1. Introduction
As it is known, heavy and highly viscous oil accounts for about 80% of global oil resources. As the density of such oils increases, the content of resinous–asphaltene compounds, heteroatoms and metals increases, which complicates their processing by standard methods [
1]. To improve processing efficiency, hydrogenation processes are used, in which polycyclic hydrocarbons and their hydrogenated derivatives, such as tetralin, anthracene and phenanthrene, play an important role. They are able to act as hydrogen donors, which makes them promising compounds for use in hydrogenation processes of heavy hydrocarbon raw materials. The use of model compounds (phenanthrene, anthracene) makes it possible to study the relationship between reactivity and the chemical structure of substances, since they fragmentarily reflect the composition of the organic mass of heavy oils, coal tar and its fractions [
2].
One of the key factors for successful processing of aromatic hydrocarbons is their fragmentation, which is usually carried out through partial hydrogenation of polycyclic aromatic structures with subsequent catalytic decomposition. The effectiveness of these processes is largely determined by the choice of catalysts that not only affect the reaction mechanism but also determine the possibility of its practical implementation [
3,
4,
5,
6,
7]. However, the question of distinguishing the contribution of the chemical structure of hydrocarbon clusters and the characteristics of the catalyst to the reactivity of the compounds is still open.
Phenanthrene, as an important component of coal tar, is of interest as a model compound for studying hydrogenation mechanisms. Its structure with condensed aromatic rings causes high stability and complexity of transformations. The nonlinear structure of phenanthrene makes the hydrogen saturation process difficult, even in the presence of known industrial catalysts. For example, the authors in [
8] showed that the conversion of phenanthrene on zeolite catalysts does not exceed 10–18%, which indicates the need to search for new approaches and catalysts.
Traditionally, platinum-based catalysts are considered the most effective [
9], but their high cost limits industrial use. In this regard, research is underway to create alternative catalysts, among which monometallic and bimetallic systems based on nickel and molybdenum, cobalt and molybdenum, as well as iron and nickel, are promising [
9,
10,
11]. For example, ref. [
12] shows that when using a nickel–molybdenum catalyst based on alumina (NiMo/Al
2O
3), the yield of hydrogenated phenanthrene products reaches 70%. In addition, the possibility of using hydrogenated products of phenanthrene, naphthalene and tetralin as hydrogen donors for the hydrogenation of solid and heavy hydrocarbon systems is being investigated [
13].
The paper [
14] reports on the positive effect of coal shale on coal liquefaction processes: its optimal content in the mixture with coal is 15%, which increases the yield of liquid products by 10%. This is due to the donor capacity of the hydrogen contained in the shale carbon matrix. The presence of donor properties in coal shale is mentioned in [
15,
16], where the authors show that the donor hydrogen activity of the organic mass of shale during thermolysis of the resin exceeds the donor ability of tetralin in this reaction.
Previous studies have demonstrated the use of metal-supported chrysotile as a catalyst in the destructive hydrogenation of phenanthrene and heavy hydrocarbon feedstocks [
17,
18,
19]. Chrysotile, as a silicate carrier with a developed specific surface, can contribute to the activation of hydrogen molecules and increase the selectivity of reactions.
The catalysts used in the study—coal shale of Shubarkol Komir JSC and chrysotile of Kostanay Minerals JSC—are natural materials while being by-products of industrial production. Their use contributes to high economic efficiency, since they do not require recycling, and also ensures environmental feasibility through the rational use of industrial waste.
This study is aimed at studying the reactivity of phenanthrene in destructive hydrogenation processes using coal shale and a chrysotile catalyst modified with nickel and titanium. The selectivity and activity of catalysts in both hydrogenation and degradation processes are also evaluated.
2. Materials and Methods
The phenanthrene model object was selected from Alfa Chemical Co., Ltd. (Zhengzhou, China). In order to determine the composition of phenanthrene and its impurities, the individual chemical composition of phenanthrene was determined. Phenanthrene composition was determined using an Agilent 7890A(Santa Clara, USA) gas chromatograph with an Agilent 5975C (Santa Clara, USA) mass selective detector. The individual chemical composition of phenanthrene is shown in
Table 1.
As a catalyst, we used the coal shale of the deposits of Shubarkol Komir JSC (Karaganda < Kazakhstan) and chrysotile/NiTi (chrysotile (Kostanay Minerals JSC, Zhitikarinskoye deposit, Kazakhstan) with applied Ni and Ti).
