Laboratory Modeling of the Bazhenov Formation Organic Matter Transformation in a Semi-Open System: A Comparison of Oil Generation Kinetics in Two Samples with Type II Kerogen
Abstract
1. Introduction
2. Geological Setting
3. Materials and Methods
3.1. Sample Collection
3.2. Hydrous Pyrolysis
3.3. Pyrolysis
3.4. Synthetic Oil Preparation for GC-MS Analysis
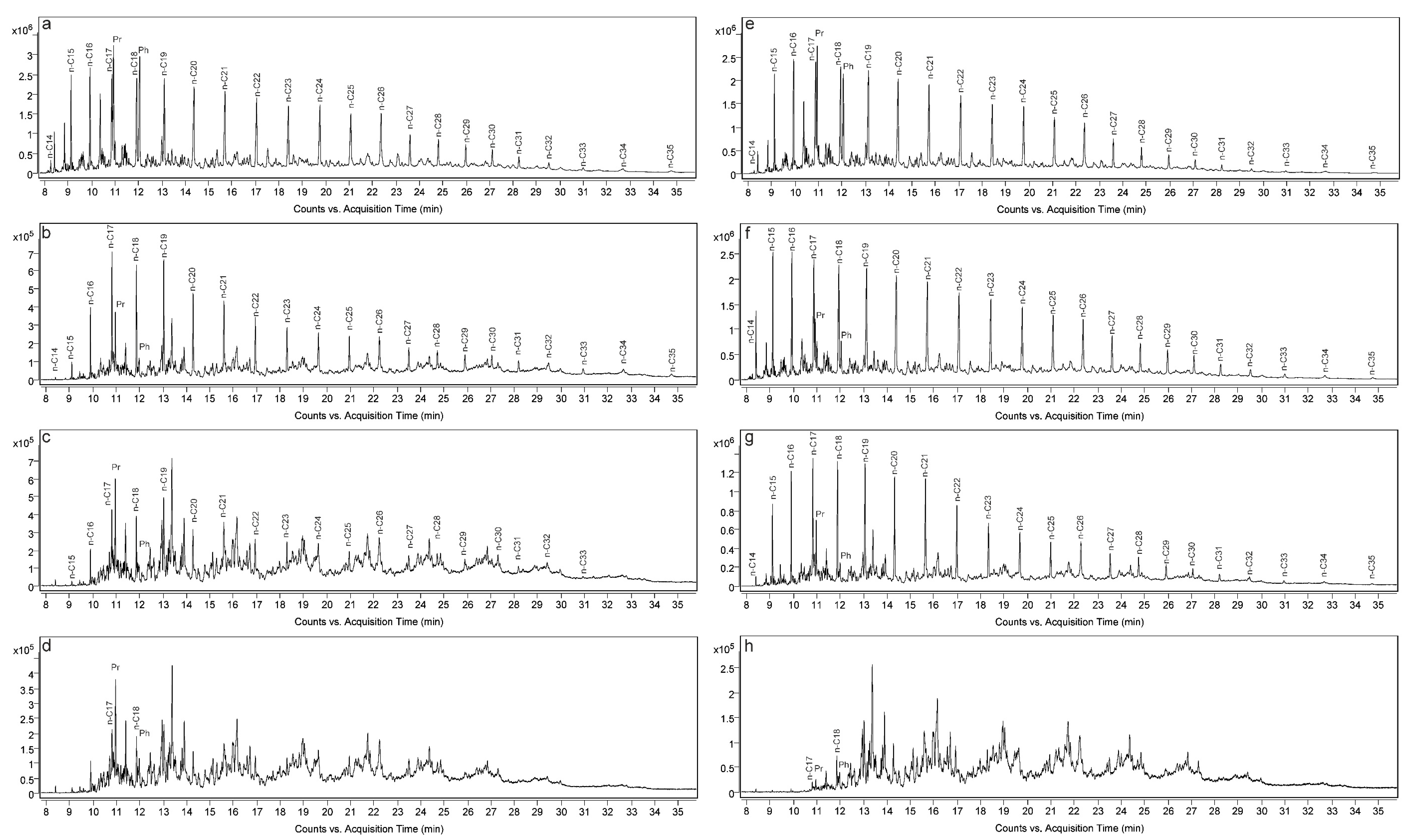
3.5. GC-MS Analysis
4. Results
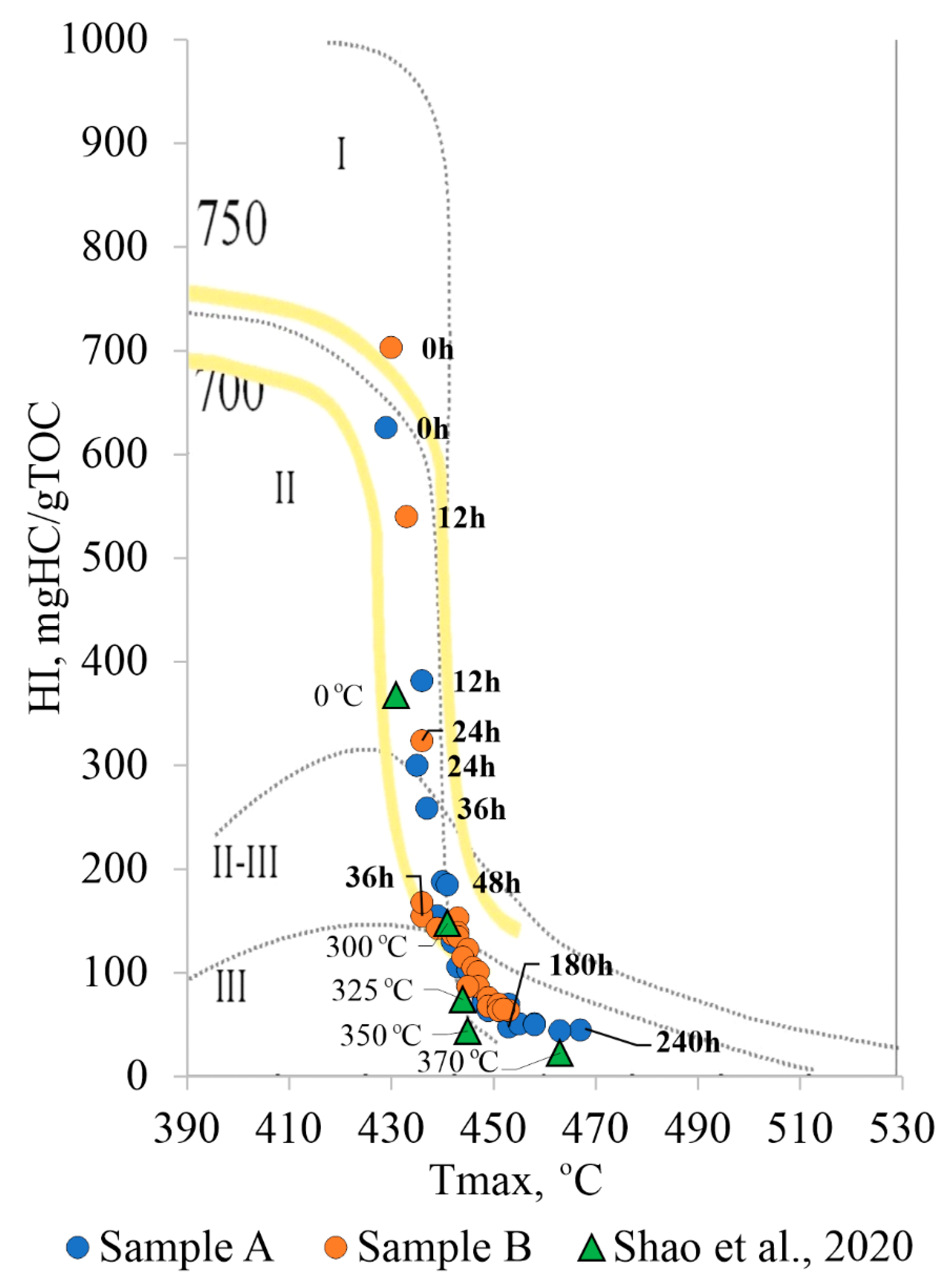
4.1. The Results of Oil Generation Laboratory Modeling in a Semi-Open System
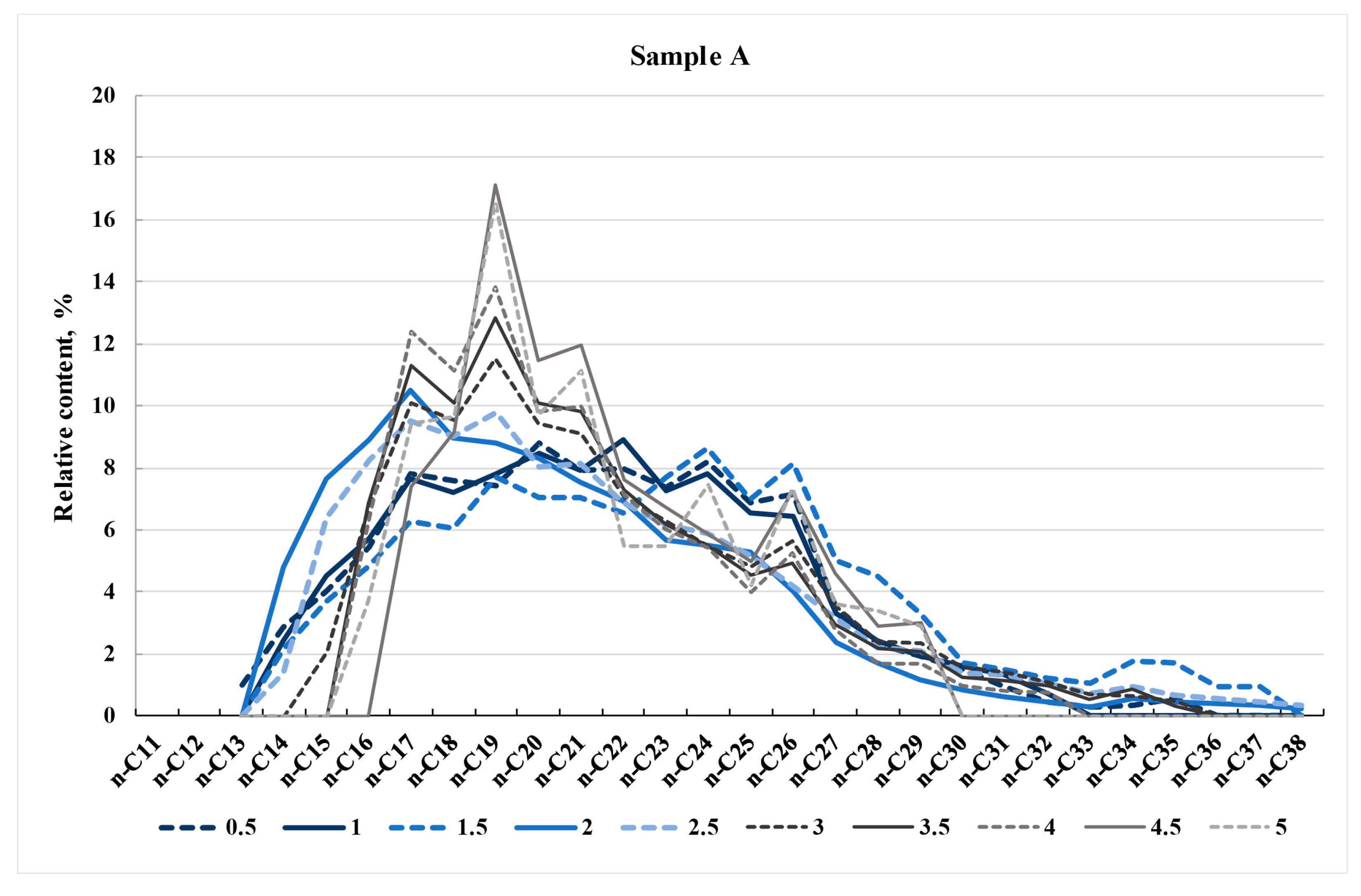
4.2. Normal Alkane Distribution in Synthetic Oils
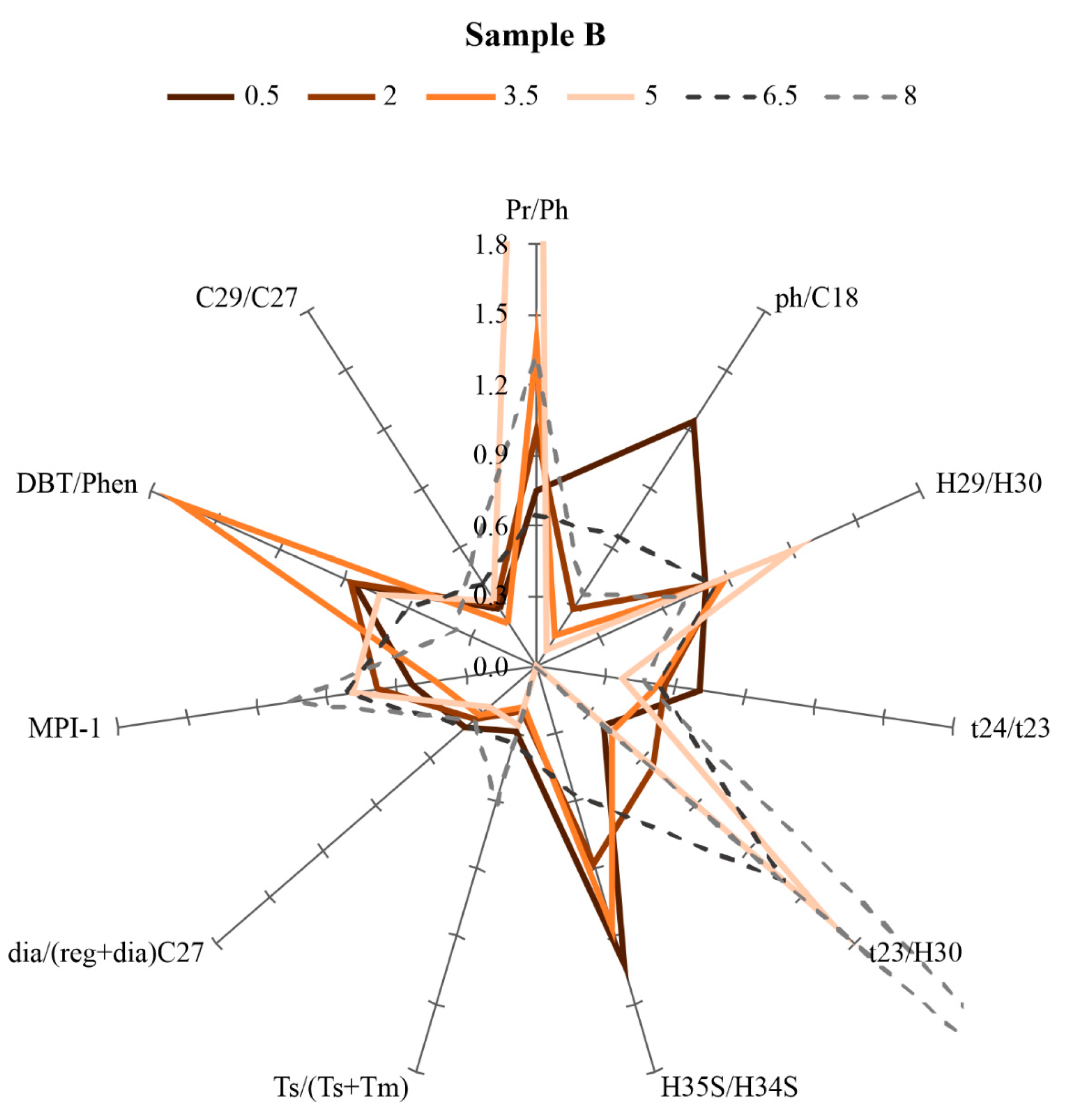
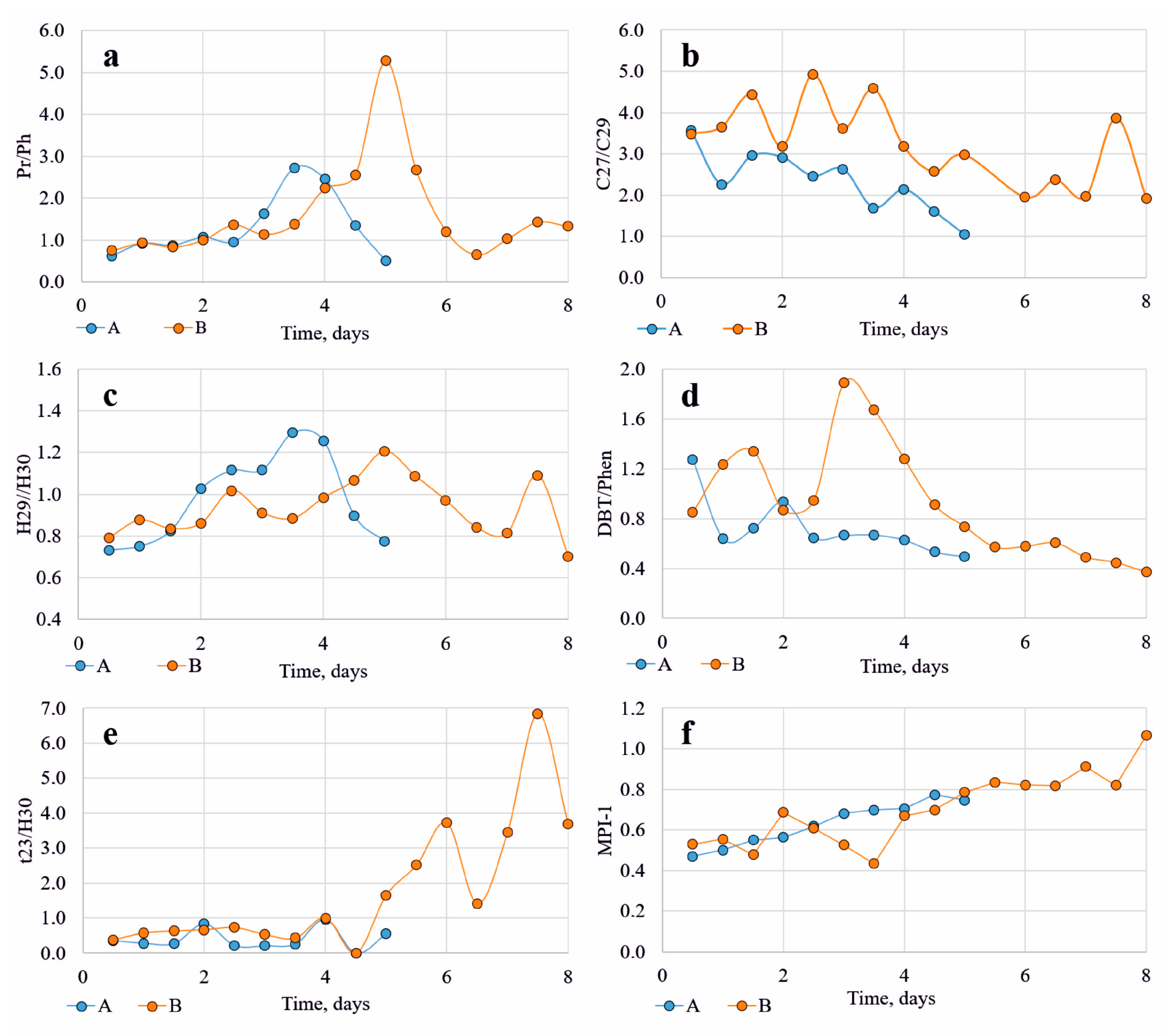
4.3. Biomarker Parameters of Synthetic Oils
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durand, B. Kerogen: Insoluble Organic Matter from Sedimentary Rocks; Editions Technip: Paris, France, 1980. [Google Scholar]
- Tegelaar, E.W.; de Leeuw, J.W.; Derenne, S.; Largeau, C. A reappraisal of kerogen formation. Geochim. Cosmochim. Acta 1989, 53, 3103–3106. [Google Scholar] [CrossRef]
- Hutton, A.; Bharati, S.; Robl, T. Chemical and Petrographic Classification of Kerogen/Macerals. Energy Fuels 1994, 8, 1478–1488. [Google Scholar] [CrossRef]
- Peters, K.; Walters, C.; Mankiewicz, P. Evaluation of kinetic uncertainty in numerical models of petroleum generation. AAPG Bull. 2006, 90, 387–403. [Google Scholar] [CrossRef]
- McFarlane, R.A.; Gentzis, T.; Goodarzi, F.; Hanna, J.V.; Vassallo, A.M. Evolution of the chemical structure of Hat Creek resinite during oxidation: A combined FT-IR photoacoustic, NMR and optical microscopic study. Int. J. Coal Geol. 1993, 22, 119–147. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, J.-B.; Yang, H.-Y.; Liu, Q. Chemical Composition and Structural Characteristics of Oil Shales and Their Kerogens Using Fourier Transform Infrared (FTIR) Spectroscopy and Solid-State 13C Nuclear Magnetic Resonance (NMR). Energy Fuels 2016, 30, 6271–6280. [Google Scholar] [CrossRef]
