Oils for Biodiesel Production. In Biodiesel Plants—Fueling the Sustainable Outlooks; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Velez, I.-S.C.; Von Bernewitz, K. Evaluation of Environmentally Acceptable Hydraulic Fluids; TARDEC-TR-13640; USA Tank Automotive Command Mobility Technology Center: Fort Belvoir, VA, USA, 1995. [Google Scholar]
- Randles, S.J.; Wilton, I.; Uk, M.; Cranjield, M.W. Environmentally considerate ester lubricants for the automotive and engineering industries. J. Synth. Lubr. 1992, 9, 145–161. [Google Scholar] [CrossRef]
- Al-Widyan, M.I.; Al-Shyoukh, A.O. Experimental evaluation of the transesterification of waste palm oil into biodiesel. Bioresour. Technol. 2002, 85, 253–256. [Google Scholar] [CrossRef]
- Chinnamma, M.; Bhasker, S.; Madhav, H.; Devasia, R.M.; Shashidharan, A.; Pillai, B.C.; Thevannoor, P. Production of coconut methyl ester (CME) and glycerol from coconut (Cocos nucifera) oil and the functional feasibility of CME as biofuel in diesel engine. Fuel 2015, 140, 4–9. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Anantapinitwatna, A.; Ngaosuwan, K.; Kiatkittipong, W.; Wongsawaeng, D.; Assabumrungrat, S. Effect of Water Content in Waste Cooking Oil on Biodiesel Production via Ester-transesterification in a Single Reactive Distillation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 559, 012014. [Google Scholar] [CrossRef]
- Okonkwo, P.A.; Omenihu, I. Production and characterization of biodiesel from mango seed oil (MSO). Niger. J. Technol. 2021, 40, 598–607. [Google Scholar] [CrossRef]
- Suraj, C.K.; Anand, K.; Sundararajan, T. Investigation of biodiesel production methods by altering free fatty acid content in vegetable oils. Biofuels 2020, 11, 587–595. [Google Scholar] [CrossRef]
