Heavy Fuel Oil Quality Dependence on Blend Composition, Hydrocracker Conversion, and Petroleum Basket
Abstract
1. Introduction
2. Materials and Methods
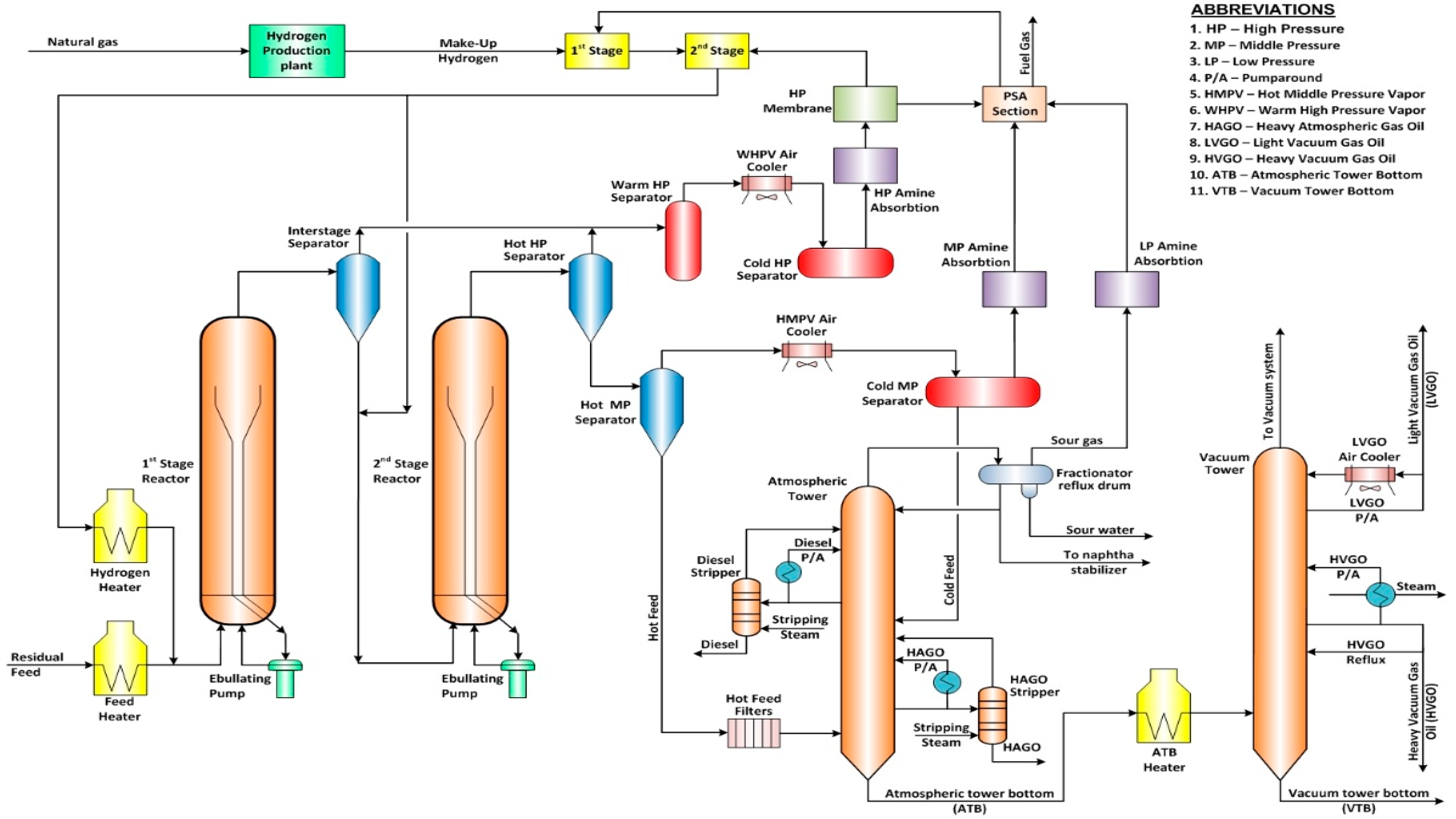
2.1. Experimental Materials
2.2. Experimental Methods
3. Results and Discussion
3.1. Investigation of Finished Heavy Fuel Oil Characteristics
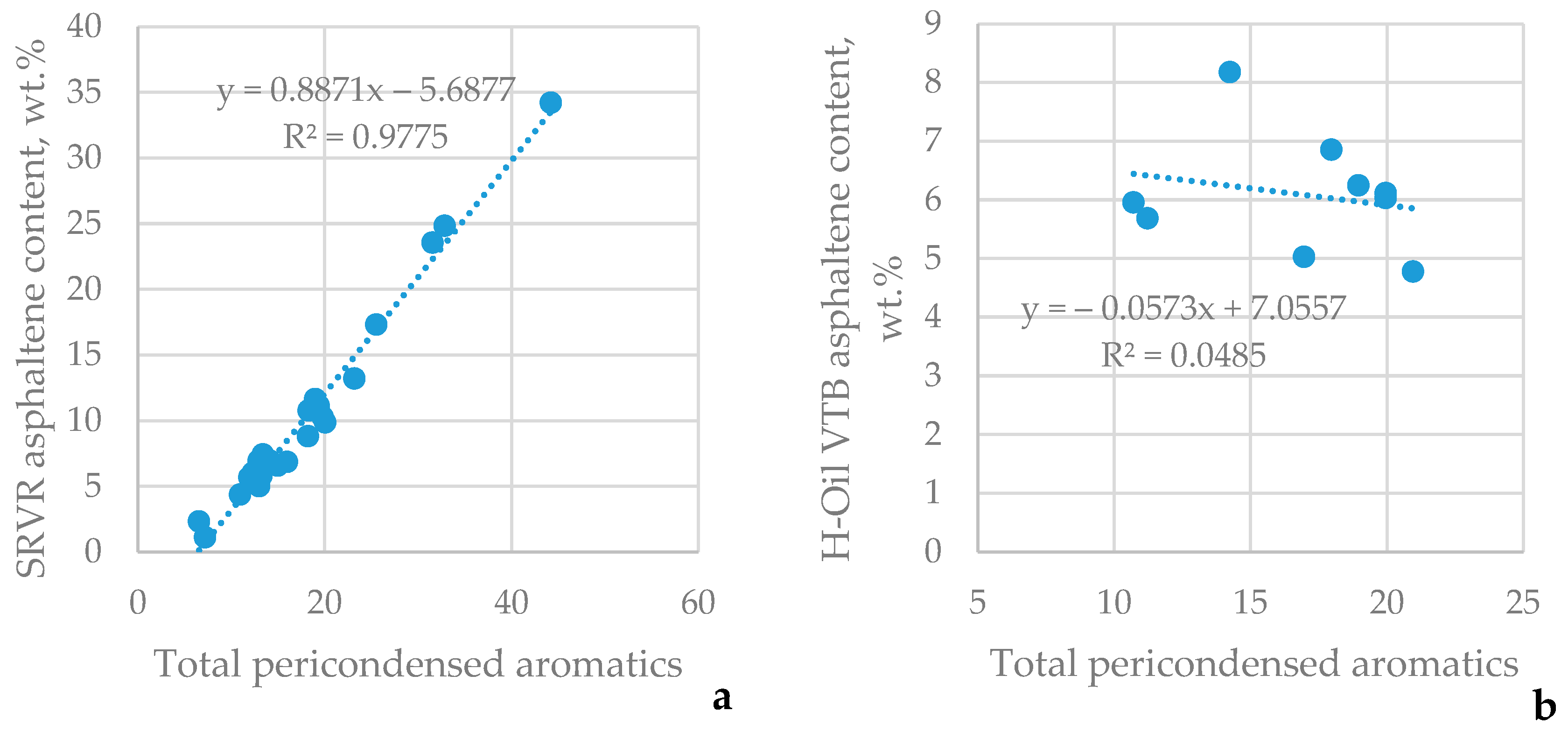
3.2. Contrasting Straight-Run Vacuum Residue Properties Against the Properties of Hydrocracked Vacuum Residues H–Oil VTBs

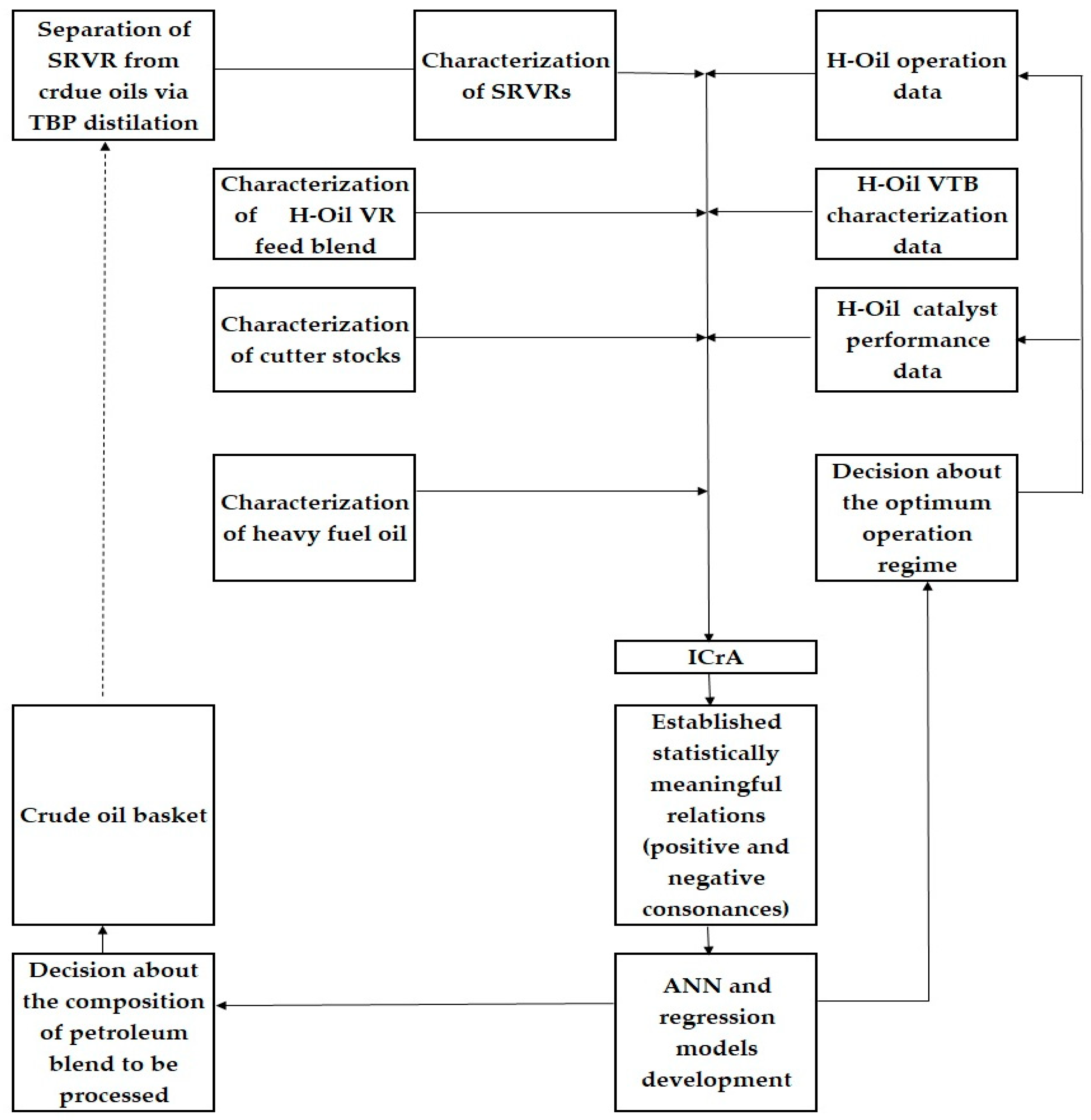
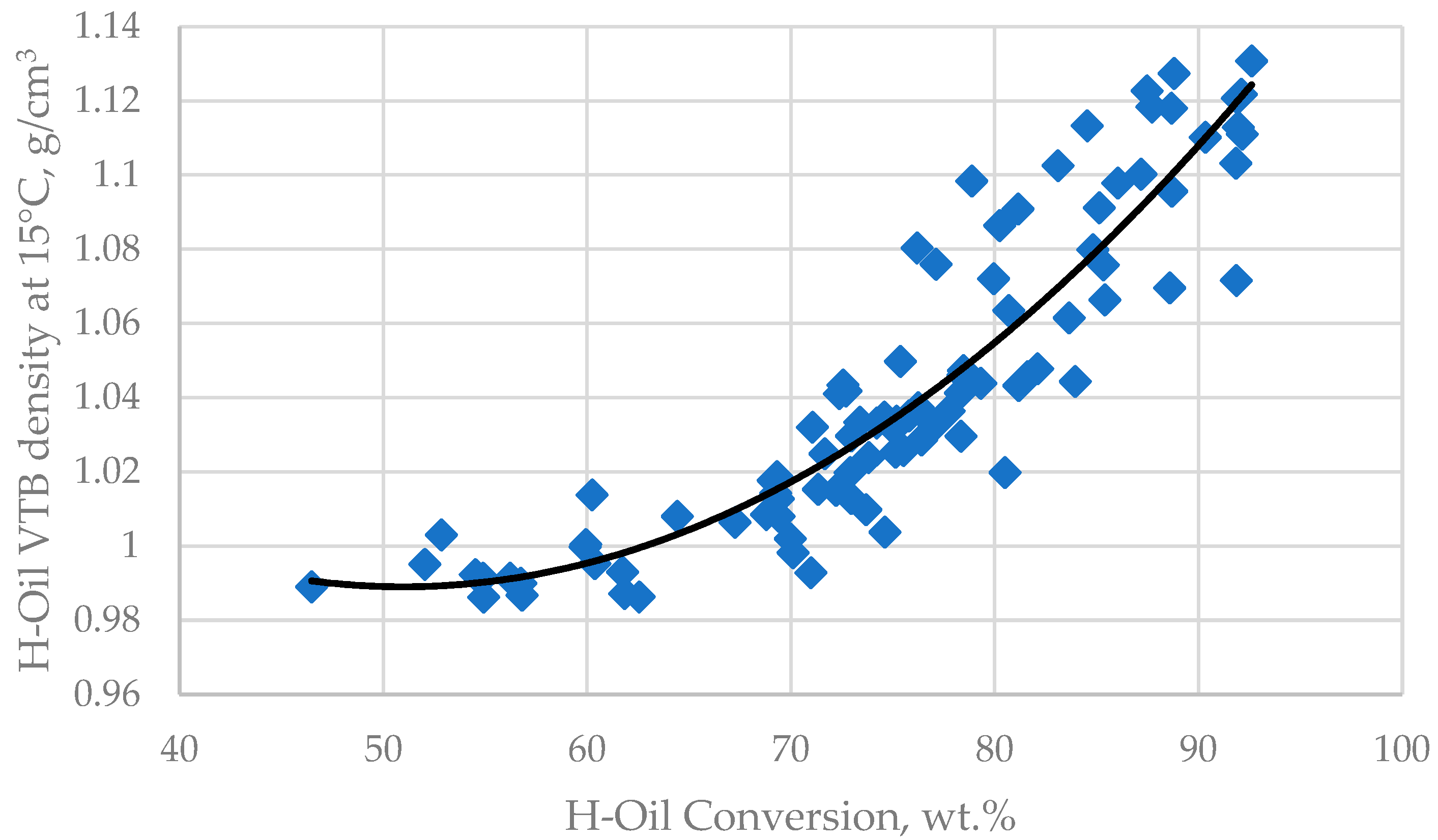
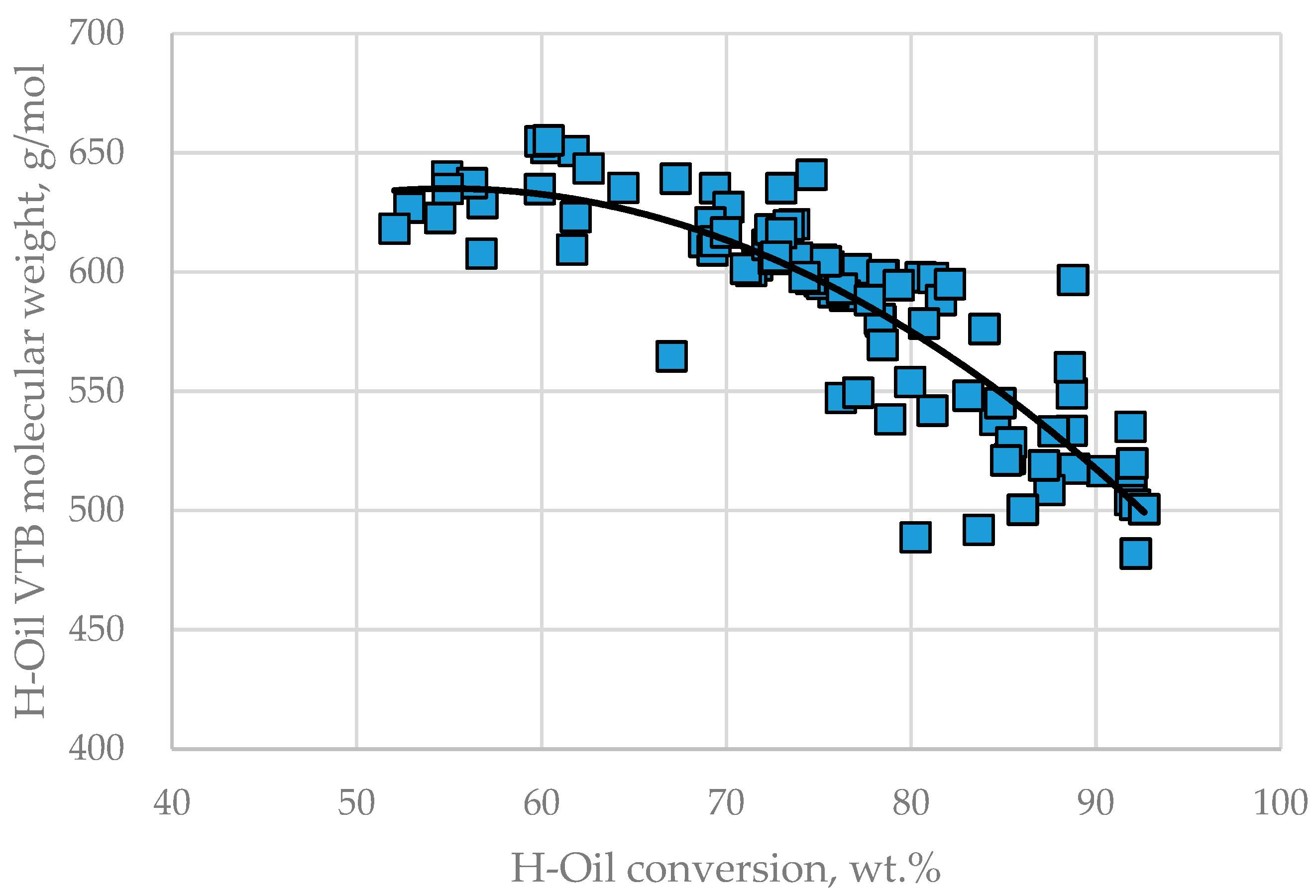
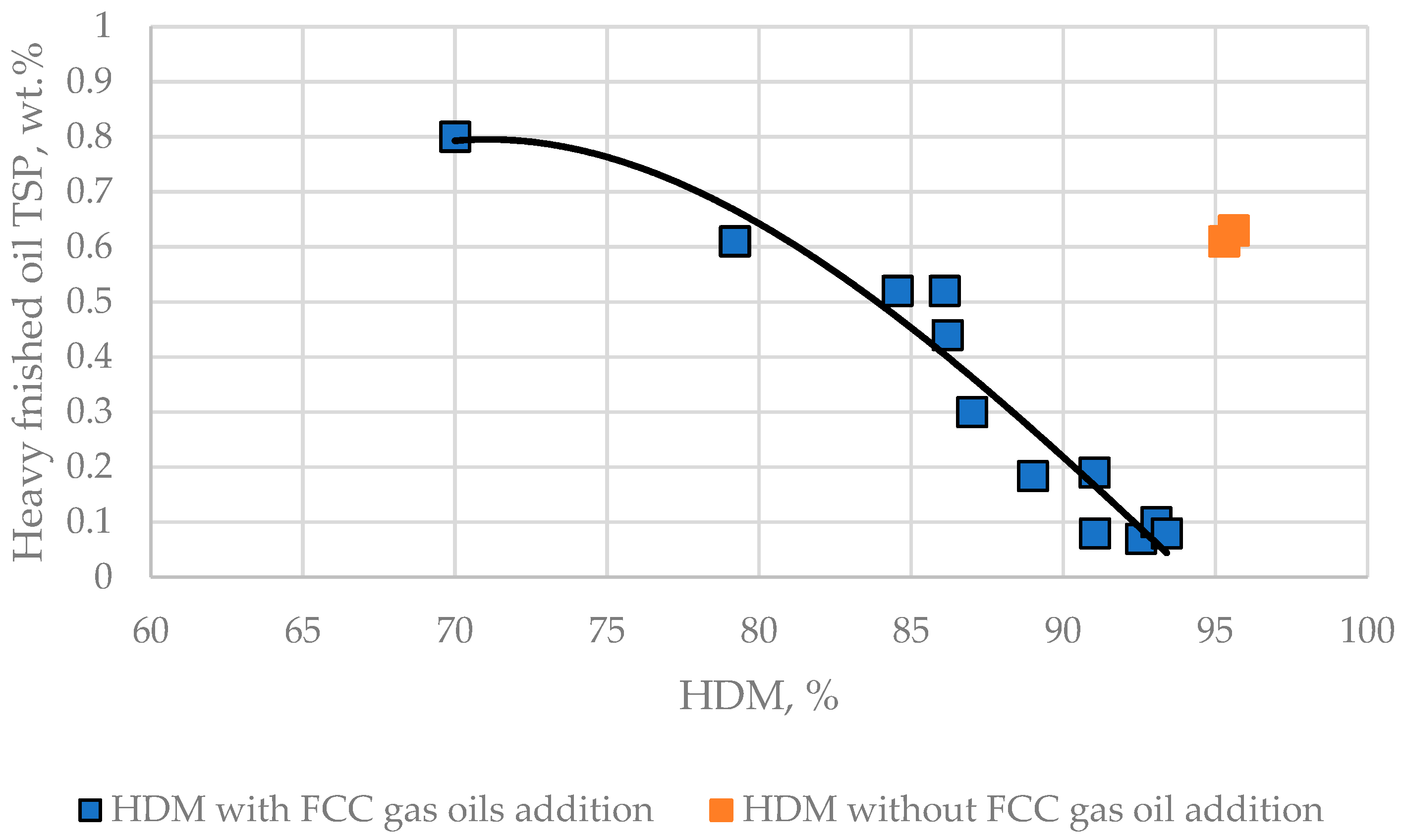
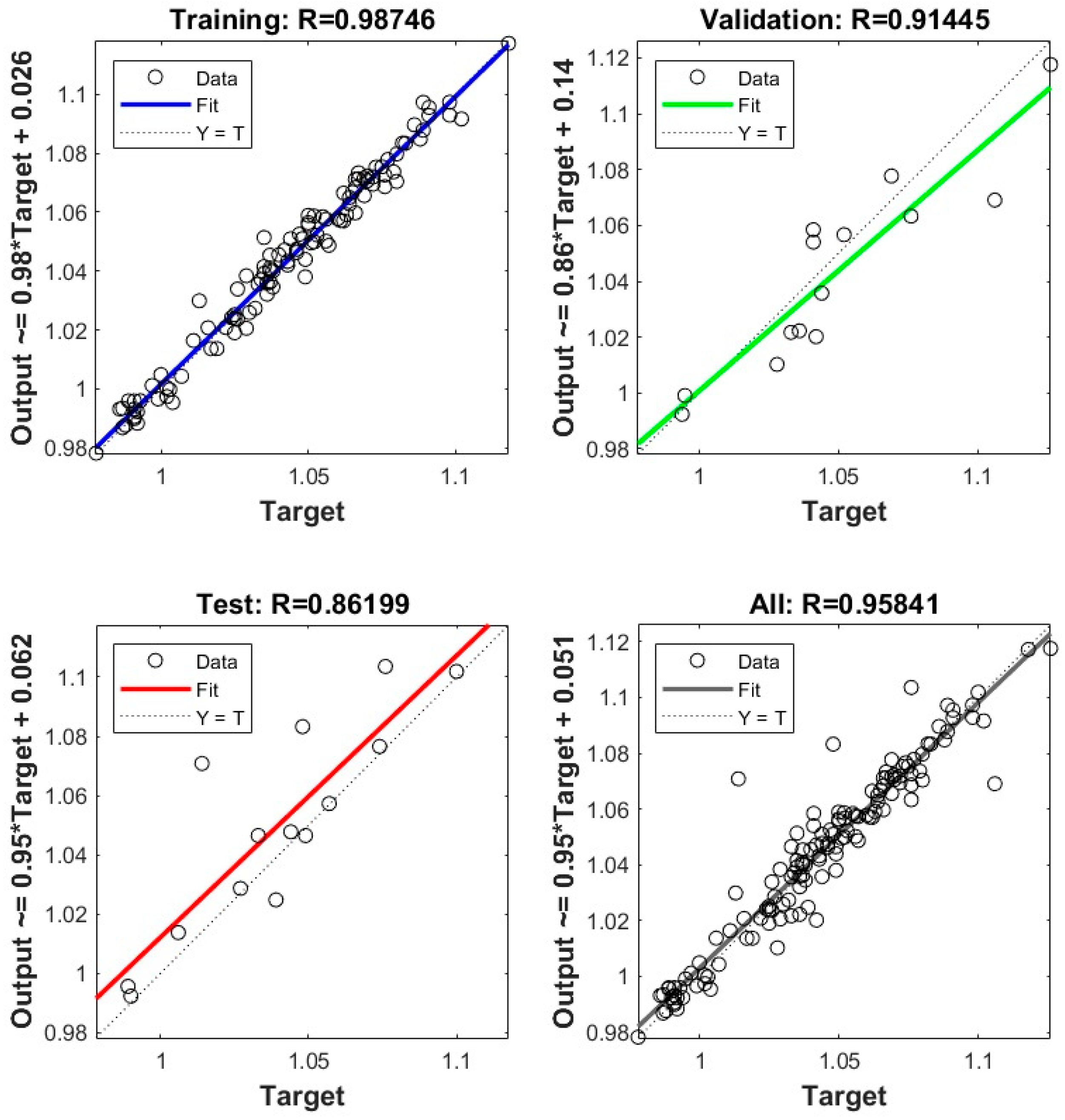
3.3. Relation of Operation Conditions, Vacuum Residue Feed Blend Characteristics of the H–Oil Hydrocracker to the VTB and PBFO Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| %AAD | Average absolute relative deviation, % |
| ∆T R-1 | ∆T of the first reactor, °C |
| ∆T R-1 | ∆T of the first reactor, °C |
| AGFU | Absorption gas fractionation unit |
| Al + Si | Contents of alumina and silicon |
| ANN | Artificial neural network |
| Asp | Asphaltenes content, wt.% |
| ATB | Atmospheric tower bottom |
| C, wt.% | Carbon content, wt.% |
| C5-asp | Content of asphaltenes insoluble in n-pentane, wt.% |
| C7-asp | Content of asphaltenes insoluble in n-heptane, wt.% |
| CAR | Catalyst addition rate, kg/t |
| CCR | Conradson carbon residue |
| CDU | Crude distillation unit |
| CGFU | Central gas fractionation unit |
| CN | Cracked Naphtha |
| Crude KV | Crude oil kinematic viscosity, mm2/s |
| D15 | Density at 15 °C |
| DE | Differential evolution |
| FBP | Final boiling point, °C |
| FCC | Fluid catalytic cracking |
| FCC-PT | Fluid catalytic cracking feed pretreater |
| Fe, ppm | Iron content, ppm |
| FG Storage | Fuel gas storage |
| FPCC | Flash point closed cup, °C |
| FPOC | Flash point open cup, °C |
| H, wt.% | Hydrogen content, wt.% |
| H/C ratio | Hydrogen to carbon atomic ratio |
| H2O | Water content, wt.% |
| HCKVGO | Hydrocracked VGO |
| HCO | Heavy cycle oil |
| HDAs | Hydrodeasphaltization |
| HDM | Hydrodemetallization |
| HDS | Hydrodesulfurization |
| Heat | Specific heat of combustion/lower |
| HFO | Heavy fuel oil |
| HN | Heavy Naphtha |
| H–oil Conv | H–oil conversion |
| HPU | Hydrogen production unit |
| HTD | Hydrotreated Diesel |
| HTK | Hydrotreated Kerosene |
| HTN | Hydrotreated Naphtha |
| HTSD | High temperature simulation distillation |
| HTVGO | Hydrotreated vacuum gas oil |
| IBP | Initial boiling point, °C |
| ICrA | Intercriteria analysis |
| Imp | Mechanical impurities content, wt.% |
| KERO | Kerosene |
| Kw-factor | The Watson characterization factor |
| LCO | Light cycle oil |
| LHSV | Liquid hourly space velocity, h-1 |
| LN | Light Naphtha |
| LPG | Liquified petroleum gas |
| LSM | Least squares method |
| MCR | Microcarbon residue content, wt.% |
| MD | Molecular dynamics |
| MTBE | Methyl tert-butyl ether |
| MW | Molecular weight, g/mol |
| N, wt.% | Nitrogen content, wt.% |
| Na, ppm | Sodium content, ppm |
| Nc | Number of carbon atoms in the average molecule of fuel |
| NG | Natural gas |
| Ni, ppm | Nickel content, ppm |
| NOxs | Nitrogen oxides |
| PBFO | Partially blended fuel oil |
| PM | Particulate matter |
| PP | Pour point, °C |
| Rec. | Content of recycle of PBFO in H–oil feed, wt.% |
| RMFs | Residue marine fuels |
| S, wt.% | Sulfur content, wt.% |
| Sa | Asphaltene solubility |
| SARA | Saturates, aromatics, resins, asphaltenes |
| SAR-AD | Automated asphaltene determinator coupled with saturates, aromatics, and resins |
| SLO | Slurry oil |
| So | Peptizing power of the maltene fraction |
| SOxs | Sulfur oxides |
| SRAR | Straight-run atmospheric residue |
| SRVRs | Straight-run vacuum residues |
| S-value | Intrinsic stability of an oil |
| T50% | Boiling point at 50% of distilled volume, °C |
| TBP | True boiling point |
| TSA | Total sediment accelerated, wt.% |
| TSE | Total sediments existent, wt.% |
| TSP | Total sediments potential, wt.% |
| ULSD | Ultra-low sulfur diesel |