- Panattoni, F.; Mitchell, J.; Fordham, E.J.; Kausik, R.; Grey, C.P.; Magusin, P.C.M.M. Combined High-Resolution Solid-State 1H/13C NMR Spectroscopy and 1H NMR Relaxometry for the Characterization of Kerogen Thermal Maturation. Energy Fuels 2021, 35, 1070–1079. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Behar, F.; Vandenbroucke, M. Chemical modelling of kerogens. Org. Geochem. 1987, 11, 15–24. [Google Scholar] [CrossRef]
- Liang, T.; Zou, Y.-R.; Zhan, Z.-W.; Lin, X.-H.; Shi, J.; Peng, P. An evaluation of kerogen molecular structures during artificial maturation. Fuel 2020, 265, 116979. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Zhang, R.; Zhang, Y. The molecular model of Marcellus shale kerogen: Experimental characterization and structure reconstruction. Int. J. Coal Geol. 2020, 246, 103833. [Google Scholar] [CrossRef]
- Siskin, M.; Scouten, C.G.; Rose, K.D.; Aczel, T.; Colgrove, S.G.; Pabst, R.E. Detailed Structural Characterization of the Organic Material in Rundle Ramsay Crossing and Green River Oil Shales. In Composition, Geochemistry and Conversion of Oil Shales; Kluwer Academic Publishers: New York, UK, 1995; pp. 143–158. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Hantschel, T.; Kauerauf, A. Fundamentals of Basin and Petroleum System Modeling; Springer Science & Business Media: Berlin, Germany, 2009. [Google Scholar]
- Behar, F.; Beaumont, V.D.E.B.; Penteado, H.L.D.B. Rock-Eval 6 Technology: Performances and Developments. Oil Gas Sci. Technol. 2001, 56, 111–134. [Google Scholar] [CrossRef]
- Burnham, A.K. Pyrolysis in Open Systems. In Global Chemical Kinetics of Fossil Fuels: How to Model Maturation and Pyrolysis; Springer International Publishing: Cham, Switzerland, 2017; pp. 107–169. [Google Scholar]
- Lewan, M.D. Laboratory Simulation of Petroleum Formation. In Organic Geochemistry: Principles and Applications; Engel, M.H., Macko, S.A., Eds.; Springer: Boston, MA, USA, 1993; pp. 419–442. [Google Scholar]
- Horsfield, B.; Disko, U.; Leistner, F. The micro-scale simulation of maturation: Outline of a new technique and its potential applications. Geol. Rundsch. 1989, 78, 361–373. [Google Scholar] [CrossRef]