The chrysotile/NiTi nanocatalyst was prepared according to the procedure described earlier in the article [
20]: chrysotile was leached with a 20% hydrochloric acid solution. Then, using the impregnation method to the limit of moisture capacity, active metals Ni and Ti were applied to the surface and inner cavities of chrysotile nanotubes with aqueous solutions of nickel (NiNO
3·6H
2O) and titanium (TiOSO
4·2H
2O) salts.
The treated samples were dried at 105 °C, followed by cooling to room temperature [
12]. After cooling, the samples were calcined for a few hours in a muffle furnace at 550 °C.
The coal shale was mechanically crushed using a jaw mill, then screened on SieveShakerOBRK-SA, manufactured by Changzhou Oubeiruike Instrument and Equipment Co., Ltd. (Xi’an, China). As a result, fractions with a particle size of less than 0.1 mm and 0.1 mm were separated, which were subsequently placed in polyethylene bags for storage. The coal shale of Shubarkol Komir JSC was studied with the following characteristics: ash content 64.6 wt%; content, wt%: Cdaf 63.08, Hdaf 7.79, Sdaf 4.68, Ndaf 1.74, Odaf 22.77; molecular formula of coal shale C44H65O12NS.
Temperature-programmable desorption (TPD) of ammonia was carried out on a Micromeritics precision chemisorption AutoChem-2920 (Santa Clara, CA, USA) analyzer in the temperature region of 100–600 °C with a heating rate of the measuring cell with the sample of 10 °C/min. The flow rate through the sample reactor was 30 cm3 (STP)/min.
The specific surface area of the coal shale was determined by the Brunauer–Emmett–Teller (BET) method (TriStar II 3020, Norcross, GA, USA), while its morphological characteristics were analyzed using scanning electron microscopy (SEM, Hitachi S-4800, Tokyo, Japan) and TEM (Joel JEM-1400Plus, Tokyo, Japan).
Experiments on the catalytic hydrogenation of phenanthrene were carried out in a high-pressure reactor made of heat-resistant stainless steel with a volume of 0.01 L with an initial hydrogen pressure of 4.0 MPa, a temperature of 420 °C and a duration of 60 min. The start of the reaction was considered to be the moment when the temperature in the reactor reached 420 °C. The weight of phenanthrene was 1 g, and the weight fraction of catalytic additives was 1% by weight of the starting compound.
Experiments on the catalytic hydrogenation of phenanthrene with pre-reduced catalysts were carried out under similar conditions. Pretreatment reduction in the coal shale and chrysotile loaded with nickel and titanium was performed in a tubular furnace (OTF-1200X-S, Zhengzhou KJ Technology Co., Ltd. (Zhengzhou, China), 2022) at 400 °C, under a gas pressure of 0.02 MPa, for 30 min.
The resulting hydrogenate was determined on a Clarus SQ 8T (Waltham, MA, USA) chromatographic mass spectrometer manufactured by PerkinElmer (Hong Kong, China), 2022.
3. Results and Discussion
As noted above, two catalysts were used to conduct experiments on the phenanthrene hydrogenation process: (1) the prepared chrysotile catalyst with nickel and titanium applied and (2) the coal shale of the Shubarkul Komir JSC deposits.
The chemical composition of chrysotile of Kostanay Minerals JSC and coal shale of Shubarkol Komir JSC is presented in
Table 2. From
Table 2 it follows that the main components of chrysotile include oxides of silicon, magnesium and iron, and coal shale consists of oxides of iron (Fe
2O
3), alumina (Al
2O
3) and titanium oxide (TiO
2).
One effective approach to creating active heterogeneous catalysts is to use naturally occurring mineral compounds with a developed texture, such as chrysotile. However, despite the layered structure and the presence of functional groups, natural chrysotile is characterized by a relatively low density of acid centers, which limits its catalytic activity in a number of acid-catalyzed processes, including the hydrogenation of polycyclic aromatic hydrocarbons (PAH), such as phenanthrene.
In this regard, it is of interest to modify chrysotile with transition metal oxides in order to increase its acidic properties and texture characteristics. The acidity of unmodified chrysotile had to be characterized in detail to justify such modification, as well as to further compare the properties of the modified and starting material.
