Study of the Effect of Alkali Metal Ions (Li+, Na+, K+) in Inhibiting the Spontaneous Combustion of Coal
Abstract
1. Introduction
2. Materials and Methods
2.1. Programmed Warming Experiment
2.1.1. Preparation of Samples
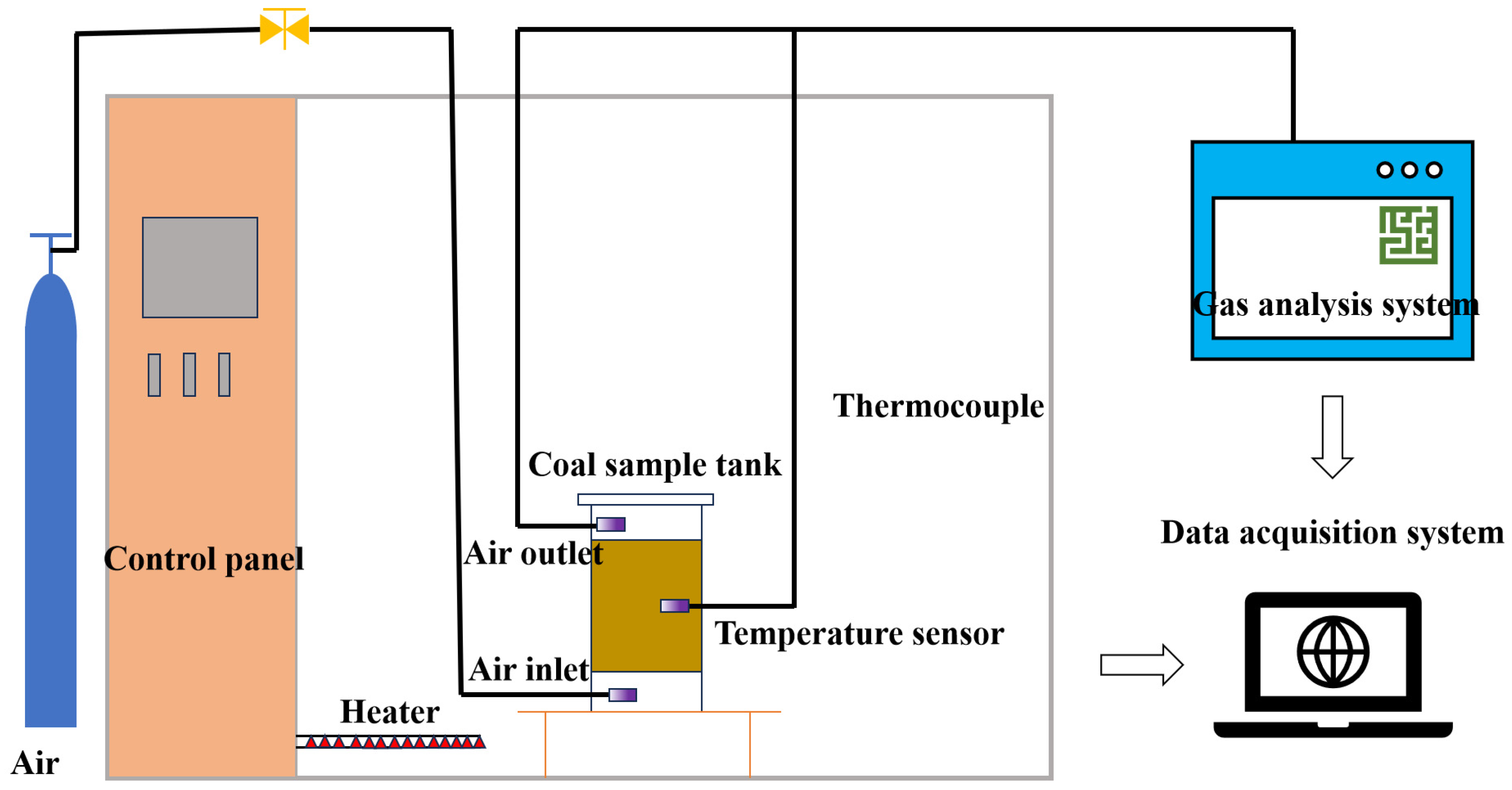
2.1.2. Program Heating Experiment
3. Results and Discussion
3.1. Indicator Gas and Oxygen Consumption Analysis
