Advancements in Synthetic Biology for Enhancing Cyanobacterial Capabilities in Sustainable Plastic Production: A Green Horizon Perspective
Abstract
1. Introduction
2. Synthetic Biology Strategies for Enhancing Cyanobacterial Plastic Production
3. Role of Cyanobacteria in Carbon Sequestration
3.1. Cyanobacteria as Photosynthetic Prokaryotes
3.2. Unique Attributes: Minimal Growth Requirements, High Photosynthetic Efficiency, and Rapid Growth Rates
| Species | Temperature (°C) | Time (h) | Genome Size (Mb) | Genome Copy No. | References |
|---|---|---|---|---|---|
| Anabaena cylindrica | 28 | 18.7 | – | 25 | [73] |
| Anabaena variabilis | 30 | – | 7.1 | 5–8 | [74] |
| 1450/10 Microcystis aeruginosa | 28 | stat.ph. a | – | 1–10 | [75] |
| Anabaena sp. Strain PCC 7120 | 28 | – | 7.2 | 8.2 | [76] |
| Prochlorococcus | – | – | 1.7 | – | [77] |
| Synechococcus elongatus PCC 7942 | 29 | 26 | 2.7 | 3.7/3.4 | [78] |
| Synechococcus elongatus PCC 6301) | 35 | 10 to >50 | 2.9 | 2–7 to >1–2 d | [79] |
| Synechococcus sp. (strain WH7803) | 26 | – | 2.5 | 3.7 e | [78] |
| Synechococcus sp. Strain WH7803 | 23 | – | 2.3 | 2–6 c | [80] |
| Parasynechococcus subtropicalis WH 7805 | 26 | 17 | 2.5 | 1 e | [80] |
| Synechococcus sp. WH 8101 | 27 | 15 | 3.3 | 1 e | [81] |
| Synechocystis sp. PCC 6803 | 23 | 23 | 3.7 | 215/56/59 c | [78] |
| Synechocystis sp. PCC 6803 | 22 | 22 | 3.8 | 143/46/47 c | [78] |
| Synechocystis sp. PCC 6803 | 31 | 15–24 | 3.9 | 16 | [82] |
| Trichodesmium erythraeumIMS101 | 26 | LDC b | 7.77 | 700 | [83] |
3.3. Lipid Content in Thylakoid Membranes and Its Significance
4. Cyanobacteria’s Contribution to Biofuel Production
4.1. Overview of Biodiesel Production from Cyanobacteria
4.2. Mitigation of Toxic Sulfur Release
4.3. Reduction of Aromatic Hydrocarbons
4.4. Combustion Potential and Sustainability
5. Polyhydroxyalkanoates (PHAs) Production by Cyanobacteria
5.1. Intracellular Energy Source and Carbon Sink
5.2. Cyanobacteria’s Role in Bioplastic Production
5.3. Comparative Analysis with Conventional Plastics
5.4. PHAs as Parallel Competitors to Petrochemical-Based Plastics
6. Environmental Benefits of Cyanobacteria
| Strain Classification | Functional Strains | Target Microcystins | Initial Microcystins Concentration | Microcystins Degradation Efficiency | References |
|---|---|---|---|---|---|
| Bacillota | Bacillus subtilis (strain 168) | Microcystin-LR | 6 μg/mL | (48 h) | [225] |
| Bacillota | Bacillus brevis LEw-1238 | Microcystin-LR | 6 μg/mL | (48 h) | [226] |
| Bacillota | Bacillus thuringiensis LEw-2010 | MICROCYSTIN-RR | 20 μg/mL | 73% (3 d) | [226] |
| Bacillota | Lysinibacillus boronitolerans strain CQ5 | Microcystin-LR | 14.10 μg/L | 90% (24 h) | [227] |
| Actinomyces | Rhodococcus cavernicola C1-24 | Microcystin-LR | 10 mg/L | 9, 10, 8, 6, days | [228] |
| Actinomyces | Brevibacterium F3 | MICROCYSTIN-RR | 10 mg/L | 9, 10, 7, 9, days | [228] |
| Actinomyces | Arthrobacter sp. C6 | Microcystin-LR | 10 mg/L | 9, 10, 8, 5 days | [228] |
| Actinomyces | Arthrobacter sp. F7 | MICROCYSTIN-LF | 10 mg/L | 9, 10, 7, 6, days | [228] |
| Actinomyces | Arthrobacter sanguinis sp. F10 | Microcystin-LR | 6 μg/mL | 98% (4 days) | [229] |
| Actinomycetes | Arthrobacter sp. strain R1 | Microcystin-LR | 6 μg/mL | (4 days) | [229] |
| Actinomyces | Arthrobacter sp. strain R6. | Microcystin-LR | 6 μg/mL | (4 days) | [229] |
| Actinomycetes | Arthrobacter sp. strain R9 | Microcystin-LR | 6 μg/mL | (4 days) | [229] |
| Actinomyces | Arthrobacter sanguinis sp. 423 | Microcystin-LR | 4 μg/L | 25.88% (10 days) | [230] |
| Actinomycetes | Arthrobacter sanguinis sp. 443 | Microcystin-LR | 4 μg/L | 17.91% (10 days) | [230] |
| Actinomycetes | Bifidobacterium BB-12 | Microcystin-LR | 110μg/L | 59.12% (3 days) | [231] |
| Actinomycetes | Bifidobacterium longum BB-46 | Microcystin-LR | 110 μg/L | 48.0% (3 days) | [231] |
| Actinomycetes | Bifidobacterium BB-420 | Microcystin-LR | 110 μg/L | 48.8% (3 days) | [231] |
| Fungus | Trichaptum abietinum 1302BG | Microcystin-LR | 0.07 mg/L | (12 h) | [232,233] |
| Fungus | Schizophyllum commune strain IUM1114-SS01 | Microcystin-LR | 20 mg/L | (2 days) | [234] |
| Fungus | ascomycete strain Trichoderma | MICROCYSTINs | 2.9 mg/L | (4 days) | [67] |
| Fungus | Winter mucus d. ursingii strain EH5 | Microcystin-LR | 0.05 mg/L | 39% (3 days) | [235] |
| Fungus | Aureobasidium pullulans KKUY0701 | MICROCYSTINs | 3 mg/L | 58.8% (1 h) | [236] |
| Proteobacteria | Sphingomonas Paucimobilis . CBA4 | MICROCYSTIN-RR | 150 μg/L | (3 days) | [237] |
| Alphaproteobacteria | Sphingomonas sp. ACM-3962 | MICROCYSTIN-RR | 15 mg/L | 2.7 mg/(L·h) | [238] |
| Proteobacteria | Pseudomonas paucimobilis | MICROCYSTIN-RR | 3 mg/L | 0.09, mg/(L·h) | [239,240] |
| Proteobacteria | Novosphingobium sp. NV-3 | Microcystin-LR | 30 mg/L 3) | 0.39 mg/(L·h) | [241] |
| Proteobacteria | Sphingomonas parapaucimobilis 7CY | MICROCYSTIN-LW | 8 mg/L | 5 days | [242] |
| Proteobacteria | Sphingomonas Y2 | Microcystin-LR | 22 mg/L | 0.26 mg/(L·h) | [243] |
| Proteobacteria | Novosphingobium sp. KKU15 | Microcystin-LR | 6 μg/mL | (4 days) | [244] |
| Proteobacteria | Novosphingobium sp. ERW19 | Microcystin-LR, | 0.2 mg/L | 0.010 mg/(L·h) | [245] |
| Proteobacteria | Novosphingobium sp. ERN07 | MICROCYSTIN-RR | 0.2 mg/L | 0.007 mg/(L·h) | [245] |
| Proteobacteria | Novosphingobium sp. KKU25s | Microcystin-LR | 30 μg/L | 1.07 μg/(L·h) | [246] |
| Alphaproteobacteria | Sphingopyxis sp. a7 | Microcystin-LR | 14.5 mg/L | 3.44 mg/(L·h) | [121] |
| Alphaproteobacteria | Sphingopyxis sp. X20 | Microcystin-LR | 7 mg/L | (2 days) | [122] |
| Alphaproteobacteria | Sphingopyxis sp. YF1 | Microcystin-LR | 15 μg/mL | 53.6 μg/(mL·h) | [102] |
| Alphaproteobacteria | Sphingopyxis sp. USTB-05 | MICROCYSTIN-LA | 3.7 μg/L | (3 days) | [247] |
| Alphaproteobacteria | Sphingosinicella microcystinivorans strain B-9 | MICROCYSTIN-RR | 2.4 mg/L | 0.06, mg/(L·h) | [239,240] |
| Alphaproteobacteria | algicidal bacterium Ochrobactrum sp. FDT5 | Microcystin-LR | 460.9 mg/L | (6 days) | [248] |
| Pseudomonadota | R. solanacearum | Microcystin-LR | 9.6 mg/L | (2 days) | [249] |
| Pseudomonadota | Bordetella sp. strain MC-LTH1 | MICROCYSTIN-RR | 11, 8 mg/L | (3 days) | [250] |
| Beta Proteobacteria | B pertussis. | Microcystin-LR | 18 μg/L | (2 days) | [251] |
| Pseudomonadota | Methylobacillus sp. | MICROCYSTIN-RR | 3.7, 4.2 mg/L | <0.26, >0.26 mg/(L·h) | [252] |
| Pseudomonadota | Paucibacter sp. KCTC 42545 | Microcystin-LR | 11.8 μg/mL | (10 h) | [253] |
| Beta Proteobacteria | Paucibacter toxinivorans DSM 16,998; | MICROCYSTIN-LY | 15 mg/L | 8, days | [228] |
| Beta Proteobacteria | Paucibacter toxinivorans IM-4) | MICROCYSTIN-YR | 0.95, mg/L | (3 days) | [119] |
| Pseudomonadota | P. toxinivorans (2C20 strain) | MICROCYSTIN-YR, | 15, μg/mL | (8 days) | [254] |
| Pseudomonadota | ComamonasLEw-2 | Microcystin-LR | 10 μg/mL | (3 days) | [226] |
| Pseudomonadota | Stenotrophomonas maltophilia . EMS | Microcystin-LR | 1.7 μg/mL | (2 days) | [255] |
| Pseudomonadota | Stenotrophomonas maltophila LEw-1278 | Microcystin-LR | 10 μg/mL | (2 days) | [226] |
| Pseudomonadota | Stenotrophomonas maltophila | Microcystin-LR | 10 μg/mL | 0.6 μg/(mL·h) | [256] |
| Gammaproteobacteria | Xanthomonas beteli | Microcystin-LR | 39.4 mg/L | 31, mg/(L·h) | [257] |
| Pseudomonadota | Stenotrophomonas mori sp. | MICROCYSTIN-LF | 5.7 μg/mL | (12 days), (14 days), (15 days) | [258] |
| Pseudomonadota | Morganella morganii | Microcystin-LR | 25 μg/L | 3.77 mg/(L·h) | [259] |
| Pseudomonadota | Pseudomonas aeruginosa UCBPP-PA14 | MICROCYSTIN-LW | 350 μg/L 3) | 85% (30 days) | [218] |
| Pseudomonadota | Arthrobacter siderocapsulatus | Microcystin-LR | 350 μg/L 3) | 37% (30 days) | [218] |
| Gammaproteobacteria | Achromobacter xylosoxidans | Microcystin-LR | 150 μg/L | 79.8% (7 days) | [260] |
| Gammaproteobacteria | Enterobacter kobei | Microcystin-LR | 5 μg/mL | 0.388 μg/(mL·h) | [261] |
| Bacillota | Lacticaseibacillus rhamnosus | Microcystin-LR | 150 μg/L | (3 days) | [231] |
| Firmicutes | Lacticaseibacillus rhamnosus Lc 705 | Microcystin-LR | 150 μg/L | (3 days) | [231] |
| Bacillota | Bacillus sp. | MICROCYSTIN-RR | 15 mg/L | (6 days) | [262] |
| Bacillota | Bacillus subtilis | MICROCYSTIN-RR | 15 mg/L | (6 days) | [262] |
| Firmicutes | B. thuringiensis sp. | MICROCYSTIN-LR | 20 mg/L | 85% (10 days) | [263] |
| Bacillota | Bacillus atrophaeus sp. | MICROCYSTIN-RR | 15 mg/L | (5 days) | [262] |
| Bacillota | Bacillus sp. Ak3 | MICROCYSTIN-RR | 25 μg/mL | 75%(5 days) | [225] |
| Firmicutes | Brevibacillus brevis LEw-1238 | MICROCYSTIN-LR | 15 μg/mL | (3 days) | [226] |
| Bacillota | Bacillus thuringiensis LEw-2010 | MICROCYSTIN-LR | 15 μg/mL | (3 days) | [228] |
| Bacillota | Lysinibacillus boronitolerans (T-10a, AB199591) | MICROCYSTIN-LR | 14.15 μg/L | (3 days) | [227] |
| Actinomycetota | Rhodococcus cavernicola C1-24 | MICROCYSTIN-LR, | 15 mg/L | 5 days | [228] |
| Actinomycetota | Brevibacterium F3 | MICROCYSTIN-LR, | 15 mg/L | 6 days | [228] |
| Actinomycetes | Arthrobacter sp. C6 | MICROCYSTIN-LR | 15 mg/L | 5 days | [228] |
| Arthrobacter sanguinis sp. F7 | MICROCYSTIN-LR | 15 mg/L | 6 days | [228] | |
| Actinomycetota | MICROCYSTIN-LY | 10 mg/L | 97 days | [228] | |
| Corynebacterium | Arthrobacter sanguinis sp. F10 | MICROCYSTIN-LR | 7 μg/mL | (4 days) | [229] |
| Corynebacterium | Arthrobacter sanguinis sp. R1 | MICROCYSTIN-LR | 7 μg/mL | (4 days) | [229] |
| Bifidobacterium | Arthrobacter sanguinis sp. R6 | MICROCYSTIN-LR | 7 μg/mL | (4 days) | [229] |
| Actinomycetes | Arthrobacter sanguinis sp. R9 | MICROCYSTIN-LR | 7 μg/mL | (4 days) | [229] |
| Bifidobacterium | Arthrobacter sp. 423 | MICROCYSTIN-LR | 55 μg/L | (10 days) | [230] |
| Actinomycetes | Arthrobacter sp. JS443 | MICROCYSTIN-LR | 5 μg/L | 15.92% 10 days) | [230] |
| Bifidobacterium | Bifidobacterium BB-12 | MICROCYSTIN-LR | 150 μg/L | (2 days) | [231] |
| Bifidobacterium | B. longum strains | MICROCYSTIN-LR | 150 μg/L | (2 days) | [231] |
| Bifidobacterium | Bifidobacterium animalis subsp. lactis 420 | MICROCYSTIN-LR | 150 μg/L | (2 days) | [231] |
6.1. Carbon Sequestration and Reduction of Carbon Footprint
6.2. Sustainable Environmental Goals and Cyanobacteria’s Contribution
7. Efficiency of Cyanobacteria in Bioplastic Production
7.1. Photosynthetic Process and Increased Production Efficiency
7.2. Limited Land Input and Acceptable Cost Implications
7.3. Advantages over Other Sources of Bioplastics
8. Achievements and Constraints in Commercialization Pathway
Challenges Faced in Scaling up Cyanobacteria-Based Production
9. Cyanobacterial Species as Sources of Green and Clean Energy
9.1. Diversity of Cyanobacterial Species
9.2. High Potential in Biofuel Production
9.3. Role in the Production of Biodegradable Plastics
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic waste: Challenges and opportunities to mitigate pollution and effective management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Bleakley, B.; Zhou, R. Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology. Molecules 2024, 29, 2534. [Google Scholar] [CrossRef] [PubMed]
- Davis, H. Plastic Matter; Duke University Press: Durham, NC, USA, 2022; ISBN 978-1-4780-1775-2. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S. Impacts of plastic pollution on ecosystem services, sustainable development goals, and need to focus on circular economy and policy interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, J.; Saturno, J.; Brooks, A.L.; Jacobs, S.; Jambeck, J.R. An emerging source of plastic pollution: Environmental presence of plastic personal protective equipment (PPE) debris related to COVID-19 in a metropolitan city. Environ. Pollut. 2021, 269, 116160. [Google Scholar] [CrossRef]
