Optimized Biochar from Chicken Manure via Hydrothermal Activation and Catalytic HTC: Properties and CO2 Reduction Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
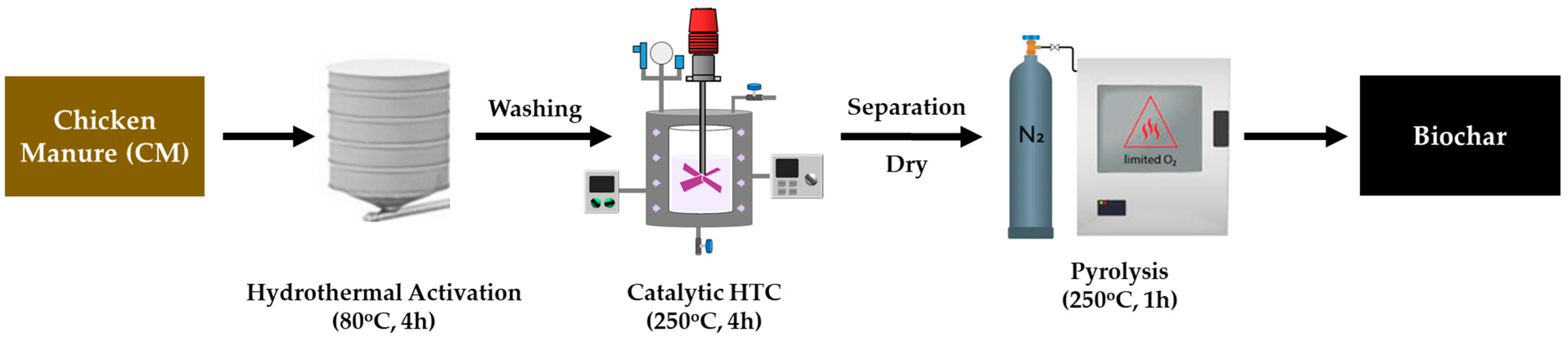
2.2. Experimental Procedure
2.3. Characterization
2.3.1. Mass Yield
2.3.2. Proximate and Elemental Analysis
2.3.3. Specific Surface Area (SSA)
2.3.4. Heavy Metal Content
2.3.5. CO2 Reduction Potential (CRP)
3. Results and Discussion
3.1. Effect of Hydrothermal Activators on Biochar
3.2. Effect of HTC Catalysts on Biochar
3.3. Comparison with Conventional Biochar Production
3.3.1. Comparison of Physicochemical Properties of Biochar
3.3.2. Comparison of Heavy Metal Distribution in Biochar
3.3.3. Comparison of Carbon Reduction Effects on Biochar
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Govoni, C.; Chiarelli, D.D.; Luciano, A.; Ottoboni, M.; Perpelek, S.N.; Pinotti, L.; Rulli, M.C. Global assessment of natural resources for chicken production. Adv. Water Resour. 2021, 154, 103987. [Google Scholar] [CrossRef]
- Aboutayeb, R.; Fijahi, S.; Hssaini, L.; Azim, K. Quality assessment of poultry manure compost: Focus on organic amendment and bioremediation roles toward sustainable agriculture. Euro-Mediterr. J. Environ. Integr. 2024, 9, 1–23. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Osman, A.I.; Ai, P.; Zhou, Z.; Meng, F.; Rooney, D.W. Bioenergy production from chicken manure: A review. Environ. Chem. Lett. 2023, 21, 2707–2727. [Google Scholar] [CrossRef]
- Park, J.H.; Yun, J.J.; Kim, S.H.; Park, J.H.; Acharya, B.S.; Cho, J.S.; Kang, S.W. Biochar improves soil properties and corn productivity under drought conditions in South Korea. Biochar 2023, 5, 66. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation; Taylor & Francis: Oxford, UK, 2024. [Google Scholar]
- Sun, L.; Gong, P.; Sun, Y.; Qin, Q.; Song, K.; Ye, J.; Zhang, H.; Zhou, B.; Xue, Y. Modified chicken manure biochar enhanced the adsorption for Cd2+ in aqueous and immobilization of Cd in contaminated agricultural soil. Sci. Total Environ. 2022, 851, 158252. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Zulkifli, N.W.M.; Ibrahim, S.; Chen, W.H.; Chong, C.T.; Ok, Y.S. Valorization of animal manure via pyrolysis for bioenergy: A review. J. Clean. Prod. 2022, 343, 130965. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Ravenni, G.; Thomsen, T.P.; Smith, A.M.; Ambye-Jensen, M.; Rohde-Nielsen, K.T.; Henriksen, U.B. Integration of a drying and pyrolysis system in a green biorefinery: Biochar product quality and impacts on the overall energy balance and climate footprint. Biomass Conv. Bioref. 2024, 14, 25143–25159. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes, and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties, and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2015, 283, 789. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, S.; Wang, S.; Niedzwiecki, L.; Baranowski, M.; Czerep, M.; Tang, C.; Kawi, S.; Wang, C.H.; Jiang, J.; et al. Hydrothermal carbonization coupled with pyrolysis: An innovative approach to digestate management. Green Energy Resour. 2023, 1, 100034. [Google Scholar] [CrossRef]