| UNIFAC model | Universal quasichemical model |
| V, ppm | Vanadium content, ppm |
| VDU | Vacuum distillation unit |
| VGO | Vacuum gas oil |
| VIS | Specific viscosity, °E |
| VLSFO | Very-low-sulfur residual marine fuel |
| VR | Vacuum residue |
| VR Aro, wt.% | Vacuum residue aromatic content, wt.% |
| VR Res, wt.% | Vacuum residue resins content, wt.% |
| VR Sat, wt.% | Vacuum residue saturates content, wt. % |
| VR Soft Point | Vacuum residue softening point, °C |
| VR Sp Gravity | Vacuum residue specific gravity |
| VR Sp. VIS | Vacuum residue specific viscosity, °E |
| VTB | Vacuum tower bottom |
| WABT | Weight average bed temperature, °C |
| WCO | Waste cooking oil |
| XE | Xylene equivalent |
References
- Li, Q.; Li, Q.; Cao, H.; Wu, J.; Wang, F.; Wang, Y. The Crack Propagation Behaviour of CO2 Fracturing Fluid in Unconventional Low Permeability Reservoirs: Factor Analysis and Mechanism Revelation. Processes 2025, 13, 159. [Google Scholar] [CrossRef]
- Yatimi, Y.; Mendil, J.; Marafi, M.; Alalou, A.; Al-Dahhan, M.H. Advancement in Heavy Oil Upgrading and Sustainable Exploration Emerging Technologies. Arab. J. Chem. 2024, 17, 105610. [Google Scholar] [CrossRef]
- Bilgili, L. A Systematic Review on the Acceptance of Alternative Marine Fuels. Renew. Sust. Energ. Rev. 2023, 182, 113367. [Google Scholar] [CrossRef]
- Bendl, J.; Saraji-Bozorgzad, M.R.; Käfer, U.; Padoan, S.; Mudan, A.; Etzien, U.; Giocastro, B.; Schade, J.; Jeong, S.; Kuhn, E.; et al. How Do Different Marine Engine Fuels and Wet Scrubbing Affect Gaseous Air Pollutants and Ozone Formation Potential from Ship Emissions? Environ. Res. 2024, 260, 119609. [Google Scholar] [CrossRef]
- Javad Ziabakhsh Ganji, M.; Ghassemi, H.; Reza Goodarzi, M. Heavy Fuel Oil Droplets: Transient Modeling of Heating to Pyrolysis Process. Fuel 2025, 381, 133521. [Google Scholar] [CrossRef]
- Fasih, H.F.; Ghassemi, H.; MazraeShahi, H.K. Experimental Investigation of Heavy Fuel Oil Gasification in an Entrained Flow Gasifier. Fuel 2023, 351, 128955. [Google Scholar] [CrossRef]
- Azimi, A.; Arabkhalaj, A.; Shahsavan Markadeh, R.; Ghassemi, H. Fully Transient Modeling of the Heavy Fuel Oil Droplets Evaporation. Fuel 2018, 230, 52–63. [Google Scholar] [CrossRef]
- Speight, J.G. Visbreaking: A Technology of the Past and the Future. Sci. Iran. 2012, 19, 569–573. [Google Scholar] [CrossRef]
- Aguilar, R.A.; Ancheyta, J. Modeling Coil and Soaker Reactors for Visbreaking. Ind. Eng. Chem. Res. 2016, 55, 912–924. [Google Scholar] [CrossRef]
- Alvarez-Majmutov, A. Exploring the Conversion Limits of Bitumen Visbreaking through a Molecular Reaction Model. Energy Fuels 2023, 37, 12685–12695. [Google Scholar] [CrossRef]
- Marafi, A.; Albazzaz, H.; Rana, M.S. Hydroprocessing of Heavy Residual Oil: Opportunities and Challenges. Catal. Today 2019, 329, 125–134. [Google Scholar] [CrossRef]
- Kao, T.C.; Lin, Y.C.; Yang, H.N.; Tsai, H.Y.; Chen, J.R. Incident Investigation of Hydrogen Explosion and Fire in a Residue Desulfurization Process. J. Loss Prev. Process Ind. 2024, 92, 105458. [Google Scholar] [CrossRef]
- Parkhomchuk, E.V.; Fedotov, K.V.; Lysikov, A.I.; Polukhin, A.V.; Vorobyeva, E.E.; Shamanaeva, I.A.; Sankova, N.N.; Shestakova, D.O.; Reshetnikov, D.M.; Volf, A.V.; et al. Catalytic Hydroprocessing of Oil Residues for Marine Fuel Production. Fuel 2023, 341, 127714. [Google Scholar] [CrossRef]
- Mitkova, M.; Stratiev, D.; Shishkova, I.; Dobrev, D. Thermal and Thermo-Catalytic Processes for Heavy Oil Conversion; Professor Marin Drinov Publishing House of Bulgarian Academy of Sciences: Sofia, Bulgaria, 2017. [Google Scholar]
- Stratiev, D.; Shishkova, I.; Dinkov, R.; Dobrev, D.; Argirov, G.; Yordanov, D. The Synergy between Ebullated Bed Vacuum Residue Hydrocracking and Fluid Catalytic Cracking Processes in Modern Refining—Commercial Experience; Professor Marin Drinov Publishing House of Bulgarian Academy of Sciences: Sofia, Bulgaria, 2022. [Google Scholar]