- Mejjad, N.; Cherif, E.K.; Rodero, A.; Krawczyk, D.A.; El Kharraz, J.; Moumen, A.; Laqbaqbi, M.; Fekri, A. Disposal behavior of used masks during the COVID-19 pandemic in the Moroccan community: Potential environmental impact. Int. J. Environ. Res. Public Health 2021, 18, 4382. [Google Scholar] [CrossRef]
- Awogbemi, O.; Von Kallon, D.V. Achieving affordable and clean energy through conversion of waste plastic to liquid fuel. J. Energy Inst. 2023, 106, 101154. [Google Scholar] [CrossRef]
- Warning, U.N.E.P.D.o.E. UNEP Year Book 2011: Emerging Issues in Our Global Environment; UNEP/Earthprint: Nairobi, Kenya, 2011. [Google Scholar]
- Stegmann, P.; Daioglou, V.; Londo, M.; van Vuuren, D.P.; Junginger, M. Plastic futures and their CO2 emissions. Nature 2022, 612, 272–276. [Google Scholar] [CrossRef]
- Nawaz, T.; Gu, L.; Gibbons, J.; Hu, Z.; Zhou, R. Bridging Nature and Engineering: Protein-Derived Materials for Bio-Inspired Applications. Biomimetics 2024, 9, 373. [Google Scholar] [CrossRef]
- Ghosh, K.; Jones, B.H. Roadmap to biodegradable plastics—Current state and research needs. ACS Sustain. Chem. Eng. 2021, 9, 6170–6187. [Google Scholar] [CrossRef]
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Strategies to achieve a carbon neutral society: A review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, T.; Fahad, S.; Zhou, R. Protein Phosphorylation Nexus of Cyanobacterial Adaptation and Metabolism. Kinases Phosphatases 2024, 2, 209–223. [Google Scholar] [CrossRef]
- Agarwal, P.; Soni, R.; Kaur, P.; Madan, A.; Mishra, R.; Pandey, J.; Singh, S.; Singh, G. Cyanobacteria as a promising alternative for sustainable environment: Synthesis of biofuel and biodegradable plastics. Front. Microbiol. 2022, 13, 939347. [Google Scholar] [CrossRef]
- Chen, H.-G.; Zhang, Y.-H.P. New biorefineries and sustainable agriculture: Increased food, biofuels, and ecosystem security. Renew. Sustain. Energy Rev. 2015, 47, 117–132. [Google Scholar] [CrossRef]
- Żymańczyk-Duda, E.; Samson, S.O.; Brzezińska-Rodak, M.; Klimek-Ochab, M. Versatile applications of cyanobacteria in biotechnology. Microorganisms 2022, 10, 2318. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, L.; Mallick, N.; Mala, J. Progress and challenges in producing polyhydroxyalkanoate biopolymers from cyanobacteria. J. Appl. Phycol. 2017, 29, 1213–1232. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Chang, J.-S.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.; Ornelas-Soto, N.; Romero-Ogawa, M.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef]
- Khan, A.Z.; Bilal, M.; Mehmood, S.; Sharma, A.; Iqbal, H.M. State-of-the-art genetic modalities to engineer cyanobacteria for sustainable biosynthesis of biofuel and fine-chemicals to meet bio–economy challenges. Life 2019, 9, 54. [Google Scholar] [CrossRef]
- Johnson, T.J.; Gibbons, J.L.; Gu, L.; Zhou, R.; Gibbons, W.R. Molecular genetic improvements of cyanobacteria to enhance the industrial potential of the microbe: A review. Biotechnol. Prog. 2016, 32, 1357–1371. [Google Scholar] [CrossRef]
- Satta, A.; Esquirol, L.; Ebert, B.E. Current metabolic engineering strategies for photosynthetic bioproduction in cyanobacteria. Microorganisms 2023, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Carr, R. B-Pinene Synthesis in Synechococcus sp. pcc 7002 Cyanobacteria: Metabolic Engineering for High-Density Biofuel Applications. Master’s Thesis, The University of Wisconsin Oshkosh, Oshkosh, WI, USA, 2017. [Google Scholar]
- Jeong, S.H.; Lee, H.J.; Lee, S.J. Recent Advances in CRISPR-Cas Technologies for Synthetic Biology. J. Microbiol. 2023, 61, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Meng, W.; Su, Y.; Qian, C.; Fu, W. Emerging technologies for advancing microalgal photosynthesis and metabolism toward sustainable production. Front. Mar. Sci. 2023, 10, 1260709. [Google Scholar] [CrossRef]
- Kumar, G.; Shekh, A.; Jakhu, S.; Sharma, Y.; Kapoor, R.; Sharma, T.R. Bioengineering of microalgae: Recent advances, perspectives, and regulatory challenges for industrial application. Front. Bioeng. Biotechnol. 2020, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Koch, M. Metabolic Engineering Strategies for an Increased PHB Production in Cyanobacteria. Ph.D. Thesis, Universität Tübingen, Tübingen, Germany, 2020. [Google Scholar]
- Choi, Y.-N.; Lee, J.W.; Kim, J.W.; Park, J.M. Acetyl-CoA-derived biofuel and biochemical production in cyanobacteria: A mini review. J. Appl. Phycol. 2020, 32, 1643–1653. [Google Scholar] [CrossRef]
- Verdú-Navarro, F.; Moreno-Cid, J.A.; Weiss, J.; Egea-Cortines, M. The advent of plant cells in bioreactors. Front. Plant Sci. 2023, 14, 1310405. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Yang, T.; Chen, R.; Luo, X. Pathway engineering of plant-derived bioactive compounds in microbes. In Engineering Biology for Microbial Biosynthesis of Plant-Derived Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2024; pp. 73–87. [Google Scholar]
- Hassan, A.I.; Saleh, H.M. Production of Amino Acids and Nucleic Acids from Genetically Engineered Microbial Cells and their Relevance to Biodegradation. Green Energy Environ. Technol. 2023. [Google Scholar] [CrossRef]
- Kastberg, L.L.B.; Ard, R.; Jensen, M.K.; Workman, C.T. Burden imposed by heterologous protein production in two major industrial yeast cell factories: Identifying sources and mitigation strategies. Front. Fungal Biol. 2022, 3, 827704. [Google Scholar] [CrossRef]
- Gudmundsson, S.; Nogales, J. Cyanobacteria as photosynthetic biocatalysts: A systems biology perspective. Mol. BioSystems 2015, 11, 60–70. [Google Scholar] [CrossRef]
- Janssen, P.J.; Lambreva, M.D.; Plumeré, N.; Bartolucci, C.; Antonacci, A.; Buonasera, K.; Frese, R.N.; Scognamiglio, V.; Rea, G. Photosynthesis at the forefront of a sustainable life. Front. Chem. 2014, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Jiang, Z.; Hassan, S.; Harrison, M.T.; Liu, K.; Khan, M.A.; Liu, H. A comprehensive review of the therapeutic potential of cyanobacterial marine bioactives: Unveiling the hidden treasures of the sea. Food Energy Secur. 2023, 12, e495. [Google Scholar] [CrossRef]
- Gu, L.; Nawaz, T.; Qiu, Y.; Wu, Y.; Zhou, R. Photosynthetic conversion of CO2 and H2O to long-chain terpene alcohol by genetically engineered N2-fixing cyanobacteria. In Photosynthesis; Elsevier: Amsterdam, The Netherlands, 2023; pp. 451–461. [Google Scholar]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Hassan, S.; Harrison, M.T.; Liu, K.; Zhou, R. Unveiling the antioxidant capacity of fermented foods and food microorganisms: A focus on cyanobacteria. J. Umm Al-Qura Univ. Appl. Sci. 2023, 10, 232–243. [Google Scholar] [CrossRef]
- Archer, M.D.; Barber, J. Photosynthesis and photoconversion. Mol. Glob. Photosynth. 2004, 2, 1–42. [Google Scholar]
- Singh, S.K.; Sundaram, S.; Kishor, K. Photosynthetic Microorganisms: Mechanism for Carbon Concentration; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Golubic, S.; Seong-Joo, L.; Browne, K.M. Cyanobacteria: Architects of sedimentary structures. In Microbial Sediments; Springer: Berlin/Heidelberg, Germany, 2000; pp. 57–67. [Google Scholar]
- Robles-Fernández, A.; Areias, C.; Daffonchio, D.; Vahrenkamp, V.C.; Sánchez-Román, M. The Role of microorganisms in the nucleation of carbonates, environmental implications and applications. Minerals 2022, 12, 1562. [Google Scholar] [CrossRef]
- Paerl, H.W. Mitigating harmful cyanobacterial blooms in a human-and climatically-impacted world. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Zhang, P.; Wu, F.; Wang, Y.; Xu, C.; Zhang, L.; An, S.; Kuzyakov, Y. Large-scale ecosystem carbon stocks and their driving factors across Loess Plateau. Carbon Neutrality 2023, 2, 5. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Singh, P.; Gupta, N.; Bhattacharjee, S.; Srivastava, A.; Parida, A.; Mishra, A.K. Cyanobacteria: A key player in nutrient cycling. In Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2024; pp. 579–596. [Google Scholar]
- Ullah, I.; Dawar, K.; Tariq, M.; Sharif, M.; Fahad, S.; Adnan, M.; Ilahi, H.; Nawaz, T.; Alam, M.; Ullah, A. Gibberellic acid and urease inhibitor optimize nitrogen uptake and yield of maize at varying nitrogen levels under changing climate. Environ. Sci. Pollut. Res. 2022, 29, 6568–6577. [Google Scholar] [CrossRef]
- Stanier, R.; Cohen-Bazire, G. Phototrophic prokaryotes: The cyanobacteria. Annu. Rev. Microbiol. 1977, 31, 225–274. [Google Scholar] [CrossRef]
- Ligrone, R.; Ligrone, R. The Chloroplast and Photosynthetic Eukaryotes. Biological Innovations That Built the World: A Four-Billion-Year Journey through Life and Earth History; Springer: Berlin/Heidelberg, Germany, 2019; pp. 269–310. [Google Scholar]
- Nevo, R.; Charuvi, D.; Tsabari, O.; Reich, Z. Composition, architecture and dynamics of the photosynthetic apparatus in higher plants. Plant J. 2012, 70, 157–176. [Google Scholar] [CrossRef]
- Sekar, N.; Ramasamy, R.P. Recent advances in photosynthetic energy conversion. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 19–33. [Google Scholar] [CrossRef]
- Assunção, J.; Amaro, H.M.; Malcata, F.X.; Guedes, A.C. Cyanobacterial pigments: Photosynthetic function and biotechnological purposes. In The Pharmacological Potential of Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–256. [Google Scholar]
- Johnson, M.P.; Wientjes, E. The relevance of dynamic thylakoid organisation to photosynthetic regulation. Biochim. Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148039. [Google Scholar] [CrossRef] [PubMed]
- Knoll, A.H. Cyanobacteria and earth history. In The Cyanobacteria: Molecular Biology, Genomics, and Evolution; Caister Academic Press: Wymondham, UK, 2008; Volume 484. [Google Scholar]
- Cole, J.J.; Hararuk, O.; Solomon, C.T. The carbon cycle: With a brief introduction to global biogeochemistry. In Fundamentals of Ecosystem Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 131–160. [Google Scholar]
- Gaysina, L.A.; Saraf, A.; Singh, P. Cyanobacteria in diverse habitats. In Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–28. [Google Scholar]
- Fischer, W.W.; Hemp, J.; Valentine, J.S. How did life survive Earth’s great oxygenation? Curr. Opin. Chem. Biol. 2016, 31, 166–178. [Google Scholar] [CrossRef]
- Dextro, R.B.; Andreote, A.P.; Vaz, M.G.; Carvalho, C.R.; Fiore, M.F. The pros and cons of axenic cultures in cyanobacterial research. Algal Res. 2024, 78, 103415. [Google Scholar] [CrossRef]
- Singhal, R.K.; Fahad, S.; Kumar, P.; Choyal, P.; Javed, T.; Jinger, D.; Singh, P.; Saha, D.; Md, P.; Bose, B. Beneficial elements: New Players in improving nutrient use efficiency and abiotic stress tolerance. Plant Growth Regul. 2023, 100, 237–265. [Google Scholar] [CrossRef]
- Wehr, J.; van Vuuren, S.J. Algae and Cyanobacteria Communities. In Wetzel’s Limnology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 463–510. [Google Scholar]
- Rabalais, N.N. Nitrogen in aquatic ecosystems. AMBIO J. Hum. Environ. 2002, 31, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Zúñiga, D.; Méndez-Zavala, A.; Solís-Quiroz, O.; Morales-Oyervides, L.; Montañez-Saénz, J.C.; Benavente-Valdés, J.R. Biology and composition of microalgae and cyanobacteria. In Sustainable Industrial Processes Based on Microalgae; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–22. [Google Scholar]
- Creed, I.F.; Bergström, A.K.; Trick, C.G.; Grimm, N.B.; Hessen, D.O.; Karlsson, J.; Kidd, K.A.; Kritzberg, E.; McKnight, D.M.; Freeman, E.C. Global change-driven effects on dissolved organic matter composition: Implications for food webs of northern lakes. Glob. Change Biol. 2018, 24, 3692–3714. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, M.; Hess, W.R. Systems and synthetic biology for the biotechnological application of cyanobacteria. Curr. Opin. Biotechnol. 2018, 49, 94–99. [Google Scholar] [CrossRef]