- Ipiales, R.P.; Sarrion, A.; Diaz, E.; Diaz-Portuondo, E.; Mohedano, A.F.; de la Rubia, A. Strategies to improve swine manure hydrochar: HCl-assisted hydrothermal carbonization versus hydrochar washing. Biomass Conv. Bioref. 2023, 13, 16467–16478. [Google Scholar] [CrossRef]
- Tran, L.T.; Hoang, H.T.; Nguyen, M.Q.; Dao, N.D.; Vu, T.H.T. Effect of FeCl3 Catalyst on Chemical Properties of Hydrochar Produced by Hydrothermal Carbonization of Dragon Fruit Stem (Hylocereus undatus). BioEnergy Res. 2022, 15, 1986–2005. [Google Scholar] [CrossRef]
- Petrović, J.; Eregović, M.; Simić, M.; Koprivica, M.; Dimitrijević, J.; Jovanović, A.; Janković Pantić, J. Hydrothermal Carbonization of Waste Biomass: A Review of Hydrochar Preparation and Environmental Application. Processes 2024, 12, 217. [Google Scholar] [CrossRef]
- Chatir, E.M.; El Hadrami, A.; Ojala, S.; Brahmi, R. Production of activated carbon with tunable porosity and surface chemistry via chemical activation of hydrochar with phosphoric acid under oxidizing atmosphere. Surf. Interfaces 2022, 30, 101849. [Google Scholar] [CrossRef]
- Allwar, A.; Febriyantr, H.Z.; Yuliantari, R. Preparation and Characterization of Hydrothermal Activated Carbon from Banana Empty Fruit Bunch with ZnCl2 Activation for Removal of Phenol in Aqueous Solution. Asian J. Appl. Sci. 2017, 11, 20. [Google Scholar] [CrossRef][Green Version]
- Kennedy, L.J.; Vijaya, J.J.; Sekaran, G. Effect of two-stage process on the preparation and characterization of porous carbon composite from rice husk by phosphoric acid activation. Ind. Eng. Chem. Res. 2004, 43, 1832–1838. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Q.; Zhao, X.; Li, G. Effect of hydrothermal carbonization temperature and time on characteristics of bio-chars from chicken manure. Trans. Chin. Soc. Agric. Eng. 2015, 31, 239–244. [Google Scholar]
- Odales-Bernal, L.; González, L.M.L.; Ghysels, S.; Lobanov, V.; De Vrieze, J.; Barrera, E.L.; Ronsse, F. Optimized hydrothermal carbonization of chicken manure and anaerobic digestion of its process water for better energy management. J. Environ. Manag. 2025, 375, 124191. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Bae, S. Hydrothermal carbonization: Modeling, final properties design and applications: A review. Energies 2018, 11, 216. [Google Scholar] [CrossRef]
- Yameen, M.Z.; Naqvi, S.R.; Juchelkova, D.; Khan, M.N.A. Harnessing the power of functionalized biochar: Progress, challenges, and future perspectives in energy, water treatment, and environmental sustainability. Biochar 2024, 6, 25. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021.
