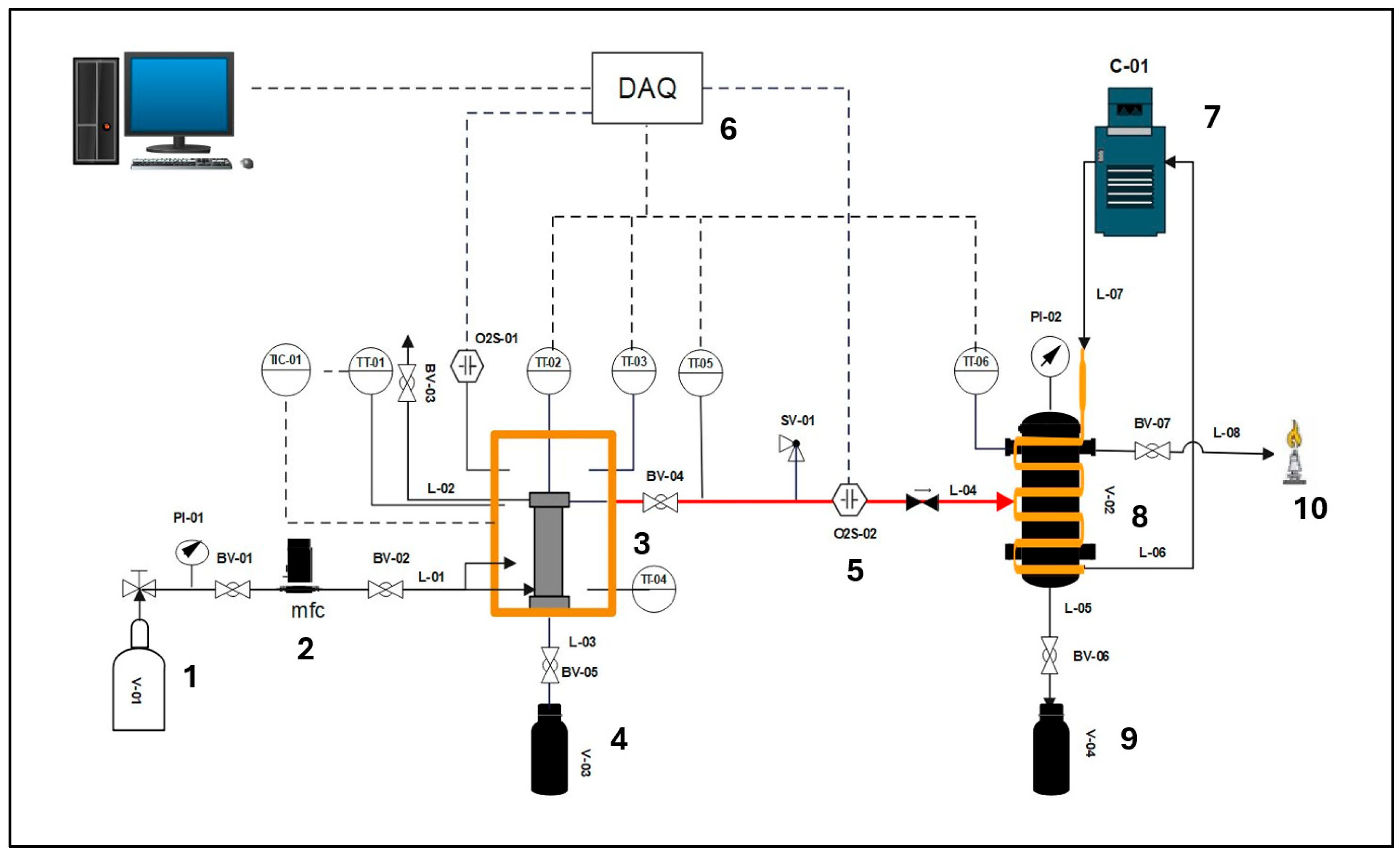
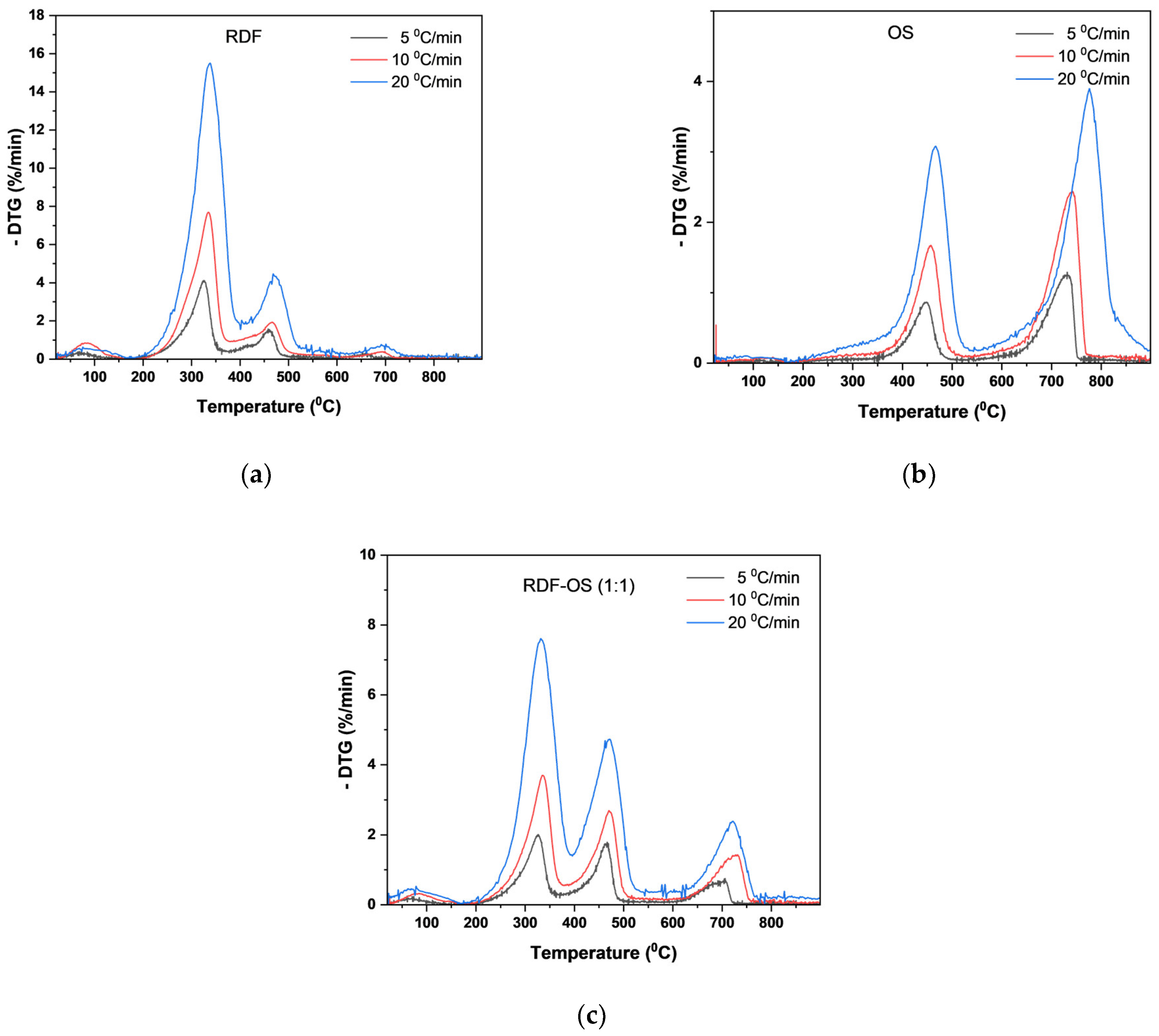
3.4.1. Proximate and Ultimate Analysis of Char
As shown in
Table 5, the RDF char produced in this study exhibited notable differences in its elemental composition compared to values reported in the literature at 650 °C [
11], particularly in terms of carbon, hydrogen, nitrogen, and sulfur content. The carbon content of RDF char in this study (50.10%) was higher than the 45.23% reported in the literature, indicating a greater retention of carbonaceous material, which enhances its potential as a solid fuel. Similarly, the hydrogen content (3.21%) was significantly higher than the 0.98% observed in the literature, suggesting that the RDF feedstock used in this study contained a higher fraction of hydrogen-rich compounds, likely originating from plastic or biomass components. In contrast, the nitrogen content (0.55%) was lower than the 1.31% reported in the literature, suggesting a reduced presence of nitrogenous compounds in the RDF feedstock, which may contribute to lower NOx emissions upon combustion. Additionally, the sulfur content (0.23%) was considerably lower than the 0.71% reported in the literature, which is advantageous in minimizing sulfur-related emissions and mitigating environmental concerns associated with RDF char utilization. These variations underscore the influence of feedstock composition and pyrolysis conditions on the physicochemical properties of RDF char, which ultimately affect its combustion characteristics, emission profile, and suitability for energy recovery applications.
The OS char exhibits drastically different properties due to the mineral-rich nature of oil shale. It has an extremely low fixed carbon content of 3.52%, coupled with high ash content (76.27%), reflecting the dominance of inorganic compounds in the residual char. The ultimate analysis shows a low carbon content (9.03%) and hydrogen content (0.16%), contributing to its very low calorific value of 0.53 MJ/kg. The sulfur content is notably higher in OS char (0.54%) compared to RDF, which can influence its environmental impact when used for energy applications.
