Enhancing Bioenergy Production from Chlorella via Salt-Induced Stress and Heat Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Microalgae Strains and Pre-Cultivation Condition
2.3. Microalgae Cultivation in a Photobioreactor
2.3.1. Photobioreactor (PBR) Setup and Operation
2.3.2. Inoculum Preparation
2.3.3. Nutrient Media Preparation and Composition
2.3.4. Salt Stress Treatment
2.3.5. Biomass Harvesting
2.4. Thermal Pretreatment
2.5. Biomass Composition Analysis
2.5.1. Protein Determination
2.5.2. Carbohydrate Determination
2.5.3. Lipid Determination
2.5.4. Fatty Acid Profile Analysis
2.5.5. Volatile Solids (VS) Analysis
2.6. Biochemical Methane Potential (BMP) Test
- mi: Mass of inoculum (g);
- mtot: Total mass in the bottle (400 g);
- VSs: Volatile solids in the substrate;
- VSi: Volatile solids in the inoculum;
- ISR: Inoculum-to-substrate ratio, defined as:
- VS: Accumulated volume of biomethane from the reactor containing the sample (substrate and inoculum);
- VI: Volume of biomethane produced by the inoculum present in the sample bottle;
- mVS,SS: Amount of organic material (substrate) contained in the sample bottle.
2.7. Energy Output (kJ) Based on the Biogas Production Potential
2.8. Energy Efficiency
2.9. Statistical Analysis
3. Results and Discussion
3.1. Biochemical Composition of Microalgae Under Different Cultivation Conditions
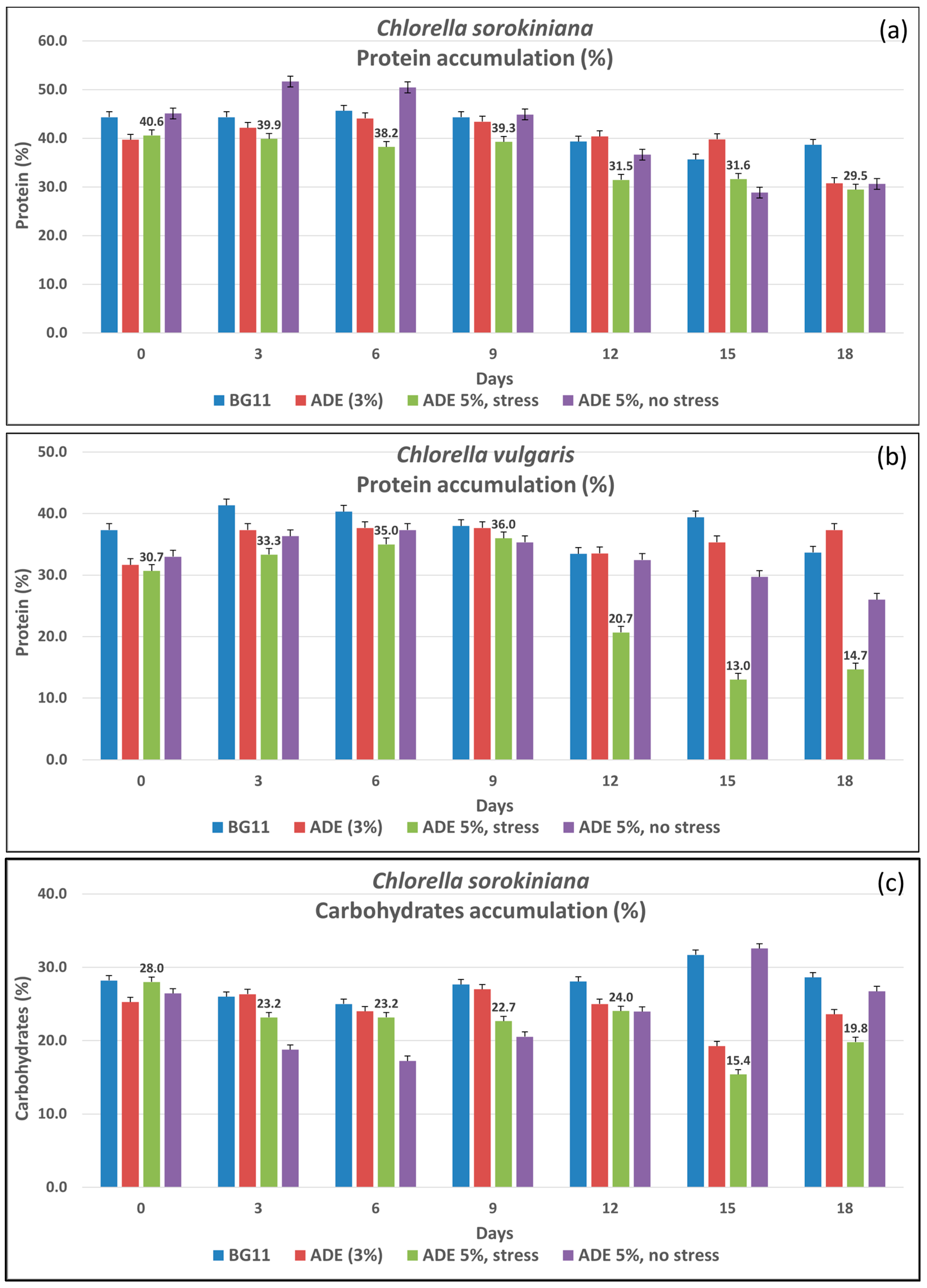
3.1.1. Protein Accumulation
3.1.2. Carbohydrates Accumulation
3.1.3. Lipids Accumulation
3.1.4. Impact of Media and Stress on Biochemical Profiles of Chlorella
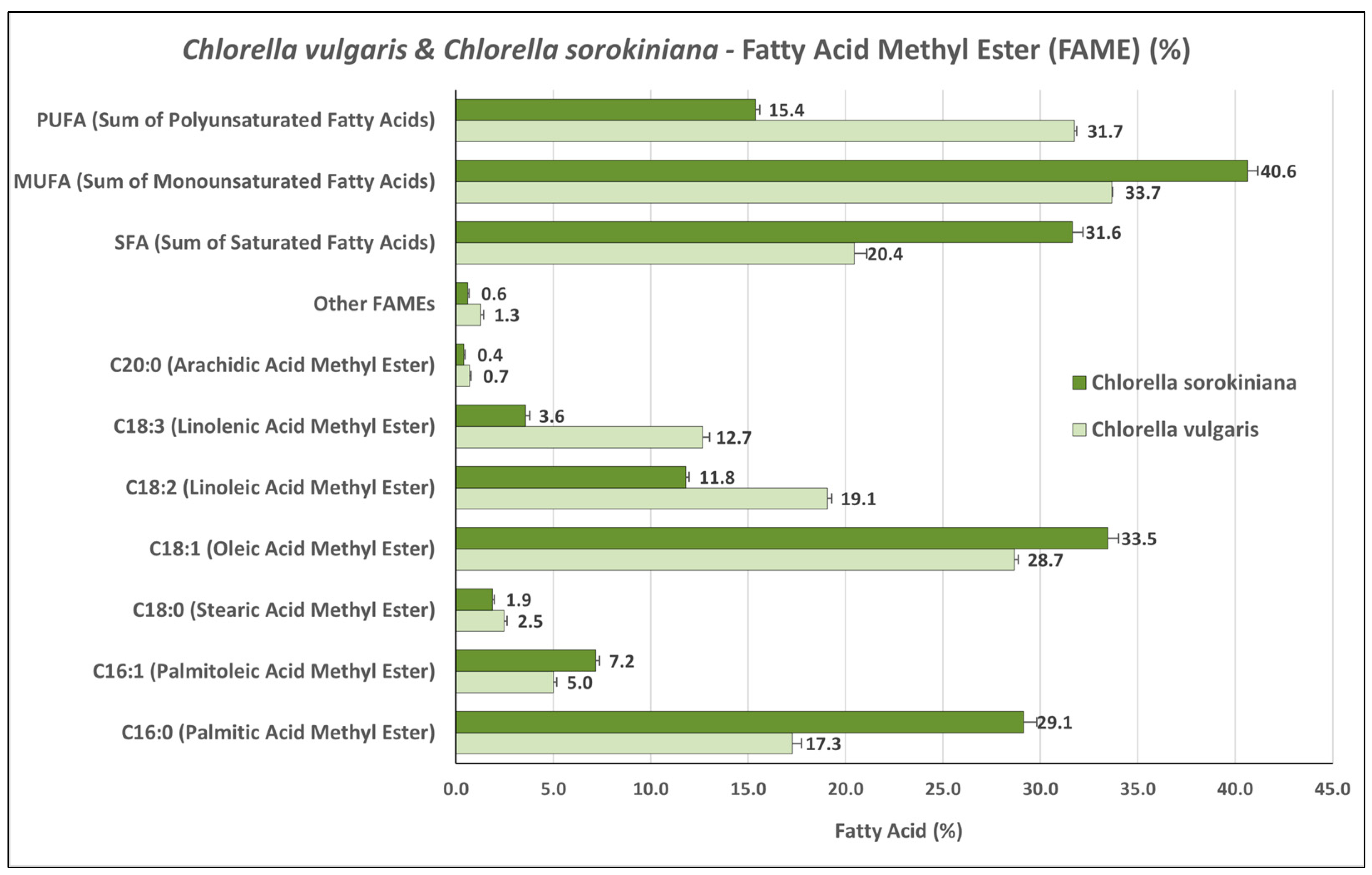
3.2. Fatty Acid Methyl Ester (FAME) Profiles
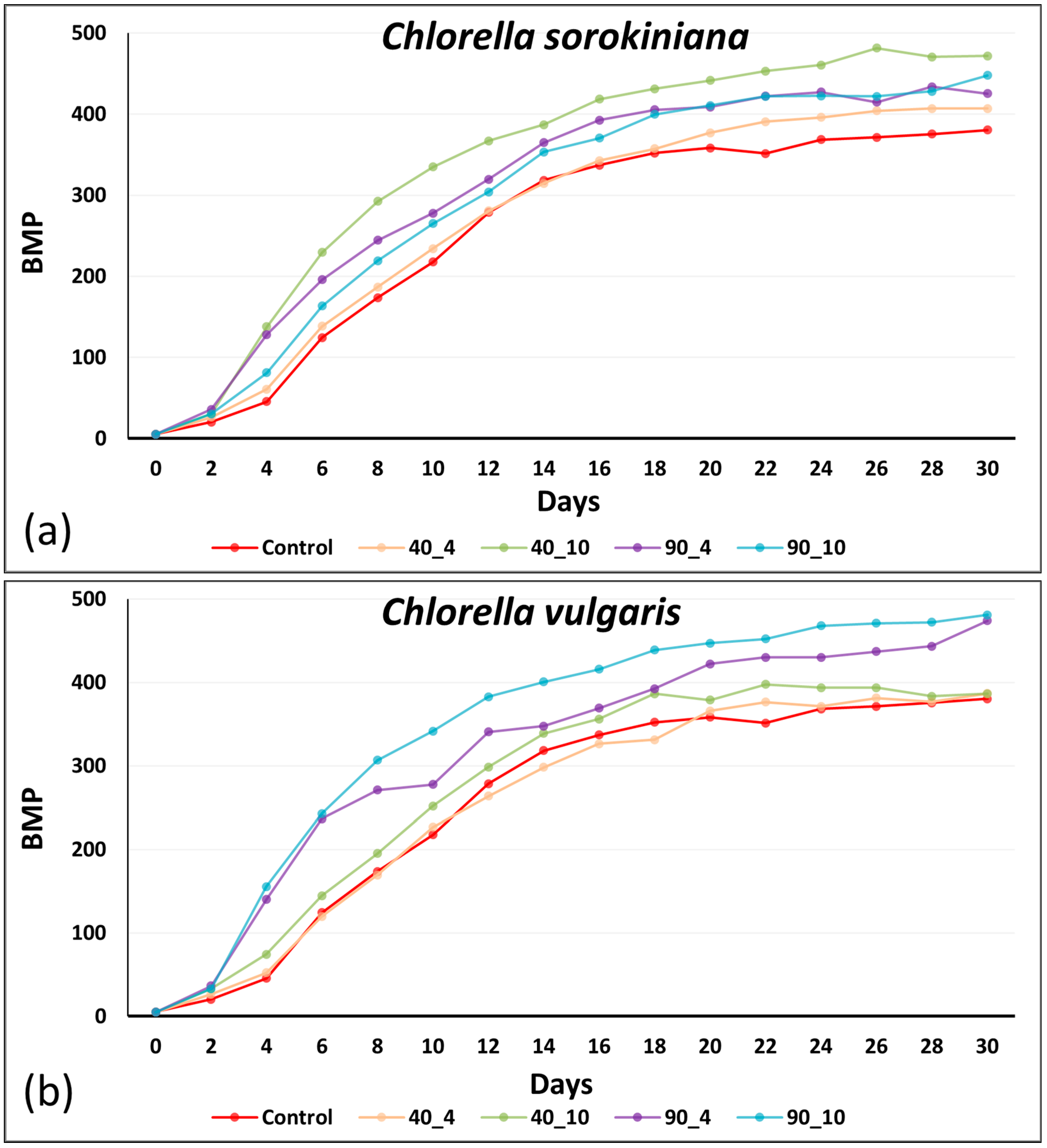
3.3. BMP: Influence of Anaerobic Digestate Effluent and Stress
3.4. Impact of Thermal Pretreatment and Energy Efficiency of Thermal Pretreatment for Enhanced Methane Production
3.5. Economic Feasibility and Scalability of Implementation in Industrial Biofuel Production Plans
3.6. Economic Viability Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendez, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. Methane Production of Thermally Pretreated Chlorella vulgaris and Scenedesmus sp. Biomass at Increasing Biomass Loads. Appl. Energy 2014, 129, 238–242. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. Biomethane Production Using Fresh and Thermally Pretreated Chlorella vulgaris Biomass: A Comparison of Batch and Semi-Continuous Feeding Mode. Ecol. Eng. 2015, 84, 273–277. [Google Scholar] [CrossRef]
- Rahman, Q.M.; Zhang, B.; Wang, L.; Shahbazi, A. A Combined Pretreatment, Fermentation and Ethanol-Assisted Liquefaction Process for Production of Biofuel from Chlorella sp. Fuel 2019, 257, 116026. [Google Scholar] [CrossRef]
- Das, P.K.; Rani, J.; Rawat, S.; Kumar, S. Microalgal Co-Cultivation for Biofuel Production and Bioremediation: Current Status and Benefits. BioEnergy Res. 2022, 15, 1–26. [Google Scholar] [CrossRef]
- Hajinajaf, N.; Mehrabadi, A.; Tavakoli, O. Practical Strategies to Improve Harvestable Biomass Energy Yield in Microalgal Culture: A Review. Biomass Bioenergy 2021, 145, 105941. [Google Scholar] [CrossRef]
- Bahrim, R.Z.K.; Wazir, N.A.; Jaapar, A.S.; Abidin, Q.H.Z.; Wahab, N.F.A.; Khairulanwar, M.K.M.; Jalil, M.M.; Bakri, F.A.M.; Harom, Z.; Nor, M.G.M.; et al. Advancing Renewable Energy Through Open Tanks Microalgae Cultivation for Biofuel Production: Opportunities, Challenges, and Innovative Solutions. In Proceedings of the International Petroleum Technology Conference, Dhahran, Saudi Arabia, 12 February 2024; p. IPTC-24530-EA. [Google Scholar]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Falfushynska, H. Advancements and Prospects in Algal Biofuel Production: A Comprehensive Review. Phycology 2024, 4, 548–575. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Al Maskari, T.; Abujazar, M.S.S.; Bashir, M.J.K.; Nassani, D.E.; Abu Amr, S.S. Biofuel Production Using Cultivated Algae: Technologies, Economics, and Its Environmental Impacts. Energies 2023, 16, 1316. [Google Scholar] [CrossRef]