- Behar, F.; Kressmann, S.; Rudkiewicz, J.L.; Vandenbroucke, M. Experimental simulation in a confined system and kinetic modelling of kerogen and oil cracking. Org. Geochem. 1992, 19, 173–189. [Google Scholar] [CrossRef]
- Wu, Y.; Ji, L.; He, C.; Zhang, Z.; Zhang, M.; Sun, L.; Su, L.; Xia, Y. The effects of pressure and hydrocarbon expulsion on hydrocarbon generation during hydrous pyrolysis of type-I kerogen in source rock. J. Nat. Gas Sci. Eng. 2016, 34, 1215–1224. [Google Scholar] [CrossRef]
- Lewan, M.D. Effects of thermal maturation on stable organic carbon isotopes as determined by hydrous pyrolysis of Woodford Shale. Geochim. Cosmochim. Acta 1983, 47, 1471–1479. [Google Scholar] [CrossRef]
- Ruble, T.E.; Lewan, M.D.; Philp, R.P. New Insights on the Green River Petroleum System in the Uinta Basin from Hydrous Pyrolysis Experiments. AAPG Bull. 2001, 85, 1333–1371. [Google Scholar] [CrossRef]
- Curtis, J.B.; Kotarba, M.J.; Lewan, M.D.; Więcław, D. Oil/source rock correlations in the Polish Flysch Carpathians and Mesozoic basement and organic facies of the Oligocene Menilite Shales: Insights from hydrous pyrolysis experiments. Org. Geochem. 2004, 35, 1573–1596. [Google Scholar] [CrossRef]
- Li, R.; Jin, K.; Lehrmann, D.J. Hydrocarbon potential of Pennsylvanian coal in Bohai Gulf Basin, Eastern China, as revealed by hydrous pyrolysis. Int. J. Coal Geol. 2008, 73, 88–97. [Google Scholar] [CrossRef]
- Spigolon, A.L.D.; Lewan, M.D.; de Barros Penteado, H.L.; Coutinho, L.F.C.; Mendonça Filho, J.G. Evaluation of the petroleum composition and quality with increasing thermal maturity as simulated by hydrous pyrolysis: A case study using a Brazilian source rock with Type I kerogen. Org. Geochem. 2015, 83–84, 27–53. [Google Scholar] [CrossRef]
- Han, H.; Guo, C.; Zhong, N.-N.; Pang, P.; Chen, S.-J.; Lu, J.-G.; Gao, Y. Pore structure evolution of lacustrine shales containing Type I organic matter from the Upper Cretaceous Qingshankou Formation, Songliao Basin, China: A study of artificial samples from hydrous pyrolysis experiments. Mar. Pet. Geol. 2019, 104, 375–388. [Google Scholar] [CrossRef]