To assess the acidic properties of coal shale and chrysotile, an analysis of ammonia desorption during programmed temperature increase (TPD-NH
3) was carried out. The results of the experiment are presented in
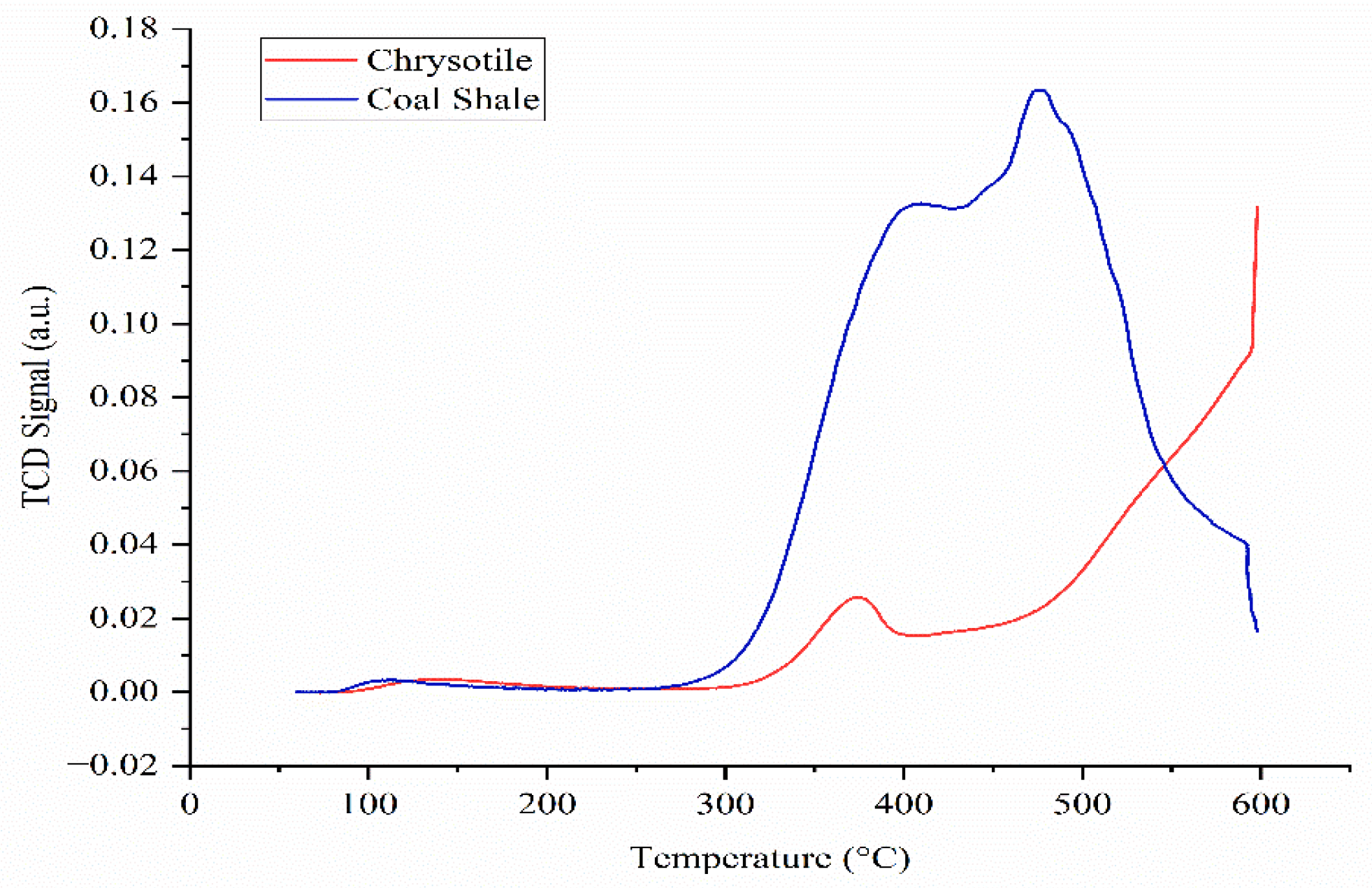
Figure 1 as a function of desorption intensity versus temperature. The TPD-NH
3 method is used to characterize the strength of acid centers of solid acids: desorption of NH
3 from centers of weak acidity occurs at lower temperatures, while desorption from strong acid centers requires higher temperatures.
Figure 1 shows thermo-programmable ammonia desorption curves for coal shale and chrysotile. Both curves show desorption in the temperature range of 100 to 600 °C, indicating the presence of acid centers of different strengths. At the same time, the curve for coal shale is characterized by a significantly higher intensity, especially in the region of 300–500 °C, while desorption for chrysotile occurs mainly at higher temperatures. This indicates the presence of predominantly high-strength acid centers in chrysotile, but in a smaller total amount compared to coal shale.