- Schuster, G.; Löffler, G.; Weigl, K.; Hofbauer, H. Biomass steam gasification-an extensive parametric modeling study. Bioresour. Technol. 2001, 77, 71–79. [Google Scholar] [CrossRef]
- ASTM D6349-21; Standard Test Method for Determination of Major and Minor Elements in Coal, Coke, and Combustion Residues from Coal Utilization Processes by Inductively Coupled Plasma–Atomic Emission Spectrometry (ICP-AES). ASTM International: West Conshohocken, PA, USA, 2021.
- Venkatesh, G.; Gopinath, K.A.; Reddy, K.S.; Reddy, B.S.; Prabhakar, M.; Srinivasarao, C.; Singh, V.K. Characterization of biochar derived from crop residues for soil amendment, carbon sequestration and energy use. Sustainability 2022, 14, 2295. [Google Scholar] [CrossRef]
- Li, S.; Tasnady, D. Biochar for Soil Carbon Sequestration: Current Knowledge, Mechanisms, and Future Perspectives. C 2023, 9, 67. [Google Scholar] [CrossRef]
- Manimekala, T.; Sivasubramanian, R.; Dar, M.A.; Dharmalingam, G. Crafting the architecture of biomass-derived activated carbon via electrochemical insights for supercapacitors: A review. RSC Adv. 2025, 15, 2490. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, W.; Mašek, O.; Chen, X.; Gu, B.; Sharma, B.K.; Cao, X. Roles of Phosphoric Acid in Biochar Formation: Synchronously Improving Carbon Retention and Sorption Capacity. J. Env. Qual. 2017, 46, 393. [Google Scholar] [CrossRef]
- Zubir, M.H.M.; Zaini, M.A.A. Twigs-derived activated carbons via H3PO4/ZnCl2 composite activation for methylene blue and congo red dyes removal. Sci. Rep. 2020, 10, 14050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, Z.; Xiong, D.; Wu, Y.; Tong, M.; Su, H.; Zhang, Z.; Liao, J. Investigation into the Structure and Properties of Biochar Co-Activated by ZnCl2 and NaHCO3 under Low Temperature Conditions. Materials 2024, 17, 942. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, W.; Shi, Z.; Yang, L.; Wen, Z.; Zi, G.; Luo, L. Effect of ZnCl2, H3PO4, and KOH activation on low-temperature NH3-denitration performance of activated carbon. CLEAN Soil Air Water 2023, 52, 2300148. [Google Scholar] [CrossRef]
- Zhang, S.; Sheng, K.; Yan, W.; Liu, J.; Shuang, E.; Yang, M.; Zhang, X. Bamboo derived hydrochar microspheres fabricated by acid-assisted hydrothermal carbonization. Chemosphere 2021, 263, 128093. [Google Scholar] [CrossRef]
- Macdermid-Watts, K.; Adewakun, E.; Norouzi, O.; Abhi, T.D.; Pradhan, R.; Dutta, A. Effects of FeCl3 Catalytic Hydrothermal Carbonization on Chemical Activation of Corn Wet Distillers’ Fiber. ACS Omega 2021, 6, 14875. [Google Scholar] [CrossRef]
- Budai, A.; Zimmerman, A.R.; Cowie, A.L.; Webber, J.B.W.; Singh, B.P.; Glaser, B.; Masiello, C.A.; Andersson, D.; Shields, F.; Lehmann, J.; et al. Biochar Carbon Stability Test Method: An Assessment of Methods to Determine Biochar Carbon Stability; International Biochar Initiative: Westerville, OH, USA, 2013; Available online: https://biochar-international.org/wp-content/uploads/2018/04/IBI_Report_Biochar_Stability_Test_Method_Final.pdf (accessed on 23 February 2025).