- Eglinton, T.I.; Douglas, A.G. Quantitative study of biomarker hydrocarbons released from kerogens during hydrous pyrolysis. Energy Fuels 1988, 2, 81–88. [Google Scholar] [CrossRef]
- Peters, K.E.; Moldowan, J.M.; Sundararaman, P. Effects of hydrous pyrolysis on biomarker thermal maturity parameters: Monterey Phosphatic and Siliceous members. Org. Geochem. 1990, 15, 249–265. [Google Scholar] [CrossRef]
- Michels, R.; Landais, P.; Torkelson, B.E.; Philp, R.P. Effects of effluents and water pressure on oil generation during confined pyrolysis and high-pressure hydrous pyrolysis. Geochim. Cosmochim. Acta 1995, 59, 1589–1604. [Google Scholar] [CrossRef]
- Lewan, M.; Henry, A. Gas: Oil Ratios for Source Rocks Containing Type-I, -II, -IIS, and -III Kerogens as Determined by Hydrous Pyrolysis; US Geological Survey: Reston, VA, USA, 1999. [Google Scholar]
- Rushdi, A.I.; BaZeyad, A.Y.; Al-Awadi, A.S.; Al-Mutlaq, K.F.; Simoneit, B.R.T. Chemical characteristics of oil-like products from hydrous pyrolysis of scrap tires at temperatures from 150 to 400 °C. Fuel 2013, 107, 578–584. [Google Scholar] [CrossRef]
- Kontorovich, A.E.; Yan, P.A.; Zamirailova, A.G.; Kostyreva, E.A.; Eder, V.G. Classification of rocks of the Bazhenov Formation. Russ. Geol. Geophys. 2016, 57, 1606–1612. [Google Scholar] [CrossRef]
- Kalmykov, A.G.; Gafurova, D.R.; Tikhonova, M.S.; Vidishcheva, O.N.; Ivanova, D.A.; Man’ko, I.E.; Korost, D.V.; Bychkov, A.Y.; Kalmykov, G.A. The Influence of the Composition of Rocks from High-Carbon Formations on the Process of Oil and Gas Generation: The Results of Laboratory Modeling. Mosc. Univ. Geol. Bull. 2021, 76, 191–203. [Google Scholar] [CrossRef]
- Savostin, G.G.; Kalmykov, A.G.; Ivanova, D.A.; Kalmykov, G.A. Experimental data on organic matter maturation investigation in one sample of the Bazhenov shale formations (Western Siberia). Mosc. Univ. Bull. 2023, 78, 805–814. [Google Scholar] [CrossRef]
- Kontorovich, A.E.; Bogorodskaya, L.I.; Borisova, L.S.; Burshtein, L.M.; Ismagilov, Z.P.; Efimova, O.S.; Kostyreva, E.A.; Lemina, N.M.; Ryzhkova, S.V.; Sozinov, S.A.; et al. Geochemistry and Catagenetic Transformations of Kerogen from the Bazhenov Horizon. Geochem. Int. 2019, 57, 621–634. [Google Scholar] [CrossRef]