- Muhammad, G.; Alam, M.A.; Mofijur, M.; Jahirul, M.I.; Lv, Y.; Xiong, W.; Ong, H.C.; Xu, J. Modern Developmental Aspects in the Field of Economical Harvesting and Biodiesel Production from Microalgae Biomass. Renew. Sustain. Energy Rev. 2021, 135, 110209. [Google Scholar] [CrossRef]
- Zhang, S.; Hou, Y.; Liu, Z.; Ji, X.; Wu, D.; Wang, W.; Zhang, D.; Wang, W.; Chen, S.; Chen, F. Electro-Fenton Based Technique to Enhance Cell Harvest and Lipid Extraction from Microalgae. Energies 2020, 13, 3813. [Google Scholar] [CrossRef]
- Lee, S.Y.; Khoiroh, I.; Vo, D.-V.N.; Senthil Kumar, P.; Show, P.L. Techniques of Lipid Extraction from Microalgae for Biofuel Production: A Review. Environ. Chem. Lett. 2021, 19, 231–251. [Google Scholar] [CrossRef]
- Marsolek, M.D.; Kendall, E.; Thompson, P.L.; Shuman, T.R. Thermal Pretreatment of Algae for Anaerobic Digestion. Bioresour. Technol. 2014, 151, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Betenbaugh, M.J.; Bouwer, E.J. The Effects of Alternative Pretreatment Strategies on Anaerobic Digestion and Methane Production from Different Algal Strains. Bioresour. Technol. 2014, 155, 366–372. [Google Scholar] [CrossRef]
- Giang, T.T.; Lunprom, S.; Liao, Q.; Reungsang, A.; Salakkam, A. Improvement of Hydrogen Production from Chlorella sp. Biomass by Acid-Thermal Pretreatment. PeerJ 2019, 7, e6637. [Google Scholar] [CrossRef]
- De Oliveira, M.C.; Bassin, I.D.; Cammarota, M.C. Microalgae and Cyanobacteria Biomass Pretreatment Methods: A Comparative Analysis of Chemical and Thermochemical Pretreatment Methods Aimed at Methane Production. Fermentation 2022, 8, 497. [Google Scholar] [CrossRef]
- Markou, G.; Ilkiv, B.; Brulé, M.; Antonopoulos, D.; Chakalis, L.; Arapoglou, D.; Chatzipavlidis, I. Methane Production through Anaerobic Digestion of Residual Microalgal Biomass after the Extraction of Valuable Compounds. Biomass Convers. Biorefinery 2022, 12, 419–426. [Google Scholar] [CrossRef]
- Da Silva, C.; Astals, S.; Peces, M.; Campos, J.L.; Guerrero, L. Biochemical Methane Potential (BMP) Tests: Reducing Test Time by Early Parameter Estimation. Waste Manag. 2018, 71, 19–24. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) Assay Method for Anaerobic Digestion Research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- Du, X.; Tao, Y.; Li, H.; Liu, Y.; Feng, K. Synergistic Methane Production from the Anaerobic Co-Digestion of Spirulina Platensis with Food Waste and Sewage Sludge at High Solid Concentrations. Renew. Energy 2019, 142, 55–61. [Google Scholar] [CrossRef]
- Passos, F.; Hom-Diaz, A.; Blanquez, P.; Vicent, T.; Ferrer, I. Improving Biogas Production from Microalgae by Enzymatic Pretreatment. Bioresour. Technol. 2016, 199, 347–351. [Google Scholar] [CrossRef]
- Córdova, O.; Passos, F.; Chamy, R. Enzymatic Pretreatment of Microalgae: Cell Wall Disruption, Biomass Solubilisation and Methane Yield Increase. Appl. Biochem. Biotechnol. 2019, 189, 787–797. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Barreiro-Vescovo, S.; De Godos, I.; Fernandez, M.; Zouhayr, A.; Ballesteros, M. Biochemical Methane Potential of Microalgae Biomass Using Different Microbial Inocula. Biotechnol. Biofuels 2018, 11, 184. [Google Scholar] [CrossRef]