- Qi, R.; Xu, Z.; Zhou, Y.; Zhang, D.; Sun, Z.; Chen, W.; Xiong, M. Clean solid fuel produced from cotton textiles waste through hydrothermal carbonization with FeCl3: Upgrading the fuel quality and combustion characteristics. Energy 2020, 214, 118926. [Google Scholar] [CrossRef]
- Sun, K.; Huang, Q.; Chi, Y.; Yan, J. Effect of ZnCl2-activated biochar on catalytic pyrolysis of mixed waste plastics for producing aromatic-enriched oil. Waste Manag. 2018, 81, 128. [Google Scholar] [CrossRef]
- Peng, J.; Kang, X.; Zhao, S.; Yin, Y.; Zhao, P.; Ragauskas, A.J.; Si, C.; Song, X. Regulating the properties of activated carbon for supercapacitors: Impact of particle size and degree of aromatization of hydrochar. Adv. Compos. Hybrid Mater. 2023, 6, 107. [Google Scholar] [CrossRef]
- Shen, C.; Hu, C.; Zhang, W.; Lin, X.; Qi, W.; Zhang, Z.; Gu, J. Acidified ZnCl2 molten salt hydrate systems as hydrolytic media for cellulose I and II nanocrystal production: From rods to spheres. Cellulose 2022, 29, 7629. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Y.; Wang, K.; Yu, H.; Yu, S.; Liu, S. High-Efficient Conversion of Cellulose to Levulinic Acid Catalyzed via Functional Brønsted–Lewis Acidic Ionic Liquids. Catal. Lett. 2021, 152, 1064. [Google Scholar] [CrossRef]
- Chambon, F.; Rataboul, F.; Pinel, C.; Cabiac, A.; Guillon, E.; Essayem, N. Cellulose hydrothermal conversion promoted by heterogeneous Brønsted and Lewis acids: Remarkable efficiency of solid Lewis acids to produce lactic acid. Appl. Catal. B Environ. 2011, 105, 171. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on activated carbons by chemical activation with FeCl3. Carbon 2020, 6, 21. [Google Scholar] [CrossRef]
- Dai, L.; Yang, B.; Li, H.; Tan, F.; Zhu, N.; Zhu, Q.; He, M.; Ran, Y.; Hu, G. A synergistic combination of nutrient reclamation from manure and resultant hydrochar upgradation by acid-supported hydrothermal carbonization. Bioresour. Technol. 2017, 243, 860. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240. [Google Scholar] [CrossRef]
- Aktar, S.; Hossain, M.A.; Rathnayake, N.; Patel, S.; Gasco, G.; Mendez, A.; De Figueiredo, C.; Surapaneni, A.; Shah, K.; Paz-Ferreiro, J. Effects of temperature and carrier gas on physico-chemical properties of biochar derived from biosolids. J. Anal. Appl. Pyrolysis 2022, 164, 105542. [Google Scholar] [CrossRef]
- Meng, J.; Wang, L.; Liu, X.; Wu, J.; Brookes, P.C.; Xu, J. Physicochemical properties of biochar produced from aerobically composted swine manure and its potential use as an environmental amendment. Bioresour. Technol. 2013, 142, 641. [Google Scholar] [CrossRef]
- National Institute of Animal Science. Improvement of Livestock Manure Treatment Efficiency and Establishment of Evaluation Criteria for Compost and Liquid Fertilizer; National Institute of Animal Science: Jeonbuk-do, Republic of Korea, 2012; Available online: https://scienceon.kisti.re.kr/srch/selectPORSrchReport.do?cn=TRKO201400000673 (accessed on 23 February 2025).
- European Biochar Foundation. European Biochar Certificate: Guidelines Version 10.3E.; European Biochar Foundation: Arbaz, Switzerland, 2023; Available online: https://www.european-biochar.org/en/ct/29-Summary-of-the-EBC-certificiation (accessed on 23 February 2025).
- World Biochar Foundation. World Biochar Certificate Guidelines; World Biochar Foundation: Geneva, Switzerland, 2023; Available online: https://www.european-biochar.org/en/ct/2-EBC-and-WBC-guidelines-documents (accessed on 23 February 2025).