- Kozlova, E.V.; Fadeeva, N.P.; Kalmykov, G.A.; Balushkina, N.S.; Pronina, N.V.; Poludetkina, E.N.; Kostenko, O.V.; Yurchenko, A.Y.; Borisov, R.S.; Bychkov, A.Y.; et al. Geochemical technique of organic matter research in deposits enrich in kerogene (the Bazhenov Formation, West Siberia). Mosc. Univ. Geol. Bull. 2015, 70, 409–418. [Google Scholar] [CrossRef]
- Leushina, E.; Mikhaylova, P.; Kozlova, E.; Polyakov, V.; Morozov, N.; Spasennykh, M. The effect of organic matter maturity on kinetics and product distribution during kerogen thermal decomposition: The Bazhenov Formation case study. J. Pet. Sci. Eng. 2021, 204, 108751. [Google Scholar] [CrossRef]
- Marunova, D.A.; Pronina, N.V.; Kalmykov, A.G.; Ivanova, D.A.; Kalmykov, G.A. Organic matter maturation stages in Tutleim formation rocks depending on maceral composition. Mosc. Univ. Bull. 2021, 1, 86–97. [Google Scholar] [CrossRef]
- Lin, R.; Patrick Ritz, G. Studying individual macerals using i.r. microspectrometry, and implications on oil versus gas/condensate proneness and “low-rank” generation. Org. Geochem. 1993, 20, 695–706. [Google Scholar] [CrossRef]
- Salter, T.L.; Watson, J.S.; Sephton, M.A. Effects of minerals (phyllosilicates and iron oxides) on the responses of aliphatic hydrocarbon containing kerogens (Type I and Type II) to analytical pyrolysis. J. Anal. Appl. Pyrolysis 2023, 170, 105900. [Google Scholar] [CrossRef]
- Bogatyreva, I.Y.; Kotochkova, Y.A.; Balushkina, N.S.; Khotylev, O.V.; Fomina, M.M.; Tyurina, N.A.; Yablonovskiy, B.I.; Kalmykov, G.A. Structural-Facies Typification of Sections of the Bazhenov High-Carbon Formation of the West Siberian Basin. Mosc. Univ. Geol. Bull. 2024, 79, 263–281. [Google Scholar] [CrossRef]
- Vishnevskaya, V.S.; Amon, E.O.; Kalmykov, G.A.; Gatovsky, Y.A. Radiolarians and Their Role in the Study of Stratigraphy and Paleogeography of Shale Oil Basins (Based on the Example of the Bazhenovo Formation in Western Siberia and the Arctic). Paleontol. J. 2024, 58, 25–39. [Google Scholar] [CrossRef]