According to the data obtained, the concentration of acid centers of chrysotile is 3.74 mmol/g, and the specific acidity is 0.15 mmol/m2. Despite the relatively low density of acid centers on the surface, chrysotile exhibits moderate acidic properties, which allow it to participate in acid catalytic reactions, especially after appropriate modification.
In turn, for coal shale, the concentration of acid centers was 12.31 mmol/g, and the specific acidity was 0.66 mmol/m2, which indicates a high density and uniform distribution of active centers. These characteristics indicate the significant catalytic potential of coal shale in acidic reactions, including the hydrogenation of polycyclic aromatic hydrocarbons such as phenanthrene.
The acid and texture characteristics of the modified chrysotile/NiTi catalyst were studied in our previously published work [
21]. According to the data obtained, the modification with nickel and titanium oxides led to a significant increase in acidity—the concentration of acid centers was 267 mmol/g. The increase in acid properties is due to the formation of new acid centers on the surface of chrysotile during the modification process. In addition, in the same study, texture characteristics were determined by the BET method: the surface area of the initial chrysotile was 39.4 m
2/g, and after modification—54 m
2/g, which indicates an improvement in porosity and availability of active centers.
Turning to the analysis of morphology, it should be noted that the structural features of the surface of the catalyst have a significant effect on its catalytic activity. In the present study, coal shale morphology was analyzed using scanning electron microscopy (SEM). As shown in
Figure 2, shale particles have micron and submicron sizes ranging from 0.2 to 0.7 μm. Such a high degree of dispersion contributes to an increase in the active surface and facilitated diffusion of reagents.
Each of the catalysts has its own advantages: chrysotile/NiTi catalyst—developed surface; shale—high availability of active centers. This makes both catalysts promising for further modification and use in heterogeneous catalysis.
Thus, the analysis of the acid and texture characteristics of catalysts allows us to conclude that there are differences in the availability and strength of acid centers, as well as in the morphological organization of the surface, which, in turn, should affect their catalytic behavior.
However, as is known from the literature [
22,
23], high catalytic activity in hydrogenation reactions is due not only to acidity and texture but also to the reducibility of metal oxides.
In this context, the reduction in metal oxides deposited on chrysotile or present in the coal shale ash fraction becomes an important factor. Pre-reduction promotes the formation of reduced metal particles that can effectively activate hydrogen molecules and thereby enhance the hydrogenation capacity of the catalysts.
In order to study the effect of reducing the active components on the catalytic activity, phenanthrene hydrogenation experiments were carried out using initial and pre-reduced catalysts based on chrysotile and coal shale. The results are shown in
Table 3.
As shown in
Table 3, in all cases there is a partial conversion of phenanthrene, the main starting compound, which is reduced from 79.12% (coal shale) to 65.38% (reduced chrysotile/NiTi). The most significant hydrogenation products are tetrahydronaphthalene (naphthalene, 1,2,3,4-tetrahydro-) and 9,10-dihydrofenanthrene, which confirm the implementation of the partial hydrogenation steps of aromatic nuclei.
Catalysts based on pre-reduced chrysotile/NiTi and shale show a significantly higher content of deep hydrogenation products (for example, tetrahydronaphthalene—up to 18.21% for pre-reduced chrysotile/NiTi and 15.72% for pre-reduced coal shale), compared to their non-reduced counterparts (10.36% and 5.94%, respectively).
The appearance of decalin (cis/trans-decalin) and indan in the reaction products is also observed only in the case of reduced catalysts, which further confirms the activation of deeper hydrogenation stages due to the reduction in metal phases.
Thus, pre-reduction in chrysotile and coal shale catalysts leads to a significant increase in their catalytic activity in the phenanthrene hydrogenation reaction. The data obtained demonstrate that the pre-activation of catalysts in a hydrogen atmosphere is an important step for the implementation of an effective hydrogenation effect, which provides a deep transformation of aromatic structures.