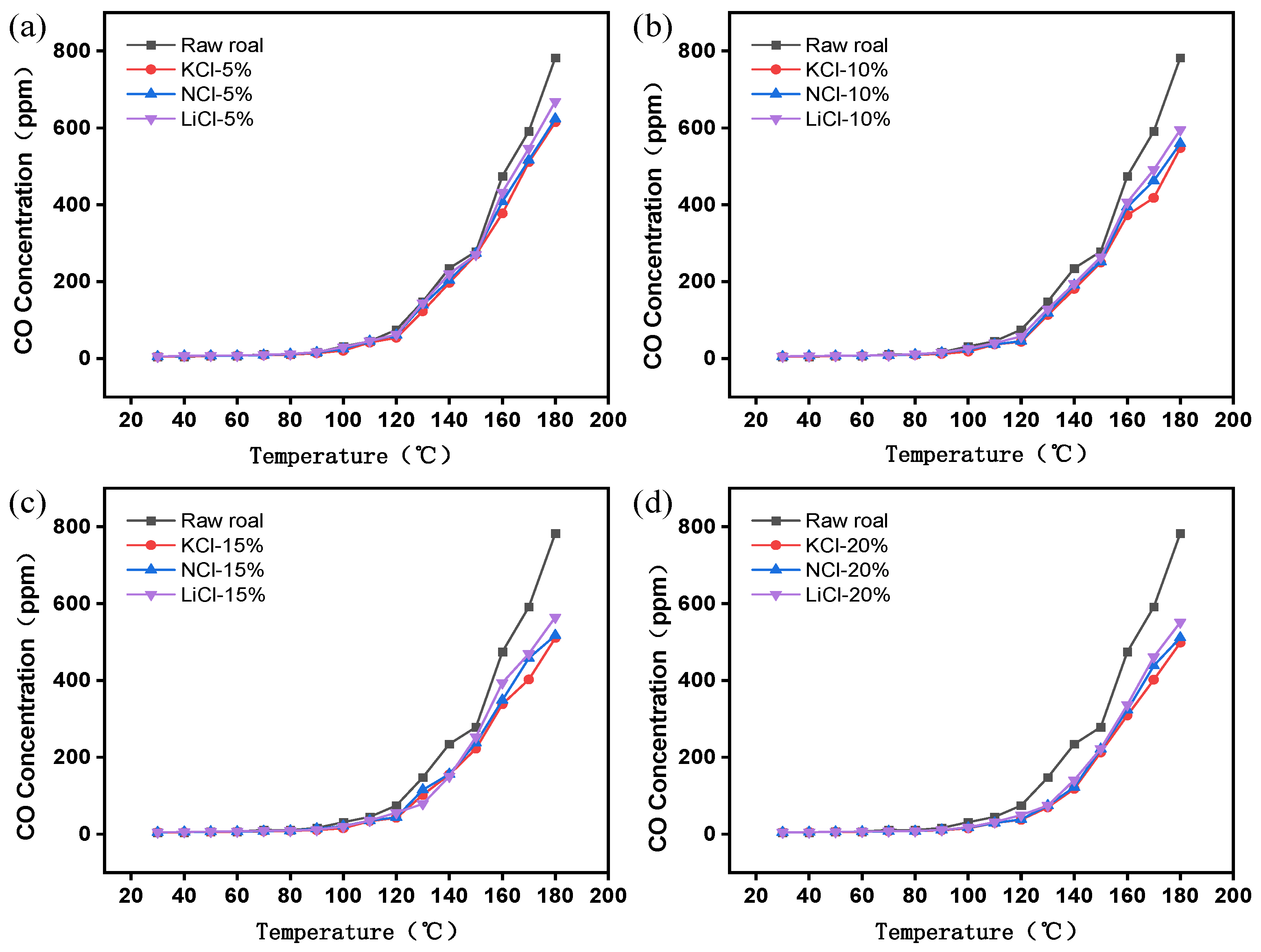
3.1.1. Analysis of Indicator Gases
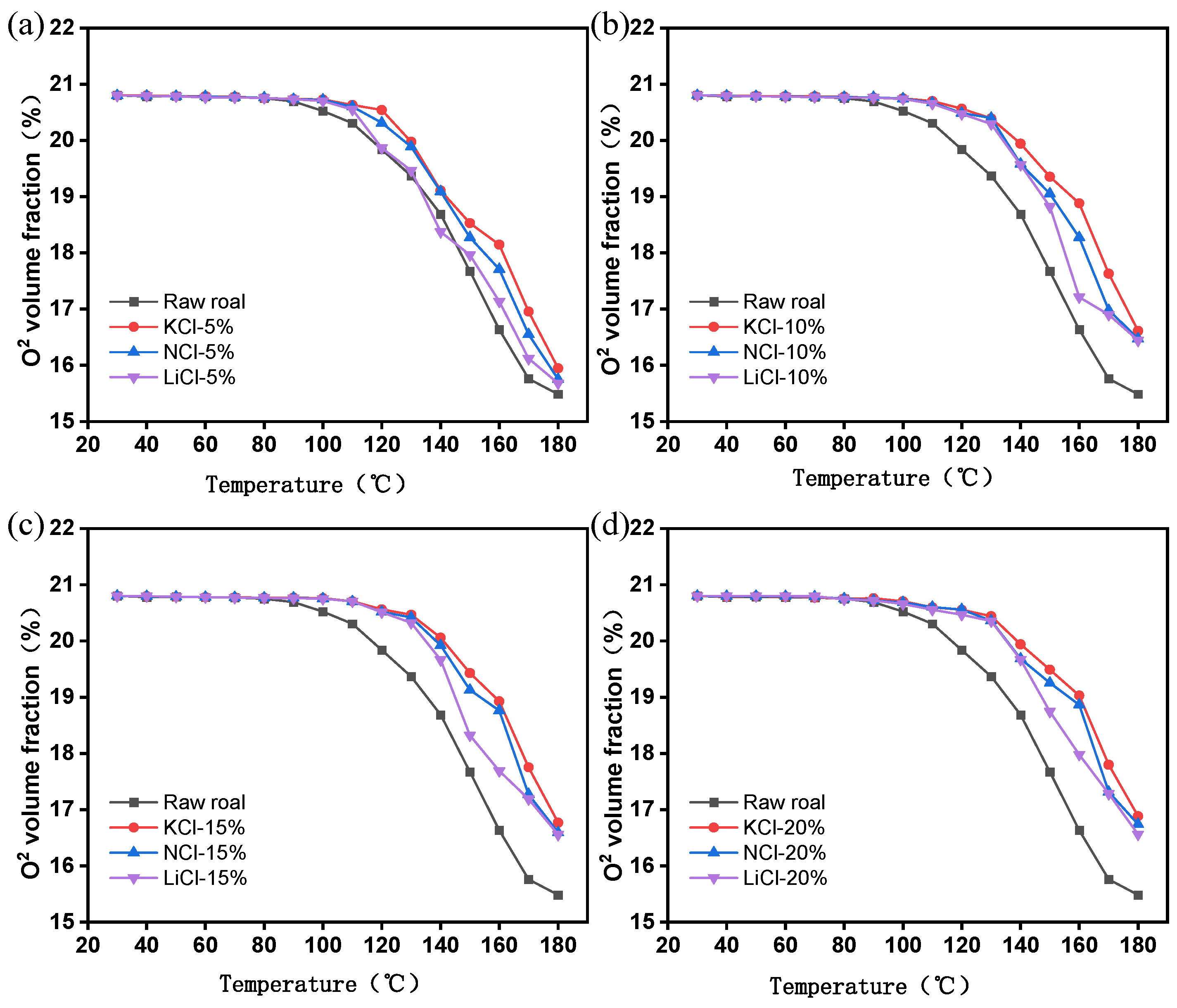
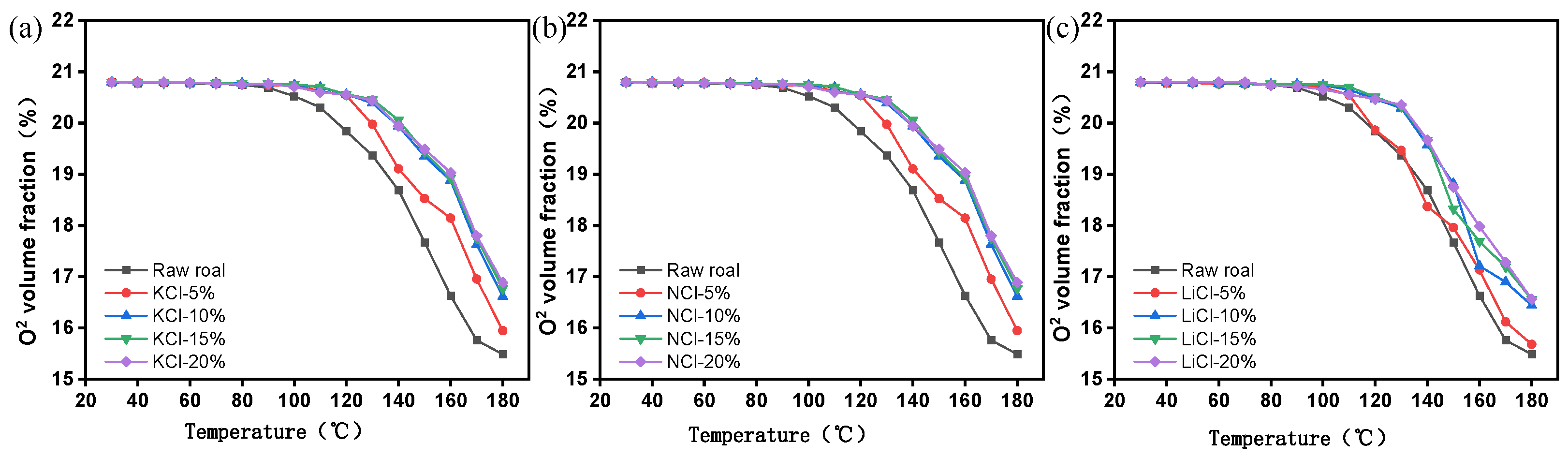
3.1.2. Oxygen Consumption Analysis
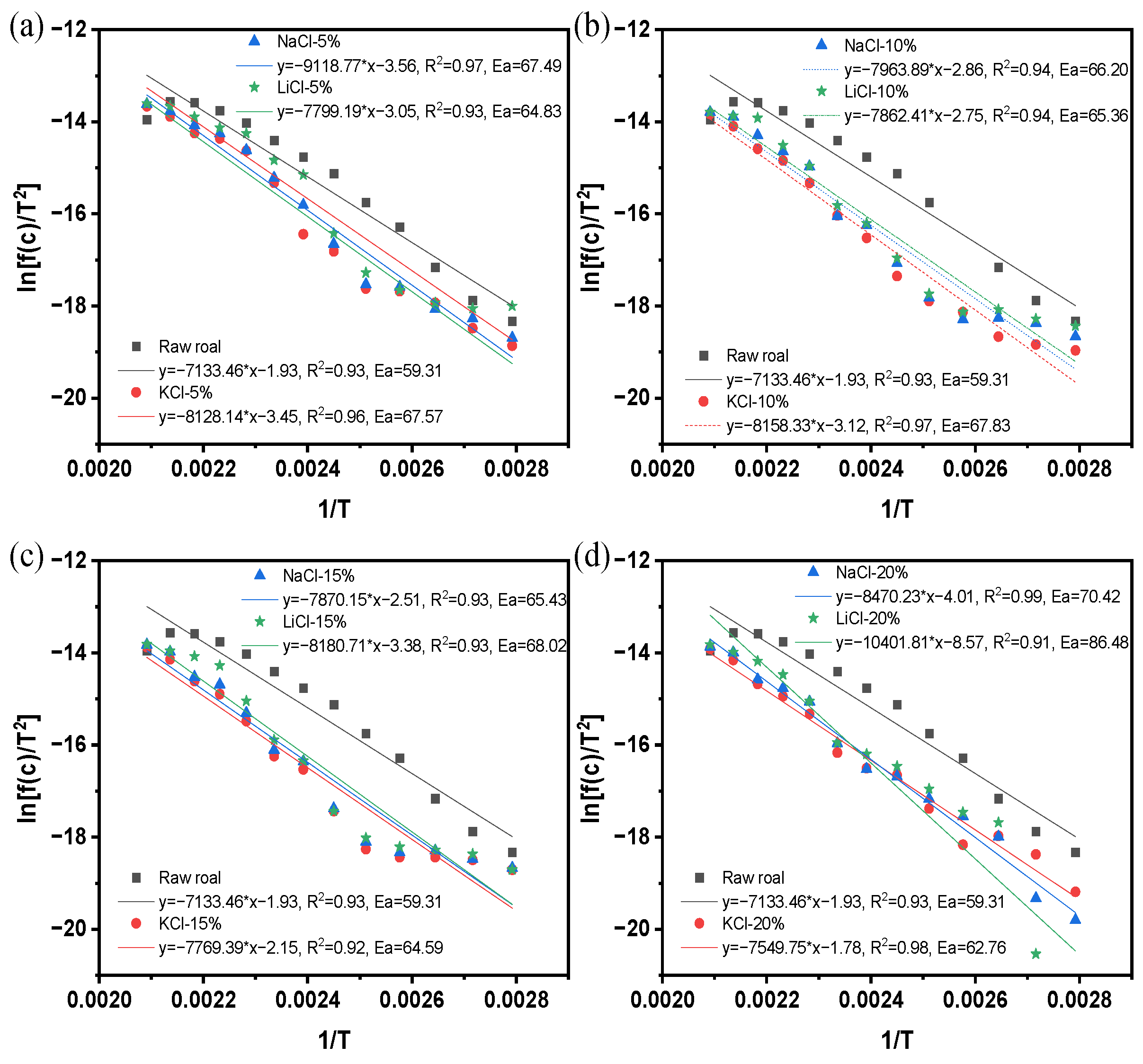
3.1.3. Calculation of Apparent Activation Energy
3.1.4. Retardation Rate
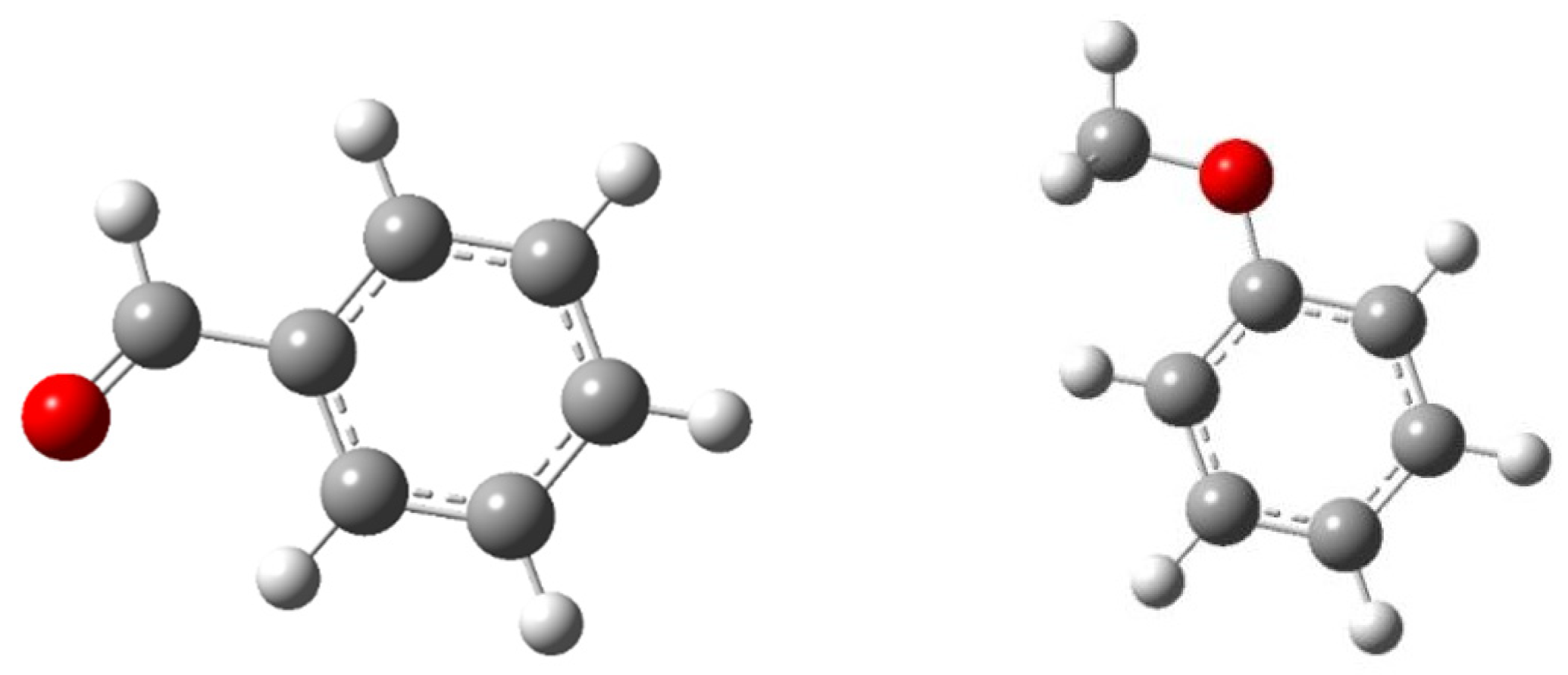
3.2. Geometric Configuration of the Simplified Structure of the Coal Molecule