- Shih, P.M.; Wu, D.; Latifi, A.; Axen, S.D.; Fewer, D.P.; Talla, E.; Calteau, A.; Cai, F.; Tandeau de Marsac, N.; Rippka, R. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 1053–1058. [Google Scholar] [CrossRef]
- Oliver, R.L.; Hamilton, D.P.; Brookes, J.D.; Ganf, G.G. Physiology, blooms and prediction of planktonic cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Springer: Berlin/Heidelberg, Germany, 2012; pp. 155–194. [Google Scholar]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Turnau, K.; Płachno, B.J.; Bień, P.; Świątek, P.; Dąbrowski, P.; Kalaji, H. Fungal symbionts impact cyanobacterial biofilm durability and photosynthetic efficiency. Curr. Biol. 2023, 33, 5257–5262.e3. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Tiwari, D.; Rai, A.N. Cyanobacteria: From Basic Science to Applications; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Butman, D.; Stackpoole, S.; Stets, E.; McDonald, C.P.; Clow, D.W.; Striegl, R.G. Aquatic carbon cycling in the conterminous United States and implications for terrestrial carbon accounting. Proc. Natl. Acad. Sci. USA 2016, 113, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.D. Macromolecular composition of spores from the filamentous cyanobacterium Anabaena cylindrica. J. Bacteriol. 1977, 129, 1154–1155. [Google Scholar] [CrossRef] [PubMed]
- Lynn, M.E.; Bantle, J.A.; Ownby, J.D. Estimation of gene expression in heterocysts of Anabaena variabilis by using DNA-RNA hybridization. J. Bacteriol. 1986, 167, 940–946. [Google Scholar] [CrossRef]
- Kurmayer, R.; Kutzenberger, T. Application of real-time PCR for quantification of microcystin genotypes in a population of the toxic cyanobacterium Microcystis sp. Appl. Environ. Microbiol. 2003, 69, 6723–6730. [Google Scholar] [CrossRef]
- Hu, B.; Yang, G.; Zhao, W.; Zhang, Y.; Zhao, J. MreB is important for cell shape but not for chromosome segregation of the filamentous cyanobacterium Anabaena sp. PCC 7120. Mol. Microbiol. 2007, 63, 1640–1652. [Google Scholar] [CrossRef]
- Vaulot, D.; Marie, D.; Olson, R.J.; Chisholm, S.W. Growth of Prochlorococcus, a photosynthetic prokaryote, in the equatorial Pacific Ocean. Science 1995, 268, 1480–1482. [Google Scholar] [CrossRef]
- Griese, M.; Lange, C.; Soppa, J. Ploidy in cyanobacteria. FEMS Microbiol. Lett. 2011, 323, 124–131. [Google Scholar] [CrossRef]
- Palacio, A.S.; Cabello, A.M.; García, F.C.; Labban, A.; Morán, X.A.G.; Garczarek, L.; Alonso-Sáez, L.; López-Urrutia, Á. Changes in population age-structure obscure the temperature-size rule in marine cyanobacteria. Front. Microbiol. 2020, 11, 2059. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.J.; Chisholm, S.W. Cell cycle regulation in marine Synechococcus sp. strains. Appl. Environ. Microbiol. 1995, 61, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, E.; Bowen, J.; Olson, R.; Chisholm, S. Effect of light on the cell cycle of a marine Synechococcus strain. Appl. Environ. Microbiol. 1989, 55, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Labarre, J.; Chauvat, F.; Thuriaux, P. Insertional mutagenesis by random cloning of antibiotic resistance genes into the genome of the cyanobacterium Synechocystis strain PCC 6803. J. Bacteriol. 1989, 171, 3449–3457. [Google Scholar] [CrossRef]
- Sargent, E.C.; Hitchcock, A.; Johansson, S.A.; Langlois, R.; Moore, C.M.; LaRoche, J.; Poulton, A.J.; Bibby, T.S. Evidence for polyploidy in the globally important diazotroph Trichodesmium. FEMS Microbiol. Lett. 2016, 363, fnw244. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Blankenship, R.E. On the interface of light-harvesting antenna complexes and reaction centers in oxygenic photosynthesis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2019, 1860, 148079. [Google Scholar] [CrossRef]
- Zhou, Y.; vom Dorp, K.; Dörman, P.; Hölzl, G. Chloroplast lipids. In Chloroplasts. Current Research and Future Trends; Caister Academic Press: Wymondham, UK, 2016; pp. 1–24. [Google Scholar]
- Yang, Z.; Su, X.; Wu, F.; Gong, Y.; Kuang, T. Photochemical activities of plant photosystem I particles reconstituted into phosphatidylglycerol liposomes. J. Photochem. Photobiol. B Biol. 2005, 78, 125–134. [Google Scholar] [CrossRef]
- Duffy, C.; Valkunas, L.; Ruban, A. Light-harvesting processes in the dynamic photosynthetic antenna. Phys. Chem. Chem. Phys. 2013, 15, 18752–18770. [Google Scholar] [CrossRef]
- Puthiyaveetil, S.; Kirchhoff, H.; Höhner, R. Structural and functional dynamics of the thylakoid membrane system. In Chloroplasts: Current Research and Future Trends; Caister Academic Press: Wymondham, UK, 2016; pp. 59–88. [Google Scholar]
- Kobayashi, K. Role of membrane glycerolipids in photosynthesis, thylakoid biogenesis and chloroplast development. J. Plant Res. 2016, 129, 565–580. [Google Scholar] [CrossRef]
- Yip, L.X.; Wang, J.; Xue, Y.; Xing, K.; Sevencan, C.; Ariga, K.; Leong, D.T. Cell-derived nanomaterials for biomedical applications. Sci. Technol. Adv. Mater. 2024, 25, 2315013. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I. Optimising photosynthesis for environmental fitness. Funct. Plant Biol. 2020, 47, iii–vii. [Google Scholar] [CrossRef] [PubMed]
- Huokko, T.; Ni, T.; Dykes, G.F.; Simpson, D.M.; Brownridge, P.; Conradi, F.D.; Beynon, R.J.; Nixon, P.J.; Mullineaux, C.W.; Zhang, P. Probing the biogenesis pathway and dynamics of thylakoid membranes. Nat. Commun. 2021, 12, 3475. [Google Scholar] [CrossRef] [PubMed]
- Douchi, D.; Si Larbi, G.; Fel, B.; Bonnanfant, M.; Louwagie, M.; Jouhet, J.; Agnely, M.; Pouget, S.; Maréchal, E. Dryland endolithic Chroococcidiopsis and temperate fresh water Synechocystis have distinct membrane lipid and photosynthesis acclimation strategies upon desiccation and temperature increase. Plant Cell Physiol. 2023, 65, pcad139. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, N.-Z. Plant unsaturated fatty acids: Multiple roles in stress response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, T.; Saud, S.; Gu, L.; Khan, I.; Fahad, S.; Zhou, R. Cyanobacteria: Harnessing the Power of Microorganisms for Plant Growth Promotion, Stress Alleviation, and Phytoremediation in the Era of Sustainable Agriculture. Plant Stress 2024, 11, 100399. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.C.; Sinha, R.P.; Hader, D.-P. Role of lipids and fatty acids in stress tolerance in cyanobacteria. Acta Protozool. 2002, 41, 297–308. [Google Scholar]
- Marchetto, F.; Santaeufemia, S.; Lebiedzińska-Arciszewska, M.; Śliwińska, M.A.; Pich, M.; Kurek, E.; Naziębło, A.; Strawski, M.; Solymosi, D.; Szklarczyk, M. Dynamic adaptation of the extremophilic red microalga Cyanidioschyzon merolae to high nickel stress. Plant Physiol. Biochem. 2024, 207, 108365. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.; Javaid, A.; Khan, I. Study of biological processes using physical principles (eg, protein folding, molecular interactions). Worldw. J. Phys. 2022, 3, 7–15. [Google Scholar]
- Zorz, J. An Integrated Approach to Improving Efficiency in Photosynthetic Microbial Systems. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2021. [Google Scholar]
- Singh, S.; Morya, R.; Jaiswal, D.K.; Keerthana, S.; Kim, S.-H.; Manimekalai, R.; de Araujo Pereira, A.P.; Verma, J.P. Innovations and advances in enzymatic deconstruction of biomass and their sustainability analysis: A review. Renew. Sustain. Energy Rev. 2024, 189, 113958. [Google Scholar] [CrossRef]
- Lu, X. A perspective: Photosynthetic production of fatty acid-based biofuels in genetically engineered cyanobacteria. Biotechnol. Adv. 2010, 28, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Eungrasamee, K.; Zhu, Z.; Liu, X.; Jantaro, S.; Lindblad, P. Lipid metabolism in cyanobacteria: Biosynthesis and utilization. In Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2024; pp. 85–116. [Google Scholar]
- Srirangan, K.; Pyne, M.E.; Chou, C.P. Biochemical and genetic engineering strategies to enhance hydrogen production in photosynthetic algae and cyanobacteria. Bioresour. Technol. 2011, 102, 8589–8604. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Sharma, N.K.; Stal, L.J. The economics of cyanobacteria-based biofuel production: Challenges and opportunities. In Cyanobacteria: An Economic Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 167–180. [Google Scholar]
- Ambrosio, R.; Rizza, L.S.; Do Nascimento, M.; Pacheco, H.G.J.; Ramos, L.M.M.; Hernandez, J.A.; Curatti, L. Promises and challenges for expanding the use of N2-fixing cyanobacteria as a fertilizer for sustainable agriculture. In Cyanobacterial Lifestyle and Its Applications in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 99–158. [Google Scholar]
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny microbes with a big impact: The role of cyanobacteria and their metabolites in shaping our future. Mar. Drugs 2016, 14, 97. [Google Scholar] [CrossRef]
- Weigmann, K. Fixing carbon: To alleviate climate change, scientists are exploring ways to harness nature’s ability to capture CO2 from the atmosphere. EMBO Rep. 2019, 20, e47580. [Google Scholar] [CrossRef]
- Johnson, T.J.; Katuwal, S.; Anderson, G.A.; Gu, L.; Zhou, R.; Gibbons, W.R. Photobioreactor cultivation strategies for microalgae and cyanobacteria. Biotechnol. Prog. 2018, 34, 811–827. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Bonilla-Petriciolet, A.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Emerging technologies for biofuel production: A critical review on recent progress, challenges and perspectives. J. Environ. Manag. 2021, 290, 112627. [Google Scholar] [CrossRef]
- Çelekli, A.; Zariç, Ö.E. Plasma-Enhanced Microalgal Cultivation: A Sustainable Approach for Biofuel and Biomass Production. In Emerging Applications of Plasma Science in Allied Technologies; IGI Global: Hershey, PA, USA, 2024; pp. 243–263. [Google Scholar]
- Work, V.H. Metabolic and Physiological Engineering of Photosynthetic Microorganisms for the Synthesis of Bioenergy Feedstocks: Development, Characterization, and Optimization. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2014. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Fernández, F.A.; Camacho, F.G.; Chisti, Y. Photobioreactors: Light regime, mass transfer, and scaleup. In Progress in Industrial Microbiology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 231–247. [Google Scholar]
- Santos-Merino, M.; Singh, A.K.; Ducat, D.C. New applications of synthetic biology tools for cyanobacterial metabolic engineering. Front. Bioeng. Biotechnol. 2019, 7, 33. [Google Scholar] [CrossRef]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae-A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Egesa, D.; Plucinski, P. Efficient extraction of lipids from magnetically separated microalgae using ionic liquids and their transesterification to biodiesel. Biomass Convers. Biorefinery 2024, 14, 419–434. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M.M. A review of enzymatic transesterification of microalgal oil-based biodiesel using supercritical technology. Enzym. Res. 2011, 2011, 468292. [Google Scholar] [CrossRef] [PubMed]
- Okoye, P.; Hameed, B. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Gülüm, M.; Yesilyurt, M.K.; Bilgin, A. The modeling and analysis of transesterification reaction conditions in the selection of optimal biodiesel yield and viscosity. Environ. Sci. Pollut. Res. 2020, 27, 10351–10366. [Google Scholar] [CrossRef]
- Singh, V.K.; Jha, S.; Rana, P.; Soni, R.; Lalnunpuii, R.; Singh, P.K.; Sinha, R.P.; Singh, G. Cyanobacteria as a Biocatalyst for Sustainable Production of Biofuels and Chemicals. Energies 2024, 17, 408. [Google Scholar] [CrossRef]