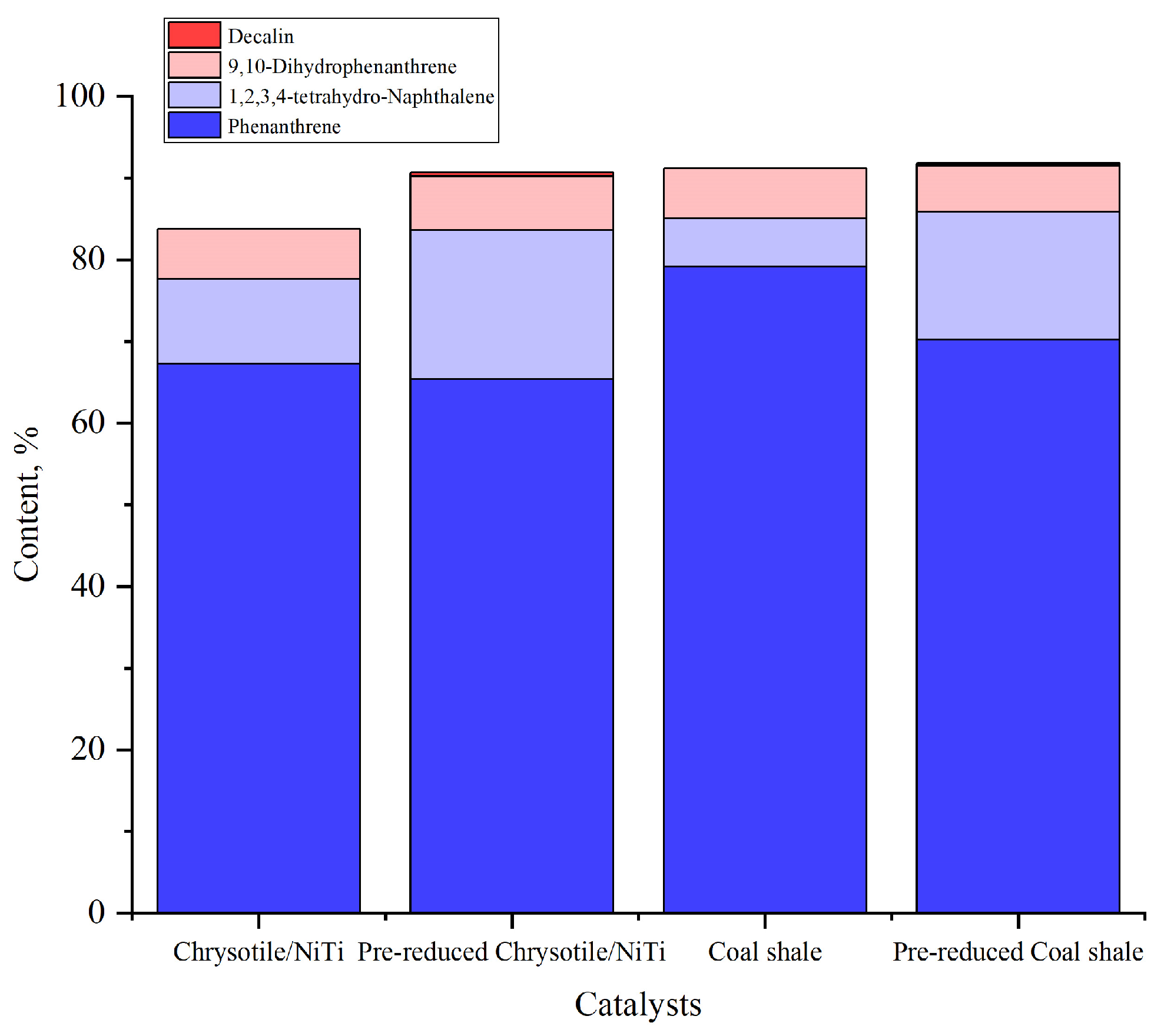
A graph illustrating the fraction of phenanthrene and key products (tetrahydronaphthalene, dihydrophenanthrene, and decalin) versus catalyst type is provided for a visual comparison of catalyst activity (
Figure 3).
Analysis of the plotted data showed a relationship between the acidity of the catalysts and their selectivity to the partial phenanthrene hydrogenation products. The catalyst with a higher concentration of acid centers, chrysotile/NiTi, provides the formation of more saturated products, such as tetrahydronaphthalene (up to 10.36%), while coal shale, with a concentration of acid centers of 12.31 mmol/g, shows less selectivity (5.94% tetrahydronaphthalene). Modification of chrysotile with the addition of nickel and titanium improves its activity in hydrogenation processes, which explains the higher selectivity of chrysotile/NiTi compared to coal shale. These differences in the acidic properties of the catalysts explain the higher efficiency of modified chrysotile in the phenanthrene hydrogenation reaction.