- Bazooyar, B.; Ghorbani, A.; Shariati, A. Physical Properties of Methyl Esters Made from Alkali-based Transesterification and Conventional Diesel Fuel. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 468–476. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A.K. Biodiesel from vegetable oils. Renew. Sustain. Energy Rev. 2014, 31, 446–471. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Peterson, C.L.; Feldman, M.; Korus, R.; Auld, D.L. Batch Type Transesterification Process for Winter Rape Oil. Appl. Eng. Agric. 1991, 7, 711–716. [Google Scholar] [CrossRef]
- Korus, R.A.; Hoffman, D.S.; Barn, N.; Peterson, C.L.; Drown, D.C. Transesterification Process to Manufacture Ethyl Ester of Rape Oil; NREL: Golden, CO, USA, 1993. [Google Scholar]
- Wright, H.J.; Segur, J.B.; Clark, H.V.; Coburn, S.K.; Langdon, E.E.; DuPuis, R.N. A report on ester interchange. Oil Soap 1944, 21, 145–148. [Google Scholar] [CrossRef]
- Bapat, A.; Pradhan, S.; Madankar, C.S. Oil and Fats as Raw Materials as Corrosion Inhibitors and Biolubricants. In Oils and Fats as Raw Materials for Industry; Wiley: Hoboken, NJ, USA, 2024; pp. 195–229. [Google Scholar] [CrossRef]
- Sharma, A.; Pradhan, S.; Chakraborty, P.; Prasad, L. Extraction of Ester-Based Biolubricants from Vegetable Oils. In Lubricants from Renewable Feedstocks; Wiley: Hoboken, NJ, USA, 2024; pp. 39–65. [Google Scholar] [CrossRef]
- Uppar, R.; Kumar, S.; Dinesha, P. Comparative evaluation of lubricant properties of jatropha and jojoba methyl ester. Cogent Eng. 2014, 11, 2334397. [Google Scholar] [CrossRef]