- ASTM D2007-19; Standard Test Method for Characteristic Groups in Rubber Extender and Processing Oils and Other Petroleum-Derived Oils by the Clay-Gel Absorption Chromatographic Method. ASTM International: West Conshohocken, PA, USA, 2024. [CrossRef]
- Palacas, J.G.; Anders, D.E.; King, J.D. South Florida Basin—A Prime Example of Carbonate Source Rocks of Petroleum. In Petroleum Geochemistry and Source Rock Potential of Carbonate Rocks; Palacas, J.G., Ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1984; Volume 18. [Google Scholar]
- ten Haven, H.L.; de Leeuw, J.W.; Sinninghe Damsté, J.S.; Schenck, P.A.; Palmer, S.E.; Zumberge, J.E. Application of biological markers in the recognition of palaeohypersaline environments. Geol. Soc. Lond. Spec. Publ. 1988, 40, 123–130. [Google Scholar] [CrossRef]
- Czochanska, Z.; Gilbert, T.D.; Philp, R.P.; Sheppard, C.M.; Weston, R.J.; Wood, T.A.; Woolhouse, A.D. Geochemical application of sterane and triterpane biomarkers to a description of oils from the Taranaki Basin in New Zealand. Org. Geochem. 1988, 12, 123–135. [Google Scholar] [CrossRef]
- Peters, K.E.; Moldowan, J.M. Effects of source, thermal maturity, and biodegradation on the distribution and isomerization of homohopanes in petroleum. Org. Geochem. 1991, 17, 47–61. [Google Scholar] [CrossRef]
- Waples, D.W.; Machihara, T. Biomarkers for Geologists: A Practical Guide to the Application of Steranes and Triterpanes in Petroleum Geology; Cambridge University Press: Cambridge, UK, 2009; Volume 129, p. 793. [Google Scholar]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide: Volume 1: Biomarkers and Isotopes in the Environment and Human History, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004; Volume 1. [Google Scholar]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide: Volume 2: Biomarkers and Isotopes in Petroleum Systems and Earth History, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004; Volume 2. [Google Scholar]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Shao, X.; Pang, X.; Li, M.; Qian, M.; Hu, T.; Li, Z.; Zhang, H.a.; Xu, Y. Hydrocarbon retention in lacustrine shales during thermal maturation: Insights from semi-open system pyrolysis. J. Pet. Sci. Eng. 2020, 184, 106480. [Google Scholar] [CrossRef]