- Qian, Q.; Machida, M.; Tatsumoto, H. Preparation of activated carbons from cattle-manure compost by zinc chloride activation. Bioresour. Technol. 2006, 98, 353–360. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Yang, G.; Wang, Q.; Li, R.; Lucia, L.A. Preparation and Characterization of Activated Carbon from Hydrochar by Phosphoric Acid Activation and its Adsorption Performance in Prehydrolysis Liquor. BioResources 2018, 12, 5928–5941. [Google Scholar] [CrossRef]
- Aktar, S.; Hossain, M.A.; Shah, K.; Mendez, A.; De Figueiredo, C.C.; Gasco, G.; Paz-Ferreiro, J. Immobilization of Heavy Metals in Biochar Derived from Biosolids: Effect of Temperature and Carrier Gas. Soil Syst. 2024, 8, 17. [Google Scholar] [CrossRef]
- Gbouri, I.; Yu, F.; Wang, X.; Wang, J.; Cui, X.; Hu, Y.; Yan, B.; Chen, G. Co-Pyrolysis of Sewage Sludge and Wetland Biomass Waste for Biochar Production: Behaviors of Phosphorus and Heavy Metals. Int. J. Environ. Res. Public Health 2022, 19, 2818. [Google Scholar] [CrossRef]
- Li, C.; Xie, S.; Wang, Y.; Jiang, R.; Wang, X.; Lv, N.; Pan, X.; Cai, G.; Yu, G.; Wang, Y. Multi-functional biochar preparation and heavy metal immobilization by co-pyrolysis of livestock feces and biomass waste. Waste Manag. 2021, 134, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Piash, M.I.; Itoh, T.; Abe, K.; Iwabuchi, K. Superior nutrient recovery and release by chicken manure-derived biochar over hydrochar and compost for soil fertilization. Geoderma Reg. 2024, 40, e00906. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Valorization of poultry manure into biochar, bio-oil and gas product by co-pyrolysis with residual biomass and the effects analysis of the feedstock on products yield and their characteristics. J. Anal. Appl. Pyrolysis 2025, 186, 106978. [Google Scholar] [CrossRef]
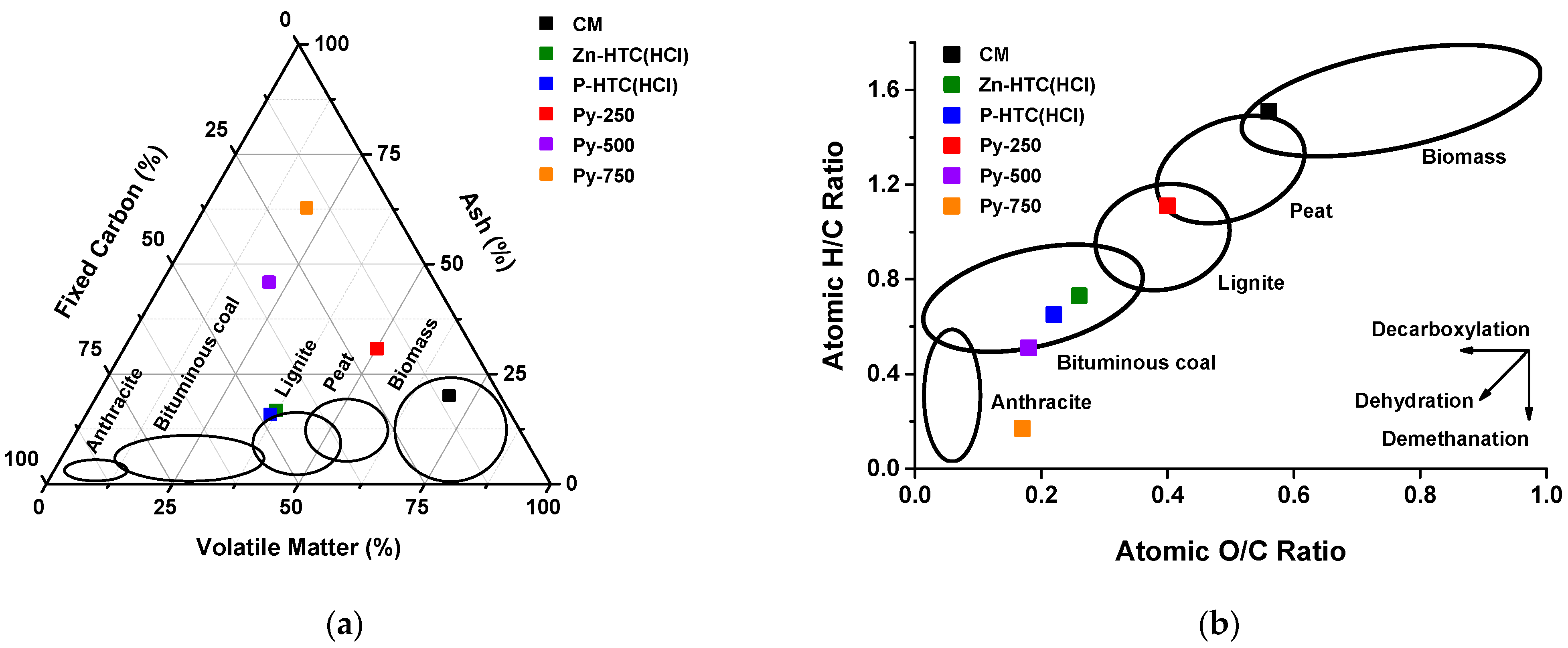