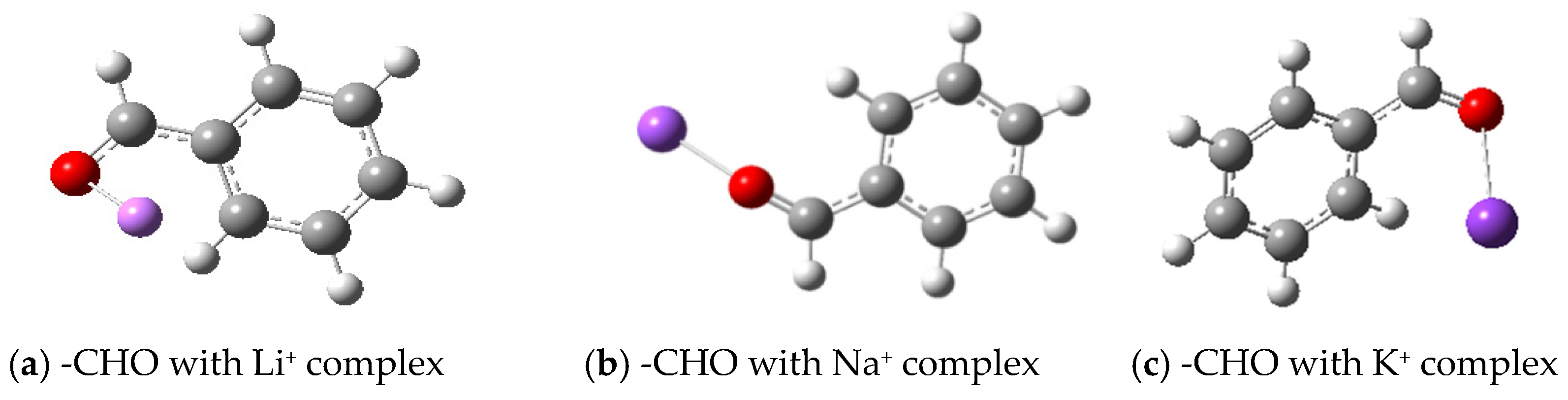
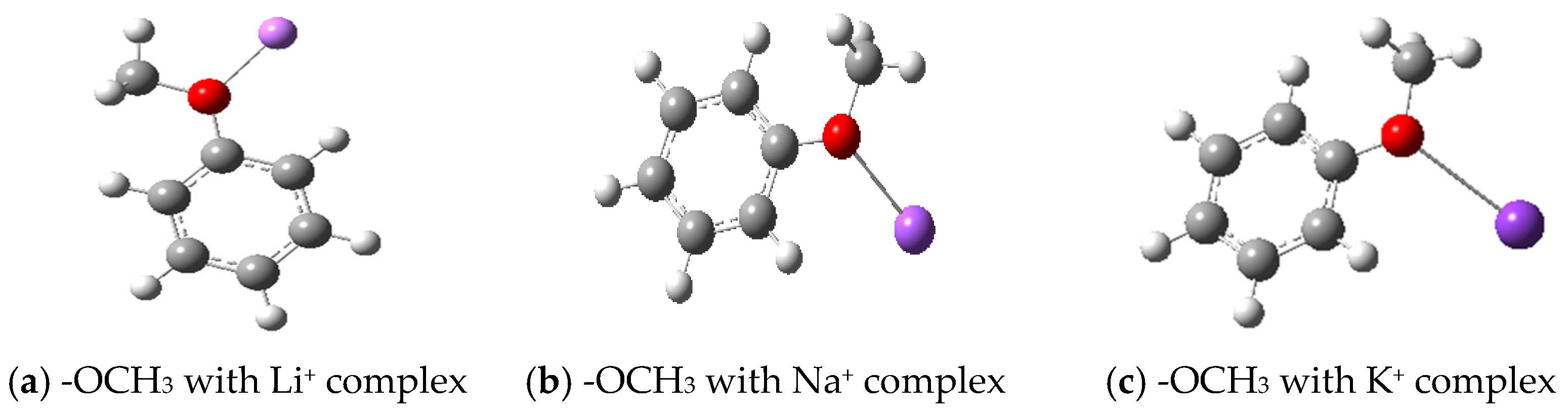
3.2.1. Geometrical Configuration of Complexes
3.2.2. Molecular Frontier Orbitals and Stability Analysis
3.2.3. Analysis of Electrostatic Charge Layout
3.2.4. Transition State Analysis
4. Conclusions
- (1)
- The results of the programmed temperature rise experiments show that the inhibition effect of different concentrations of the retardants on spontaneous combustion of coal is different, and the inhibition effect will be enhanced with the increase in the concentration of the retardants, and the inhibition effect tends to level off when the concentration of the retardants is more than 15%, and the inhibition effect of KCl is the best under the same concentration.