- Gaurav, K.; Neeti, K.; Singh, R. Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green Technol. Sustain. 2023, 2, 100060. [Google Scholar] [CrossRef]
- Jiao, H.; Tsigkou, K.; Elsamahy, T.; Pispas, K.; Sun, J.; Manthos, G.; Schagerl, M.; Sventzouri, E.; Al-Tohamy, R.; Kornaros, M. Recent advances in sustainable hydrogen production from microalgae: Mechanisms, challenges, and future perspectives. Ecotoxicol. Environ. Saf. 2024, 270, 115908. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.A.; Soudagar, M.E.M.; Fattah, I.R. Current state and perspectives on transesterification of triglycerides for biodiesel production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Lin, F.; Xu, M.; Ramasamy, K.K.; Li, Z.; Klinger, J.L.; Schaidle, J.A.; Wang, H. Catalyst deactivation and its mitigation during catalytic conversions of biomass. ACS Catal. 2022, 12, 13555–13599. [Google Scholar] [CrossRef]
- Trunschke, A. Prospects and challenges for autonomous catalyst discovery viewed from an experimental perspective. Catal. Sci. Technol. 2022, 12, 3650–3669. [Google Scholar] [CrossRef]
- Yahaya, M.S. Development of Solid Acid Catalysts for Biodiesel Production from High Free Fatty Acid Feedstock; University of Malaya: Kuala Lumpur, Malaysia, 2015. [Google Scholar]
- Jatoi, A.S.; Hashmi, Z.; Anjum, A.; Bhatti, Z.A.; Siyal, S.H.; Mazari, S.; Akhter, F.; Mubarak, N.; Iqbal, A. Overview of bioelectrochemical approaches for sulfur reduction: Current and future perspectives. Biomass Convers. Biorefinery 2021, 13, 12333–12348. [Google Scholar] [CrossRef]
- Modiri, S.; Sharafi, H.; Alidoust, L.; Hajfarajollah, H.; Haghighi, O.; Azarivand, A.; Zamanzadeh, Z.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Lipid production and mixotrophic growth features of cyanobacterial strains isolated from various aquatic sites. Microbiology 2015, 161 Pt 3, 662–673. [Google Scholar] [CrossRef]
- Etim, A.O.; Jisieike, C.F.; Ibrahim, T.H.; Betiku, E. Biodiesel and its properties. In Production of Biodiesel from Non-Edible Sources; Elsevier: Amsterdam, The Netherlands, 2022; pp. 39–79. [Google Scholar]
- Atadashi, I.; Aroua, M.; Aziz, A.A. Biodiesel separation and purification: A review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Avagyan, A.B.; Singh, B. Biodiesel: Feedstocks, Technologies, Economics and Barriers. Assessment of Environmental Impact in Producing and Using Chains; Springer: Singapore, 2019. [Google Scholar]
- Babadi, A.A.; Rahmati, S.; Fakhlaei, R.; Barati, B.; Wang, S.; Doherty, W.; Ostrikov, K.K. Emerging technologies for biodiesel production: Processes, challenges, and opportunities. Biomass Bioenergy 2022, 163, 106521. [Google Scholar] [CrossRef]
- Kawale, H.D.; Kishore, N. Production of hydrocarbons from a green algae (Oscillatoria) with exploration of its fuel characteristics over different reaction atmospheres. Energy 2019, 178, 344–355. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K.; Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. An overview of total petroleum hydrocarbons. In Total Petroleum Hydrocarbons: Environmental Fate, Toxicity, and Remediation; Springer: Cham, Switzerland, 2020; pp. 1–27. [Google Scholar]
- Farrokh, P.; Sheikhpour, M.; Kasaeian, A.; Asadi, H.; Bavandi, R. Cyanobacteria as an eco-friendly resource for biofuel production: A critical review. Biotechnol. Prog. 2019, 35, e2835. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Salerno, A.; Dias, M.; Reiterer, A.; Braunegg, G. Modern biotechnological polymer synthesis: A review. Food Technol. Biotechnol. 2010, 48, 255–269. [Google Scholar]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Environ. Sci. 2011, 4, 83–99. [Google Scholar] [CrossRef]
- Günay, M.E.; Türker, L.; Tapan, N.A. Significant parameters and technological advancements in biodiesel production systems. Fuel 2019, 250, 27–41. [Google Scholar] [CrossRef]
- Ndaya, D.M. Biodiesel Production From Candlenut And Calodendrum Capense Seeds: Process Design And Technological Assessment. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2013. [Google Scholar]
- Wang, J.; Singer, S.D.; Souto, B.A.; Asomaning, J.; Ullah, A.; Bressler, D.C.; Chen, G. Current progress in lipid-based biofuels: Feedstocks and production technologies. Bioresour. Technol. 2022, 351, 127020. [Google Scholar] [CrossRef] [PubMed]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An overview to process design, simulation and sustainability evaluation of biodiesel production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.A. Coordinated Response and Regulation of Carotenogenesis in Thermosynechococcus elongatus (BP-1): Implications for Commercial Application. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2014. [Google Scholar]
- Piloto-Rodríguez, R.; Sánchez-Borroto, Y.; Melo-Espinosa, E.A.; Verhelst, S. Assessment of diesel engine performance when fueled with biodiesel from algae and microalgae: An overview. Renew. Sustain. Energy Rev. 2017, 69, 833–842. [Google Scholar] [CrossRef]
- Sitther, V.; Tabatabai, B.; Fathabad, S.G.; Gichuki, S.; Chen, H.; Arumanayagam, A.C.S. Cyanobacteria as a biofuel source: Advances and applications. In Advances in Cyanobacterial Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 269–289. [Google Scholar]
- Elgarahy, A.M.; Eloffy, M.; Hammad, A.; Saber, A.N.; El-Sherif, D.M.; Mohsen, A.; Abouzid, M.; Elwakeel, K.Z. Hydrogen production from wastewater, storage, economy, governance and applications: A review. Environ. Chem. Lett. 2022, 20, 3453–3504. [Google Scholar] [CrossRef]
- Hassan, H.; Ansari, F.A.; Ingle, K.N.; Singh, K.; Bux, F. Commercial products and environmental benefits of algal diversity. In Biodiversity and Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 475–502. [Google Scholar]
- Francisco, E.C.; Neves, D.B.; Jacob-Lopes, E.; Franco, T.T. Microalgae as feedstock for biodiesel production: Carbon dioxide sequestration, lipid production and biofuel quality. J. Chem. Technol. Biotechnol. 2010, 85, 395–403. [Google Scholar] [CrossRef]
- Zhang, A.; Carroll, A.L.; Atsumi, S. Carbon recycling by cyanobacteria: Improving CO2 fixation through chemical production. FEMS Microbiol. Lett. 2017, 364, fnx165. [Google Scholar] [CrossRef]
- Arias, D.M.; Uggetti, E.; García, J. Assessing the potential of soil cyanobacteria for simultaneous wastewater treatment and carbohydrate-enriched biomass production. Algal Res. 2020, 51, 102042. [Google Scholar] [CrossRef]
- Xu, H.; Ou, L.; Li, Y.; Hawkins, T.R.; Wang, M. Life cycle greenhouse gas emissions of biodiesel and renewable diesel production in the United States. Environ. Sci. Technol. 2022, 56, 7512–7521. [Google Scholar] [CrossRef] [PubMed]
- Mathimani, T.; Baldinelli, A.; Rajendran, K.; Prabakar, D.; Matheswaran, M.; van Leeuwen, R.P.; Pugazhendhi, A. Review on cultivation and thermochemical conversion of microalgae to fuels and chemicals: Process evaluation and knowledge gaps. J. Clean. Prod. 2019, 208, 1053–1064. [Google Scholar] [CrossRef]
- Wang, H.; Peng, X.; Zhang, H.; Yang, S.; Li, H. Microorganisms-promoted biodiesel production from biomass: A review. Energy Convers. Manag. X 2021, 12, 100137. [Google Scholar] [CrossRef]
- Yang, X.; Mukherjee, S.; O’Carroll, T.; Hou, Y.; Singh, M.R.; Gauthier, J.A.; Wu, G. Achievements, Challenges, and Perspectives on Nitrogen Electrochemistry for Carbon-Neutral Energy Technologies. Angew. Chem. 2023, 135, e202215938. [Google Scholar] [CrossRef]
- Borah, D.; Rout, J.; Nooruddin, T. Phycoremediation and water reuse in bioenergy production from algae and cyanobacteria in relevance to sustainable development goals. In Water, the Environment and the Sustainable Development Goals; Elsevier: Amsterdam, The Netherlands, 2024; pp. 375–406. [Google Scholar]
- Gomes Gradíssimo, D.; Pereira Xavier, L.; Valadares Santos, A. Cyanobacterial polyhydroxyalkanoates: A sustainable alternative in circular economy. Molecules 2020, 25, 4331. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wang, M.; Song, L.; Yu, D.; Zhou, S.; Li, Y.; Li, H.; Han, X. Polyhydroxybutyrate production by recombinant Escherichia coli based on genes related to synthesis pathway of PHB from Massilia sp. UMI-21. Microb. Cell Factories 2023, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Shishatskaya, E.I.; Volova, T.G. Biosynthesis of Poly (3-hydroxybutyrate-co-4-hydroxybutyrate) from Different 4-Hydroxybutyrate Precursors by New Wild-Type Strain Cupriavidus necator IBP/SFU-1. Processes 2023, 11, 1423. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Di Serio, M. In vivo and post-synthesis strategies to enhance the properties of PHB-based materials: A review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef]
- Zheng, L.Z.; Li, Z.; Tian, H.-L.; Li, M.; Chen, G.-Q. Molecular cloning and functional analysis of (R)-3-hydroxyacyl-acyl carrier protein: Coenzyme A transacylase from Pseudomonas mendocina LZ. FEMS Microbiol. Lett. 2005, 252, 299–307. [Google Scholar] [CrossRef]
- Kassab, E.; Mehlmer, N.; Brueck, T. GFP Scaffold-Based Engineering for the Production of Unbranched Very Long Chain Fatty Acids in Escherichia coli With Oleic Acid and Cerulenin Supplementation. Front. Bioeng. Biotechnol. 2019, 7, 408. [Google Scholar] [CrossRef]
- Mendhulkar, V.D.; Shetye, L.A. Synthesis of biodegradable polymer polyhydroxyalkanoate (PHA) in cyanobacteria Synechococcus elongates under mixotrophic nitrogen-and phosphate-mediated stress conditions. Ind. Biotechnol. 2017, 13, 85–93. [Google Scholar] [CrossRef]
- Riaz, S.; Rhee, K.Y.; Park, S.J. Polyhydroxyalkanoates (PHAs): Biopolymers for biofuel and biorefineries. Polymers 2021, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Bergsma, S.; Colpa, D.I.; Euverink, G.-J.W.; Krooneman, J. Polyhydroxyalkanoates (PHAs) synthesis and degradation by microbes and applications towards a circular economy. J. Environ. Manag. 2023, 341, 118033. [Google Scholar] [CrossRef]
- Kamravamanesh, D.; Lackner, M.; Herwig, C. Bioprocess engineering aspects of sustainable polyhydroxyalkanoate production in cyanobacteria. Bioengineering 2018, 5, 111. [Google Scholar] [CrossRef]
- Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA production—Review of recent advances and a summary of three years’ working experience running a pilot plant. Bioengineering 2017, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green bioplastics as part of a circular bioeconomy. Trends Plant Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Kuzhiumparambil, U.; Pernice, M.; Ralph, P.J. Cyanobacterial polyhydroxybutyrate for sustainable bioplastic production: Critical review and perspectives. J. Environ. Chem. Eng. 2020, 8, 104007. [Google Scholar] [CrossRef]
- Yadav, B.; Pandey, A.; Kumar, L.R.; Tyagi, R.D. Bioconversion of waste (water)/residues to bioplastics-A circular bioeconomy approach. Bioresour. Technol. 2020, 298, 122584. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.M.; Paradise, C.J. Photosynthesis; Momentum Press: New York, NY, USA, 2016. [Google Scholar]
- Tiwari, S.; Patel, A.; Pandey, N.; Singh, G.; Pandey, A.; Prasad, S.M. Photosynthesis and Energy Flow in Cyanobacteria. In Ecophysiology and Biochemistry of Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 2022; pp. 49–64. [Google Scholar]
- Razeghifard, R. Natural and Artificial Photosynthesis: Solar Power as an Energy Source; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Norena-Caro, D.; Benton, M.G. Cyanobacteria as photoautotrophic biofactories of high-value chemicals. J. CO2 Util. 2018, 28, 335–366. [Google Scholar] [CrossRef]
- Gayathri, R.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Vodovnik, M.; Vijayalakshmi, S. A review on biological carbon sequestration: A sustainable solution for a cleaner air environment, less pollution and lower health risks. J. King Saud Univ.-Sci. 2021, 33, 101282. [Google Scholar] [CrossRef]