| Sample | FC (wt.%) | VM (wt.%) | Ash (wt.%) | FR | C (wt.%) | H (wt.%) | N (wt.%) | O (wt.%) | S (wt.%) | H/C | O/C | SSA (m2/g) | HHV (MJ/kg) | Yield (wt.%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CM | 10.1 (0.5) | 69.8 (1.5) | 20.1 (0.3) | 0.14 (0.01) | 40.4 (0.3) | 5.1 (0.1) | 3.3 (0.1) | 30.1 (0.5) | 1.0 (0.1) | 1.51 (0.05) | 0.56 (0.01) | 0.70 | 15.90 (0.16) | - |
| Control | 27.1 (1.1) | 31.3 (0.5) | 41.6 (1.0) | 0.87 (0.09) | 39.4 (0.1) | 2.7 (0.1) | 4.2 (0.2) | 11.7 (0.4) | 0.4 (0.1) | 0.82 (0.02) | 0.22 (0.02) | 4.18 | 14.44 (0.11) | 47.3 (2.1) |
| Zn-HTC | 40.5 (1.6) | 32.0 (0.4) | 27.5 (0.6) | 1.27 (0.03) | 47.7 (0.1) | 3.1 (0.1) | 4.2 (0.1) | 17.2 (0.4) | 0.3 (0.1) | 0.78 (0.01) | 0.27 (0.02) | 8.79 | 17.47 (0.14) | 43.6 (1.8) |
| P-HTC | 36.6 (1.6) | 34.1 (0.6) | 29.3 (0.5) | 1.07 (0.01) | 45.6 (0.2) | 3.0 (0.1) | 4.7 (0.1) | 16.8 (0.4) | 0.6 (0.1) | 0.79 (0.01) | 0.28 (0.01) | 7.91 | 16.66 (0.19) | 36.1 (1.2) |
| Sample | FC (wt.%) | VM (wt.%) | Ash (wt.%) | FR | C (wt.%) | H (wt.%) | N (wt.%) | O (wt.%) | S (wt.%) | H/C | O/C | SSA (m2/g) | HHV (MJ/kg) | Yield (wt.%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn-HTC(HCl) | 46.1 (0.5) | 37.2 (0.3) | 16.7 (0.1) | 1.24 (0.01) | 56.2 (0.3) | 3.4 (0.2) | 3.8 (0.1) | 19.6 (0.4) | 0.3 (0.1) | 0.73 (0.01) | 0.26 (0.01) | 33.19 | 20.73 (0.21) | 31.0 (0.3) |
| Zn-HTC(Fe) | 43.3 (1.1) | 35.8 (0.4) | 20.9 (0.8) | 1.21 (0.02) | 54.0 (0.2) | 3.0 (0.2) | 4.1 (0.1) | 17.7 (0.3) | 0.3 (0.1) | 0.67 (0.02) | 0.25 (0.01) | 24.61 | 19.65 (0.10) | 23.3 (0.8) |
| P-HTC(HCl) | 47.7 (0.5) | 36.5 (0.2) | 15.8 (0.1) | 1.31 (0.04) | 59.5 (0.2) | 3.2 (0.1) | 3.7 (0.1) | 17.4 (0.5) | 0.4 (0.1) | 0.65 (0.01) | 0.22 (0.01) | 19.00 | 21.94 (0.11) | 33.3 (0.2) |
| P-HTC(Fe) | 45.7 (0.6) | 35.4 (0.6) | 18.9 (0.3) | 1.29 (0.01) | 56.8 (0.1) | 3.4 (0.1) | 3.6 (0.1) | 16.9 (0.4) | 0.4 (0.1) | 0.72 (0.01) | 0.22 (0.01) | 14.26 | 21.19 (0.13) | 25.0 (1.0) |