- Peters, K.; Clarke, M.E.; Dasgupta, U.; MacCaffrey, M.E.; Lee, C.Y. Recognition of an Infracambrian source rock based on biomarkers in the Baghewala-1 oil, India. AAPG Bull. 1995, 79, 1481–1494. [Google Scholar] [CrossRef]
- Lockhart, R.S.; Meredith, W.; Love, G.D.; Snape, C.E. Release of bound aliphatic biomarkers via hydropyrolysis from Type II kerogen at high maturity. Org. Geochem. 2008, 39, 1119–1124. [Google Scholar] [CrossRef]
- Lewan, M.; Birdwell, J. Application of Uniaxial Confining-Core Clamp with Hydrous Pyrolysis in Petrophysical and Geochemical Studies of Source Rocks at Various Thermal Maturities. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Denver, CO, USA, 12–14 August 2013. [Google Scholar]
- Arysanto, A.; Burnaz, L.; Zheng, T.; Littke, R. Geochemical and petrographic evaluation of hydrous pyrolysis experiments on core plugs of Lower Toarcian Posidonia Shale: Comparison of artificial and natural thermal maturity series. Int. J. Coal Geol. 2024, 284, 104459. [Google Scholar] [CrossRef]
- Mackenzie, A.S.; McKenzie, D. Isomerization and aromatization of hydrocarbons in sedimentary basins formed by extension. Geol. Mag. 1983, 120, 417–470. [Google Scholar] [CrossRef]
- Suzuki, N. Estimation of maximum temperature of mudstone by two kinetic parameters; epimerization of sterane and hopane. Geochim. Cosmochim. Acta 1984, 48, 2273–2282. [Google Scholar] [CrossRef]
- Rullkötter, J.; Marzi, R. Natural and artificial maturation of biological markers in a Toarcian shale from northern Germany. Org. Geochem. 1988, 13, 639–645. [Google Scholar] [CrossRef]
- Marzi, R.; Rullkötter, J.; Perriman, W.S. Application of the change of sterane isomer ratios to the reconstruction of geothermal histories: Implications of the results of hydrous pyrolysis experiments. Org. Geochem. 1990, 16, 91–102. [Google Scholar] [CrossRef]
- Ulmishek, G.F. Petroleum Geology and Resources of the West Siberian Basin, Russia. U.S. Geol. Surv. Bull. 2001, 2201-G, 49. [Google Scholar] [CrossRef]