- Hu, C.; Ai, J.; Ma, L.; Wen, P.; Fan, M.; Zhou, F.; Liu, W. Ester Oils Prepared from Fully Renewable Resources and Their Lubricant Base Oil Properties. ACS Omega 2021, 6, 16343–16355. [Google Scholar] [CrossRef]
- Sanjurjo, C.; Rodríguez, E.; Viesca, J.L.; Battez, A.H. Influence of Molecular Structure on the Physicochemical and Tribological Properties of Biolubricants: A Review. Lubricants 2023, 11, 380. [Google Scholar] [CrossRef]
- Nomède-Martyr, N.; Vitulin, M.; Joseph, H.; Thomas, P. Moringa oil with graphite and hexagonal boron nitride particles as additives for lubrication. Diam. Relat. Mater. 2022, 124, 108930. [Google Scholar] [CrossRef]
- Su, Y.; Gong, L.; Chen, D. An Investigation on Tribological Properties and Lubrication Mechanism of Graphite Nanoparticles as Vegetable Based Oil Additive. J. Nanomater. 2015, 1, 276753. [Google Scholar] [CrossRef]
- Huang, H.D.; Tu, J.P.; Gan, L.P.; Li, C.Z. An investigation on tribological properties of graphite nanosheets as oil additive. Wear 2006, 261, 140–144. [Google Scholar] [CrossRef]
- Spence, D.A. The hertz contact problem with finite friction. J. Elast. 1975, 5, 297–319. [Google Scholar] [CrossRef]
- Kamaronzaman, M.F.F.; Kahar, H.; Hassan, N.; Hanafi, M.F.; Sapawe, N. Analysis of biodiesel product derived from waste cooking oil using fourier transform infrared spectroscopy. Mater. Today. Proc. 2020, 31, 329–332. [Google Scholar] [CrossRef]
- Ortega, M.F.; Donoso, D.; Bousbaa, H.; Bolonio, D.; Ballesteros, R.; García-Martínez, M.J.; Lapuerta, M.; Canoira, L. Optimized Production of Fatty Acid Ethyl Esters (FAEE) from Waste Frying Oil by Response Surface Methodology. Waste Biomass Valorization 2021, 12, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Ekeoma, M.O.; Wirnkor, A.; Emmanuel, C.; Ngozi, E.; Ezichi, M.-N.I. Production and Characterization of Biodiesel from Coconut (Cocos nucifera) Oil. Chem. Res. J. 2023, 8, 45–53. [Google Scholar]