| Sample | FC (wt.%) | VM (wt.%) | Ash (wt.%) | FR | C (wt.%) | H (wt.%) | N (wt.%) | O (wt.%) | S (wt.%) | H/C | O/C | SSA (m2/g) | HHV (MJ/kg) | Yield (wt.%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn-HTC(HCl) | 46.1 (0.5) | 37.2 (0.3) | 16.7 (0.1) | 1.24 (0.01) | 56.2 (0.3) | 3.4 (0.2) | 3.8 (0.1) | 19.6 (0.4) | 0.3 (0.1) | 0.73 (0.01) | 0.26 (0.01) | 33.19 | 20.73 (0.21) | 31.0 (0.3) |
| P-HTC(HCl) | 47.7 (0.5) | 36.5 (0.2) | 15.8 (0.1) | 1.31 (0.04) | 59.5 (0.2) | 3.2 (0.1) | 3.7 (0.1) | 17.4 (0.5) | 0.4 (0.1) | 0.65 (0.01) | 0.22 (0.01) | 19.00 | 21.94 (0.11) | 33.3 (0.2) |
| Py-250 | 19.1 (0.2) | 50.1 (0.6) | 30.8 (0.2) | 0.38 (0.01) | 40.0 (0.3) | 3.7 (0.1) | 4.1 (0.2) | 21.1 (0.2) | 0.3 (0.1) | 1.11 (0.02) | 0.40 (0.01) | 1.77 | 14.93 (0.35) | 71.2 (2.6) |
| Py-500 | 32.9 (0.4) | 21.2 (0.1) | 45.9 (0.3) | 1.55 (0.03) | 40.1 (0.3) | 1.7 (0.1) | 2.8 (0.1) | 9.4 (0.2) | 0.1 (0.1) | 0.51 (0.01) | 0.18 (0.01) | 14.73 | 13.79 (0.19) | 42.7 (3.3) |
| Py-750 | 17.0 (0.2) | 20.2 (0.1) | 62.8 (0.5) | 0.84 (0.01) | 28.2 (0.3) | 0.4 (0.1) | 1.9 (0.1) | 6.4 (0.1) | 0.3 (0.1) | 0.17 (0.01) | 0.17 (0.01) | 119.85 | 8.28 (0.09) | 31.6 (2.1) |
| Sample | As (mg/kg) | Cd (mg/kg) | Pb (mg/kg) | Cr (mg/kg) | Cu (mg/kg) | Ni (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|---|---|---|
| Global Standard 1 | 13 | 1.5 | 120 | 90 | 100 | 50 | 400 |
| CM | 0.51 (0.01) | <0.10 | <1.5 | 5.4 (0.6) | 248 (4) | 7.0 (0.4) | 306 (12) |
| Zn-HTC(HCl) | 0.88 (0.01) | <0.10 | <1.5 | 6.2 (0.5) | 222 (2) | 6.3 (0.5) | 12569 (132) |
| P-HTC(HCl) | 0.98 (0.01) | <0.10 | 2.3 (0.1) | 59.7 (0.9) | 143 (6) | 40.3 (1.2) | 71 (6) |
| Py-250 | 1.43 (0.02) | <0.10 | 1.8 (0.1) | 17.9 (0.8) | 395 (9) | 12.9 (0.9) | 661 (16) |
| Py-500 | 7.51 (0.01) | <0.10 | 2.9 (0.1) | 66.9 (1.6) | 466 (8) | 45.6 (1.1) | 2191 (56) |
| Py-750 | 9.42 (0.01) | <0.10 | 3.2 (0.1) | 97.4 (2.1) | 633 (9) | 55.0 (1.7) | 2331 (38) |