- Cho, S.; Park, S.; Seon, J.; Yu, J.; Lee, T. Evaluation of Thermal, Ultrasonic and Alkali Pretreatments on Mixed-Microalgal Biomass to Enhance Anaerobic Methane Production. Bioresour. Technol. 2013, 143, 330–336. [Google Scholar] [CrossRef]
- Wang, M.; Lee, E.; Dilbeck, M.P.; Liebelt, M.; Zhang, Q.; Ergas, S.J. Thermal Pretreatment of Microalgae for Biomethane Production: Experimental Studies, Kinetics and Energy Analysis. J. Chem. Technol. Biotechnol. 2017, 92, 399–407. [Google Scholar] [CrossRef]
- Almarashi, J.Q.M.; El-Zohary, S.E.; Ellabban, M.A.; Abomohra, A.E.-F. Enhancement of Lipid Production and Energy Recovery from the Green Microalga Chlorella vulgaris by Inoculum Pretreatment with Low-Dose Cold Atmospheric Pressure Plasma (CAPP). Energy Convers. Manag. 2020, 204, 112314. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Autohydrolysis and Alkaline Pretreatment Effect on Chlorella vulgaris and Scenedesmus sp. Methane Production. Energy 2014, 78, 48–52. [Google Scholar] [CrossRef]
- Mahdy, A.; Ballesteros, M.; González-Fernández, C. Enzymatic Pretreatment of Chlorella vulgaris for Biogas Production: Influence of Urban Wastewater as a Sole Nutrient Source on Macromolecular Profile and Biocatalyst Efficiency. Bioresour. Technol. 2016, 199, 319–325. [Google Scholar] [CrossRef]
- Carrillo-Reyes, J.; Barragán-Trinidad, M.; Buitrón, G. Biological Pretreatments of Microalgal Biomass for Gaseous Biofuel Production and the Potential Use of Rumen Microorganisms: A Review. Algal Res. 2016, 18, 341–351. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A Critical Review on Anaerobic Digestion of Microalgae and Macroalgae and Co-Digestion of Biomass for Enhanced Methane Generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef]
- Dos Santos Ferreira, J.; De Oliveira, D.; Maldonado, R.R.; Kamimura, E.S.; Furigo, A. Enzymatic Pretreatment and Anaerobic Co-Digestion as a New Technology to High-Methane Production. Appl. Microbiol. Biotechnol. 2020, 104, 4235–4246. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial Characteristics in Anaerobic Digestion Process of Food Waste for Methane Production–A Review. Bioresour. Technol. 2018, 248, 29–36. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K. Anaerobic Co-Digestion on Improving Methane Production from Mixed Microalgae (Scenedesmus sp., Chlorella sp.) and Food Waste: Kinetic Modeling and Synergistic Impact Evaluation. Chem. Eng. J. 2016, 299, 332–341. [Google Scholar] [CrossRef]
- Lizzul, A.M.; Hellier, P.; Purton, S.; Baganz, F.; Ladommatos, N.; Campos, L. Combined Remediation and Lipid Production Using Chlorella Sorokiniana Grown on Wastewater and Exhaust Gases. Bioresour. Technol. 2014, 151, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Juntila, D.J.; Bautista, M.A.; Monotilla, W. Biomass and Lipid Production of a Local Isolate Chlorella Sorokiniana under Mixotrophic Growth Conditions. Bioresour. Technol. 2015, 191, 395–398. [Google Scholar] [CrossRef]
- Church, J.; Hwang, J.-H.; Kim, K.-T.; McLean, R.; Oh, Y.-K.; Nam, B.; Joo, J.C.; Lee, W.H. Effect of Salt Type and Concentration on the Growth and Lipid Content of Chlorella vulgaris in Synthetic Saline Wastewater for Biofuel Production. Bioresour. Technol. 2017, 243, 147–153. [Google Scholar] [CrossRef]
- Rismani, S.; Shariati, M. Changes of the Total Lipid and Omega-3 Fatty Acid Contents in Two Microalgae Dunaliella salina and Chlorella vulgaris Under Salt Stress. Braz. Arch. Biol. Technol. 2017, 60, e17160555. [Google Scholar] [CrossRef]
- Abdellaoui, N.; Kim, M.J.; Choi, T.J. Transcriptome Analysis of Gene Expression in Chlorella Vulgaris under Salt Stress. World J. Microbiol. Biotechnol. 2019, 35, 141. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H.; Karuna, M.S.L. Effect of Salinity Stress on Growth, Lipid Productivity, Fatty Acid Composition, and Biodiesel Properties in Acutodesmus Obliquus and Chlorella vulgaris. Environ. Sci. Pollut. Res. 2017, 24, 13437–13451. [Google Scholar] [CrossRef]