- Miao, X.; Li, R.; Yao, H. Effective acid-catalyzed transesterification for biodiesel production. Energy Convers. Manag. 2009, 50, 2680–2684. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Benseñor, M.; Med, S.P.; Ergün, H.; Frattarelli, D.A.C.; Aranda, J.; Frattarelli, J.P.-C.; Biomed, J.P.-C.; Pierre, C.; Schmidt, R.; Brenneis, C.; et al. Production of biodiesel from babassu oil using methanol-ethanol blends. Eclética Química 2010, 35, 47–54. [Google Scholar] [CrossRef]
- Nomède-Martyr, N.; Bilas, P.; Mathieu, G.; Bercion, Y.; Joseph, H.; Thomas, P. Moringa Oil and Carbon Phases of Different Shapes as Additives for Lubrication. Lubricants 2024, 12, 358. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Estellé, P. Viscosity, tribological and physicochemical features of ZnO and MoS2 diesel oil-based nanofluids: An experimental study. Fuel 2021, 293, 120481. [Google Scholar] [CrossRef]
- Nomede-Martyr, N.; Philippe, B.; Philippe, T.; Georges, M.; Laurence, R. Tribological performances of graphite and hexagonal boron nitride particles in the presence of liquid. J. Tribol. 2021, 43, 071401. [Google Scholar] [CrossRef]
- Bahari, A.; Lewis, R.; Slatter, T. Friction and Wear Phenomena of Vegetable Oil–Based Lubricants with Additives at Severe Sliding Wear Conditions. Tribol. Trans. 2018, 61, 207–219. [Google Scholar] [CrossRef]
- Salih, N.; Salimon, J. A review on eco-friendly green biolubricants from renewable and sustainable plant oil sources. Biointerface Res. Appl. Chem. 2021, 11, 13303–13327. [Google Scholar] [CrossRef]