- Ershov, M.A.; Savelenko, V.D.; Makhmudova, A.E.; Rekhletskaya, E.S.; Makhova, U.A.; Kapustin, V.M.; Mukhina, D.Y.; Abdellatief, T.M.M. Technological Potential Analysis and Vacant Technology Forecasting in Properties and Composition of Low-Sulfur Marine Fuel Oil (VLSFO and ULSFO) Bunkered in Key World Ports. J. Mar. Sci. Eng. 2022, 10, 1828. [Google Scholar] [CrossRef]
- Kumar, K.; Tripathi, D.; Shekhar, I.; Thapliyal, M.; Srivastava, M. Feasibility Study of the Preparation of RFO from Deasphalted Pitch. Mater. Today Proc. 2022, 76, 146–152. [Google Scholar] [CrossRef]
- Gulyaeva, L.A.; Lobashova, M.M.; Mitusova, T.N.; Shmel’kova, O.I.; Khavkin, V.A.; Nikul’shin, P.A. Production of Low -Sulfur Marine Fuel. Chem. Technol. Fuels Oils 2020, 55, 704–711. [Google Scholar] [CrossRef]
- Kondrasheva, N.K.; Rudko, V.A.; Kondrashev, D.O.; Shakleina, V.S.; Smyshlyaeva, K.I.; Konoplin, R.R.; Shaidulina, A.A.; Ivkin, A.S.; Derkunskii, I.O.; Dubovikov, O.A. Application of a Ternary Phase Diagram to Describe the Stability of Residual Marine Fuel. Energy Fuels 2019, 33, 4671–4675. [Google Scholar] [CrossRef]
- Yan, Y.; Prado, G.H.C.; De Klerk, A. Storage Stability of Products from Visbreaking of Oilsands Bitumen. Energy Fuels 2020, 34, 9585–9598. [Google Scholar] [CrossRef]
- Abdellatief, T.M.M.; Ershov, M.A.; Abdelkareem, M.A.; Mustafa, A.; Jamil, F.; Kapustin, V.M.; Makhova, U.A.; Chernysheva, E.A.; Savelenko, V.D.; Klimov, N.A.; et al. A Unifying Methodology for Gasoline-Grade Biofuel from Several Renewable and Sustainable Gasoline Additives. PSEP 2024, 190, 1386–1402. [Google Scholar] [CrossRef]
- Abdellatief, T.M.M.; Ershov, M.A.; Makhmudova, A.E.; Kapustin, V.M.; Makhova, U.A.; Klimov, N.A.; Chernysheva, E.A.; Ali Abdelkareem, M.; Mustafa, A.; Olabi, A.G. Novel Variants Conceptional Technology to Produce Eco-Friendly Sustainable High Octane-Gasoline Biofuel Based on Renewable Gasoline Component. Fuel 2024, 366, 131400. [Google Scholar] [CrossRef]
- Organization of the Petroleum Exporting Countries. 2023 World Oil Outlook 2045; OPEC Secretariat: Vienna, Austria, 2023. [Google Scholar]
- Bilgili, L.; Ölçer, A.I. IMO 2023 Strategy-Where Are We and What’s next? Mar. Policy 2024, 160, 105953. [Google Scholar] [CrossRef]
- Qin, X.; Ji, Y.; Cai, G.; Wang, T.; Du, Y.; Mu, G.; Zhang, J.; Duan, X.; Pu, X.; Han, X.; et al. Molecular Level Simulation and Analysis of Removal of Sulfur, Nitrogen and Carbon Residue in Residual Oil Hydrotreating Process. Chem. Eng. J. 2025, 508, 161176. [Google Scholar] [CrossRef]
- Umana, B.; Zhang, N.; Smith, R. Development of Vacuum Residue Hydrodesulphurization-Hydrocracking Models and Their Integration with Refinery Hydrogen Networks. Ind. Eng. Chem. Res. 2016, 55, 2391–2406. [Google Scholar] [CrossRef]
- Plain, C.; Benazzi, E.; Guillaume, D. Residue Desulphurisation and Conversion. PTQ 2006, Q2, 57–63. [Google Scholar]
- Panariti, N.; Rispoli, G. The First EST Commercial Unit: Achieving the Goal of Residue Conversion. In Proceedings of the 13th International Bottom of the Barrel Conference, Istanbul, Turkey, 13–14 May 2015. [Google Scholar]
- Shishkova, I.; Stratiev, D.; Sotirov, S. Petroleum Chemistry and Processing Investigated by the Use of Intercriteria Analysis; Professor Marin Drinov Publishing House of Bulgarian Academy of Sciences: Sofia, Bulgaria, 2024; pp. 124–141. [Google Scholar]
- Alonso, F.; Ancheyta, J.; Centeno, G.; Marroquín, G.; Rayo, P.; Silva-Rodrigo, R. Effect of Reactor Configuration on the Hydrotreating of Atmospheric Residue. Energy Fuels 2019, 33, 1649–1658. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, Q.A.; Cao, A.N.T.; Ernest, T.; Nguyen, T.B.; Pham, P.T.H.; Nguyen, T.M. Hydrodemetallization of Heavy Oil: Recent Progress, Challenge, and Future Prospects. J. Pet. Sci. Eng. 2022, 216, 110762. [Google Scholar] [CrossRef]
- Sundaram, K.M.; Mukherjee, U.; Baldassari, M. Thermodynamic Model of Sediment Deposition in the LC-FINING Process. Energy Fuels 2008, 22, 3226–3236. [Google Scholar] [CrossRef]
- Chabot, J.; Shiflett, W. Residuum Hydrocracking: Chemistry and Catalysis. PTQ 2019, Q3, 1–9. [Google Scholar]