- Tuit, C.; Waterbury, J.; Ravizza, G. Diel variation of molybdenum and iron in marine diazotrophic cyanobacteria. Limnol. Oceanogr. 2004, 49, 978–990. [Google Scholar] [CrossRef]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef]
- Koller, M. A review on established and emerging fermentation schemes for microbial production of polyhydroxyalkanoate (PHA) biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef]
- Singh, A.K.; Mallick, N. Advances in cyanobacterial polyhydroxyalkanoates production. FEMS Microbiol. Lett. 2017, 364, fnx189. [Google Scholar] [CrossRef]
- Lau, N.-S.; Matsui, M.; Abdullah, A.A.-A. Cyanobacteria: Photoautotrophic microbial factories for the sustainable synthesis of industrial products. BioMed Res. Int. 2015, 2015, 754934. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-Y.; Teng, W.-K.; Zhao, L.; Hu, C.-X.; Zhou, Y.-K.; Han, B.-P.; Song, L.-R.; Shu, W.-S. Comparative genomics reveals insights into cyanobacterial evolution and habitat adaptation. ISME J. 2021, 15, 211–227. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V.A. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Maiti, S.K. Recent progress and challenges in cyanobacterial autotrophic production of polyhydroxybutyrate (PHB), a bioplastic. J. Environ. Chem. Eng. 2021, 9, 105379. [Google Scholar]
- Pandey, A.; Adama, N.; Adjallé, K.; Blais, J.-F. Sustainable applications of polyhydroxyalkanoates in various fields: A critical review. Int. J. Biol. Macromol. 2022, 221, 1184–1201. [Google Scholar] [CrossRef]
- Mukherjee, A.; Koller, M. Microbial polyHydroxyAlkanoate (PHA) biopolymers—Intrinsically natural. Bioengineering 2023, 10, 855. [Google Scholar] [CrossRef]
- Law, K.L.; Narayan, R. Reducing environmental plastic pollution by designing polymer materials for managed end-of-life. Nat. Rev. Mater. 2022, 7, 104–116. [Google Scholar] [CrossRef]
- Koller, M. “Bioplastics from microalgae”—Polyhydroxyalkanoate production by cyanobacteria. In Handbook of Microalgae-Based Processes and Products; Academic Press: Cambridge, MA, USA, 2020; pp. 597–645. [Google Scholar]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Odediran, E.T.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- Farrelly, D.J.; Everard, C.D.; Fagan, C.C.; McDonnell, K.P. Carbon sequestration and the role of biological carbon mitigation: A review. Renew. Sustain. Energy Rev. 2013, 21, 712–727. [Google Scholar] [CrossRef]
- Muradov, N.; Muradov, N. Industrial utilization of CO2: A win–win solution. In Liberating Energy from Carbon: Introduction to Decarbonization; Springer: New York, NY, USA, 2014; pp. 325–383. [Google Scholar]
- Ali, S.; Isha; Chang, Y. -C. Ecotoxicological Impact of Bioplastics Biodegradation: A Comprehensive Review. Processes 2023, 11, 3445. [Google Scholar] [CrossRef]
- Obruča, S.; Dvořák, P.; Sedláček, P.; Koller, M.; Sedlář, K.; Pernicová, I.; Šafránek, D. Polyhydroxyalkanoates synthesis by halophiles and thermophiles: Towards sustainable production of microbial bioplastics. Biotechnol. Adv. 2022, 58, 107906. [Google Scholar] [CrossRef]
- Nikolaivits, E.; Pantelic, B.; Azeem, M.; Taxeidis, G.; Babu, R.; Topakas, E.; Brennan Fournet, M.; Nikodinovic-Runic, J. Progressing plastics circularity: A review of mechano-biocatalytic approaches for waste plastic (re) valorization. Front. Bioeng. Biotechnol. 2021, 9, 696040. [Google Scholar] [CrossRef] [PubMed]
- Rasul, I.; Azeem, F.; Siddique, M.H.; Muzammil, S.; Rasul, A.; Munawar, A.; Afzal, M.; Ali, M.A.; Nadeem, H. Algae biotechnology: A green light for engineered algae. In Algae Based Polymers, Blends, and Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 301–334. [Google Scholar]
- Iles, A.; Martin, A.; Rosen, C.M. Undoing Chemical Industry Lock-ins: Polyvinyl Chloride and Green Chemistry. In Ethics of Chemistry: From Poison Gas to Climate Engineering; World Scientific: Singapore, 2021; pp. 279–316. [Google Scholar]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Agboola, O.; Babatunde, D.E.; Fayomi, O.S.I.; Sadiku, E.R.; Popoola, P.; Moropeng, L.; Yahaya, A.; Mamudu, O.A. A review on the impact of mining operation: Monitoring, assessment and management. Results Eng. 2020, 8, 100181. [Google Scholar] [CrossRef]
- Blaser, K.K. The Triple Bottom Line of Sustainable Fashion. Master’s Thesis, Ohio University, Athens, OH, USA, 2017. [Google Scholar]
- Jacob-Lopes, E.; Zepka, L.Q.; Queiroz, M.I. Cyanobacteria and carbon sequestration. In Cyanobacteria: An Economic Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 65–71. [Google Scholar]
- Andrady, A.L. Plastics and Environmental Sustainability; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Ajao, V.O. Biomaterials from Renewable Sources: Biosurfactants and Biopolymers. Master’s Thesis, Universidade do Algarve, Faro, Portugal, 2015. [Google Scholar]
- Mah, A. Plastic Unlimited: How Corporations Are Fuelling the Ecological Crisis and What We Can Do about It; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Kandpal, V.; Jaswal, A.; Santibanez Gonzalez, E.D.; Agarwal, N. Circular Economy Principles: Shifting Towards Sustainable Prosperity. In Sustainable Energy Transition: Circular Economy and Sustainable Financing for Environmental, Social and Governance (ESG) Practices; Springer: Berlin/Heidelberg, Germany, 2024; pp. 125–165. [Google Scholar]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Poltronieri, P.; Kumar, P. Polyhydroxyalkanoates (PHAs) in industrial applications. Handb. Ecomater. 2017, 4, 2843–2872. [Google Scholar]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Phung Hai, T.A.; Tessman, M.; Neelakantan, N.; Samoylov, A.A.; Ito, Y.; Rajput, B.S.; Pourahmady, N.; Burkart, M.D. Renewable polyurethanes from sustainable biological precursors. Biomacromolecules 2021, 22, 1770–1794. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.; Nguyen, L.N.; Zdarta, J.; Nga, T.T.; Nghiem, L.D. Blue-green algae in surface water: Problems and opportunities. Curr. Pollut. Rep. 2020, 6, 105–122. [Google Scholar] [CrossRef]
- Dick, G.J.; Grim, S.L.; Klatt, J.M. Controls on O2 production in cyanobacterial mats and implications for Earth’s oxygenation. Annu. Rev. Earth Planet. Sci. 2018, 46, 123–147. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Zhou, G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 2015, 6, 701. [Google Scholar] [CrossRef]
- Singh, S.K.; Sundaram, S.; Kishor, K.; Singh, S.K.; Sundaram, S.; Kishor, K. Photosynthetic microorganism-based CO2 mitigation system: Integrated approaches for global sustainability. Photosynthetic Microorganisms: Mechanism For Carbon Concentration; Springer: Cham, Switzerland, 2014; pp. 83–123. [Google Scholar]
- Paul, S.; Parvez, S.S.; Goswami, A.; Banik, A. Exopolysaccharides from agriculturally important microorganisms: Conferring soil nutrient status and plant health. Int. J. Biol. Macromol. 2024, 262, 129954. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Park, C.-S.; Han, M.-S. Pseudomonas aeruginosa UCBPP-PA14 a useful bacterium capable of lysing Microcystis aeruginosa cells and degrading microcystins. J. Appl. Phycol. 2012, 24, 1517–1525. [Google Scholar] [CrossRef]
- Monib, A.W.; Niazi, P.; Barai, S.M.; Sawicka, B.; Baseer, A.Q.; Nikpay, A.; Fahmawi, S.M.S.; Singh, D.; Alikhail, M.; Thea, B. Nitrogen Cycling Dynamics: Investigating Volatilization and its Interplay with N2 Fixation. J. Res. Appl. Sci. Biotechnol. 2024, 3, 17–31. [Google Scholar] [CrossRef]
- Gholamhosseinian, A.; Sepehr, A.; Asgari Lajayer, B.; Delangiz, N.; Astatkie, T. Biological soil crusts to keep soil alive, rehabilitate degraded soil, and develop soil habitats. In Microbial Polymers: Applications and Ecological Perspectives; Springer: Singapore, 2021; pp. 289–309. [Google Scholar]
- Mona, S.; Kumar, V.; Deepak, B.; Kaushik, A. Cyanobacteria: The eco-friendly tool for the treatment of industrial wastewaters. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Springer: Singapore, 2020; pp. 389–413. [Google Scholar]
- Sadvakasova, A.K.; Kossalbayev, B.D.; Zayadan, B.K.; Kirbayeva, D.K.; Alwasel, S.; Allakhverdiev, S.I. Potential of cyanobacteria in the conversion of wastewater to biofuels. World J. Microbiol. Biotechnol. 2021, 37, 140. [Google Scholar] [CrossRef]
- Ullah, H.; Nagelkerken, I.; Goldenberg, S.U.; Fordham, D.A. Climate change could drive marine food web collapse through altered trophic flows and cyanobacterial proliferation. PLoS Biol. 2018, 16, e2003446. [Google Scholar] [CrossRef] [PubMed]
- Wicker, R.J. Bioprospecting for Nordic Photosynthetic Consortia for Wastewater Treatment, Carbon Capture, and Value Creation. Ph.D. Thesis, Lappeenranta-Lahti University of Technology LUT, Lappeenranta, Finland, 2023. [Google Scholar]
- Boonbangkeng, D.; Thiemsorn, W.; Ruangrit, K.; Pekkoh, J. Promoting the simultaneous removal of Microcystis bloom and microcystin-RR by Bacillus sp. AK3 immobilized on floating porous glass pellets. J. Appl. Phycol. 2022, 34, 1513–1525. [Google Scholar] [CrossRef]
- Krishnan, A.; Zhang, Y.-Q.; Mou, X. Isolation and characterization of microcystin-degrading bacteria from Lake Erie. Bull. Environ. Contam. Toxicol. 2018, 101, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Chen, Z.; Dong, X.; Shen, H.; Su, P.; Mao, L.; Zhang, W. Biodegradation kinetics of microcystins-LR crude extract by Lysinibacillus boronitolerans strain CQ5. Ann. Microbiol. 2019, 69, 1259–1266. [Google Scholar] [CrossRef]
- Lawton, L.; Welgamage, A.; Manage, P.; Edwards, C. Novel bacterial strains for the removal of microcystins from drinking water. Water Sci. Technol. 2011, 63, 1137–1142. [Google Scholar] [CrossRef]
- Manage, P.M.; Edwards, C.; Singh, B.K.; Lawton, L.A. Isolation and identification of novel microcystin-degrading bacteria. Appl. Environ. Microbiol. 2009, 75, 6924–6928. [Google Scholar] [CrossRef] [PubMed]
- Benegas, G.R.S.; Bernal, S.P.F.; de Oliveira, V.M.; Passarini, M.R.Z. Antimicrobial activity against Microcystis aeruginosa and degradation of microcystin-LR by bacteria isolated from Antarctica. Environ. Sci. Pollut. Res. 2021, 28, 52381–52391. [Google Scholar] [CrossRef]
- Nybom, S.M.; Salminen, S.J.; Meriluoto, J.A. Removal of microcystin-LR by strains of metabolically active probiotic bacteria. FEMS Microbiol. Lett. 2007, 270, 27–33. [Google Scholar] [CrossRef]
- Jia, Y.; Du, J.; Song, F.; Zhao, G.; Tian, X. A fungus capable of degrading microcystin-LR in the algal culture of Microcystis aeruginosa PCC7806. Appl. Biochem. Biotechnol. 2012, 166, 987–996. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, C.; Zhao, G.; Guo, P.; Tian, X. The possibility of using cyanobacterial bloom materials as a medium for white rot fungi. Lett. Appl. Microbiol. 2012, 54, 96–101. [Google Scholar] [CrossRef]
- Bawazeer, S.; Rauf, A.; Nawaz, T.; Khalil, A.A.; Javed, M.S.; Muhammad, N.; Shah, M.A. Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities. Green Process. Synth. 2021, 10, 882–892. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Hertel, S.; Pflugmacher, S. Uptake and biotransformation of pure commercial microcystin-LR versus microcystin-LR from a natural cyanobacterial bloom extract in the aquatic fungus Mucor hiemalis. Biotechnol. Lett. 2017, 39, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.A.; Alamri, S.; Hashem, M.; Mostafa, Y. Growth inhibition of Microcystis aeruginosa and adsorption of microcystin toxin by the yeast Aureobasidium pullulans, with no effect on microalgae. Environ. Sci. Pollut. Res. 2020, 27, 38038–38046. [Google Scholar] [CrossRef] [PubMed]