- (2)
- Metal ions form complexes with active groups, reduce the chemical activity of active groups, enhance the antioxidant capacity of coal, and effectively prevent spontaneous combustion of coal. Through Gaussian software simulation, the geometrical configuration, electronic structure and energy changes in the complexes were analyzed, revealing that the coordination bond formed between the metal ions and oxygen atoms reduces the contact between the reactive groups and oxygen, and delays the oxidation reaction. The analysis of frontier orbitals and electrostatic charge layouts further confirmed that the addition of metal ions reduced the activity of the reactive groups and improved the stability of the complexes. Transition state analysis showed that the addition of metal ions raised the reaction energy threshold, making the coal oxidation reaction more difficult. Therefore, its application value includes that it can effectively reduce the risk of spontaneous coal combustion and reduce the occurrence of fire and explosion accidents in coal mines; inhibiting spontaneous coal combustion reduces the loss of coal resources and improves the utilization rate of the resources; reduces the emission of hazardous gases (e.g., CO) produced by spontaneous combustion of coal and reduces the pollution to the environment; and reduces the loss of accidents and resource losses, and reduces the economic losses of the coal mining enterprises.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, B.; Zhong, X.; Wang, D.; Xin, H.; Shi, Q. Research Progress on Characteristics and Prevention and Control Technologies of Coal Spontaneous Combustion Process. Coal Sci. Technol. 2021, 49, 66–99. [Google Scholar]
- Wang, S.; Liu, L.; Zhu, M.; Shen, Y.; Shi, Q.; Sun, Q.; Fang, Z.; Ruan, S.; He, W.; Yang, P.; et al. New Ideas for Green and Low-Carbon Development of Coal under the “Dual Carbon” Goal. J. China Coal Soc. 2024, 49, 152–171. [Google Scholar]
- Dai, B.; Wang, D.; Cao, Y.; Liu, X.; Luo, D.; Xia, M.; Kong, X.; Wang, P. Review of China’s Energy Industry in 2023 and Outlook for 2024. Contemp. Pet. Petrochem. 2024, 32, 1–7. [Google Scholar]
- Pei, X.; Yao, Z.; Wang, L.; Shao, H.; Wang, K.; Wu, Z. Virtual simulation experimental system for mine fire monitoring and prevention based on Unity3D. China Saf. Sci. J. 2024, 34, 109–116. [Google Scholar]
- Guo, J.; Research Group on China’s Coal Industry Prosperity Index, Beijing. Research Report on the Economic Situation of China’s Coal Industry in 2023–2024. China Coal 2024, 50, 12–20. [Google Scholar]
- Wang, Q. Strengthening the Foundation of Coal as the Main Energy Source. China Mining News, 5 March 2024; p. 2. [Google Scholar]
- Xue, W.; Jiang, L. Corporate Management and High-Quality Development of Coal Enterprises under the Constraint of “Double Carbon”. Coal Technol. 2023, 42, 251–254. [Google Scholar]