- Kuzmin, K.A.; Sultanbekov, R.R.; Khromova, S.M.; Vovk, M.A.; Rudko, V.A. Establishing the Influence of Recycled Used Oil on the Sedimentation Stability of Residual Marine Fuel. Fuel 2025, 389, 134625. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Islamov, S.; Mardashov, D.; Beloglazov, I.; Hemmingsen, T. Research of the Influence of Marine Residual Fuel Composition on Sedimentation Due to Incompatibility. J. Mar. Sci. Eng. 2021, 9, 1067. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Beloglazov, I.; Islamov, S.; Ong, M.C. Exploring of the Incompatibility of Marine Residual Fuel: A Case Study Using Machine Learning Methods. Energies 2021, 14, 8422. [Google Scholar] [CrossRef]
- Vráblík, A.; Schlehöfer, D.; Dlasková Jaklová, K.; Hidalgo Herrador, J.M.; Černý, R. Comparative Study of Light Cycle Oil and Naphthalene as an Adequate Additive to Improve the Stability of Marine Fuels. ACS Omega 2022, 7, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Efimov, I.; Smyshlyaeva, K.I.; Povarov, V.G.; Buzyreva, E.D.; Zhitkov, N.V.; Vovk, M.A.; Rudko, V.A. UNIFAC Residual Marine Fuels Stability Prediction from NMR and Elemental Analysis of SARA Components. Fuel 2023, 352, 129014. [Google Scholar] [CrossRef]
- Smyshlyaeva, K.I.; Rudko, V.A.; Kuzmin, K.A.; Povarov, V.G. Asphaltene Genesis Influence on the Low-Sulfur Residual Marine Fuel Sedimentation Stability. Fuel 2022, 328, 125291. [Google Scholar] [CrossRef]
- Mitusova, T.N.; Kondrasheva, N.K.; Lobashova, M.M.; Ershov, M.A.; Rudko, V.A.; Titarenko, M.A. Determination and Improvement of Stability of High-Viscosity Marine Fuels. Chem. Technol. Fuels Oils 2018, 53, 842–845. [Google Scholar] [CrossRef]
- Vermeire, M.; Heyberger, B. Report no.11/19 Study to Evaluate Test Methods to Assess the Stability and Compatibility of Marine Fuels in View of the IMO MARPOL Annex VI Regulation 14.1.3 for 2020 Sulphur Requirements. 2019. Available online: https://www.concawe.eu/wp-content/uploads/Rpt_19-11.pdf (accessed on 10 April 2025).
- ASTM D7157; Standard Test Method for Determination of Intrinsic Stability of Asphaltene-Containing Residues, Heavy Fuel Oils, and Crude Oils (n-Heptane Phase Separation; Optical Detection). ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D7112-24; Standard Test Method for Determining Stability and Compatibility of Heavy Fuel Oils and Crude Oils by Heavy Fuel Oil Stability Analyzer (Optical Detection). ASTM: West Conshohocken, PA, USA, 2024.
- ASTM D7060−20; Standard Test Method for Determination of the Maximum Flocculation Ratio and Peptizing Power in Residual and Heavy Fuel Oils (Optical Detection Method). ASTM: West Conshohocken, PA, USA, 2020.
- Zhou, D.; Wei, H.; Xue, S.; Qiu, Y.; Wu, S.; Yu, H. Investigating the Compatibility of Various Components in Marine Low-Sulfur Fuel Oil by Molecular Dynamics Simulations. Hindawi J. Chem. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Acosta-López, J.G.; de Lasa, H. Artificial Intelligence for Hybrid Modeling in Fluid Catalytic Cracking (FCC). Processes 2024, 12, 61. [Google Scholar] [CrossRef]
- Ghosh, D.; Moreira, J.; Mhaskar, P. Application of data-driven modeling approaches to industrial hy-droprocessing units. Chem. Eng. Res. Des. 2022, 177, 123–135. [Google Scholar] [CrossRef]
- Iplik, E.; Aslanidou, I.; Kyprianidis, K. Hydrocracking: A Perspective towards Digitalization. Sustainability 2020, 12, 7058. [Google Scholar] [CrossRef]
- Elkamel, A.; Al-Ajmi, A.; Fahim, M. Modeling the hydrocracking process using artificial neural networks. Pet. Sci. Technol. 1999, 17, 931–954. [Google Scholar] [CrossRef]
- Al-Zaidi, B.Y.; Al-Shathr, A.; Shehab, A.K.; Shakor, Z.M.; Majdi, H.S.; AbdulRazak, A.A.; McGregor, J. Hydroisomerisa-tion and Hydrocracking of n-Heptane: Modelling and Optimisation Using a Hybrid Artificial Neural Network–Genetic Algorithm (ANN–GA). Catalysts 2023, 13, 1125. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, H.; Jeon, G.; Kim, Y. Neural network models for atmospheric residue desulfurization using real plant data with novel training strategies. Comput. Chem. Eng. 2023, 177, 108333. [Google Scholar] [CrossRef]