The next stage of our research was to establish the donor capacity of the organic mass and the catalytic effect of the mineral part of coal shale under conditions that simulate thermocatalytic processes that occur without an external source of hydrogen.
To this end, experiments were carried out on the thermolysis of phenanthrene in an inert gas (nitrogen) atmosphere with the addition of coal shale. For comparison, the reaction in a hydrogen atmosphere was also investigated. The data obtained are presented in
Table 4.
Analysis of
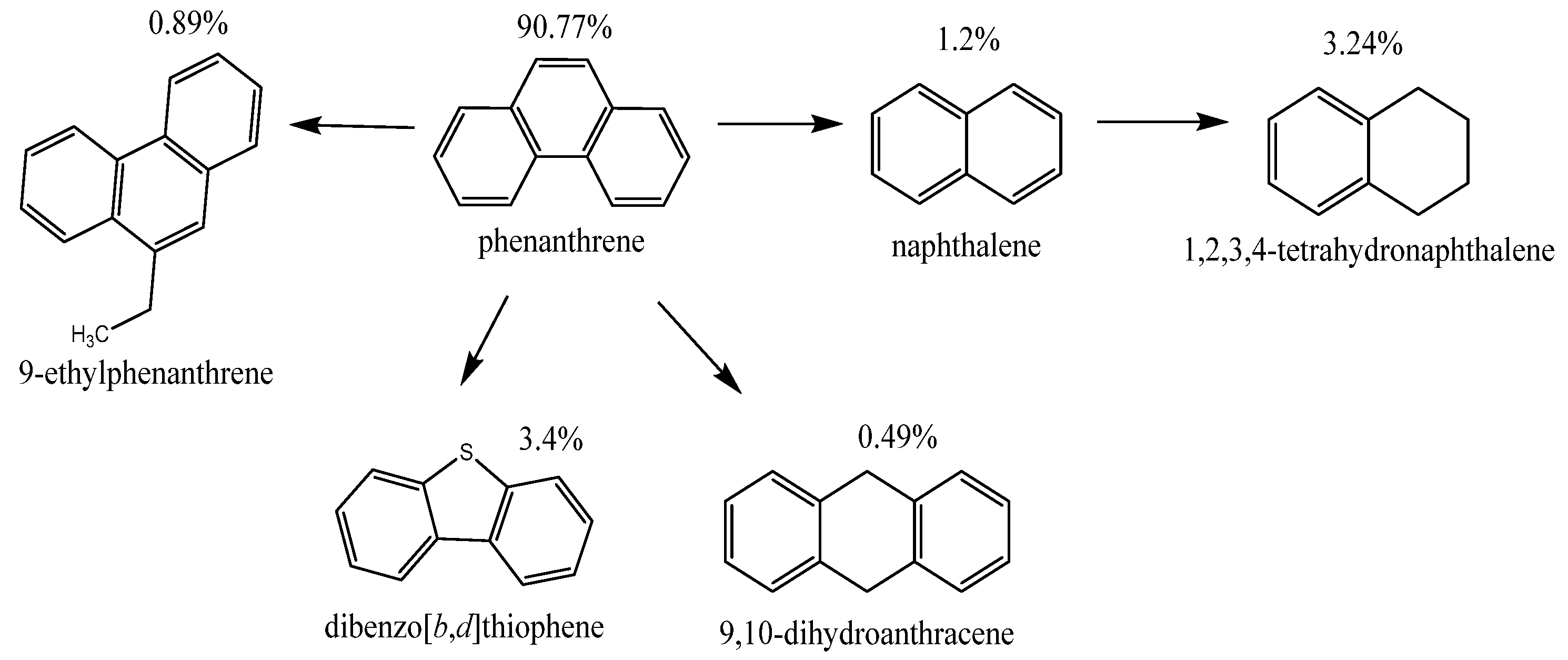
Table 4 shows that under conditions of catalytic thermolysis in N₂ medium, the formation of several products of partial conversion of phenanthrene is observed, among which the main ones are tetralin (3.24%)—the product of partial hydrogenation of the aromatic ring; naphthalene (1.2%)—the product of dehydrocyclization or aromatization; 9-ethylphenanthrene (0.89%); and 9,10-dihydroanthracene (0.49%)—isomerization and partial saturation products of the aromatic ring.
The conversion of phenanthrene in a nitrogen atmosphere was about 9.2%, which confirms the limited but clearly pronounced reaction even in the absence of an external source of hydrogen. These data indicate that coal shale is capable of performing a donor function, probably due to hydrogen released from organic fragments of its composition during thermolysis.