- Valeria, A.M.; Ricardo, E.J.; Stephan, P.; Alberto, W.D. Degradation of microcystin-RR by Sphingomonas sp. CBA4 isolated from San Roque reservoir (Córdoba–Argentina). Biodegradation 2006, 17, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.J.; Bourne, D.G.; Blakeley, R.L.; Doelle, H. Degradation of the cyanobacterial hepatotoxin microcystin by aquatic bacteria. Nat. Toxins 1994, 2, 228–235. [Google Scholar] [CrossRef]
- Tsuji, K.; Asakawa, M.; Anzai, Y.; Sumino, T.; Harada, K.-i. Degradation of microcystins using immobilized microorganism isolated in an eutrophic lake. Chemosphere 2006, 65, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, S.; Kato, H.; Mizuno, M.; Tsuji, K.; Harada, K.-i. Bacterial degradation of microcystins and nodularin. Chem. Res. Toxicol. 2005, 18, 591–598. [Google Scholar] [CrossRef]
- Somdee, T.; Thunders, M.; Ruck, J.; Lys, I.; Allison, M.; Page, R. Degradation of [Dha7] MC-LR by a Microcystin Degrading Bacterium Isolated from Lake Rotoiti, New Zealand. Int. Sch. Res. Not. 2013, 2013, 596429. [Google Scholar] [CrossRef]
- Ishii, H.; Nishijima, M.; Abe, T. Characterization of degradation process of cyanobacterial hepatotoxins by a gram-negative aerobic bacterium. Water Res. 2004, 38, 2667–2676. [Google Scholar] [CrossRef]
- Park, H.D.; Sasaki, Y.; Maruyama, T.; Yanagisawa, E.; Hiraishi, A.; Kato, K. Degradation of the cyanobacterial hepatotoxin microcystin by a new bacterium isolated from a hypertrophic lake. Environ. Toxicol. Int. J. 2001, 16, 337–343. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Massey, I.Y.; Peng, T.; Yang, F. Immobilization of Microbes for Biodegradation of Microcystins: A Mini Review. Toxins 2022, 14, 573. [Google Scholar] [CrossRef]
- Zeng, Y.-H.; Cai, Z.-H.; Zhu, J.-M.; Du, X.-P.; Zhou, J. Two hierarchical LuxR-LuxI type quorum sensing systems in Novosphingobium activate microcystin degradation through transcriptional regulation of the mlr pathway. Water Res. 2020, 183, 116092. [Google Scholar] [CrossRef] [PubMed]
- Phujomjai, Y.; Somdee, A.; Somdee, T. Biodegradation of microcystin [Dha7] MC-LR by a novel microcystin-degrading bacterium in an internal airlift loop bioreactor. Water Sci. Technol. 2016, 73, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Hoefel, D.; Saint, C.P.; Newcombe, G. Isolation and identification of a novel microcystin-degrading bacterium from a biological sand filter. Water Res. 2007, 41, 4685–4695. [Google Scholar] [CrossRef]
- Jing, W.; Sui, G.; Liu, S. Characteristics of a microcystin-LR biodegrading bacterial isolate: Ochrobactrum sp. FDT5. Bull. Environ. Contam. Toxicol. 2014, 92, 119–122. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, H.; Pan, G. Microbial degradation of microcystin-LR by Ralstonia solanacearum. Environ. Technol. 2011, 32, 1779–1787. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, Y.; Sun, R.; Wei, H.; Li, Y.; Yin, L.; Pu, Y. Biodegradation of microcystin-LR and-RR by a novel microcystin-degrading bacterium isolated from Lake Taihu. Biodegradation 2014, 25, 447–457. [Google Scholar] [CrossRef]
- Mou, X.; Lu, X.; Jacob, J.; Sun, S.; Heath, R. Metagenomic identification of bacterioplankton taxa and pathways involved in microcystin degradation in Lake Erie. PLoS ONE 2013, 8, e61890. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.B.; Yang, J.D.; Zhou, W.; Yin, Y.F.; Chen, J.; Shi, Z.Q. Isolation of a Methylobacillus sp. that degrades microcystin toxins associated with cyanobacteria. New Biotechnol. 2009, 26, 205–211. [Google Scholar] [CrossRef] [PubMed]
- You, D.-J.; Chen, X.-G.; Xiang, H.-Y.; Ouyang, L.; Yang, B. Isolation, identification and characterization of a microcystin-degrading bacterium Paucibacter sp. strain CH. Huan Jing Ke Xue=Huanjing Kexue 2014, 35, 313–318. [Google Scholar]
- Santos, A.A.; Soldatou, S.; de Magalhães, V.F.; Azevedo, S.M.; Camacho-Muñoz, D.; Lawton, L.A.; Edwards, C. Degradation of multiple peptides by Microcystin-Degrader Paucibacter toxinivorans (2C20). Toxins 2021, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, L.B.; Zhou, W.; Yan, S.H.; Yang, J.D.; Xue, Y.F.; Shi, Z.Q. Degradation of microcystin-LR and RR by a Stenotrophomonas sp. strain EMS isolated from Lake Taihu, China. Int. J. Mol. Sci. 2010, 11, 896–911. [Google Scholar] [CrossRef]
- Yang, F.; Massey, I.Y.; Guo, J.; Yang, S.; Pu, Y.; Zeng, W.; Tan, H. Microcystin-LR degradation utilizing a novel effective indigenous bacterial community YFMCD1 from Lake Taihu. J. Toxicol. Environ. Health Part A 2018, 81, 184–193. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, Y.; Yin, L.; Zhu, G.; Liang, G.; Pu, Y. Microcystin-degrading activity of an indigenous bacterial strain Stenotrophomonas acidaminiphila MC-LTH2 isolated from Lake Taihu. PLoS ONE 2014, 9, e86216. [Google Scholar] [CrossRef]
- Idroos, F.S.; De Silva, B.; Manage, P.M. Biodegradation of microcystin analogues by Stenotrophomonas maltophilia isolated from Beira Lake Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2017, 45, 91–99. [Google Scholar] [CrossRef]
- Eleuterio, L.; Batista, J.R. Biodegradation studies and sequencing of microcystin-LR degrading bacteria isolated from a drinking water biofilter and a fresh water lake. Toxicon 2010, 55, 1434–1442. [Google Scholar] [CrossRef]
- Crettaz-Minaglia, M.; Fallico, M.; Aranda, O.; Juarez, I.; Pezzoni, M.; Costa, C.; Andrinolo, D.; Giannuzzi, L. Effect of temperature on microcystin-LR removal and lysis activity on Microcystis aeruginosa (cyanobacteria) by an indigenous bacterium belonging to the genus Achromobacter. Environ. Sci. Pollut. Res. 2020, 27, 44427–44439. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Feng, H.; Li, X.; Yi, X.; Guo, J.; Clara, T.; Yang, F. Anaerobic degradation of microcystin-LR by an indigenous bacterial Enterobacter sp. YF3. J. Toxicol. Environ. Health Part A 2019, 82, 1120–1128. [Google Scholar] [CrossRef]
- Alamri, S.A. Biodegradation of microcystin-RR by Bacillus flexus isolated from a Saudi freshwater lake. Saudi J. Biol. Sci. 2012, 19, 435–440. [Google Scholar] [CrossRef]
- Massey, I.Y.; Yang, F. A mini review on microcystins and bacterial degradation. Toxins 2020, 12, 268. [Google Scholar] [CrossRef]
- Eloka-Eboka, A.C.; Bwapwa, J.K.; Maroa, S. Biomass for CO2 sequestration. Encycl. Renew. Sustain. Mater. 2019, 1, 4252. [Google Scholar]
- Sarma, M.K.; Kaushik, S.; Goswami, P. Cyanobacteria: A metabolic power house for harvesting solar energy to produce bio-electricity and biofuels. Biomass Bioenergy 2016, 90, 187–201. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z. Advances in the biological fixation of carbon dioxide by microalgae. J. Chem. Technol. Biotechnol. 2021, 96, 1475–1495. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Anu, K.; Sneha, V.; Busheera, P.; Muhammed, J.; Augustine, A. Mangroves in environmental engineering: Harnessing the multifunctional potential of Nature’s coastal architects for sustainable ecosystem management. Results Eng. 2024, 21, 101765. [Google Scholar]
- Goodchild-Michelman, I.M.; Church, G.M.; Schubert, M.G.; Tang, T.-C. Light and carbon: Synthetic biology toward new cyanobacteria-based living biomaterials. Mater. Today Bio 2023, 19, 100583. [Google Scholar] [CrossRef]
- Vasile, N.S.; Cordara, A.; Usai, G.; Re, A. Computational analysis of dynamic light exposure of unicellular algal cells in a flat-panel photobioreactor to support light-induced CO2 bioprocess development. Front. Microbiol. 2021, 12, 639482. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.-H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts-A critical review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.-L.; Xu, L.; Cui, L.; Fu, G.-Y.; Xu, X.-W. Metagenome-based analysis of carbon-fixing microorganisms and their carbon-fixing pathways in deep-sea sediments of the southwestern Indian Ocean. Mar. Genom. 2023, 70, 101045. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.P.; Salmaso, N.; Paerl, H.W. Mitigating harmful cyanobacterial blooms: Strategies for control of nitrogen and phosphorus loads. Aquat. Ecol. 2016, 50, 351–366. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumar, A.; Singh, V.K.; Shrivistava, A.K. Advances in Cyanobacterial Biology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Boucot, A.; Gray, J. A critique of Phanerozoic climatic models involving changes in the CO2 content of the atmosphere. Earth-Sci. Rev. 2001, 56, 1–159. [Google Scholar] [CrossRef]
- Mani, A.; Budd, T.; Maine, E. Emissions-Intensive and Trade-Exposed Industries: Technological Innovation and Climate Policy Solutions to Achieve Net-Zero Emissions by 2050. RSC Sustain. 2024, 2, 903–927. [Google Scholar] [CrossRef]
- Mantzouki, E.; Visser, P.M.; Bormans, M.; Ibelings, B.W. Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquat. Ecol. 2016, 50, 333–350. [Google Scholar] [CrossRef]
- Olivares, J.; Bedmar, E.J.; Sanjuán, J. Biological nitrogen fixation in the context of global change. Mol. Plant-Microbe Interact. 2013, 26, 486–494. [Google Scholar] [CrossRef]
- Timsina, J. Preface to” Fertilizer Application on Crop Yield”. Fertilizer Application on Crop Yield; MDPI: Basel, Switzerland, 2019. [Google Scholar]
- Alvarez, A.L.; Weyers, S.L.; Gardner, R.D. Cyanobacteria-based soil amendments in the soil-plant system: Effects of inoculations on soil nutrient and microbial dynamics under spring wheat growth. Algal Res. 2024, 77, 103326. [Google Scholar] [CrossRef]
- Maestre, F.T.; Bowker, M.A.; Cantón, Y.; Castillo-Monroy, A.P.; Cortina, J.; Escolar, C.; Escudero, A.; Lázaro, R.; Martínez, I. Ecology and functional roles of biological soil crusts in semi-arid ecosystems of Spain. J. Arid Environ. 2011, 75, 1282–1291. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F. Future role of bioenergy. In The Role of Bioenergy in the Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 435–547. [Google Scholar]
- Rony, Z.I.; Rasul, M.; Jahirul, M.; Mofijur, M. Harnessing marine biomass for sustainable fuel production through pyrolysis to support United Nations’ Sustainable Development Goals. Fuel 2024, 358, 130099. [Google Scholar] [CrossRef]
- Gregucci, D.; Nazir, F.; Calabretta, M.M.; Michelini, E. Illuminating Progress: The Contribution of Bioluminescence to Sustainable Development Goal 6—Clean Water and Sanitation—Of the United Nations 2030 Agenda. Sensors 2023, 23, 7244. [Google Scholar] [CrossRef]
- Lane, C.E. Bioplastic Production in Cyanobacteria and Consensus Degenerate PCR Probe Design. Ph.D. Thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 2015. [Google Scholar]
- Tan, C.; Xu, P.; Tao, F. Carbon-negative synthetic biology: Challenges and emerging trends of cyanobacterial technology. Trends Biotechnol. 2022, 40, 1488–1502. [Google Scholar] [CrossRef]
- Koller, M. Production, properties, and processing of microbial polyhydroxyalkanoate (PHA) biopolyesters. In Microbial and Natural Macromolecules; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–55. [Google Scholar]
- Reisoglu, Ş.; Aydin, S. Microalgae as a Promising Candidate for Fighting Climate Change and Biodiversity Loss; IntechOpen: London, UK, 2023. [Google Scholar]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro-and microplastics biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef] [PubMed]
- El-Shafei, H.A.; Abd El-Nasser, N.H.; Kansoh, A.L.; Ali, A.M. Biodegradation of disposable polyethylene by fungi and Streptomyces species. Polym. Degrad. Stab. 1998, 62, 361–365. [Google Scholar] [CrossRef]
- Delacuvellerie, A.; Cyriaque, V.; Gobert, S.; Benali, S.; Wattiez, R. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J. Hazard. Mater. 2019, 380, 120899. [Google Scholar] [CrossRef]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Kunlere, I.O.; Fagade, O.E.; Nwadike, B.I. Biodegradation of low density polyethylene (LDPE) by certain indigenous bacteria and fungi. Int. J. Environ. Stud. 2019, 76, 428–440. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef]