- Song, W.; Mahalec, V.; Long, J.; Yang, M.; Qian, F. Modeling the Hydrocracking Process with Deep Neural Networks. Ind. Eng. Chem. Res. 2020, 59, 3077–3090. [Google Scholar] [CrossRef]
- Zheng, Q.; Fan, Y.; Zhou, Z.; Jiang, H.; Zhou, X. Research on Product Yield Prediction and Benefit of Tuning Diesel Hy-drogenation Conversion Device Based on Data-Driven System. Energies 2023, 16, 5332. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, N.; Wang, H.; Ping, L. Soft chemical analyzer development using adaptive least-squares support vector re-gression with selective pruning and variable moving window size. Ind. Eng. Chem. Res. 2009, 48, 5731–5741. [Google Scholar] [CrossRef]
- Shokri, S.; Marvast, M.A.; Sadeghi, M.T.; Narasimhan, S. Combination of data rectification techniques and soft sensor model for robust prediction of sulfur content in HDS process. J. Taiwan Inst. Chem. E 2016, 58, 117–126. [Google Scholar] [CrossRef]
- Li, X.; Chan, C.W.; Nguyen, H.H. Application of the Neural Decision Tree approach for prediction of petroleum pro-duction. J. Pet. Sci. Eng. 2013, 104, 11–16. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, K.; Yuan, X.; Cao, Y.; Li, L.; Koivo, H.N. A novel sliding window PCA-IPF based steady-state detection framework and its industrial application. IEEE Access 2018, 6, 20995–21004. [Google Scholar] [CrossRef]
- Li, Z.; Qin, K.; Zhang, Y.; Yang, P.; Lou, Y.; Li, M. PSO-Optimized Data-Driven and Mechanism Hybrid Model to Enhance Prediction of Industrial Hydrocracking Product Yields Under Data Constraints. Processes 2025, 13, 1118. [Google Scholar] [CrossRef]
- ASTM D2892–24; Standard Test Method for Distillation of Crude Petroleum (15-Theoretical Plate Column). ASTM: West Conshohocken, PA, USA, 2024.
- ASTM D5236–23; Standard Test Method for Distillation of Heavy Hydrocarbon Mixtures (Vacuum Potstill Method). ASTM: West Conshohocken, PA, USA, 2023.
- BDS EN ISO 3675:2004; Crude petroleum and liquid petroleum products—Laboratory determination of density - Hydrometer method. Bulgarian Institute for Standardization: Sofia, Bulgaria, 2004.
- ASTM D4294-24; Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry. ASTM: West Conshohocken, PA, USA, 2024.
- ASTM D6560-22; Standard Test Method for Determination of Asphaltenes (Heptane Insolubles) in Crude Petroleum and Petroleum Products. ASTM: West Conshohocken, PA, USA, 2022.
- EN ISO 10370-14; Petroleum products—Determination of carbon residue—Micro method. ISO: Geneva, Switzerland, 2014.
- ASTM D1665-20; Standard Test Method for Engler Specific Viscosity of Tar Products. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM D5291-21; Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Petroleum Products and Lubricants. ASTM: West Conshohocken, PA, USA, 2021.
- IP 501-19; Determination of Aluminium, Silicon, Vanadium, Nickel, Iron, Sodium, Calcium, Zinc and Phosphorus in Residual Fuel Oil by Ashing, Fusion and Inductively Coupled Plasma Emission Spectrometry. EI: London, UK, 2019.
- ASTM D7169-23; Standard Test Method for Boiling Point Distribution of Samples with Residues Such as Crude Oils and Atmospheric and Vacuum Residues by High Temperature Gas Chromatography. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM D95-23e1; Standard Test Method for Water in Petroleum Products and Bituminous Materials by Distillation. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM D473-22; Standard Test Method for Sediment in Crude Oils and Fuel Oils by the Extraction Method. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D92-24; Standard Test Method for Flash and Fire Points by Cleveland Open Cup Tester. ASTM: West Conshohocken, PA, USA, 2024.
- ASTM D93-20; Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM D97-17b(2022); Standard Test Method for Pour Point of Petroleum Products. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D482-19; Standard Test Method for Ash from Petroleum Products. ASTM: West Conshohocken, PA, USA, 2019.
- ASTM D4809-18; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter (Precision Method). ASTM: West Conshohocken, PA, USA, 2018.