- Psachoulia, P.; Schortsianiti, S.-N.; Lortou, U.; Gkelis, S.; Chatzidoukas, C.; Samaras, P. Assessment of Nutrients Recovery Capacity and Biomass Growth of Four Microalgae Species in Anaerobic Digestion Effluent. Water 2022, 14, 221. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimation and Toxicity of High Ammonium Concentrations to Unicellular Algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Psachoulia, P.; Chatzidoukas, C.; Samaras, P. Study of Chlorella Sorokiniana Cultivation in an Airlift Tubular Photobioreactor Using Anaerobic Digestate Substrate. Water 2024, 16, 485. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Y.; Cheng, D.; Gao, J.; Kong, L.; Zhao, Q.; Wei, W.; Sun, Y. Exploring Stress Tolerance Mechanism of Evolved Freshwater Strain Chlorella Sp. S30 under 30 g/L Salt. Bioresour. Technol. 2018, 250, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Nakanishi, A.; Kato, Y.; Yamasaki, H.; Chang, J.-S.; Misawa, N.; Hirose, Y.; Minagawa, J.; Hasunuma, T.; Kondo, A. Dynamic Metabolic Profiling Together with Transcription Analysis Reveals Salinity-Induced Starch-to-Lipid Biosynthesis in Alga Chlamydomonas Sp. JSC4. Sci. Rep. 2017, 7, 45471. [Google Scholar] [CrossRef]
- Srivastava, G.; Nishchal; Goud, V.V. Salinity Induced Lipid Production in Microalgae and Cluster Analysis (ICCB 16-BR_047). Bioresour. Technol. 2017, 242, 244–252. [Google Scholar] [CrossRef]
- Wang, T.; Ge, H.; Liu, T.; Tian, X.; Wang, Z.; Guo, M.; Chu, J.; Zhuang, Y. Salt Stress Induced Lipid Accumulation in Heterotrophic Culture Cells of Chlorella Protothecoides: Mechanisms Based on the Multi-Level Analysis of Oxidative Response, Key Enzyme Activity and Biochemical Alteration. J. Biotechnol. 2016, 228, 18–27. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Yang, H.; Liao, Q.; Fu, Q.; Liu, Z. Hydrothermal Treatment of Chlorella sp.: Influence on Biochemical Methane Potential, Microbial Function and Biochemical Metabolism. Bioresour. Technol. 2019, 289, 121746. [Google Scholar] [CrossRef]
- Schwenzfeier, A.; Wierenga, P.A.; Gruppen, H. Isolation and Characterization of Soluble Protein from the Green Microalgae Tetraselmis sp. Bioresour. Technol. 2011, 102, 9121–9127. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Schmid, M.; Stengel, D.B. Lipids and Fatty Acids in Algae: Extraction, Fractionation into Lipid Classes, and Analysis by Gas Chromatography Coupled with Flame Ionization Detector (GC-FID). In Natural Products from Marine Algae; Stengel, D.B., Connan, S., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1308, pp. 173–190. ISBN 978-1-4939-2683-1. [Google Scholar]
- Standard Methods Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 2540 Solids. In Standard Methods for the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Weik, M.H. Computer Science and Communications Dictionary; Springer US: Boston, MA, USA, 2001; ISBN 978-0-7923-8425-0. [Google Scholar]
- Çengel, Y.A.; Boles, M.A.; Kanoğlu, M. Thermodynamics: An Engineering Approach, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2019; ISBN 978-1-259-82267-4. [Google Scholar]
- Haji Abolhasani, M.; Safavi, M.; Goodarzi, M.T.; Kassaee, S.M.; Azin, M. Statistical Optimization of Medium with Response Surface Methodology for Biomass Production of a Local Iranian Microalgae Picochlorum sp. RCC486. Adv. Res. Microb. Metab. Technol. 2018, 1, 39–49. [Google Scholar] [CrossRef]