- Chen, J.; Peng, P.a. A comparative study of free and bound bitumens from different maturesource rocks with Type III kerogens. Org. Geochem. 2017, 112, 1–15. [Google Scholar] [CrossRef]
- Lewan, M.D. Evaluation of petroleum generation by hydrous phrolysis experimentation. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1985, 315, 123–134. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalmykov, A.G.; Levkina, V.V.; Tikhonova, M.S.; Savostin, G.G.; Makhnutina, M.L.; Vidishcheva, O.N.; Volkov, D.S.; Pirogov, A.V.; Proskurnin, M.A.; Kalmykov, G.A. Laboratory Modeling of the Bazhenov Formation Organic Matter Transformation in a Semi-Open System: A Comparison of Oil Generation Kinetics in Two Samples with Type II Kerogen. Fuels 2025, 6, 22. https://doi.org/10.3390/fuels6020022
Kalmykov AG, Levkina VV, Tikhonova MS, Savostin GG, Makhnutina ML, Vidishcheva ON, Volkov DS, Pirogov AV, Proskurnin MA, Kalmykov GA. Laboratory Modeling of the Bazhenov Formation Organic Matter Transformation in a Semi-Open System: A Comparison of Oil Generation Kinetics in Two Samples with Type II Kerogen. Fuels. 2025; 6(2):22. https://doi.org/10.3390/fuels6020022
Chicago/Turabian StyleKalmykov, Anton G., Valentina V. Levkina, Margarita S. Tikhonova, Grigorii G. Savostin, Mariia L. Makhnutina, Olesya N. Vidishcheva, Dmitrii S. Volkov, Andrey V. Pirogov, Mikhail A. Proskurnin, and Georgii A. Kalmykov. 2025. "Laboratory Modeling of the Bazhenov Formation Organic Matter Transformation in a Semi-Open System: A Comparison of Oil Generation Kinetics in Two Samples with Type II Kerogen" Fuels 6, no. 2: 22. https://doi.org/10.3390/fuels6020022
APA StyleKalmykov, A. G., Levkina, V. V., Tikhonova, M. S., Savostin, G. G., Makhnutina, M. L., Vidishcheva, O. N., Volkov, D. S., Pirogov, A. V., Proskurnin, M. A., & Kalmykov, G. A. (2025). Laboratory Modeling of the Bazhenov Formation Organic Matter Transformation in a Semi-Open System: A Comparison of Oil Generation Kinetics in Two Samples with Type II Kerogen. Fuels, 6(2), 22. https://doi.org/10.3390/fuels6020022