These findings are supported by a study of Huadian oil shale and its shale char, which reported a significant reduction in carbon content from 27.33% in raw oil shale to 17.88% in shale char, along with a drastic increase in ash content from 48.24% to 73.79% after pyrolysis. Additionally, the study noted a decrease in volatile matter from 36.21% to 16.16% and an increase in fixed carbon from 4.02% to 9.84%, indicating the partial retention of carbonaceous material after pyrolysis [
63]. These findings align well with the results obtained in this study, further reinforcing the high ash content and low energy potential of OS char.
Similarly, another study of shale char properties reported comparable trends. The study found that shale char exhibited a carbon content of 17.99%, a hydrogen content of 0.88%, and an oxygen content of 5.42%, confirming the low organic composition of OS char. The ash content was measured at 73.44%, while volatile matter decreased significantly to 16.08% after pyrolysis, highlighting the loss of organic components. The fixed carbon content increased to 9.79%, further supporting the partial retention of carbonaceous material [
64].
These findings align well with the results obtained in this study, reinforcing the high ash content, low carbon retention, and poor energy potential of OS char. The comparative analysis highlights that despite slight variations in values, the general trend remains consistent across multiple studies—showing that OS char is predominantly mineral-rich with limited fuel applications, unless further treated or blended with other feedstocks to enhance its energy properties.