A significantly higher conversion is observed when the reaction is carried out in an atmosphere of molecular hydrogen (H2). Under these conditions, the yield of the main hydrogenation products, tetrahydronaphthalene (5.94%) and dihydrophenanthrene (6.11%), increases significantly, and the content of the starting phenanthrene decreases to 79.12%. The appearance of additional products, such as butylbenzene, methylindane and naphthalene-type dehydrated compounds, indicates the active progress of saturation and structural rearrangement.
The conversion of phenanthrene in hydrogen increases to ≈21%, which confirms the key role of active hydrogen in the reaction process. An increase in the yield of saturated compounds in a hydrogen atmosphere allows us to conclude that coal shale exhibits both donor and catalytic activity: it not only contributes to the release of hydrogen from its own structure but is also able to activate molecular hydrogen, increasing the conversion of phenanthrene.
According to the literature data [
23], during the thermolysis of phenanthrene in an inert atmosphere, its conversion is 7%. The addition of hydrogen and a catalytic additive leads to an increase in conversion to 28%, and the addition of tetralin as a hydrogen donor contributes to a further increase in conversion to 36%. These data confirm the importance of the presence of an active hydrogen source to increase the efficiency of the process. The results of the thermolysis of phenanthrene in an inert atmosphere reported in the literature are consistent with the data obtained in
Table 4.
Thus, in an inert gas atmosphere (N2), coal shale provides limited but appreciable phenanthrene conversion, confirming the presence of an internal hydrogen source. Under hydrogenation conditions in a hydrogen (H2) atmosphere, coal shale acts as both a hydrogen donor and a catalyst, activating molecular hydrogen and converting it to atomic hydrogen, which significantly stimulates the conversion of phenanthrene to hydrogenated products.
To visualize the main pathways of phenanthrene transformation during hydrogenation, reaction schemes were developed. These schemes are based on phenanthrene hydrogenation products obtained using the coal shale catalyst (
Table 4).
In a nitrogen (N
2) atmosphere, isomerization of phenanthrene to anthracene can compete with hydrogenation reactions. With a lack of active hydrogen, some of the molecules remain in a stable aromatic form, which contributes to the formation of the phenanthrene isomer—anthracene, which we can observe in
Figure 4. In addition to the phenanthrene isomerization product, we observe the product of radical ethylation and aromatization—ethyl phenanthrene.
The conversion of the phenanthrene to naphthalene and tetrahydronaphthalene is a multi-step process involving sequential saturation of aromatic rings to form intermediates. According to the literature data [
24], the hydrogenation of the phenanthrene to naphthalene on a Ni-Mo/HY catalyst occurs through the octahydrophenanthrene step, followed by the formation of 5,6-butyltetrahydronaphthalene, which is further transformed into naphthalene and tetralin. These reactions take place under the influence of acidic centers of zeolite HY, which promote the activation of carbon bonds, as well as metallic Ni-Mo centers that provide effective hydrogenation and structural rearrangement of the molecule. Coal shale, having acidic properties similar to natural zeolite, is able to catalyze the conversion of phenanthrene to naphthalene and tetrahydronaphthalene even in an inert medium, which confirms its catalytic activity and donor abilities.
Dibenzothiophene, a component of phenanthrene, is conserved in the products of the catalytic thermolysis of the phenanthrene.