- Cheadle, C.; Vawter, M.P.; Freed, W.J.; Becker, K.G. Analysis of Microarray Data Using Z Score Transformation. J. Mol. Diagn. 2003, 5, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. A Zipf-Plot Based Normalization Method for High-Throughput RNA-Seq Data. PLoS ONE 2020, 15, e0230594. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Chen, P.; Min, M.; Chen, Y.; Zhu, J.; Ruan, R.R. Anaerobic Digested Dairy Manure as a Nutrient Supplement for Cultivation of Oil-Rich Green Microalgae Chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella Strains Calorific Values When Grown in Low Nitrogen Medium. Enzyme Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Feng, P.; Deng, Z.; Fan, L.; Hu, Z. Lipid Accumulation and Growth Characteristics of Chlorella zofingiensis under Different Nitrate and Phosphate Concentrations. J. Biosci. Bioeng. 2012, 114, 405–410. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid Productivity as a Key Characteristic for Choosing Algal Species for Biodiesel Production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Growth Characteristics of Chlorella Sorokiniana in a Photobioreactor during the Utilization of Different Forms of Nitrogen at Various Temperatures. Plants 2022, 11, 1086. [Google Scholar] [CrossRef]
- Koutra, E.; Mastropetros, S.G.; Ali, S.S.; Tsigkou, K.; Kornaros, M. Assessing the Potential of Chlorella vulgaris for Valorization of Liquid Digestates from Agro-Industrial and Municipal Organic Wastes in a Biorefinery Approach. J. Clean. Prod. 2021, 280, 124352. [Google Scholar] [CrossRef]
- Mastropetros, S.G.; Koutra, E.; Amouri, M.; Aziza, M.; Ali, S.S.; Kornaros, M. Comparative Assessment of Nitrogen Concentration Effect on Microalgal Growth and Biochemical Characteristics of Two Chlorella Strains Cultivated in Digestate. Mar. Drugs 2022, 20, 415. [Google Scholar] [CrossRef]
- Choix, F.J.; de-Bashan, L.E.; Bashan, Y. Enhanced Accumulation of Starch and Total Carbohydrates in Alginate-Immobilized Chlorella spp. Induced by Azospirillum Brasilense: II. Heterotrophic Conditions. Enzyme Microb. Technol. 2012, 51, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, D.; Yuan, Y.; Zhou, L.; Li, X.; Wu, T.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving Carbohydrate and Starch Accumulation in Chlorella sp. AE10 by a Novel Two-Stage Process with Cell Dilution. Biotechnol. Biofuels 2017, 10, 75. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Ho, S.-H.; Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Characterization and Optimization of Carbohydrate Production from an Indigenous Microalga Chlorella vulgaris FSP-E. Bioresour. Technol. 2013, 135, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Chen, C.-Y.; Chang, J.-S. Effect of Light Intensity and Nitrogen Starvation on CO2 Fixation and Lipid/Carbohydrate Production of an Indigenous Microalga Scenedesmus Obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef]
- Chia, M.A.; Lombardi, A.T.; da Graça Gama Melão, M.; Parrish, C.C. Combined Nitrogen Limitation and Cadmium Stress Stimulate Total Carbohydrates, Lipids, Protein and Amino Acid Accumulation in Chlorella vulgaris (Trebouxiophyceae). Aquat. Toxicol. 2015, 160, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Tale, M.P.; Devi Singh, R.; Kapadnis, B.P.; Ghosh, S.B. Effect of Gamma Irradiation on Lipid Accumulation and Expression of Regulatory Genes Involved in Lipid Biosynthesis in Chlorella sp. J. Appl. Phycol. 2018, 30, 277–286. [Google Scholar] [CrossRef]
- Xia, A.; Murphy, J.D. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Mickan, B.S.; Moheimani, N.R. Chlorella sp. Growth under Batch and Fed-Batch Conditions with Effluent Recycling When Treating the Effluent of Food Waste Anaerobic Digestate. J. Appl. Phycol. 2019, 31, 3545–3556. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, C.; Li, Z. Microalgal Cultivation with Biogas Slurry for Biofuel Production. Bioresour. Technol. 2016, 220, 629–636. [Google Scholar] [CrossRef]