- Guo, J.; Liu, H.; Jin, Y.; Cai, G.; Liu, Y.; Yang, P. Summary of underground hidden coal spontaneous combustion fire source detection methods and prospect of new technologies. China Saf. Sci. J. 2022, 32, 111–119. [Google Scholar]
- Cheng, L.; Sun, J. Statistical and Regularity Analysis of Coal Mine Accidents in China from 2016 to 2022. Coal Eng. 2023, 55, 125–129. [Google Scholar]
- Deng, J.; Wang, J.; Ren, S.; Wang, C.; Qu, G.; Ma, L. Research and Prospect on Identification and Detection Technology of High-Temperature Points of Spontaneous Combustion of Coal in Goaf Areas. J. China Coal Soc. 2024, 49, 885–901. [Google Scholar]
- Huang, F.; Cui, C.; Deng, C. Study on the movement of overburden and the dangerous area of spontaneous combustion of coal in goaf during bivariate coal seam mining. Coal Mine Saf. 2022, 53, 42–47+55. [Google Scholar]
- Li, Y. Mechanism and Early Warning Method of Gas Explosion Induced by High Temperature of Spontaneous Combustion in Goaf. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2024. [Google Scholar]
- Qin, B.; Ma, D. Research Progress and Challenges in the Prevention and Control of Coal Spontaneous Combustion and Gas Complex Disasters in Goaf Areas. J. China Coal Soc. 2025, 50, 392–408. [Google Scholar]
- Li, Z.; Zhang, M.; Yang, Z.; Liu, Y.; Ding, C.; Huang, G. The effect of fault structures on coal structure and spontaneous combustion characteristics. J. China Coal Soc. 2023, 48, 1246–1254. [Google Scholar]
- Ji, Y.; Zhang, Y.; Wang, H.; Huang, Z.; Shao, Z.; Gao, Y.; Yin, Y.; Murali, M. The variation law of oxygen-containing groups during the oxidation and temperature increase of lignite. Min. Res. Dev. 2020, 40, 75–80. [Google Scholar]
- Wang, S. Reflections on the Dominant Position of Coal as the Main Energy Source in China and Green Mining. China Coal 2020, 46, 11–16. [Google Scholar]
- Deng, J.; Li, Y.; Zhang, Y.; Yang, C.; Zhang, J.; Shi, X. The effect of hydroxyl (-OH) on the oxidation reaction characteristics of side-chain active groups in coal spontaneous combustion. J. China Coal Soc. 2020, 45, 232–240. [Google Scholar]
- He, S.; Qi, J.; Zhou, C.; Tang, Y.; Liu, H. Quantum Chemical Study on the Inhibition of Oxygen-Containing Functional Groups Activity in Coal by Metal Ions (Na+, Ba2+, Ca2+). Coal Mine Saf. 2023, 54, 149–155. [Google Scholar]
- Qi, X.; Li, Y.; Chen, L.; Tang, J.; Xin, H.; Liang, Z. Reaction Mechanism of Aldehyde Groups during Coal Self-Heating. ACS Omega 2020, 5, 23184–23192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Huo, Y.; Fang, S.; He, X.; Wang, W.; Zhang, Y. Quantum Chemical Calculation of Original Aldehyde Groups Reaction Mechanism in Coal Spontaneous Combustion. Energy Fuels 2020, 34, 14776–14785. [Google Scholar] [CrossRef]