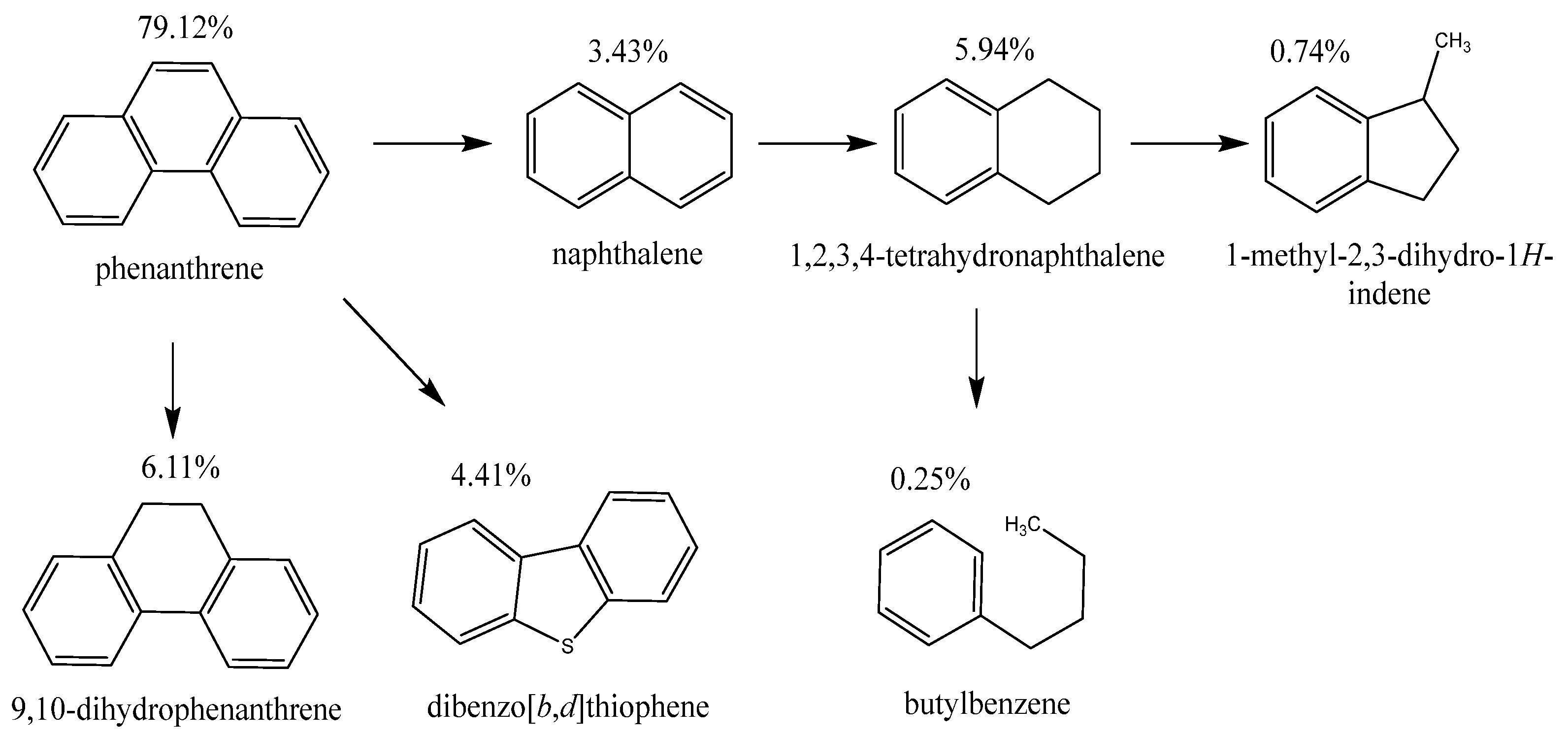
Figure 5 depicts the hydrogenation scheme of the phenanthrene with the coal shale in a hydrogen atmosphere. According to this scheme, the first stage of hydrogenation involves the partial saturation of one of phenanthrene’s aromatic rings, resulting in the formation of 9,10-dihydrophenanthrene. The formation of 9,10-dihydrophenanthrene signifies the initial step in the breakdown of the aromatic system.
In addition to the hydrogenated phenanthrene derivatives, bipolycyclic compounds are formed, such as naphthalene, which is then hydrogenated to naphthalene, 1,2,3,4-tetrahydro-, followed by indan, 1-methyl- and benzene, butyl-. Experimental data show that the content of naphthalene and tetrahydronaphthalene in a hydrogen medium when using the coal shale catalyst is doubled compared to the reaction in a nitrogen medium. In the presence of hydrogen, the coal shale catalyst exhibits higher activity, promoting not only saturation of aromatic rings but also subsequent redistribution reactions.
The scheme of phenanthrene hydrogenation in pre-reduced coal shale has common features with the scheme of phenanthrene reduction in hydrogen medium (
Figure 6).
However, the appearance of trans-decalin indicates a deeper hydrogenation of the phenanthrene on the pre-reduced coal shale. The scheme also illustrates the formation of alkyl-substituted compounds (e.g., phenanthrene, 4,5-dimethyl-, and ethylphenanthrene). This suggests the possibility of alkylation and the ability of the coal shale to activate hydrocarbon side chains.
Thus, chrysotile/NiTi and the coal shale exhibit catalytic properties for the complex hydrogenation processes of the phenanthrene, including the hydrogenolysis of aromatic systems, isomerization, and the formation of new hydrocarbon structures.