The RDF-OS co-pyrolysis char exhibits intermediate properties, reflecting a blend of organic and inorganic characteristics from both RDF and OS. The volatile matter content decreases to 18.57%, suggesting interactions between RDF and OS during pyrolysis. The fixed carbon content (13.03%) is lower than RDF but higher than OS, indicating that OS dilution affects carbon retention. The ash content is 68.39%, significantly higher than RDF but slightly lower than OS, confirming the dominant influence of OS minerals in the blend.
The ultimate analysis shows that RDF-OS char has moderate carbon content (18.65%) and a hydrogen content of 0.56%, suggesting a balance between RDF’s carbon-rich nature and OS’s mineral-heavy composition. The calorific value (4.90 MJ/kg), while lower than RDF, is notably higher than OS, suggesting potential applications in energy recovery.
These results demonstrate that co-pyrolysis offers a way to balance energy content and mineral composition, making RDF-OS char a viable material for fuel applications while mitigating RDF waste and utilizing underutilized fossil resources.
3.4.2. X-Ray Photoelectron Spectroscopy Analysis of Char
X-ray photoelectron spectroscopy (XPS) was utilized to investigate the surface chemical composition and bonding states of chars derived from the pyrolysis of RDF, OS, and their mixture (RDF-OS). The results provide insight into the elemental transformations, mineral interactions, and retention of different functionalities during pyrolysis.