- Ali, H.E.A.; El-fayoumy, E.A.; Rasmy, W.E.; Soliman, R.M.; Abdullah, M.A. Two-Stage Cultivation of Chlorella vulgaris Using Light and Salt Stress Conditions for Simultaneous Production of Lipid, Carotenoids, and Antioxidants. J. Appl. Phycol. 2021, 33, 227–239. [Google Scholar] [CrossRef]
- Yadav, D.K.; Yadav, M.; Rani, P.; Yadav, A.; Bhardwaj, N.; Bishnoi, N.R.; Singh, A. Screening of Best Growth Media for Chlorella vulgaris Cultivation and Biodiesel Production. Biofuels 2024, 15, 271–277. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Cell Growth, Lipid Production and Productivity in Photosynthetic Microalga Chlorella vulgaris under Different Nitrogen Concentrations and Culture Media Replacement. Recent Pat. Food Nutr. Agric. 2018, 9, 142–151. [Google Scholar] [CrossRef]
- Dahiya, S.; Chowdhury, R.; Tao, W.; Kumar, P. Biomass and Lipid Productivity by Two Algal Strains of Chlorella Sorokiniana Grown in Hydrolysate of Water Hyacinth. Energies 2021, 14, 1411. [Google Scholar] [CrossRef]
- Mohan Singh, H.; Tyagi, V.V.; Kothari, R.; Azam, R.; Singh Slathia, P.; Singh, B. Bioprocessing of Cultivated Chlorella Pyrenoidosa on Poultry Excreta Leachate to Enhance Algal Biomolecule Profile for Resource Recovery. Bioresour. Technol. 2020, 316, 123850. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Dhar, D.W.; Saxena, S. Influence of Nutrient Formulations on Growth, Lipid Yield, Carbon Partitioning and Biodiesel Quality Potential of Botryococcus sp. and Chlorella sp. Environ. Sci. Pollut. Res. 2019, 26, 7589–7600. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, J.; Pei, H.; Pan, J.; Jiang, L.; Hou, Q.; Han, F. Cultivation of Microalgae Using Anaerobically Digested Effluent from Kitchen Waste as a Nutrient Source for Biodiesel Production. Renew. Energy 2018, 115, 276–287. [Google Scholar] [CrossRef]
- Magdaong, J.B.; Ubando, A.T.; Culaba, A.B.; Chang, J.S.; Chen, W.H. Effect of Aeration Rate and Light Cycle on the Growth Characteristics of Chlorella Sorokiniana in a Photobioreactor. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 012112. [Google Scholar] [CrossRef]
- Chai, S.; Shi, J.; Huang, T.; Guo, Y.; Wei, J.; Guo, M.; Li, L.; Dou, S.; Liu, L.; Liu, G. Characterization of Chlorella Sorokiniana Growth Properties in Monosaccharide-Supplemented Batch Culture. PLoS ONE 2018, 13, e0199873. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, M.; Liu, B.; Wen, F.; Yang, Z.; Liu, J. Transcriptome and Metabolome Profiling of a Novel Isolate Chlorella Sorokiniana G32 (Chlorophyta) Displaying Enhanced Starch Accumulation at High Growth Rate Under Mixotrophic Condition. Front. Microbiol. 2022, 12, 760307. [Google Scholar] [CrossRef]
- Cecchin, M.; Benfatto, S.; Griggio, F.; Mori, A.; Cazzaniga, S.; Vitulo, N.; Delledonne, M.; Ballottari, M. Molecular Basis of Autotrophic vs Mixotrophic Growth in Chlorella Sorokiniana. Sci. Rep. 2018, 8, 6465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pei, H.; Chen, S.; Jiang, L.; Hou, Q.; Yang, Z.; Yu, Z. Salinity-Induced Cellular Cross-Talk in Carbon Partitioning Reveals Starch-to-Lipid Biosynthesis Switching in Low-Starch Freshwater Algae. Bioresour. Technol. 2018, 250, 449–456. [Google Scholar] [CrossRef] [PubMed]
- El-Adl, M.; Deyab, M.A.; Khafaga, M.M.; Ahmed, S.E.-S.A. The Freshwater Alga Chlorella Sorokiniana Tolerates Salt Stress via Modulating Metabolites and Minerals. Sci. J. Damietta Fac. Sci. 2024, 14, 44–51. [Google Scholar] [CrossRef]