- Qin, B.; Lu, Y.; Yin, S.; Cao, K.; Wang, M. Prevention and control technique of complex disaster caused by gas andspontaneous combustion for fully-mechanized sublevel caving face in close-distance seams. J. Min. Saf. Eng. 2013, 30, 311–316. [Google Scholar]




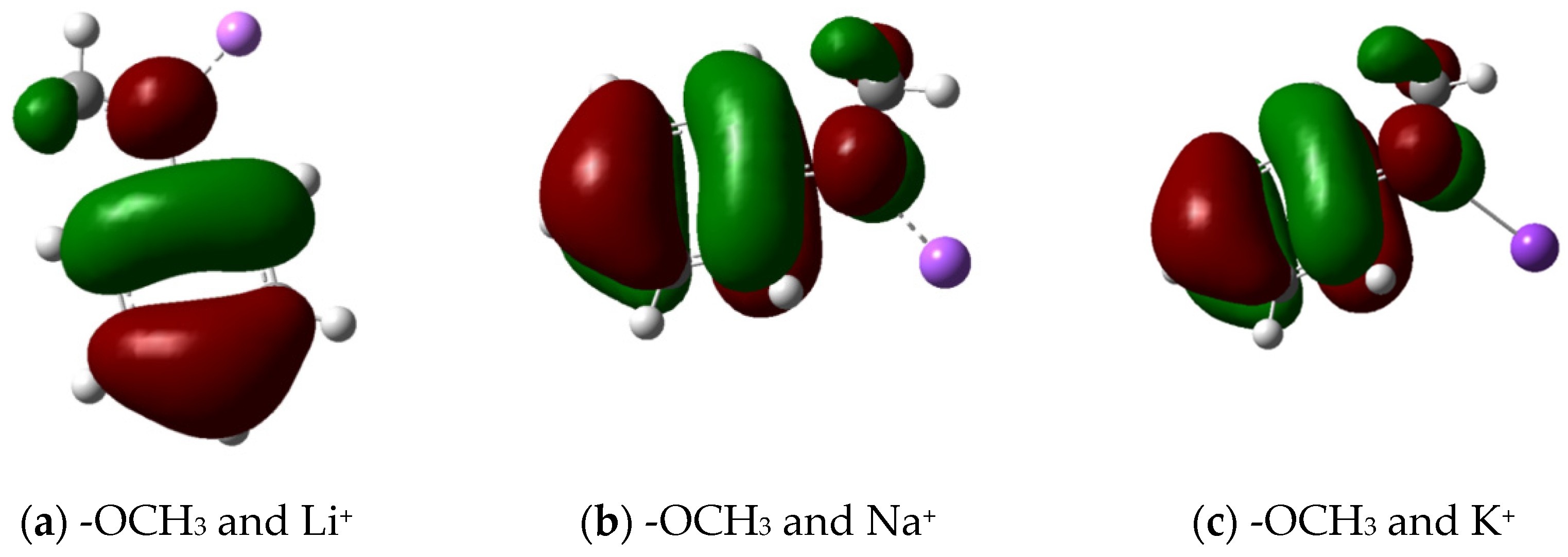
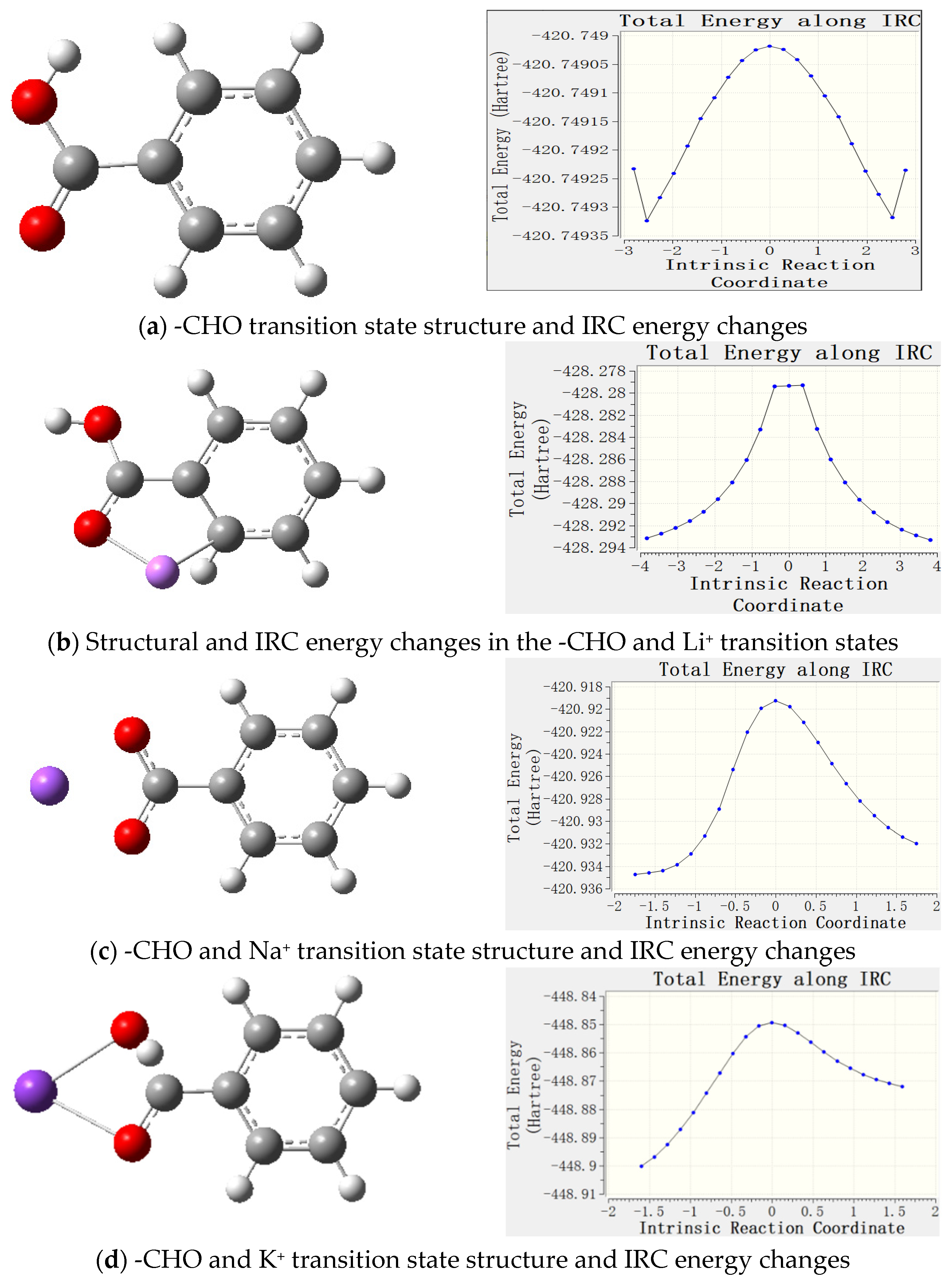
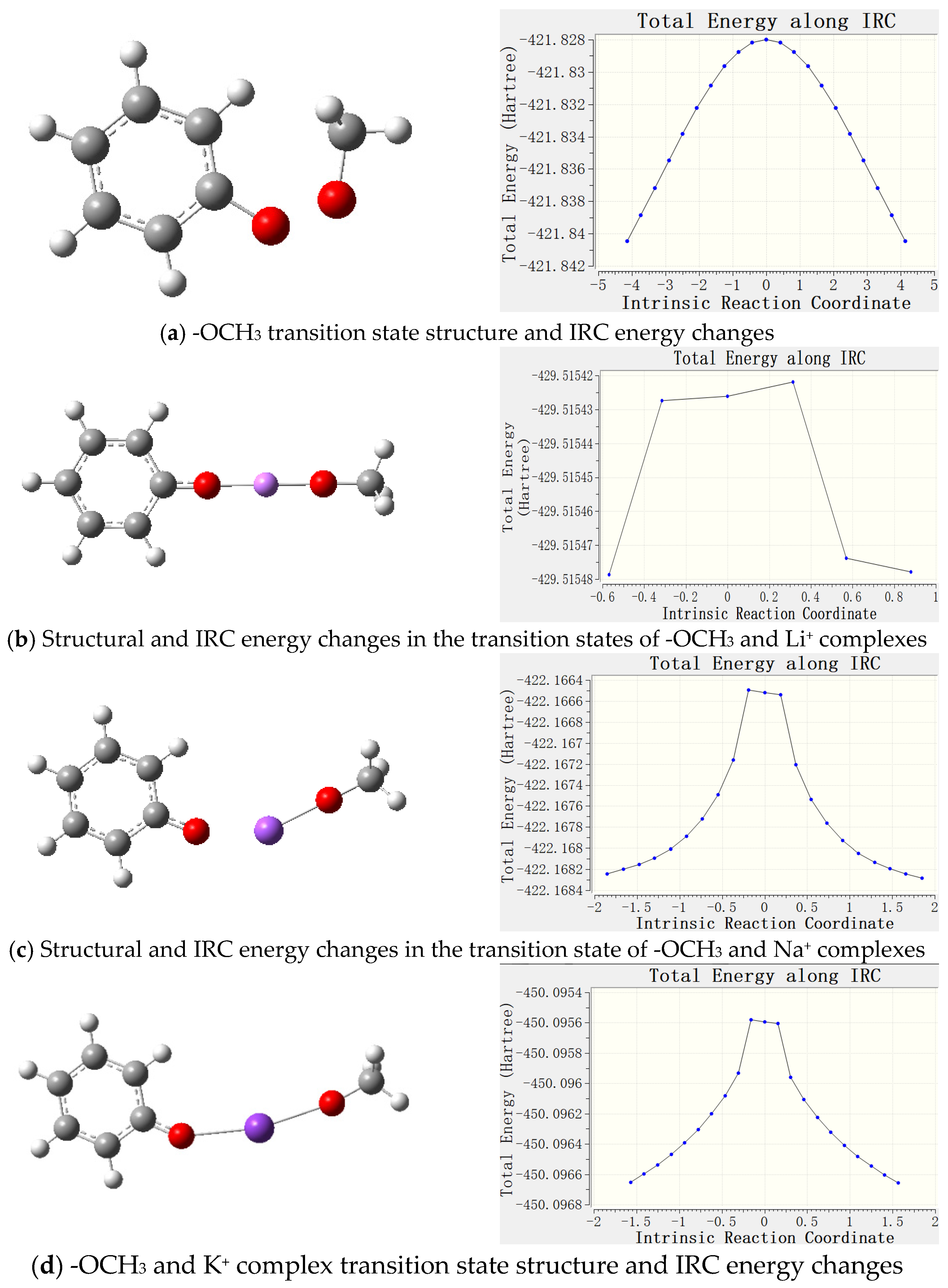
| Benzaldehyde | Anisole | ||||
|---|---|---|---|---|---|
| Atomic Number | Atomic Species | Atomic Charge | Atomic Number | Atomic Species | Atomic Charge |
| 1 | C | −0.09771 | 1 | C | −0.16107 |
| 2 | C | −0.08583 | 2 | C | 0.15881 |
| 3 | C | −0.09033 | 3 | C | −0.13709 |
| 4 | C | −0.11735 | 4 | C | −0.11082 |
| 5 | C | −0.10332 | 5 | C | −0.13218 |
| 6 | C | −0.11838 | 6 | C | −0.10821 |
| 7 | H | 0.12082 | 7 | H | 0.11764 |
| 8 | H | 0.11898 | 8 | H | 0.11823 |
| 9 | H | 0.12786 | 9 | O | −0.26099 |
| 10 | H | 0.12116 | 10 | H | 0.12254 |
| 11 | H | 0.12033 | 11 | H | 0.11826 |
| 12 | C | 0.19701 | 12 | H | 0.11760 |
| 13 | O | −0.26437 | 13 | C | −0.15821 |
| 14 | H | 0.07112 | 14 | H | 0.11393 |
| 15 | H | 0.10078 | |||
| 16 | H | 0.10078 | |||
| Ligand and Central Atom | Atomic | Key Length/nm |
|---|---|---|
| -CHO and Li+ | O (13)-Li+ (15) | 1.79189 |
| -CHO and Na+ | O (13)-Na+ (15) | 2.13588 |
| -CHO and K+ | O (13)-K+ (15) | 2.49810 |
| -OCH3 and Li+ | O (9)-Li+ (17) | 1.96442 |
| -OCH3 and Na+ | O (9)-Na+ (17) | 2.49489 |
| -OCH3 and K+ | O (9)-K+ (17) | 2.96479 |
| Ligand and Central Atom | Atomic | Key Angle/° |
|---|---|---|
| -CHO and Li+ | C (12)-O (13)-Li+ (15) | 87.60935 |
| -CHO and Na+ | C (12)-O (13)-Na+ (15) | 147.85904 |
| -CHO and K+ | C (12)-O (13)-K+ (15) | 99.48480 |
| -OCH3 and Li+ | C (2)-O (9)-Li+ (17) | 113.77708 |
| -OCH3 and Na+ | C (2)-O (9)-Na+ (17) | 115.05559 |
| -OCH3 and K+ | C (2)-O (9)-K+ (17) | 114.62615 |
| Structure Name | EHOMO/eV | ELUMO/eV | ΔE/eV |
|---|---|---|---|
| -CHO active group | −6.943620 | −1.712075 | 5.231545 |
| -CHO and Li+ complexes | −2.814183 | −1.699162 | 1.115021 |
| -CHO complex with Na+ | −2.890001 | −2.057976 | 0.832025 |
| -CHO with K+ complex | −2.577066 | −1.611542 | 0.965524 |
| -OCH3 active group | −5.854907 | 0.109031 | 5.963938 |
| -OCH3 and Li+ complexes | −2.539168 | −0.659273 | 1.879895 |
| -OCH3 and Na+ complexes | −2.793568 | −1.164018 | 1.62955 |
| -OCH3 and K+ complexes | −2.452604 | −1.212991 | 1.239613 |
| Atomic Species and Serial Number | Atomic Charge/e | Atomic Species and Serial Number | Atomic Charge/e | Atomic Species and Serial Number | Atomic Charge/e |
|---|---|---|---|---|---|
| C (1) | −0.03930 | C (1) | −0.06555 | C (1) | −0.05810 |
| C (2) | −0.10891 | C (2) | −0.09929 | C (2) | −0.08889 |
| C (3) | −0.09567 | C (3) | −0.05677 | C (3) | −0.08210 |