The XPS analysis provides key parameters, including binding energy (BE), which refers to the energy required to remove an electron from a specific atomic orbital and helps identify the chemical state of elements. The height (CPS, counts per second) represents the maximum intensity of a spectral peak, indicating the relative abundance of an element. Full width at half maximum (FWHM, eV) describes the broadness of the peak at half of its maximum intensity, reflecting the chemical environment and sample uniformity [
65]. Lastly, peak area (Area (P) CPS·eV) represents the integrated intensity of the peak, providing a more accurate measure of elemental concentration compared to peak height alone
Table 6.
As depicted in
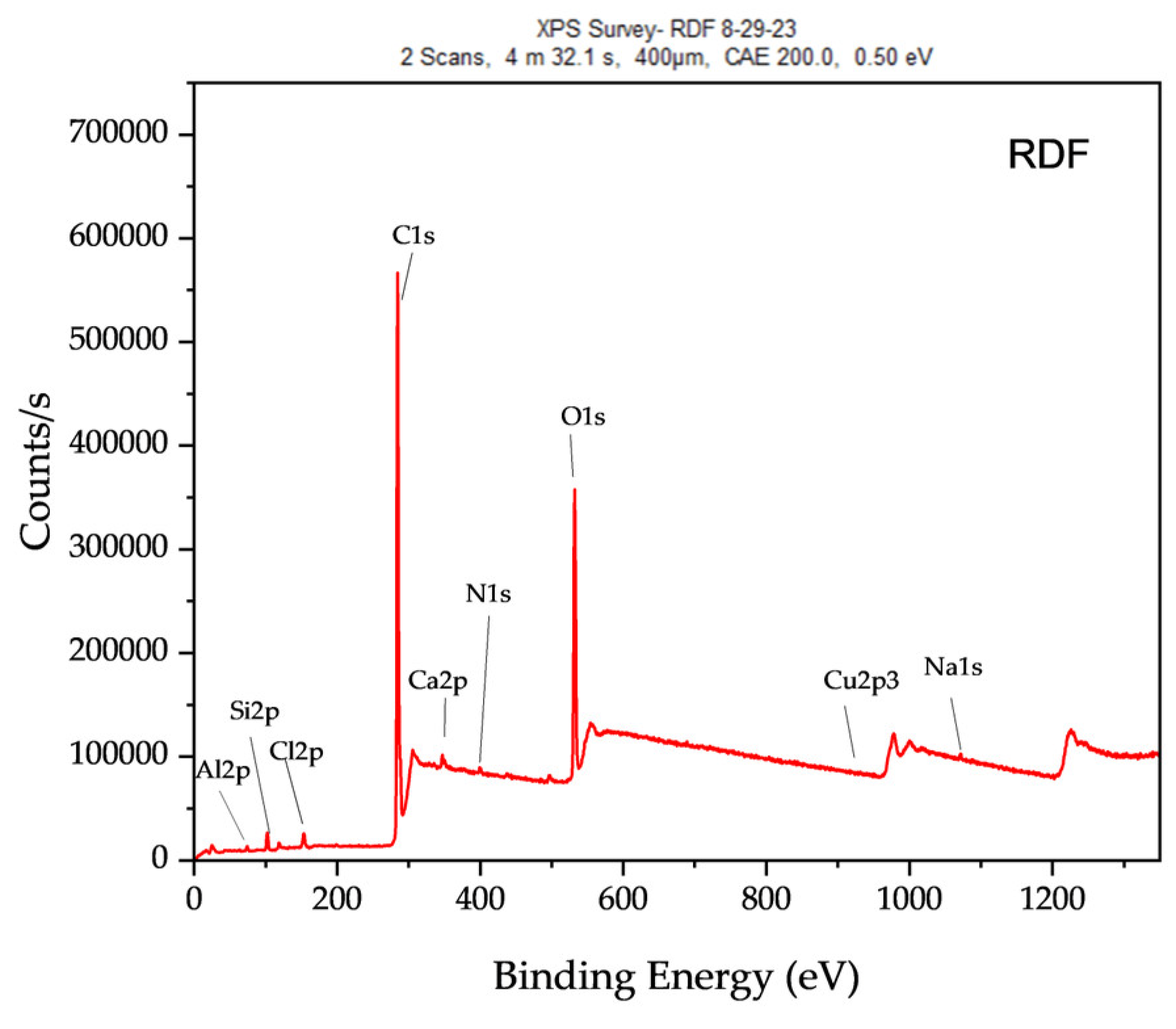
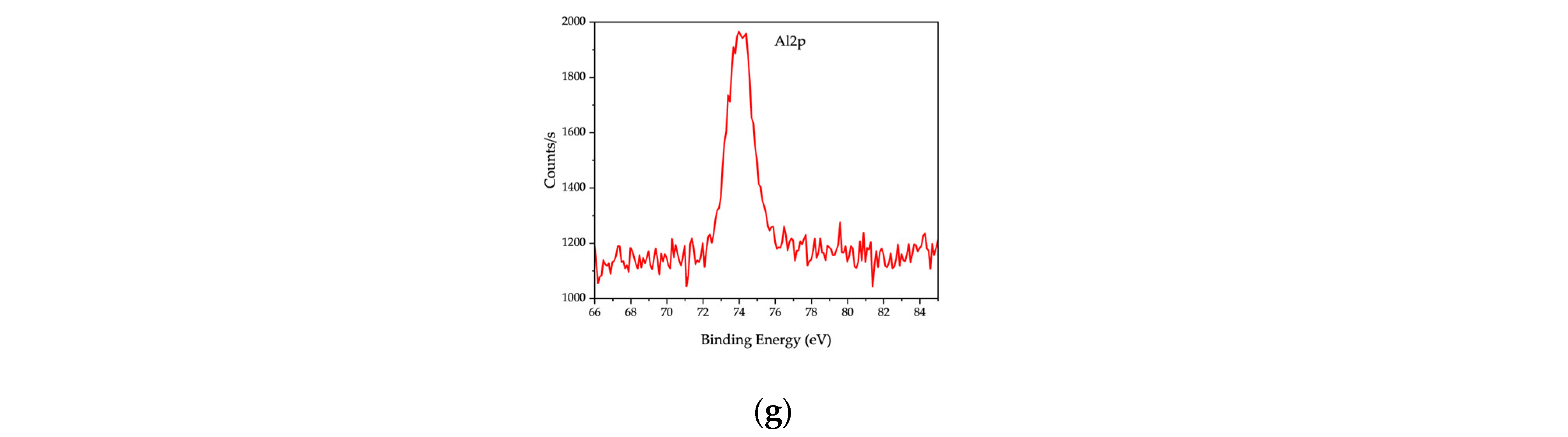
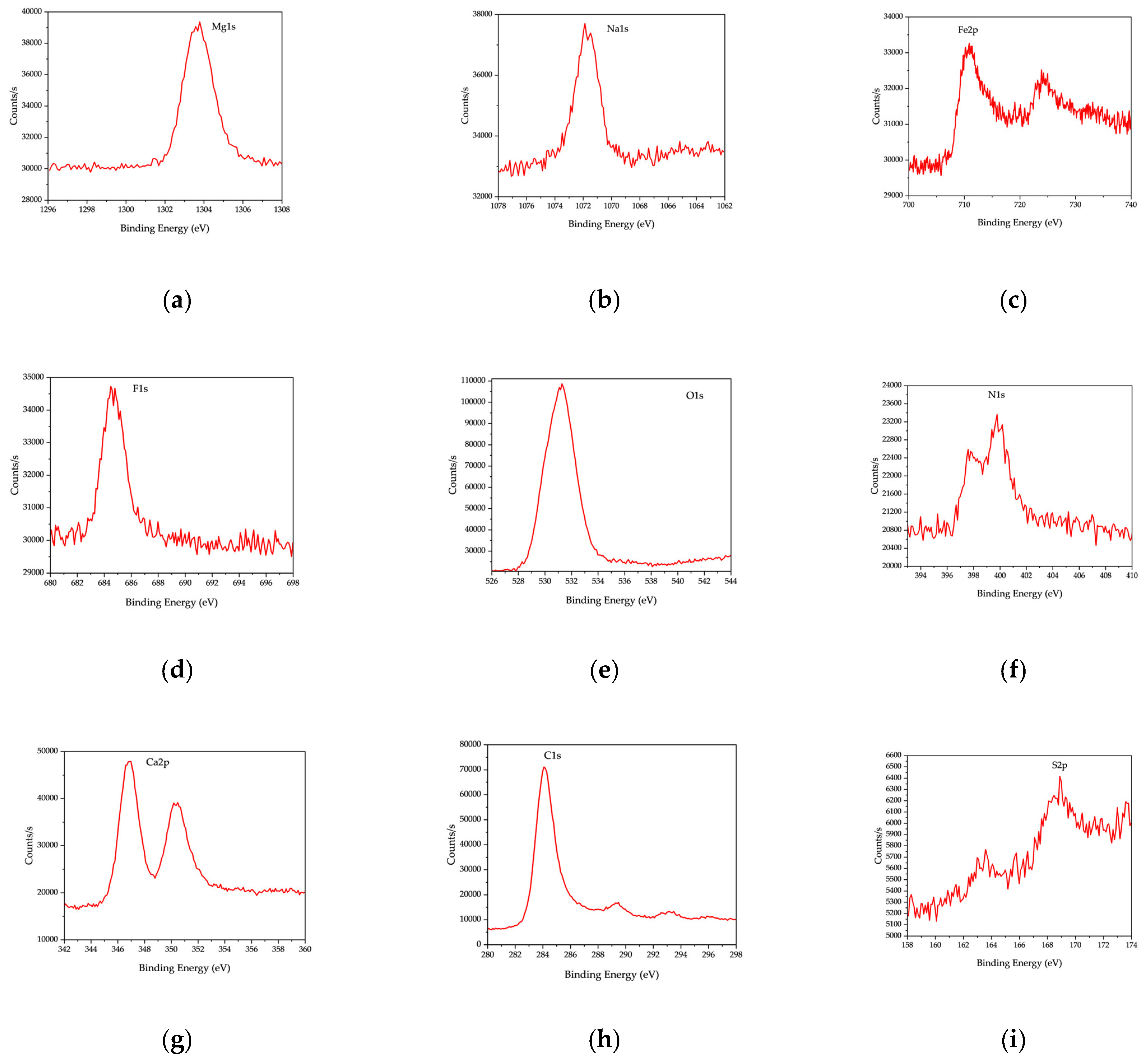
Figure 11 and
Table 7, the XPS spectrum of RDF char revealed carbon (C 1s, ~285 eV) as the dominant peak (66.44%), indicating a high carbon content, characteristic of char derived from organic-rich feedstocks. The presence of oxygen (O 1s, ~21.43%) suggests surface oxygen-containing functional groups, such as carbonyl, hydroxyl, and carboxyl species, which influence RDF char’s reactivity and adsorption capabilities.
In addition to carbon and oxygen, the XPS spectrum identified several inorganic elements, including silicon (Si 2p, 5.26%), aluminum (Al 2p, 2.6%), calcium (Ca 2p, 2.02%), nitrogen (N 1s, 1.38%), sodium (Na 1s, 0.47%), chlorine (Cl 2p, 0.31%), and copper (Cu 2p, 0.09%). These elements originate from inorganic additives, salts, and residual metals in RDF feedstock, influencing the surface chemistry of the resulting char. The presence of chlorine is particularly significant, as it suggests potential implications for catalytic activity and environmental emissions when RDF char is utilized in thermochemical applications.