| Sample | H/C | Yield (wt.%) | FC (wt.%) | MRT (years) | BC+100 (wt.%) | TPC (gcarbon/kgbiochar) | CRP (gCO2-eq/kgbiochar) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Zn-HTC(HCl) | 0.73 (0.01) | 31.0 (0.3) | 46.1 (0.5) | 441 (34) | 60.3 (6.0) | 142.9 (14.3) | 419.2 (22.0) | This study |
| P-HTC(HCl) | 0.65 (0.01) | 33.3 (0.2) | 47.7 (0.5) | 571 (37) | 65.2 (6.6) | 158.8 (15.9) | 465.9 (26.3) | |
| Py-250 | 1.11 (0.02) | 71.2 (2.6) | 19.1 (0.2) | 129 (26) | 36.6 (4.7) | 136.0 (11.7) | 398.9 (29.1) | |
| Py-500 | 0.51 (0.01) | 42.7 (3.3) | 32.9 (0.4) | 884 (92) | 73.7 (9.2) | 140.5 (10.3) | 412.1 (28.4) | |
| Py-750 | 0.17 (0.01) | 31.6 (2.1) | 17.0 (0.2) | 2611 (161) | 94.5 (9.4) | 53.7 (5.8) | 157.6 (16.0) | |
| CM Biochar-300 | 0.95 | 68.8 | 19.2 | 215 | 46.5 | 132.1 | 387.5 | [55] |
| CM Biochar-550 | 0.61 | 37.2 | 26.8 | 639 | 67.4 | 99.7 | 292.4 | [56] |
| CM Biochar-600 | 0.28 | 35.6 | 37.5 | 1837 | 87.8 | 133.5 | 391.6 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.-Y.; Ho, T.T.-T.; Nadeem, A.; Kim, S.-S.; Choe, K.; Lee, J.-Y. Optimized Biochar from Chicken Manure via Hydrothermal Activation and Catalytic HTC: Properties and CO2 Reduction Potential. Fuels 2025, 6, 41. https://doi.org/10.3390/fuels6020041
Yoo S-Y, Ho TT-T, Nadeem A, Kim S-S, Choe K, Lee J-Y. Optimized Biochar from Chicken Manure via Hydrothermal Activation and Catalytic HTC: Properties and CO2 Reduction Potential. Fuels. 2025; 6(2):41. https://doi.org/10.3390/fuels6020041
Chicago/Turabian StyleYoo, Seong-Yeun, Thi. Thu-Trang Ho, Ahmad Nadeem, Seong-Su Kim, Kangil Choe, and Jai-Young Lee. 2025. "Optimized Biochar from Chicken Manure via Hydrothermal Activation and Catalytic HTC: Properties and CO2 Reduction Potential" Fuels 6, no. 2: 41. https://doi.org/10.3390/fuels6020041
APA StyleYoo, S.-Y., Ho, T. T.-T., Nadeem, A., Kim, S.-S., Choe, K., & Lee, J.-Y. (2025). Optimized Biochar from Chicken Manure via Hydrothermal Activation and Catalytic HTC: Properties and CO2 Reduction Potential. Fuels, 6(2), 41. https://doi.org/10.3390/fuels6020041