| C (4) | −0.14645 | C (4) | −0.13812 | C (4) | −0.15115 |
| C (5) | −0.02334 | C (5) | −0.06281 | C (5) | −0.03738 |
| C (6) | −0.13938 | C (6) | −0.13696 | C (6) | −0.13908 |
| H (7) | 0.11812 | H (7) | 0.11861 | H (7) | 0.11527 |
| H (8) | 0.11404 | H (8) | 0.11458 | H (8) | 0.11141 |
| H (9) | 0.11887 | H (9) | 0.11631 | H (9) | 0.11035 |
| H (10) | 0.11896 | H (10) | 0.11855 | H (10) | 0.11507 |
| H (11) | 0.11267 | H (11) | 0.11557 | H (11) | 0.11035 |
| C (12) | 0.24561 | C (12) | 0.26908 | C (12) | 0.24040 |
| O (13) | −0.27817 | O (13) | −0.34111 | O (13) | −0.28830 |
| H (14) | 0.08283 | H (14) | 0.07415 | H (14) | 0.06900 |
| Li (15) | 0.42012 | Na (15) | 0.47377 | K (15) | 0.47313 |
| Atomic Species and Serial Number | Atomic Charge/e | Atomic Species and Serial Number | Atomic Charge/e | Atomic Species and Serial Number | Atomic Charge/e |
|---|---|---|---|---|---|
| C (1) | −0.15354 | C (1) | −0.15507 | C (1) | −0.15626 |
| C (2) | 0.15495 | C (2) | 0.15548 | C (2) | 0.15585 |
| C (3) | −0.12970 | C (3) | −0.13643 | C (3) | −0.13886 |
| C (4) | −0.10638 | C (4) | −0.10666 | C (4) | −0.10709 |
| C (5) | −0.12451 | C (5) | −0.12706 | C (5) | −0.12807 |
| C (6) | −0.10545 | C (6) | −0.10522 | C (6) | −0.10519 |
| H (7) | 0.12115 | H (7) | 0.12011 | H (7) | 0.11993 |
| H (8) | 0.12143 | H (8) | 0.12055 | H (8) | 0.12034 |
| O (9) | −0.29901 | O (9) | −0.28278 | O (9) | −0.27710 |
| H (10) | 0.12021 | H (10) | 0.12126 | H (10) | 0.12027 |
| H (11) | 0.12274 | H (11) | 0.12117 | H (11) | 0.12083 |
| H (12) | 0.12120 | H (12) | 0.12012 | H (12) | 0.11993 |
| C (13) | −0.15298 | C (13) | −0.15535 | C (13) | −0.15636 |
| H (14) | 0.11653 | H (14) | 0.11532 | H (14) | 0.11466 |
| H (15) | 0.10925 | H (15) | 0.10728 | H (15) | 0.10649 |
| H (16) | 0.10925 | H (16) | 0.10426 | H (16) | 0.10285 |
| Li (17) | 0.47487 | Na (17) | 0.48301 | K (17) | 0.48779 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Peng, H.; Peng, R.; Cui, C. Study of the Effect of Alkali Metal Ions (Li+, Na+, K+) in Inhibiting the Spontaneous Combustion of Coal. Fuels 2025, 6, 31. https://doi.org/10.3390/fuels6020031
Liu Y, Peng H, Peng R, Cui C. Study of the Effect of Alkali Metal Ions (Li+, Na+, K+) in Inhibiting the Spontaneous Combustion of Coal. Fuels. 2025; 6(2):31. https://doi.org/10.3390/fuels6020031
Chicago/Turabian StyleLiu, Yunqiu, Hongjie Peng, Ran Peng, and Chuanbo Cui. 2025. "Study of the Effect of Alkali Metal Ions (Li+, Na+, K+) in Inhibiting the Spontaneous Combustion of Coal" Fuels 6, no. 2: 31. https://doi.org/10.3390/fuels6020031
APA StyleLiu, Y., Peng, H., Peng, R., & Cui, C. (2025). Study of the Effect of Alkali Metal Ions (Li+, Na+, K+) in Inhibiting the Spontaneous Combustion of Coal. Fuels, 6(2), 31. https://doi.org/10.3390/fuels6020031