The catalytic potential of RDF char has been demonstrated in previous studies. Li and Williams [
66] highlighted that RDF-derived char can function as an effective catalyst in steam reforming, enhancing hydrogen production and syngas quality due to its high metal content, including Ca, Al, and Fe. Kusz et al. investigated the catalytic properties of RDF-derived char for tar decomposition, showing that iron-enriched char (Fe 6–11 wt%) significantly enhances CO and H
2 production while reducing CO
2 and CH
4 emissions [
67]. These findings reinforce the potential multi-functionality of RDF char, not only as a carbonaceous adsorbent but also as a low-cost catalyst for hydrogen production and syngas upgrading.
As shown in
Figure 12, the XRD analysis of OS char reveals a composition rich in quartz (SiO
2), calcite (CaCO
3), muscovite (KAl
2(AlSi
3O
10)(OH)
2), kaolinite (Al
2Si
2O
5(OH)
4), and iron sulfides (FeS/FeS
2). These results indicate that OS char retains a significant fraction of its original mineral matrix post-pyrolysis. The dominance of quartz and calcite aligns with previous studies highlighting the high silica and carbonate content of oil shale residues. Li and Ma (2016) reported similar findings, where XRD analysis demonstrated the persistence of quartz and calcite as major mineral phases in oil shale char due to their thermal stability [
63].
The presence of iron sulfides suggests incomplete oxidation of pyrite during pyrolysis, leading to FeS retention in the char. This observation is consistent with the findings of Fan et al., who identified pyrite-derived FeS phases in shale char that subsequently transformed into Fe
2O
3 (hematite) under oxidative conditions. Moreover, the persistence of muscovite and kaolinite suggests that the clay mineral structure of OS remains relatively stable under pyrolysis conditions, with phase transitions occurring at higher temperatures. Fan et al. further demonstrated that the decomposition of kaolinite begins at around 500 °C, with muscovite undergoing gradual transformation at temperatures exceeding 700 °C [
64].
Additionally, the mineral matrix significantly influences the pyrolysis behavior of OS char. The interaction between inorganic minerals and organic matter can alter the thermal decomposition pathways, delaying the release of volatile components and influencing the oxidation reactions of residual carbon. Studies have shown that inorganic components, such as carbonate-associated cations, may act as oxidation catalysts, enhancing the reactivity of shale char during combustion.