- Skariyachan, S.; Patil, A.A.; Shankar, A.; Manjunath, M.; Bachappanavar, N.; Kiran, S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym. Degrad. Stab. 2018, 149, 52–68. [Google Scholar] [CrossRef]
- Muhonja, C.N.; Makonde, H.; Magoma, G.; Imbuga, M. Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE 2018, 13, e0198446. [Google Scholar] [CrossRef]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. New Biotechnol. 2019, 52, 35–41. [Google Scholar] [CrossRef]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Biodegradation of polyvinyl chloride plastic films by enriched anaerobic marine consortia. Mar. Environ. Res. 2020, 158, 104949. [Google Scholar] [CrossRef]
- Kumari, A.; Chaudhary, D.R.; Jha, B. Destabilization of polyethylene and polyvinylchloride structure by marine bacterial strain. Environ. Sci. Pollut. Res. 2019, 26, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ding, M.; Yuan, Y. Current advances in biodegradation of polyolefins. Microorganisms 2022, 10, 1537. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Aravinthan, A.; Arkatkar, A.; Juwarkar, A.A.; Doble, M. Synergistic growth of Bacillus and Pseudomonas and its degradation potential on pretreated polypropylene. Prep. Biochem. Biotechnol. 2016, 46, 109–115. [Google Scholar] [CrossRef]
- Habib, S.; Iruthayam, A.; Abd Shukor, M.Y.; Alias, S.A.; Smykla, J.; Yasid, N.A. Biodeterioration of untreated polypropylene microplastic particles by Antarctic bacteria. Polymers 2020, 12, 2616. [Google Scholar] [CrossRef]
- Sekhar, V.C.; Nampoothiri, K.M.; Mohan, A.J.; Nair, N.R.; Bhaskar, T.; Pandey, A. Microbial degradation of high impact polystyrene (HIPS), an e-plastic with decabromodiphenyl oxide and antimony trioxide. J. Hazard. Mater. 2016, 318, 347–354. [Google Scholar] [CrossRef]
- Mohan, A.J.; Sekhar, V.C.; Bhaskar, T.; Nampoothiri, K.M. Microbial assisted high impact polystyrene (HIPS) degradation. Bioresour. Technol. 2016, 213, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Thakur, I.S. Study of optimization of wastewater contaminant removal along with extracellular polymeric substances (EPS) production by a thermotolerant Bacillus sp. ISTVK1 isolated from heat shocked sewage sludge. Bioresour. Technol. 2016, 213, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xin, X.; Shi, X.; Zhang, Y. A polystyrene-degrading Acinetobacter bacterium isolated from the larvae of Tribolium castaneum. Sci. Total Environ. 2020, 726, 138564. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Rybak, J.; Wróbel, M.; Leluk, K.; Mirończuk, A.M. A comprehensive assessment of microbiome diversity in Tenebrio molitor fed with polystyrene waste. Environ. Pollut. 2020, 262, 114281. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.-M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 2. Role of gut microorganisms. Environ. Sci. Technol. 2015, 49, 12087–12093. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lee, H.M.; Yu, H.C.; Jeon, E.; Lee, S.; Li, J.; Kim, D.-H. Biodegradation of polystyrene by Pseudomonas sp. isolated from the gut of superworms (larvae of Zophobas atratus). Environ. Sci. Technol. 2020, 54, 6987–6996. [Google Scholar] [CrossRef]
- Shah, Z.; Gulzar, M.; Hasan, F.; Shah, A.A. Degradation of polyester polyurethane by an indigenously developed consortium of Pseudomonas and Bacillus species isolated from soil. Polym. Degrad. Stab. 2016, 134, 349–356. [Google Scholar] [CrossRef]
- Rafiemanzelat, F.; Jafari, M.; Emtiazi, G. Study of biological degradation of new poly (ether-urethane-urea) s containing cyclopeptide moiety and PEG by Bacillus amyloliquefaciens isolated from soil. Appl. Biochem. Biotechnol. 2015, 177, 842–860. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, A.H.; Tibpromma, S.; Dai, D.; Xu, R.; Suwannarach, N.; Stephenson, S.L.; Dao, C.; Karunarathna, S.C. A review of the fungi that degrade plastic. J. Fungi 2022, 8, 772. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jin, T.; Xu, X.; Yin, X.; Zhang, D.; Geng, M.; Pang, C.; Luo, G.; Xiong, L.; Peng, J. Screening and degradation characteristics of plastic-degrading microorganisms in film-mulched vegetable soil. Int. Biodeterior. Biodegrad. 2024, 186, 105686. [Google Scholar] [CrossRef]
- Ojha, N.; Pradhan, N.; Singh, S.; Barla, A.; Shrivastava, A.; Khatua, P.; Rai, V.; Bose, S. Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci. Rep. 2017, 7, 39515. [Google Scholar] [CrossRef] [PubMed]
- Vivi, V.K.; Martins-Franchetti, S.M.; Attili-Angelis, D. Biodegradation of PCL and PVC: Chaetomium globosum (ATCC 16021) activity. Folia Microbiol. 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Piwowarska, D.; Festinger, N.; Chruściel, J.J. Risks Associated with the Presence of Polyvinyl Chloride in the Environment and Methods for Its Disposal and Utilization. Materials 2023, 17, 173. [Google Scholar] [CrossRef]
- Shimpi, N.; Borane, M.; Mishra, S.; Kadam, M.; Sonawane, S. Biodegradation of isotactic polypropylene (iPP)/poly (lactic acid)(PLA) and iPP/PLA/nano calcium carbonates using phanerochaete chrysosporium. Adv. Polym. Technol. 2018, 37, 522–530. [Google Scholar] [CrossRef]
- Paço, A.; Duarte, K.; da Costa, J.P.; Santos, P.S.; Pereira, R.; Pereira, M.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Srivastava, N.; Singh, T.; Tiwary, D.; Mishra, P.K. Biodegradation of thermally treated low density polyethylene by fungus Rhizopus oryzae NS 5. 3 Biotech 2017, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, S.; Bontempi, E.; Ducoli, S.; Vethaak, A.D.; Dey, A.; Federici, S. Biotechnological methods to remove microplastics: A review. Environ. Chem. Lett. 2023, 21, 1787–1810. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Saud, S.; Zhou, R. Biochar-Assisted Remediation of Contaminated Soils Under Changing Climate; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Sheik, S.; Chandrashekar, K.; Swaroop, K.; Somashekarappa, H. Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int. Biodeterior. Biodegrad. 2015, 105, 21–29. [Google Scholar] [CrossRef]
- Khan, S.; Nadir, S.; Shah, Z.U.; Shah, A.A.; Karunarathna, S.C.; Xu, J.; Khan, A.; Munir, S.; Hasan, F. Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ. Pollut. 2017, 225, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Zhang, T.; Liu, P.; Bian, J.; Zheng, Y.; Yuan, Y.; Li, Q.; Liang, Q.; Qi, Q. Biodegradation of polyurethane by the microbial consortia enriched from landfill. Appl. Microbiol. Biotechnol. 2023, 107, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Tripathi, S.; Poluri, K.M. Microalgal-based bioenergy: Strategies, prospects, and sustainability. Energy Fuels 2022, 36, 14584–14612. [Google Scholar] [CrossRef]
- Larkum, A.W. Light-harvesting in cyanobacteria and eukaryotic algae: An overview. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms; Springer: Cham, Switzerland, 2020; pp. 207–260. [Google Scholar]
- Sachse, M. Regulation of the Calvin Cycle in the Diatom Phaeodactylum tricornutum. Ph.D. Thesis, Universität Konstanz, Konstanz, Germany, 2013. [Google Scholar]
- Perera-Castro, A.V.; Flexas, J. Recent advances in understanding and improving photosynthesis. Fac. Rev. 2020, 9, 5. [Google Scholar] [CrossRef]
- Peck, B. The Chemistry of Photosynthesis. 2022, Bibliotex.
- Sanfilippo, J.E.; Garczarek, L.; Partensky, F.; Kehoe, D.M. Chromatic acclimation in cyanobacteria: A diverse and widespread process for optimizing photosynthesis. Annu. Rev. Microbiol. 2019, 73, 407–433. [Google Scholar] [CrossRef]
- Kirst, H.; Formighieri, C.; Melis, A. Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 1653–1664. [Google Scholar] [CrossRef]
- Khetkorn, W.; Khanna, N.; Incharoensakdi, A.; Lindblad, P. Metabolic and genetic engineering of cyanobacteria for enhanced hydrogen production. Biofuels 2013, 4, 535–561. [Google Scholar] [CrossRef]
- KhokharVoytas, A.; Shahbaz, M.; Maqsood, M.F.; Zulfiqar, U.; Naz, N.; Iqbal, U.Z.; Sara, M.; Aqeel, M.; Khalid, N.; Noman, A. Genetic modification strategies for enhancing plant resilience to abiotic stresses in the context of climate change. Funct. Integr. Genom. 2023, 23, 283. [Google Scholar] [CrossRef] [PubMed]
- Kitchener, R.L.; Grunden, A.M. Methods for enhancing cyanobacterial stress tolerance to enable improved production of biofuels and industrially relevant chemicals. Appl. Microbiol. Biotechnol. 2018, 102, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Nwoba, E.G.; Parlevliet, D.A.; Laird, D.W.; Alameh, K.; Moheimani, N.R. Light management technologies for increasing algal photobioreactor efficiency. Algal Res. 2019, 39, 101433. [Google Scholar] [CrossRef]
- Orr, D.J.; Pereira, A.M.; da Fonseca Pereira, P.; Pereira-Lima, Í.A.; Zsögön, A.; Araújo, W.L. Engineering photosynthesis: Progress and perspectives. F1000Research 2017, 6, 1891. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Khoo, K.S.; Chew, K.W.; Devadas, V.V.; Phang, S.J.; Lim, H.R.; Rajendran, S.; Show, P.L. Advancement of renewable energy technologies via artificial and microalgae photosynthesis. Bioresour. Technol. 2022, 363, 127830. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. Genetic engineering for modern agriculture: Challenges and perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef]
- Stephens, S.; Mahadevan, R.; Allen, D.G. Engineering photosynthetic bioprocesses for sustainable chemical production: A review. Front. Bioeng. Biotechnol. 2021, 8, 610723. [Google Scholar] [CrossRef]
- Jingura, R.M.; Matengaifa, R.; Musademba, D.; Musiyiwa, K. Characterisation of land types and agro-ecological conditions for production of Jatropha as a feedstock for biofuels in Zimbabwe. Biomass Bioenergy 2011, 35, 2080–2086. [Google Scholar] [CrossRef]
- Knoot, C.J.; Ungerer, J.; Wangikar, P.P.; Pakrasi, H.B. Cyanobacteria: Promising biocatalysts for sustainable chemical production. J. Biol. Chem. 2018, 293, 5044–5052. [Google Scholar] [CrossRef]
- Abo-Shady, A.M.; Osman, M.E.-A.H.; Gaafar, R.M.; Ismail, G.A.; El-Nagar, M.M. Cyanobacteria as a Valuable Natural Resource for Improved Agriculture, Environment, and Plant Protection. Water Air Soil Pollut. 2023, 234, 313. [Google Scholar] [CrossRef]
- Dange, P.; Gawas, S.; Pandit, S.; Mekuto, L.; Gupta, P.K.; Shanmugam, P.; Patil, R.; Banerjee, S. Trends in photobioreactor technology for microalgal biomass production along with wastewater treatment: Bottlenecks and breakthroughs. In An Integration of Phycoremediation Processes in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 135–154. [Google Scholar]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P. Emerging technologies in algal biotechnology: Toward the establishment of a sustainable, algae-based bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Rajvanshi, M.; Gautam, K.; Manjre, S.; Krishna Kumar, G.R.; Kumar, C.; Govindachary, S.; Dasgupta, S. Stoichiometrically balanced nutrient management using a newly designed nutrient medium for large scale cultivation of Cyanobacterium aponinum. J. Appl. Phycol. 2019, 31, 2779–2789. [Google Scholar] [CrossRef]
- do Vale Barreto Figueiredo, M.; do Espírito Santo Mergulhão, A.C.; Sobral, J.K.; de Andrade Lira Junior, M.; de Araújo, A.S.F. Biological nitrogen fixation: Importance, associated diversity, and estimates. In Plant Microbe Symbiosis: Fundamentals and Advances; Springer: Berlin/Heidelberg, Germany, 2013; pp. 267–289. [Google Scholar]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Mishra, V.; Mudgal, N.; Rawat, D.; Poria, P.; Mukherjee, P.; Sharma, U.; Kumria, P.; Pani, B.; Singh, M.; Yadav, A. Integrating microalgae into textile wastewater treatment processes: Advancements and opportunities. J. Water Process Eng. 2023, 55, 104128. [Google Scholar] [CrossRef]