- IP 375; Petroleum Products—Total Sediment in Residual Fuel Oils—Part 1: Determination by Hot Filtration. EI: London, UK, 2021.
- IP 390-17; Petroleum Products—Total Sediment in Residual Fuel Oils—Part 2: Determination using Standard Procedures for Ageing. EI: London, UK.
- Atanassov, K.; Mavrov, D.; Atanassova, V. Intercriteria Decision Making: A New Approach for Multicriteria Decision Making, Based on Index Matrices and Intuitionistic Fuzzy Sets. In Issues in Intuitionistic Fuzzy Sets and Generalized Nets, 11; Atanassov, K., Kacprzyk, J., Krawczak, M., Szmidt, E., Eds.; Warsaw School of Information Technology: Warsaw, Poland, 2014; pp. 1–8. [Google Scholar]
- Atanassov, K.; Atanassova, V.; Gluhchev, G. Intercriteria Analysis: Ideas and Problems. Notes Intuitionistic Fuzzy Sets 2015, 21, 81–88. [Google Scholar]
- Mavrov, D. Software for InterCriteria Analysis: Implementation of the Main Algorithm. Notes Intuitionistic Fuzzy Sets 2015, 21, 77–86. [Google Scholar]
- Mavrov, D. Software for Intercriteria Analysis: Working with the Results. Annu. Inform. Sect. Union. Sci. Bulg. 2015, 8, 37–44. [Google Scholar]
- Ikonomov, N.; Vassilev, P.; Roeva, O. ICrAData - Software for InterCriteria Analysis. Int. J. Bioautomation 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Stratiev, D.; Nenov, S.; Shishkova, I.; Georgiev, B.; Argirov, G.; Dinkov, R.; Yordanov, D.; Atanassova, V.; Vassilev, P.; Atanassov, K. Commercial investigation of the ebullated bed vacuum residue hydrocracking in the conversion range 55–93%. ACS Omega 2020, 51, 33290–33304. [Google Scholar] [CrossRef]
- Félix, G.; Ancheyta, J. Regular Solution Model to Predict the Asphaltenes Flocculation and Sediments Formation during Hydrocracking of Heavy Oil. Fuel 2020, 260, 116160. [Google Scholar] [CrossRef]
- Stanislaus, A.; Hauser, A.; Marafi, M. Investigation of the Mechanism of Sediment Formation in Residual Oil Hydrocracking Process through Characterization of Sediment Deposits. Catal. Today 2005, 109, 167–177. [Google Scholar] [CrossRef]
- Shishkova, I.; Stratiev, D.; Kirov, P.; Dinkov, R.; Sotirov, S.; Sotirova, E.; Bureva, V.; Atanassov, K.; Toteva, V.; Vasilev, S.; et al. Root Cause Analysis for Observed Increased Sedimentation in a Commercial Residue Hydrocracker. Processes 2025, 13, 674. [Google Scholar] [CrossRef]
- Kunnas, J.; Ovaskainen, O.; Respini, M. Mitigate Fouling in Ebullated Bed Hydrocrackers. Hydrocarb. Process 2010, 10, 59–64. [Google Scholar]
- Respini, M.; Ekres, S.; Wright, B.; Žajdlík, R. Strategies to Control Sediment and Coke in a Hydrocracker. PTQ 2013, Q2, 1–11. [Google Scholar]
- Marafi, M.; Al-Barood, A.; Stanislaus, A. Effect of Diluents in Controlling Sediment Formation During Catalytic Hydrocracking of Kuwait Vacuum Residue. Pet. Sci. Technol. 2005, 23, 899–908. [Google Scholar] [CrossRef]
- Tirado, A.; Ancheyta, J. Batch Reactor Study of the Effect of Aromatic Diluents to Reduce Sediment Formation during Hydrotreating of Heavy Oil. Energy Fuels 2018, 32, 60–66. [Google Scholar] [CrossRef]
- Wiehe, I.A. Process Chemistry of Petroleum Macromolecules, 1st ed.; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2008; pp. 223–224. [Google Scholar]
- Alonso, F.; Castillo, J.A.; Ancheyta, J.; Torres-Mancera, P. Evaluation of the Effect of Addition Order on the Compatibility of Binary Crude Oil Blending. Energy Fuels 2024, 38, 23358–23366. [Google Scholar] [CrossRef]
- Buch, L.; Groenzin, H.; Buenrostro-Gonzalez, E.; Andersen, S.I.; Lira-Galeana, C.; Mullins, O.C. Molecular Size of Asphaltene Fractions Obtained from Residuum Hydrotreatment. Fuel 2003, 82, 1075–1084. [Google Scholar] [CrossRef]
- Buenrostro-Gonzalez, E.; Andersen, S.I.; Garcia-Martinez, J.A.; Lira-Galeana, C. Solubility/Molecular Structure Relationships of Asphaltenes in Polar and Nonpolar Media. Energy Fuels 2002, 16, 732–741. [Google Scholar] [CrossRef]
- Adams, J.J.; Rovani, J.F.; Planche, J.P.; Loveridge, J.; Literati, A.; Shishkova, I.; Palichev, G.; Kolev, I.; Atanassov, K.; Nenov, S.; et al. SAR-AD Method to Characterize Eight SARA Fractions in Various Vacuum Residues and Follow Their Transformations Occurring during Hydrocracking and Pyrolysis. Processes 2023, 11, 1220. [Google Scholar] [CrossRef]
- Redelius, P.; Soenen, H. Relation between bitumen chemistry and performance. Fuel 2015, 140, 34–43. [Google Scholar] [CrossRef]
- Goosens, A.G. Prediction of molecular weight of petroleum fractions. Ind. Eng.Chem. Res. 1996, 35, 985–988. [Google Scholar] [CrossRef]
- Kotzakoulakis, K.; George, S.C. A Simple and Flexible Correlation for Predicting the Viscosity of Crude Oils. J. Pet. Sci. Eng. 2017, 158, 416–423. [Google Scholar] [CrossRef]
- Sinha, U.; Dindoruk, B.; Soliman, M.Y. Physics Augmented Correlations and Machine Learning Methods to Accurately Calculate Dead Oil Viscosity Based on the Available Inputs. SPE J. 2022, 27, 1–14. [Google Scholar] [CrossRef]
- Ancheyta, J.; Centeno, G.; Trejo, F.; Marroquín, G. Changes in asphaltene properties during hydrotreating of heavy crudes. Energy Fuel 2003, 17, 1233–1238. [Google Scholar] [CrossRef]
- Takahashi, T.; Higashi, H.; Kai, T. Development of a new hydrodemetallization catalyst for deep desulfurization of atmospheric residue and the effect of reaction temperature on catalyst deactivation. Catal. Today 2005, 104, 76–85. [Google Scholar] [CrossRef]
- Stratiev, D.; Dinkov, R.; Shishkova, I.; Yordanov, D. Can we manage the process of asphaltene precipitation during the production of IMO 2020 fuel oil? Erdöl Erdgas Kohle 2020, 136, 32–39. [Google Scholar]
- Mountainland, D.; Rueter, M. Using HCAT® Technology with Vacuum Bottoms Recycle. In Proceedings of the 15th International Bottom of the Barrel Technology Conference, Dubrovnik, Croatia, 18–19 May 2017. [Google Scholar]
- Stratiev, D.; Shishkova, I.; Argirov, G.; Dinkov, R.; Ivanov, M.; Sotirov, S.; Sotirova, E.; Bureva, V.; Nenov, S.; Atanassov, K.; et al. Roles of Catalysts and Feedstock in Optimizing the Performance of Heavy Fraction Conversion Processes: Fluid Catalytic Cracking and Ebullated Bed Vacuum Residue Hydrocracking. Catalysts 2024, 14, 616. [Google Scholar] [CrossRef]







| High Temperature Simulated Distillation (HTSD) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Crude oil | SG | MCR, wt.% | Sulfur, wt.% | Vis, °E | Saturate, wt.% | Aromatics, wt.% | Resins, wt.% | C7-asp, wt.% | C5-asp, wt.% | SP, °C | IBP | 10% | 30% | 50% | 70% | 90% | 95% | FBP | Recovery,% |