These findings highlight the importance of mineralogical composition in shaping the thermal stability and reaction mechanisms of OS char, reinforcing the role of quartz, calcite, and iron sulfides as key indicators of oil shale residue transformation.
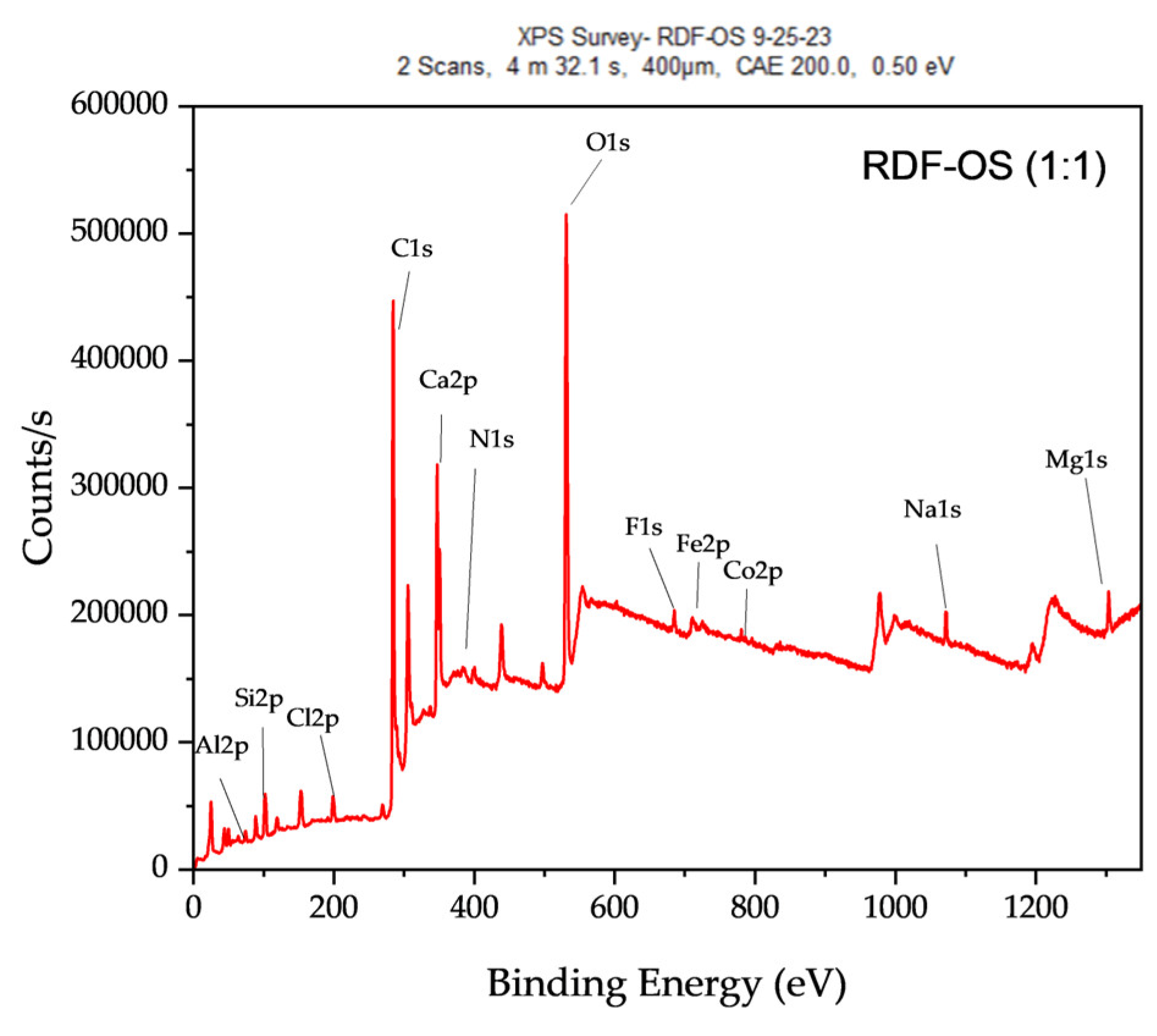
The XRD spectrum of RDF-OS char (
Figure 13) reflects a hybrid composition, with quartz and calcite remaining dominant. The presence of muscovite and kaolinite suggests that stable aluminosilicate phases persist post-pyrolysis, contributing to the structural stability of the char. The incorporation of transition metals from OS such as Fe and Co indicates potential catalytic properties, while the detection of NaCl and MgO suggests that certain inorganic salts from RDF remain stable in the co-pyrolyzed product.
The elemental composition of RDF-OS char reveals an intermediate carbon content (38.67%), bridging the gap between RDF (66.44%) and OS (33.68%). The increased calcium concentration (14.61%) reflects the mineral influence of OS, while the presence of cobalt (0.94%) suggests incorporation from RDF waste components, potentially enhancing catalytic activity. Additionally, chlorine (2.7%) is detected in RDF-OS char but absent in OS char, indicating retention from RDF waste.
The high-resolution XPS spectra for RDF, OS, and RDF-OS chars (
Figure 14,
Figure 15 and
Figure 16) provide deeper insights into the electronic states of key elements. The interpretation of the elemental peaks was based on the research studies referenced in [
66,
68,
69,
70,
71,
72,
73,
74] as summarized in
Table 8.
In
Figure 14, the deconvoluted C1s spectrum of RDF char shows distinct peaks corresponding to graphitic carbon (C=C, ~284.3 eV), oxygenated carbon (C–O, ~286 eV), and carboxyl groups (O–C=O, ~289 eV). Similarly,
Figure 15 reveals that OS char contains significant Si–O (102–104 eV) and metal–oxide (M–O) interactions, indicating the strong mineral matrix of oil shale [
63,
65]. The RDF-OS char spectrum in
Figure 16 exhibits features from both RDF and OS, confirming the hybrid nature of the material, with peaks corresponding to C=C (graphitic), Si–O (silicates), and Ca–O (carbonates).
As shown in
Table 7 and
Table 8, the XPS spectrum of RDF char revealed carbon (C 1s, Peak BE: 284.56 eV, 1,492,940.18 CPS·eV) as the dominant peak, indicating a high carbon content, characteristic of char derived from organic-rich feedstocks. The presence of oxygen (O 1s, Peak BE: 532.05 eV, 880,964.53 CPS·eV) suggests surface oxygen-containing functional groups.