- Cyriac, F.; Tee, X.Y.; Poornachary, S.K.; Chow, P.S. Influence of structural factors on the tribological performance of organic friction modifiers. Friction 2021, 9, 380–400. [Google Scholar] [CrossRef]
- Chowdary, K.; Kotia, A.; Lakshmanan, V.; Elsheikh, A.H.; Ali, M.K.A. A review of the tribological and thermophysical mechanisms of bio-lubricants based nanomaterials in automotive applications. J. Mol. Liq. 2021, 339, 116717. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wen, P.; Ma, L.; Fan, M.; Dong, R.; Zhang, C. Low-viscosity oligoether esters (OEEs) as high-efficiency lubricating oils: Insight on their structure–lubricity relationship. Friction 2024, 12, 1133–1153. [Google Scholar] [CrossRef]
- Crespo, A.; Mazuyer, D.; Morgado, N.; Tonck, A.; Georges, J.M.; Cayer-Barrioz, J. Methodology to Characterize Rheology, Surface Forces and Friction of Confined Liquids at the Molecular Scale Using the ATLAS Apparatus. Tribol. Lett. 2017, 65, 138. [Google Scholar] [CrossRef]

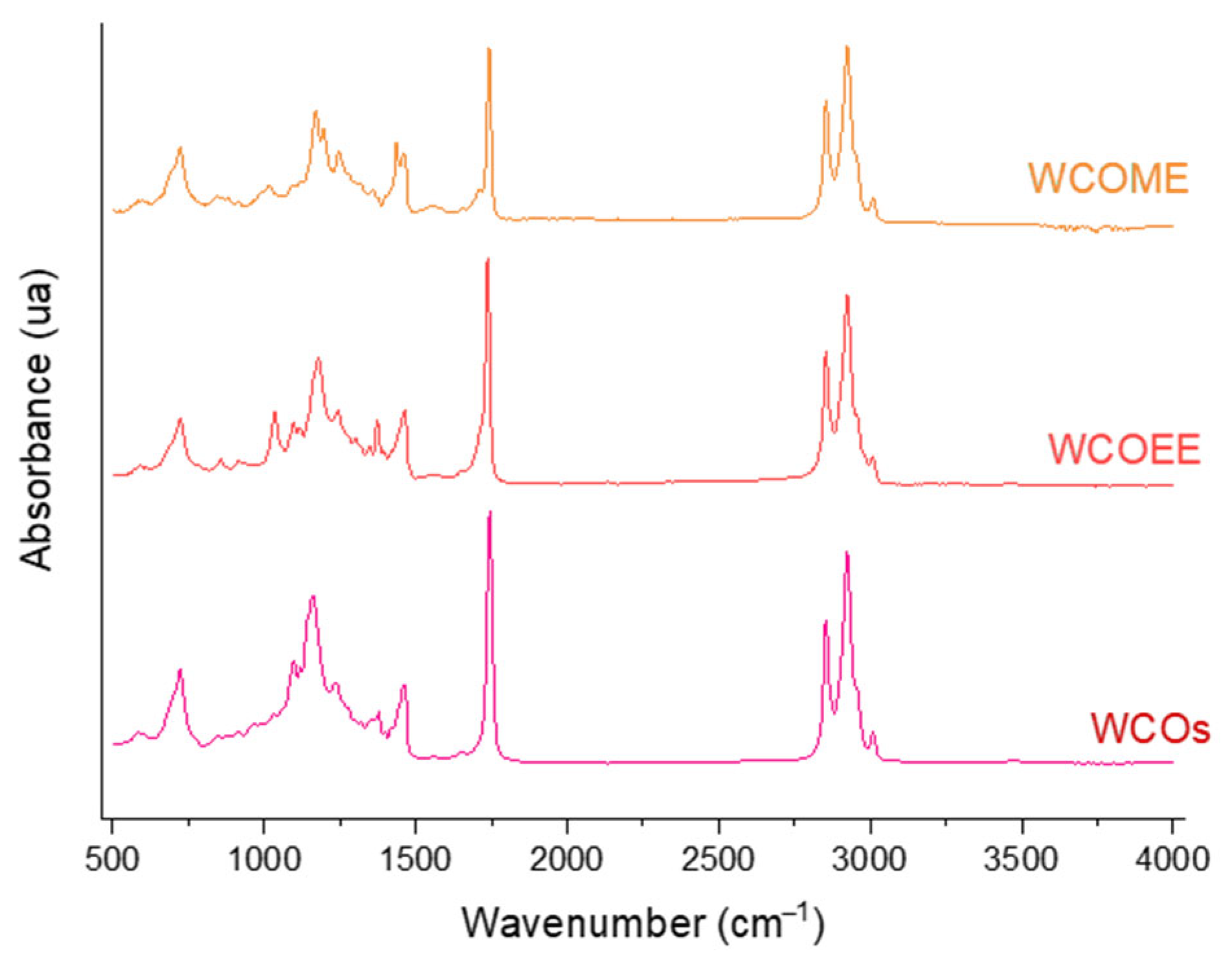
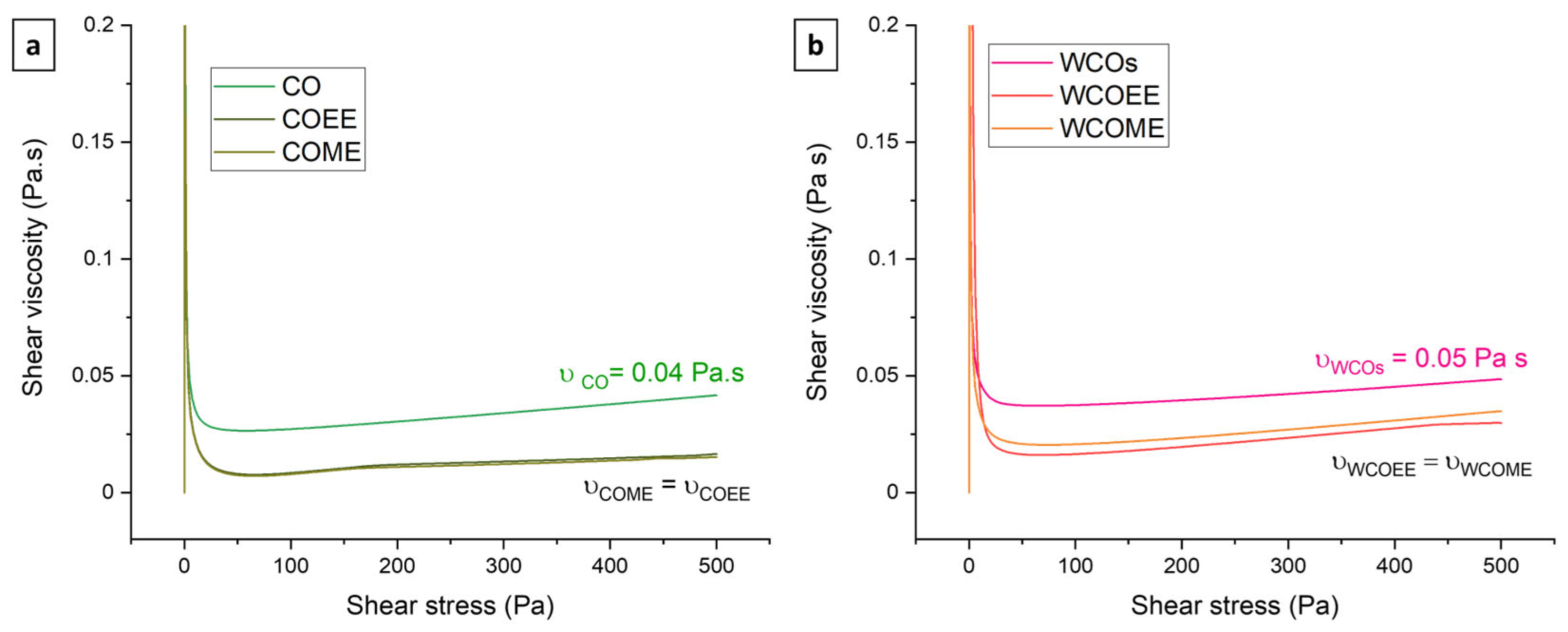
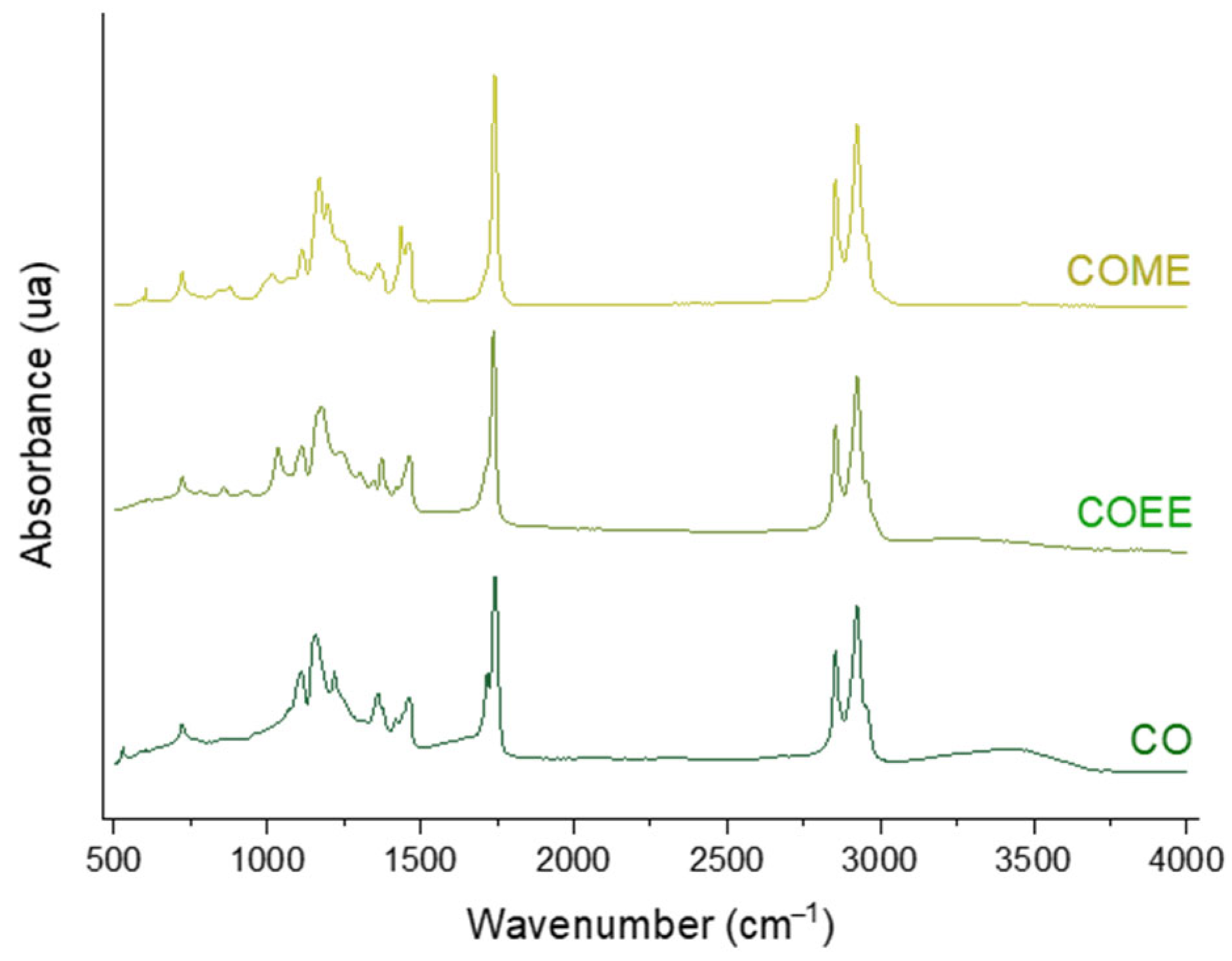
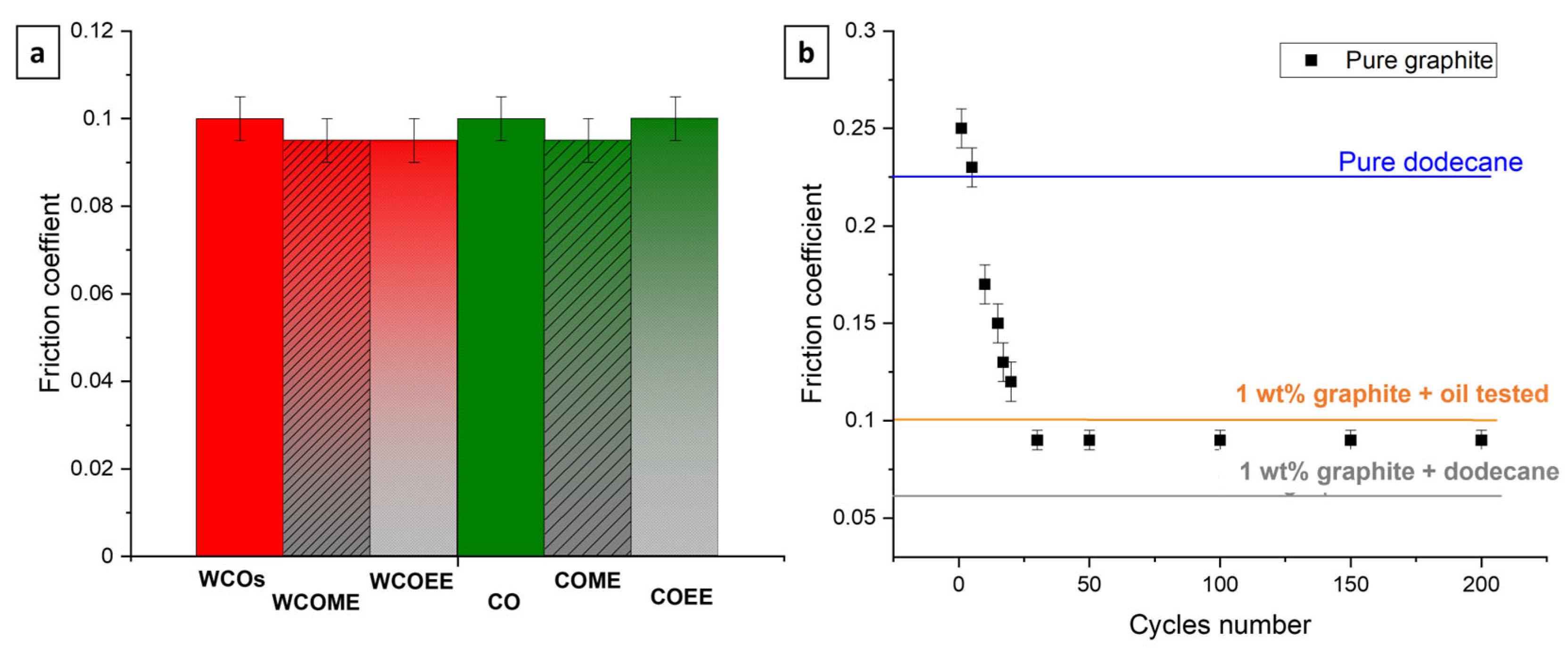

| Ethyl Ester of Waste Cooking Oils (WCOEE) | Methyl Ester of Waste Cooking Oils (WCOME) | ||||
|---|---|---|---|---|---|
| Composition | % | Composition | % | ||
| E-11-Hexadecenoic Hexadecanoic Linoleic Ethyloleate Octadecanoic | C18 C20 C20 C20 C20 | 0.16 7.99 50.21 34.16 4.47 | Hexadecanoic 9,12-Octadecadienoic 8-Octadecenoic Methylstearate Cis-11-Eicosenioc Methyl-18-methylnonadecanoate | C17 C19 C19 C19 C21 C21 | 7.73 52 32.39 4.67 0.25 0.34 |