- Yun, C.-J.; Hwang, K.-O.; Han, S.-S.; Ri, H.-G. The Effect of Salinity Stress on the Biofuel Production Potential of Freshwater Microalgae Chlorella vulgaris YH703. Biomass Bioenergy 2019, 127, 105277. [Google Scholar] [CrossRef]
- Li, H.; Tan, J.; Mu, Y.; Gao, J. Lipid Accumulation of Chlorella sp. TLD6B from the Taklimakan Desert under Salt Stress. PeerJ 2021, 9, e11525. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.; Murthy, G. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel Production from Heterotrophic Microalgal Oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential Lipid and Fatty Acid Profiles of Photoautotrophic and Heterotrophic Chlorella zofingiensis: Assessment of Algal Oils for Biodiesel Production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef]
- Talebi, A.F.; Mohtashami, S.K.; Tabatabaei, M.; Tohidfar, M.; Bagheri, A.; Zeinalabedini, M.; Hadavand Mirzaei, H.; Mirzajanzadeh, M.; Malekzadeh Shafaroudi, S.; Bakhtiari, S. Fatty Acids Profiling: A Selective Criterion for Screening Microalgae Strains for Biodiesel Production. Algal Res. 2013, 2, 258–267. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” Biodiesel: Optimizing Fatty Ester Composition to Improve Fuel Properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Menegazzo, M.L.; Ulusoy-Erol, H.B.; Hestekin, C.N.; Hestekin, J.A.; Fonseca, G.G. Evaluation of the Yield, Productivity, and Composition of Fatty Acids Methyl Esters (FAME) Obtained from the Lipidic Fractions Extracted from Chlorella Sorokiniana by Using Ultrasound and Agitation Combined with Solvents. Biofuels 2022, 13, 519–526. [Google Scholar] [CrossRef]
- Wang, M.; Sahu, A.K.; Rusten, B.; Park, C. Anaerobic Co-Digestion of Microalgae Chlorella sp. and Waste Activated Sludge. Bioresour. Technol. 2013, 142, 585–590. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Thermal Pretreatment to Improve Methane Production of Scenedesmus Biomass. Biomass Bioenergy 2012, 40, 105–111. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic Digestion of Microalgae as a Necessary Step to Make Microalgal Biodiesel Sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Rambo, I.M.; Dombrowski, N.; Constant, L.; Erdner, D.; Baker, B.J. Metabolic Relationships of Uncultured Bacteria Associated with the Microalgae Gambierdiscus. Environ. Microbiol. 2020, 22, 1764–1783. [Google Scholar] [CrossRef]
- Wang, M.; Kuo-Dahab, W.C.; Dolan, S.; Park, C. Kinetics of Nutrient Removal and Expression of Extracellular Polymeric Substances of the Microalgae, Chlorella sp. and Micractinium sp., in Wastewater Treatment. Bioresour. Technol. 2014, 154, 131–137. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Timmers, R.A.; Ballesteros, M.; González-Fernández, C. Enhancing Methane Production of Chlorella vulgaris via Thermochemical Pretreatments. Bioresour. Technol. 2013, 149, 136–141. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Influence of Hydrothermal Pretreatment on Microalgal Biomass Anaerobic Digestion and Bioenergy Production. Water Res. 2015, 68, 364–373. [Google Scholar] [CrossRef]
- Williams, P.J.l.B.; Laurens, L.M.L. Microalgae as Biodiesel & Biomass Feedstocks: Review & Analysis of the Biochemistry, Energetics & Economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–Bacteria Interactions: Evolution, Ecology and Emerging Applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]

| Composition (mg/L) | ADE | 3% ADE | 5% ADE | BG-11 |
|---|---|---|---|---|
| N-NH4 | 3536 ± 36 | 107 ± 1.08 | 175.4 ± 1.8 | n.d. 1 |
| N-NO3 | 92 ± 8.1 | 2.77 ± 0.24 | 4.6 ± 0.41 | 247.84 |
| TN | 3920 ± 66 | 117.6 ± 1.98 | 195 ± 3.3 | 247.84 |
| P | 81.4 ± 5.8 | 2.1 ± 0.17 | 4.2 ± 0.29 | 5.50 |
| Organic N | 292 ± 21.9 | 7.83 ± 0.68 | 15 ± 1.1 | n.d. |
| COD | 24,200 ± 153 | 726 ± 4.59 | 1210 ± 7.65 | n.d. |