| 1 | Arab Heavy | 1.040 | 23.6 | 5.8 | 206 | 12.4 | 61.9 | wt.5 | wt.% | 32.9 | 51.2 | 524 | 567 | 614 | 653 | 686 | 761 | 85.1 | ||
| 2 | Arab Light | 1.029 | 18.7 | 4.9 | 15.9 | 64.7 | 7.3 | 12.1 | 18.8 | 32.3 | 498 | 549 | 592 | 629 | 664 | 712 | 734 | 740 | 96.6 | |
| 3 | Arab Med. | 1.031 | 20.7 | 5.4 | 94.8 | 11.8 | 68.3 | 5.3 | 14.6 | 25.5 | 44.7 | 513 | 560 | 600 | 633 | 663 | 691 | 84.0 | ||
| 4 | Aseng | 0.984 | 14.2 | 0.6 | 32.7 | 48.5 | 15.2 | 3.7 | 10 | 28 | 523 | 556 | 588 | 619 | 656 | 712 | 776 | 776 | 95.3 | |
| 5 | Azeri Light | 0.967 | 9.5 | 0.5 | 17.3 | 40.2 | 50.1 | 8.4 | 1.4 | 5.4 | 30.2 | 483 | 526 | 567 | 605 | 644 | 650 | 73.1 | ||
| 6 | Basrah H | 1.071 | 28.9 | 7.1 | 487 | 12.3 | 54.1 | 5.8 | 27.7 | 37.0 | 68.6 | 488 | 537 | 588 | 626 | 643 | 62.6 | |||
| 7 | Basrah L | 1.052 | 23.8 | 5.9 | 127.5 | 12.3 | 64.8 | 4.9 | 18 | 27.7 | 50.3 | 507 | 560 | 603 | 637 | 666 | 710 | 713 | 91.0 | |
| 8 | Basrah Medium | 1.057 | 24.2 | 6.82 | 203 | 22.3 | 30.2 | 502 | 553 | 598 | 634 | 665 | 711 | 714 | 91.8 | |||||
| 9 | Bonga | 0.968 | 12.8 | 0.74 | 35.3 | 26.4 | 59 | 13.9 | 0.70 | |||||||||||
| 10 | CPC | 0.981 | 16 | 2.1 | 22.5 | 44.6 | 40.8 | 10.3 | 3.4 | 11 | 25.2 | 487 | 518 | 551 | 584 | 625 | 646 | 79.1 | ||
| 11 | El Bouri | 1.050 | 25.5 | 3.3 | 139.2 | 12 | 57.9 | 12.6 | 17.5 | 27.3 | 45 | 478 | 523 | 568 | 610 | 642 | 66.2 | |||
| 12 | El Sharara | 0.976 | 13.1 | 0.39 | 18.3 | 10.1 | 17.0 | 504 | 542 | 573 | 601 | 637 | 692 | 713 | 756 | 99.4 | ||||
| 13 | Es Sider | 0.999 | 13.8 | 1.096 | 31 | 10.2 | 19.0 | 505 | 551 | 591 | 628 | 661 | 711 | 733 | 739 | 96.1 | ||||
| 14 | Forties | 0.990 | 14.8 | 2.5 | 28.7 | 60.3 | 3.8 | 7.2 | 9.8 | 28.9 | 517 | 559 | 596 | 633 | 672 | 738 | 779 | 93.4 | ||
| 15 | Gulf of Suez | 1.024 | 19.7 | 3.30 | 82.2 | 22.1 | 32.0 | 498 | 556 | 599 | 637 | 671 | 718 | 90.2 | ||||||
| 16 | Helm | 1.054 | 3.013 | 422.3 | 27.0 | 41.3 | 507 | 554 | 598 | 634 | 665 | 703 | 87.1 | |||||||
| 17 | Imported AR_july 2024 | 1.047 | 20.8 | 6.30 | 19.2 | 24.9 | ||||||||||||||
| 18 | Iranian H | 1.050 | 23.9 | 5.2 | 17 | 52.6 | 5 | 25.4 | 36.2 | 61.9 | ||||||||||
| 19 | Johan Sverdrup | 1.023 | 18.26 | 1.77 | 138 | 16.4 | 27.4 | 514 | 557 | 600 | 641 | 679 | 716 | 87.3 | ||||||
| 20 | Kazakh H | 0.990 | 17.1 | 1.7 | 33 | 50.2 | 5.7 | 11.1 | 17.8 | 27.8 | 410 | 549 | 592 | 632 | 672 | 731 | 771 | 93.1 | ||
| 21 | Kzakh/Kumkol blend | 0.990 | 17.1 | 1.195 | 15.75 | |||||||||||||||
| 22 | KBT | 1.067 | 26.9 | 6.4 | 129.3 | 12.3 | 53.6 | 9.2 | 24.9 | 32.4 | 62.4 | |||||||||
| 23 | KEB | 1.037 | 23.3 | 5.7 | 15 | 64.2 | 4.2 | 16.6 | 25.7 | 47.8 | 514 | 560 | 606 | 647 | 682 | 718 | 87.1 | |||
| 24 | KEBCO | 1.020 | 16.3 | 3.23 | 35 | 14.4 | 18.9 | 504 | 551 | 591 | 627 | 660 | 708 | 728 | 735 | 97 | ||||
| 25 | Kirkuk | 1.054 | 25.2 | 5.9 | 120.8 | 15.2 | 55.4 | 5 | 24.3 | 33.1 | 58.1 | 513 | 558 | 603 | 645 | 680 | 709 | 84.7 | ||
| 26 | LSCO | 0.993 | 14 | 1.58 | 23.8 | 25 | 61.1 | 6.1 | 7.8 | 15.5 | 28.9 | 508 | 553 | 585 | 592 | 668 | 719 | 730 | 91.8 | |
| 27 | Okwuibome | 0.975 | 12.9 | 0.497 | 509 | 553 | 585 | 616 | 652 | 707 | 760 | 811 | 97.9 | |||||||
| 28 | Payara Gold | 1.001 | 13.0 | 1.43 | 39.8 | 8.1 | 13.2 | 502 | 550 | 591 | 629 | 663 | 710 | 727 | 94.7 | |||||
| 29 | Prinos | 1.108 | 32.8 | 9.14 | 12.6 | 50.6 | 6.8 | 30 | 38.8 | 69.2 | 491 | 539 | 574 | 613 | 649 | 663 | 78.8 | |||
| 30 | RasGharib | 1.059 | 25.1 | 5.6 | 14.7 | 49.7 | 9.6 | 26 | 34.9 | 75.8 | 505 | 558 | 606 | 640 | 670 | 675 | 73.9 | |||
| 31 | Rhemoura | 1.041 | 23.7 | 1.8 | 42 | 19.7 | 49.8 | 7.3 | 23.2 | 31.3 | 51.1 | 487 | 533 | 577 | 617 | 650 | 67.0 | |||
| 32 | Sepia | 0.998 | 13.8 | 0.75 | 58 | 8.5 | 17.1 | 510 | 561 | 606 | 641 | 673 | 717 | 740 | 779 | 100.6 | ||||
| 33 | SGC | 1.050 | 22.9 | 5.09 | 15 | 55.9 | 7.3 | 21.8 | 28.4 | 58.4 | 490 | 538 | 588 | 627 | 655 | 690 | 88.2 | |||
| 34 | Tartaruga | 1.008 | 16.3 | 1.35 | 77 | 14.3 | 22.4 | 502 | 553 | 597 | 635 | 669 | 708 | 87.9 | ||||||
| 35 | Tempa rossa | 1.120 | 34.3 | 9.3 | 2.2 | 48.4 | 12.6 | 36.8 | 46.8 | 100 | 531 | 576 | 627 | 659 | 690 | 696 | 74.4 | |||
| 36 | TEN_Oct.2024 | 0.981 | 11.6 | 1.06 | 15.8 | 1.2 | 5.0 | 491 | 544 | 586 | 625 | 663 | 714 | 741 | 817 | 99.8 | ||||
| 37 | Unity Gold | 0.979 | 14.7 | 1.32 | 36.5 | 10.9 | 15.7 | 503 | 550 | 589 | 626 | 662 | 711 | 729 | 94.1 | |||||
| 38 | Urals | 0.997 | 17.5 | 3 | 47.5 | 25.6 | 52.5 | 7.8 | 14.1 | 17.6 | 40.1 | 497 | 553 | 595 | 631 | 663 | 710 | 718 | 93.3 | |
| 39 | Val’Dagri | 1.052 | 21.4 | 6 | 79.5 | 11.7 | 73.5 | 6.4 | 8.5 | 19.5 | 43.7 | 488 | 550 | 592 | 630 | 663 | 707 | 732 | 856 | 107 |
| 40 | Varandey | 0.990 | 15.1 | 1.7 | 24.8 | 33.5 | 47.6 | 11.3 | 7.6 | 13.5 | 43.8 | 520 | 559 | 598 | 635 | 674 | 738 | 764 | 92.2 | |
| 41 | Western Desert | 1.052 | 19.0 | 1.31 | 60 | 17.9 | 24.7 | 510 | 547 | 585 | 622 | 663 | 717 | 726 | 92.6 | |||||
| FCC LCO | FCC HCO | FCC SLO | H–Oil Diesel | |
|---|---|---|---|---|
| Density at 15 °C, g/cm3 | ||||
| Kinematic viscosity at 80 °C, mm2/s | 1.42 | 4.42 | 33.35 | 3.56 |
| HTSD, ASTM D-7169, wt.% | 0.9399 | 1.0147 | 1.1008 | 0.872 |
| 0.5 | 158 | 200 | 247 | |
| 5 | 189 | 257 | 311 | |
| 10 | 200 | 273 | 325 | 201 |
| 30 | 224 | 306 | 359 | |
| 50 | 245 | 322 | 393 | 269 |
| 70 | 264 | 339 | 433 | |
| 90 | 292 | 372 | 525 | 330 |
| 95 | 308 | 393 | 594 | |
| 99.5 | 380 | 460 | 701 | |
| Sulfur, wt.% | 0.2 | 0.8 | 1.2 | 0.2 |
| SARA composition, wt.% | ||||
| Saturates | 19.9 | 18.2 | 15.1 | 45.3 |
| Aromatics | 77.1 | 75.1 | 50.7 | 54.7 |
| Resins | 0 | 5.4 | 27.6 | 0 |
| Asphaltenes | 0 | 0 | 3.5 | 0 |
| Kw-factor | 10.4 | 10.09 | 9.65 | 11.37 |
| TSE, % | 0 | 0 | 0.07 | 0 |
| Property | Standard method |
|---|---|
| Density, kg/m3 | BDS EN ISO 3675 [61] |
| Sulfur content wt.% | ASTM D 4294 [62] |
| Asphaltene (C7, and C5) content, wt.% | ASTM D 6560 [63] |
| Microcarbon content, wt.% | EN ISO 10370 [64] |
| Specific viscosity, °E | ASTM D 1665 [65] |
| Carbon content, wt.% | ASTM D 5291 [66] |
| Hydrogen content, wt.% | ASTM D 5291 [66] |
| Nitrogen content, wt.% | ASTM D 5291 [66] |
| Nickel, ppm | IP 501 [67] |
| Vanadium, ppm | IP 501 [67] |
| Sodium, ppm | IP 501 [67] |
| Iron, ppm | IP 501 [67] |
| High-temperature simulation distillation (HTSD) | ASTM D 7169 [68] |
| Water content, wt.% | ASTM D 95 [69] |
| Mechanical impurities content, wt.% | ASTM D 473 [70] |
| Flash point open cup, °C | ASTM D 92 [71] |
| Flash point closed cup, °C | ASTM D 93 [72] |
| Pour point, °C | ASTM D 97 [73] |