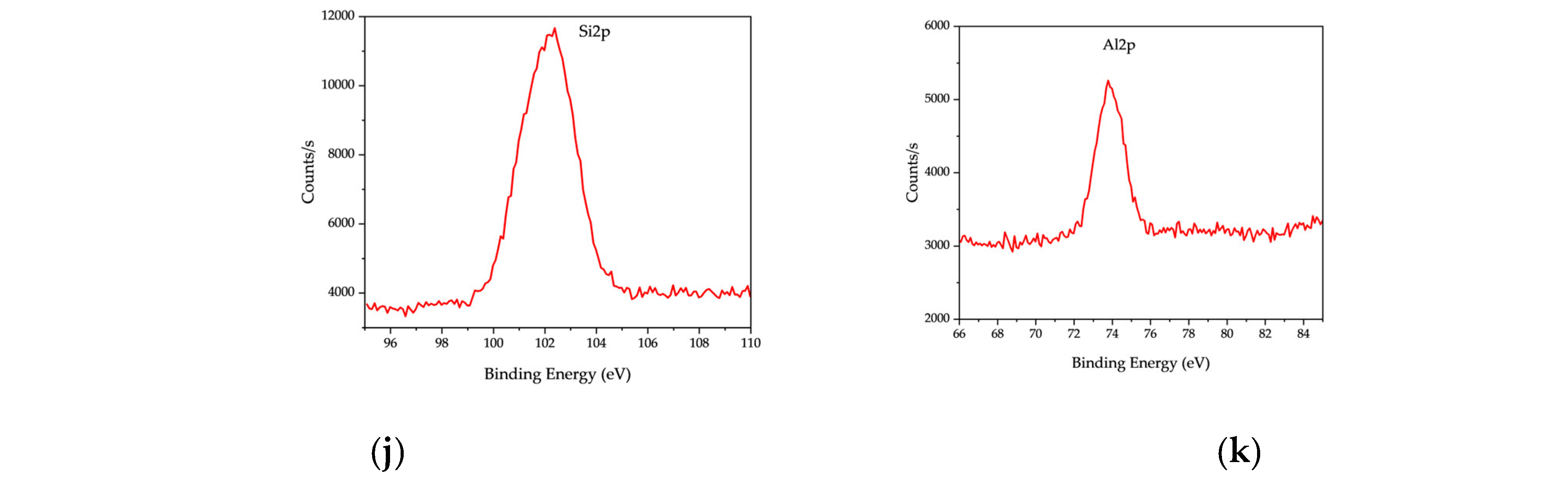
In addition to carbon and oxygen, the XPS spectrum identified several inorganic elements, including silicon (Si 2p, Peak BE: 102.35 eV, 51,176.12 CPS·eV), aluminum (Al 2p, Peak BE: 74.2 eV, 16,615.05 CPS·eV), calcium (Ca 2p, Peak BE: 347.03 eV, 78452.75 CPS·eV), nitrogen (N 1s, Peak BE: 399.19 eV, 41,707.04 CPS·eV), sodium (Na 1s, Peak BE: 1071.46 eV, 27,284.72 CPS·eV), chlorine (Cl 2p, Peak BE: 198.97 eV, 6894.71 CPS·eV), and copper (Cu 2p, Peak BE: 931.78 eV, 3355.88 CPS·eV). These elements originate from inorganic additives, salts, and residual metals in RDF feedstock, influencing the surface chemistry of the resulting char.
The XPS analysis of OS char reveals a composition rich in oxygen (O 1s, Peak BE: 531.24 eV, 1,752,819.16 CPS·eV) and carbon (C 1s, Peak BE: 284.3 eV, 1,146,424.63 CPS·eV), along with significant mineral contributions. The high calcium (Ca 2p, Peak BE: 346.98 eV, 789,727.96 CPS·eV) and silicon (Si 2p, Peak BE: 102.24 eV, 166,824.74 CPS·eV) content suggests that OS char retains a significant fraction of its original mineral matrix post-pyrolysis.
The XPS spectrum of RDF-OS char reflects a hybrid composition, with oxygen (O 1s, Peak BE: 531.09 eV, 1,251,433.6 CPS·eV) and carbon (C 1s, Peak BE: 284.4 eV, 1,186,146.21 CPS·eV) as the dominant components. The presence of calcium (Ca 2p, Peak BE: 347.07 eV, 770,523.38 CPS·eV), silicon (Si 2p, Peak BE: 102.01 eV, 115,972.21 CPS·eV), and magnesium (Mg 1s, Peak BE: 1303.68 eV, 127,953.39 CPS·eV) suggests that OS minerals influenced the char composition, while elements from RDF, such as chlorine (Cl 2p, Peak BE: 684.94 eV, 92,691.99 CPS·eV) and sodium (Na 1s, Peak BE: 1072.04 eV, 84,360.57 CPS·eV), were retained in the final char.
These results demonstrate that co-pyrolysis leads to a redistribution of elements between RDF and OS, affecting the char’s composition and potential applications. The presence of transition metals from OS, such as Fe, suggests that RDF-OS char could possess catalytic properties, while stable aluminosilicate phases contribute to its structural integrity post-pyrolysis. The retention of chlorine and sodium in RDF-OS char, as indicated in
Table 7, suggests potential environmental implications when used in thermochemical applications.
Overall, the XPS results, in combination with the functional group analysis, confirm the unique chemical characteristics of RDF, OS, and RDF-OS chars, emphasizing their potential use in energy and catalytic applications.