| Ca | 369 ± 3.1 | 11.07 ± 0.09 | 18.45 ± 0.16 | 9.81 |
| Fe | 54 ± 1.4 | 1.62 ± 0.04 | 2.71 ± 0.07 | 1.28 |
| Mg | 225 ± 5.3 | 6.75 ± 0.16 | 11.25 ± 0.27 | 6.98 |
| Mn | 6.33 ± 0.35 | 0.19 ± 0.01 | 0.32 ± 0.02 | 0.50 |
| Na | 1884.6 ± 4.5 | 56.54 ± 0.14 | 94.23 ± 0.23 | 212.28 |
| Cl | 1633.6 ± 5.9 | 49.01 ± 0.18 | 81.68 ± 0.3 | 18.02 |
| K | 3161 ± 2.7 | 94.83 ± 0.08 | 158.05 ± 0.14 | 13.70 |
| Cu | 2 ± 0.03 | 0.06 ± 0.00 | 0.10 ± 0.00 | 0.02 |
| EC | 49.7 dS/m | 2.17 dS/m | 3.15 dS/m | n.a. 2 |
| pH | 8.3 | 8.0 | 8.1 | 7.1 |
| Cultivation Conditions | Proteins (%) | Carbohydrates (%) | Lipids (%) |
|---|---|---|---|
| CSO BG11 | 35.67 ± 4.0 1 ab 2 | 31.70 ± 8.3 a | 16.37 ± 1.8 e |
| CSO ADE3 | 39.80 ± 3.6 a | 19.25 ± 1.0 b | 24.07 ± 1.6 d |
| CSO ADE5_stress | 31.63 ± 1.4 bc | 15.40 ± 0.7 bc | 36.67 ± 1.1 bc |
| CSO ADE5_nostress | 28.83 ± 1.2 c | 32.57 ± 0.5 a | 22.00 ± 0.7 d |
| CVU BG11 | 39.40 ± 1.0 a | 29.82 ± 2.5 a | 16.37 ± 1.8 e |
| CVU ADE3 | 35.33 ± 1.3 ab | 12.13 ± 1.8 c | 37.53 ± 0.4 b |
| CVU ADE5_stress | 13.00 ± 1.0 d | 20.37 ± 5.5 b | 51.57 ± 1.4 a |
| CVU ADE5_nostress | 29.70 ± 4.3 bc | 19.81 ± 5.5 b | 34.87 ± 0.7 c |
| Mean | 31.67 | 22.63 | 29.93 |
| LSD 3 | 6.00 | 6.27 | 2.08 |
| Cultivation Conditions | mL(biogas)/gVS |
|---|---|
| CSO BG11 | 407.1 ± 2.0 c 1 |
| CSO ADE3 | 399.3 ± 3.4 d |
| CSO ADE5_stress | 418.3 ± 3.9 b |
| CSO ADE5_nostress | 407.9 ± 5.1 c |
| CVU BG11 | 414.5 ± 1.4 b |
| CVU ADE3 | 408.1 ± 0.6 c |
| CVU ADE5_stress | 432.8 ± 2.4 a |
| CVU ADE5_nostress | 409.2 ± 1.2 c |
| Means | 412.1 |
| LSD 2 | 4.8 |
| Cultivation Conditions | BMP (mL biogas/gVS) | Energy Input (kJ) | Energy Output (kJ) | Energy Efficiency (%) |
|---|---|---|---|---|
| CSO 40_4 | 407 ± 6.5 e 1 | 250.8 | 14,570.6 | 2.67 ± 0.003 a 2 |
| CSO 40_10 | 472 ± 2.5 b | 627.0 | 16,897.6 | 1.24 ± 0.005 c |
| CSO 90_4 | 425 ± 1.5 d | 1085.0 | 15,215.0 | 0.93 ± 0.003 f |
| CSO 90_10 | 448 ± 2.1 c | 2712.5 | 16,038.4 | 0.49 ± 0.002 h |
| CVU 40_4 | 380 ± 3.2 f | 250.8 | 13,604.0 | 2.54 ± 0.017 b |
| CVU 40_10 | 387 ± 3.1 f | 627.0 | 13,848.6 | 1.01 ± 0.007 e |
| CVU 90_4 | 474 ± 3.6 b | 1085.0 | 16,969.2 | 1.03 ± 0.006 d |
| CVU 90_10 | 481 ± 8.0 a | 2712.5 | 17,219.8 | 0.55 ± 0.002 g |
| Control_25 °C− 3 | 380 ± 1.9 f | - | - | - 5 |
| Means | 434 | 1.31 | ||
| LSD 4 | - | 0.01 |
| Cultivation Conditions | Energy Input (EUR) | Energy Output (EUR) | Net Profit (EUR) |
|---|---|---|---|
| CSO, 40 °C, 4 h | EUR 0.020 | EUR 1.168 | EUR 1.148 |
| CSO, 90 °C, 10 h | EUR 0.217 | EUR 1.381 | EUR 1.164 |
| CVU, 40 °C, 4 h | EUR 0.020 | EUR 1.168 | EUR 1.148 |
| CVU, 90 °C, 10 h | EUR 0.217 | EUR 1.381 | EUR 1.164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfetsas, T.; Ghoghoberidze, S.; Samaras, P.; Falaras, P.; Kotsopoulos, T. Enhancing Bioenergy Production from Chlorella via Salt-Induced Stress and Heat Pretreatment. Fuels 2025, 6, 23. https://doi.org/10.3390/fuels6020023
Sfetsas T, Ghoghoberidze S, Samaras P, Falaras P, Kotsopoulos T. Enhancing Bioenergy Production from Chlorella via Salt-Induced Stress and Heat Pretreatment. Fuels. 2025; 6(2):23. https://doi.org/10.3390/fuels6020023
Chicago/Turabian StyleSfetsas, Themistoklis, Sopio Ghoghoberidze, Petros Samaras, Polycarpos Falaras, and Thomas Kotsopoulos. 2025. "Enhancing Bioenergy Production from Chlorella via Salt-Induced Stress and Heat Pretreatment" Fuels 6, no. 2: 23. https://doi.org/10.3390/fuels6020023
APA StyleSfetsas, T., Ghoghoberidze, S., Samaras, P., Falaras, P., & Kotsopoulos, T. (2025). Enhancing Bioenergy Production from Chlorella via Salt-Induced Stress and Heat Pretreatment. Fuels, 6(2), 23. https://doi.org/10.3390/fuels6020023