| Coconut Oil Ethyl Ester (COEE) | Coconut Oil Methyl Ester (COME) | ||||
|---|---|---|---|---|---|
| Composition | % | Composition | % | ||
| Hexanoic Octanoic Decanoic Dodecanoic Tetradecanoic Hexadecanoic Octadecanoic Linoleic Ethyloleate | C8 C10 C12 C14 C16 C18 C20 C20 C20 | 0.50 7.74 7.03 39.83 19.37 9.71 4.40 1.05 5.78 | Octanoic Decanoic Dodecanoic Methyltetradecanoate 14-Methylpentadecanoic 10,13-Octadecadienoic 9-Octadecenoic Methylstearate | C9 C11 C13 C15 C17 C19 C19 C19 | 7.54 7.20 39.72 20.27 10.68 1.19 6.72 4.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adou, A.I.; Brelle, L.; Marote, P.; Sylvestre, M.; Cebriàn-Torrejòn, G.; Nomede-Martyr, N. Physicochemical Properties of Coconut and Waste Cooking Oils for Biofuel Production and Lubrication. Fuels 2025, 6, 57. https://doi.org/10.3390/fuels6030057
Adou AI, Brelle L, Marote P, Sylvestre M, Cebriàn-Torrejòn G, Nomede-Martyr N. Physicochemical Properties of Coconut and Waste Cooking Oils for Biofuel Production and Lubrication. Fuels. 2025; 6(3):57. https://doi.org/10.3390/fuels6030057
Chicago/Turabian StyleAdou, Ahissan Innocent, Laura Brelle, Pedro Marote, Muriel Sylvestre, Gerardo Cebriàn-Torrejòn, and Nadiège Nomede-Martyr. 2025. "Physicochemical Properties of Coconut and Waste Cooking Oils for Biofuel Production and Lubrication" Fuels 6, no. 3: 57. https://doi.org/10.3390/fuels6030057
APA StyleAdou, A. I., Brelle, L., Marote, P., Sylvestre, M., Cebriàn-Torrejòn, G., & Nomede-Martyr, N. (2025). Physicochemical Properties of Coconut and Waste Cooking Oils for Biofuel Production and Lubrication. Fuels, 6(3), 57. https://doi.org/10.3390/fuels6030057