| Ash content, wt.% | ASTM D 482 [74] |
| Specific heat of combustion/lower | ASTM D 4809 [75] |
| Total sediment existent, wt.% | IP 375 [76] |
| Total sediment potential, wt.% | IP 390 [77] |
| Total sediment accelerated, wt.% | IP 390 [77] |
| Characteristics of Finished Heavy Fuel Oil | Min | Max | Average |
|---|---|---|---|
| Density, g/cm3 | 0.9574 | 1.0489 | 1.007 |
| Specific viscosity, °E | 4.75 | 14.97 | 11.7 |
| Sulfur content wt.% | 0.7 | 2.21 | 1.3 |
| Water content, wt.% | 0.01 | 0.7 | 0.1 |
| Mechanical impurities content, wt.% | 0.01 | 0.9 | 0.1 |
| Flash point open cup, °C | 94 | 212 | 126.7 |
| Flash point closed cup, °C | 97 | 206 | 159.6 |
| Pour point, °C | 0 | 21 | 11.3 |
| Ash content, wt.% | 0.011 | 0.099 | 0.0 |
| Specific heat of combustion/lower | 39.374 | 41.406 | 40.3 |
| Total sediment existent, wt.% | 0.01 | 0.47 | 0.08 |
| Total sediment potential, wt.% | 0.01 | 0.8 | 0.17 |
| Total sediment accelerated, wt.% | 0.02 | 0.7 | 0.12 |
| Vanadium content, ppm | 25 | 170 | 63.3 |
| Content of aluminum and silicon, ppm | 25 | 244 | 75.7 |
| Microcarbon content, wt.% | 8 | 23.1 | 14.8 |
| Asphaltene (C7) content, wt.% | 2.4 | 15.3 | 7.0 |
| Content of components in the heavy fuel oil blend, wt.% | |||
| VTB from H–oil | 36.9 | 92.4 | 71.1 |
| LCO from FCC | 0.0 | 18.8 | 2.3 |
| HCO from FCC | 1.7 | 62.6 | 19.8 |
| Slurry oil from FCC | 0.0 | 21.7 | 5.2 |
| SRAR from CDU | 0.0 | 2.0 | 0.1 |
| Diesel from H–Oil/CDU | 0.0 | 20.1 | 0.6 |
| VGO | 0.0 | 5.2 | 0.2 |
| SRVR from VDU | 0.0 | 13.2 | 1.7 |
| μ | D15 | Ash | Heat | TSE | TSP | TSA | Al + Si | CCR | Asp |
|---|---|---|---|---|---|---|---|---|---|
| D15 | 1.00 | 0.65 | 0.12 | 0.38 | 0.48 | 0.44 | 0.70 | 0.88 | 0.78 |
| Ash | 0.65 | 1.00 | 0.33 | 0.46 | 0.51 | 0.49 | 0.75 | 0.65 | 0.63 |
| Heat | 0.12 | 0.33 | 1.00 | 0.55 | 0.49 | 0.52 | 0.26 | 0.13 | 0.20 |
| TSE | 0.38 | 0.46 | 0.55 | 1.00 | 0.76 | 0.81 | 0.48 | 0.37 | 0.44 |
| TSP | 0.48 | 0.51 | 0.49 | 0.76 | 1.00 | 0.84 | 0.56 | 0.47 | 0.53 |
| TSA | 0.44 | 0.49 | 0.52 | 0.81 | 0.84 | 1.00 | 0.53 | 0.43 | 0.49 |
| Al + Si | 0.70 | 0.75 | 0.26 | 0.48 | 0.56 | 0.53 | 1.00 | 0.69 | 0.67 |
| CCR | 0.88 | 0.65 | 0.13 | 0.37 | 0.47 | 0.43 | 0.69 | 1.00 | 0.79 |
| Asp | 0.78 | 0.63 | 0.20 | 0.44 | 0.53 | 0.49 | 0.67 | 0.79 | 1.00 |
| υ | D15 | Ash | Heat | TSE | TSP | TSA | Al + Si | MCR | Asp |
|---|---|---|---|---|---|---|---|---|---|
| D15 | 0.00 | 0.32 | 0.85 | 0.53 | 0.47 | 0.49 | 0.26 | 0.09 | 0.18 |
| Ash | 0.32 | 0.00 | 0.64 | 0.45 | 0.44 | 0.44 | 0.25 | 0.31 | 0.33 |
| Heat | 0.85 | 0.64 | 0.00 | 0.36 | 0.45 | 0.41 | 0.70 | 0.83 | 0.77 |
| TSE | 0.53 | 0.45 | 0.36 | 0.00 | 0.13 | 0.09 | 0.42 | 0.54 | 0.47 |
| TSP | 0.47 | 0.44 | 0.45 | 0.13 | 0.00 | 0.09 | 0.38 | 0.48 | 0.42 |
| TSA | 0.49 | 0.44 | 0.41 | 0.09 | 0.09 | 0.00 | 0.40 | 0.51 | 0.45 |
| Al + Si | 0.26 | 0.25 | 0.70 | 0.42 | 0.38 | 0.40 | 0.00 | 0.27 | 0.29 |
| MCR | 0.09 | 0.31 | 0.83 | 0.54 | 0.48 | 0.51 | 0.27 | 0.00 | 0.17 |
| Asp | 0.18 | 0.33 | 0.77 | 0.47 | 0.42 | 0.45 | 0.29 | 0.17 | 0.00 |
| H–Oil Feed | Min | Max | H–Oil VTB | Min | Max | H–Oil PBFO | Min | Max | Average |
|---|---|---|---|---|---|---|---|---|---|
| D15, g/cm3 | 0.9255 | 1.0655 | D15, g/cm3 | 0.983 | 1.1308 | D15, g/cm3 | 0.9654 | 1.0829 | |
| S, wt.% | 1.96 | 4.6 | S, wt.% | 0.542 | 2.14 | S, wt.% | 0.49 | 1.85 | |
| T50%, °C | 469 | 621 | T50%, °C | 554 | 608 | Viscosity at 80 °C, mm2/s | 41.3 | 320.5 | |
| MW, g/mol | 389 | 694 | MW, g/mol | 482 | 655 | HCO in PBFO, wt.% | 0 | 54.6 | 27.6 |
| Nc | 28 | 49 | Nc | 36 | 48 | LCO in PBFO, wt.% | 0 | 46.5 | 2.3 |
| N, wt.% | 0.21 | 0.66 | N, wt.% | 0.36 | 0.91 | SLO in PBFO, wt.% | 0 | 12.6 | 2.9 |
| H, wt.% | 9.37 | 11.65 | H, wt.% | 7.7 | 11.8 | VTB in PBFO, wt.% | 40 | 88 | 67.0 |
| C, wt.% | 80.97 | 89.8 | C, wt.% | 85.2 | 91.6 | ||||
| MCR, wt.% | 6.2 | 22.5 | MCR, wt.% | 15.5 | 47.2 | ||||
| H/C ratio | 1.33 | 1.65 | H/C ratio | 1.05 | 1.58 | ||||
| C7 asp., wt.% | 3.40 | 18.00 | C7 asp., wt.% | 5.20 | 28.20 | ||||
| C5 asp., wt.% | 4.60 | 29.50 | C5 asp., wt.% | 8.80 | 57.10 | ||||
| V, ppm | 59 | 255 | V, ppm | 22 | 265 | ||||
| Ni, ppm | 8 | 84 | Ni, ppm | 6 | 91 | ||||
| Na, ppm | 8 | 46 | Na, ppm | 3 | 35 | ||||
| Fe, ppm | 10 | 105 | Fe, ppm | 2 | 243 | ||||
| FCC SLO in H–Oil feed, wt.% | 0.0 | 20.4 | H–oil ATB TSE, wt.% | 0.0 | 0.6 | PBFO TSP, wt.% | 0.02 | 3.37 | |
| HCO in H–Oil feed, wt.% | 0.0 | 10.2 | |||||||
| PBFO Recycle in H–Oil feed, wt.% | 0.0 | 25.1 | |||||||
| H–Oil Operating conditions | |||||||||
| WABT, °C | 405 | 436 | |||||||
| LHSV, h−1 | 0.10 | 0.25 | |||||||
| Catalyst addition rate, kg/t | 0.5 | 1.6 | |||||||
| ΔT-R1/ΔT-R2 | 0.9 | 3.1 | |||||||
| HDM, % | 48.1 | 96.3 | |||||||
| H–Oil Conversion, wt.% | 46.5 | 92.6 | |||||||
| Property | Min | Max |
|---|---|---|
| Density at 15 °C, g/cm3 | 0.987 | 1.042 |
| MCR, wt.% | 14.6 | 24.1 |
| Sulfur, wt.% | 1.6 | 5.3 |
| Nitrogen, wt.% | 0.3 | 0.7 |
| Saturates, wt.% | 10.3 | 23.2 |
| Aromatics, wt.% | 66.0 | 77.6 |
| Resins, wt.% | 3.8 | 8.8 |
| C7 asphaltenes, wt.% | 3.9 | 11.9 |
| C5 asphaltenes, wt.% | 8.1 | 20.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotirov, S.; Sotirova, E.; Dinkov, R.; Stratiev, D.; Shiskova, I.; Kolev, I.; Argirov, G.; Georgiev, G.; Bureva, V.; Atanassov, K.; et al. Heavy Fuel Oil Quality Dependence on Blend Composition, Hydrocracker Conversion, and Petroleum Basket. Fuels 2025, 6, 43. https://doi.org/10.3390/fuels6020043
Sotirov S, Sotirova E, Dinkov R, Stratiev D, Shiskova I, Kolev I, Argirov G, Georgiev G, Bureva V, Atanassov K, et al. Heavy Fuel Oil Quality Dependence on Blend Composition, Hydrocracker Conversion, and Petroleum Basket. Fuels. 2025; 6(2):43. https://doi.org/10.3390/fuels6020043
Chicago/Turabian StyleSotirov, Sotir, Evdokia Sotirova, Rosen Dinkov, Dicho Stratiev, Ivelina Shiskova, Iliyan Kolev, Georgi Argirov, Georgi Georgiev, Vesselina Bureva, Krassimir Atanassov, and et al. 2025. "Heavy Fuel Oil Quality Dependence on Blend Composition, Hydrocracker Conversion, and Petroleum Basket" Fuels 6, no. 2: 43. https://doi.org/10.3390/fuels6020043
APA StyleSotirov, S., Sotirova, E., Dinkov, R., Stratiev, D., Shiskova, I., Kolev, I., Argirov, G., Georgiev, G., Bureva, V., Atanassov, K., Nikolova, R., Veli, A., Nenov, S., Stratiev, D. D., & Vasilev, S. (2025). Heavy Fuel Oil Quality Dependence on Blend Composition, Hydrocracker Conversion, and Petroleum Basket. Fuels, 6(2), 43. https://doi.org/10.3390/fuels6020043