- Yadav, S.; Rai, S.; Rai, R.; Shankar, A.; Singh, S.; Rai, L. Cyanobacteria: Role in agriculture, environmental sustainability, biotechnological potential and agroecological impact. In Plant-Microbe Interactions in Agro-Ecological Perspectives: Volume 2: Microbial Interactions and Agro-Ecological Impacts; Springer: Singapore, 2017; pp. 257–277. [Google Scholar]
- Sakarika, M.; Sventzouri, E.; Pispas, K.; Ali, S.; Kornaros, M. Production of biopolymers from microalgae and cyanobacteria. In Algal Systems for Resource Recovery from Waste and Wastewater; IWA Publishing: London, UK, 2023; p. 207. [Google Scholar]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Yap, X.Y.; Gew, L.T.; Khalid, M.; Yow, Y.-Y. Algae-Based Bioplastic for Packaging: A Decade of Development and Challenges (2010–2020). J. Polym. Environ. 2023, 31, 833–851. [Google Scholar] [CrossRef]
- Arias, D.M.; Ortíz-Sánchez, E.; Okoye, P.U.; Rodríguez-Rangel, H.; Ortega, A.B.; Longoria, A.; Domínguez-Espíndola, R.; Sebastian, P. A review on cyanobacteria cultivation for carbohydrate-based biofuels: Cultivation aspects, polysaccharides accumulation strategies, and biofuels production scenarios. Sci. Total Environ. 2021, 794, 148636. [Google Scholar] [CrossRef]
- Kadri, M.S.; Singhania, R.R.; Anisha, G.S.; Gohil, N.; Singh, V.; Patel, A.K.; Patel, A.K. Microalgal lutein: Advancements in production, extraction, market potential, and applications. Bioresour. Technol. 2023, 389, 129808. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Costa, A.; Encarnação, T.; Tavares, R.; Todo Bom, T.; Mateus, A. Bioplastics: Innovation for Green Transition. Polymers 2023, 15, 517. [Google Scholar] [CrossRef] [PubMed]
- Kollmen, J.; Strieth, D. The beneficial effects of cyanobacterial co-culture on plant growth. Life 2022, 12, 223. [Google Scholar] [CrossRef]
- Govindasamy, R.; Gayathiri, E.; Sankar, S.; Venkidasamy, B.; Prakash, P.; Rekha, K.; Savaner, V.; Pari, A.; Thirumalaivasan, N.; Thiruvengadam, M. Emerging Trends of Nanotechnology and Genetic Engineering in Cyanobacteria to Optimize Production for Future Applications. Life 2022, 12, 2013. [Google Scholar] [CrossRef] [PubMed]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels:‘Promising’alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Nutrient removal and biomass production: Advances in microalgal biotechnology for wastewater treatment. Crit. Rev. Biotechnol. 2018, 38, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A systematic review of industrial wastewater management: Evaluating challenges and enablers. J. Environ. Manag. 2023, 348, 119230. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.L.; Fisher, M.T. The Art of Scalability: Scalable Web Architecture, Processes, and Organizations for the Modern Enterprise; Addison-Wesley Professional: Boston, MA, USA, 2015. [Google Scholar]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Kang, S.-M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.-K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, J.; Zhang, S.; Liang, J. Cyanobacterial blooms management: A modified optimization model for interdisciplinary research. Ecol. Model. 2023, 484, 110480. [Google Scholar] [CrossRef]
- Kumar, R.; Lalnundiki, V.; Shelare, S.D.; Abhishek, G.J.; Sharma, S.; Sharma, D.; Kumar, A.; Abbas, M. An investigation of the environmental implications of bioplastics: Recent advancements on the development of environmentally friendly bioplastics solutions. Environ. Res. 2023, 244, 117707. [Google Scholar] [CrossRef]
- Bello, I.A. Development of Soy Formulate and Bioprocessing for Biological Ammonia Production via Hyperammonia Bacteria Fermentation. Ph.D. Thesis, North Dakota State University, Fargo, ND, USA, 2023. [Google Scholar]
- Nobles, D.R., Jr. Cellulose in the Cyanobacteria. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2006. [Google Scholar]
- Huang, F.; Li, M.; Siffalovic, P.; Cao, G.; Tian, J. From scalable solution fabrication of perovskite films towards commercialization of solar cells. Energy Environ. Sci. 2019, 12, 518–549. [Google Scholar] [CrossRef]
- Bernard, O. Hurdles and challenges for modelling and control of microalgae for CO2 mitigation and biofuel production. J. Process Control 2011, 21, 1378–1389. [Google Scholar] [CrossRef]
- Pruvost, J.; Le Borgne, F.; Artu, A.; Cornet, J.-F.; Legrand, J. Industrial photobioreactors and scale-up concepts. In Advances in Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 257–310. [Google Scholar]
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Zhang, X.; Zhao, D.; Liu, H.; Zhou, C.; Wang, R. Urban ecological infrastructure: An integrated network for ecosystem services and sustainable urban systems. J. Clean. Prod. 2017, 163, S12–S18. [Google Scholar] [CrossRef]
- Bosma, R.H.; Verdegem, M.C. Sustainable aquaculture in ponds: Principles, practices and limits. Livest. Sci. 2011, 139, 58–68. [Google Scholar] [CrossRef]
- Page, M.A.; MacAllister, B.A.; Urban, A.; MacAllister, I.E.; White, C.S.; Pokrzywinski, K.L.; Martinez-Guerra, E.; Grasso, C.; Kennedy, A.J.; Thomas, C.C. Harmful Algal Bloom Interception, Treatment, and Transformation System,” HABITATS”: Pilot Research Study Phase I-Summer 2019; US Army Engineer Research and Development Center, Construction Engineering: Vicksburg, MS, USA, 2020. [Google Scholar]
- Kayode-Ajala, O. Establishing cyber resilience in developing countries: An exploratory investigation into institutional, legal, financial, and social challenges. Int. J. Sustain. Infrastruct. Cities Soc. 2023, 8, 1–10. [Google Scholar]
- Bade, S.O.; Tomomewo, O.S.; Meenakshisundaram, A.; Ferron, P.; Oni, B.A. Economic, social, and regulatory challenges of green hydrogen production and utilization in the US: A review. Int. J. Hydrog. Energy 2023, 49, 314–335. [Google Scholar] [CrossRef]
- Price, S. Developing Next Generation Algae Bioplastic Technology. Ph.D. Thesis, University of Technology Sydney, Sydney, Australia, 2022. [Google Scholar]
- Dixon, C.; Wilken, L.R. Green microalgae biomolecule separations and recovery. Bioresour. Bioprocess. 2018, 5, 14. [Google Scholar] [CrossRef]
- Noble, R.D.; Agrawal, R. Separations research needs for the 21st century. Ind. Eng. Chem. Res. 2005, 44, 2887–2892. [Google Scholar] [CrossRef]
- Poumadère, M.; Bertoldo, R.; Samadi, J. Public perceptions and governance of controversial technologies to tackle climate change: Nuclear power, carbon capture and storage, wind, and geoengineering. Wiley Interdiscip. Rev. Clim. Chang. 2011, 2, 712–727. [Google Scholar] [CrossRef]
- McGinn, M.M. Inclusive Outreach and Public Engagement Guide; Race and Social Justice Initiative: Seattle, DC, USA, 2009. [Google Scholar]
- Kamravamanesh, D.; Kiesenhofer, D.; Fluch, S.; Lackner, M.; Herwig, C. Scale-up challenges and requirement of technology-transfer for cyanobacterial poly (3-hydroxybutyrate) production in industrial scale. Int. J. Biobased Plast. 2019, 1, 60–71. [Google Scholar] [CrossRef]
- Bansal, S.; Mahendiratta, S.; Kumar, S.; Sarma, P.; Prakash, A.; Medhi, B. Collaborative research in modern era: Need and challenges. Indian J. Pharmacol. 2019, 51, 137. [Google Scholar] [PubMed]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)—Prospects and challenges. Algal Res. 2013, 2, 79–97. [Google Scholar] [CrossRef]
- Baunach, M.; Guljamow, A.; Miguel-Gordo, M.; Dittmann, E. Harnessing the potential: Advances in cyanobacterial natural product research and biotechnology. Nat. Prod. Rep. 2024, 41, 347–369. [Google Scholar] [CrossRef]
- Johnson, T.J.; Jahandideh, A.; Johnson, M.D.; Fields, K.H.; Richardson, J.W.; Muthukumarappan, K.; Cao, Y.; Gu, Z.; Halfmann, C.; Zhou, R. Producing next-generation biofuels from filamentous cyanobacteria: An economic feasibility analysis. Algal Res. 2016, 20, 218–228. [Google Scholar] [CrossRef]
- Majidian, P.; Tabatabaei, M.; Zeinolabedini, M.; Naghshbandi, M.P.; Chisti, Y. Metabolic engineering of microorganisms for biofuel production. Renew. Sustain. Energy Rev. 2018, 82, 3863–3885. [Google Scholar] [CrossRef]
- Yadav, I.; Rautela, A.; Kumar, S. Approaches in the photosynthetic production of sustainable fuels by cyanobacteria using tools of synthetic biology. World J. Microbiol. Biotechnol. 2021, 37, 1–17. [Google Scholar]
- Tanvir, R.U.; Zhang, J.; Canter, T.; Chen, D.; Lu, J.; Hu, Z. Harnessing solar energy using phototrophic microorganisms: A sustainable pathway to bioenergy, biomaterials, and environmental solutions. Renew. Sustain. Energy Rev. 2021, 146, 111181. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Pandey, A. Cyanobacterial hydrogen production—A step towards clean environment. Int. J. Hydrogen Energy 2012, 37, 139–150. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, S.; Chhabra, T.; Tariq, S.; Javed, M.S.; Li, H.; Naqvi, S.R.; Rajendran, S.; Luque, R.; Ahmad, I. Synergic impact of renewable resources and advanced technologies for green hydrogen production: Trends and perspectives. Int. J. Hydrogen Energy 2024, 67, 788–806. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.F.; Ren, Y.P.; Wang, X.H.; Jian, C. Electricity generation from Taihu Lake cyanobacteria by sediment microbial fuel cells. J. Chem. Technol. Biotechnol. 2012, 87, 1567–1573. [Google Scholar] [CrossRef]
- Indrama, T.; Tiwari, O.; Bandyopadhyay, T.K.; Mondal, A.; Bhunia, B. Cyanobacteria: A biocatalyst in microbial fuel cell for sustainable electricity generation. In Waste to Sustainable Energy; CRC Press: Boca Raton, FL, USA, 2019; pp. 125–140. [Google Scholar]
- Hallenbeck, P.C.; Grogger, M.; Veverka, D. Recent advances in microbial electrocatalysis. Electrocatalysis 2014, 5, 319–329. [Google Scholar] [CrossRef]
- Kumar, M.; Sundaram, S.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review. Bioresour. Technol. 2018, 247, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- López-Pacheco, I.Y.; Rodas-Zuluaga, L.I.; Fuentes-Tristan, S.; Castillo-Zacarías, C.; Sosa-Hernández, J.E.; Barceló, D.; Iqbal, H.M.; Parra-Saldívar, R. Phycocapture of CO2 as an option to reduce greenhouse gases in cities: Carbon sinks in urban spaces. J. CO2 Util. 2021, 53, 101704. [Google Scholar] [CrossRef]
- Lin, W.-R.; Tan, S.-I.; Hsiang, C.-C.; Sung, P.-K.; Ng, I.-S. Challenges and opportunity of recent genome editing and multi-omics in cyanobacteria and microalgae for biorefinery. Bioresour. Technol. 2019, 291, 121932. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef]
- Prabha, S.; Vijay, A.K.; Paul, R.R.; George, B. Cyanobacterial biorefinery: Towards economic feasibility through the maximum valorization of biomass. Sci. Total Environ. 2022, 814, 152795. [Google Scholar] [CrossRef]
- Quintana, N.; Van der Kooy, F.; Van de Rhee, M.D.; Voshol, G.P.; Verpoorte, R. Renewable energy from Cyanobacteria: Energy production optimization by metabolic pathway engineering. Appl. Microbiol. Biotechnol. 2011, 91, 471–490. [Google Scholar] [CrossRef]
- Kashyap, A.K.; Dubey, S.K.; Jain, B.P. Cyanobacterial diversity concerning the extreme environment and their bioprospecting. In Cyanobacterial Lifestyle and Its Applications in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–22. [Google Scholar]
- Meshram, B.G.; Chaugule, B.B. An introduction to cyanobacteria: Diversity and potential applications. In The Role of Photosynthetic Microbes in Agriculture and Industry; Nova Science Publishers: Hauppauge, NY, USA, 2018; p. 1. [Google Scholar]
- Chakdar, H.; Pabbi, S. Cyanobacterial phycobilins: Production, purification, and regulation. In Frontier Discoveries and Innovations in Interdisciplinary Microbiology; Springer: New Delhi, India, 2016; pp. 45–69. [Google Scholar]
- Hamilton, T.L.; Bryant, D.A.; Macalady, J.L. The role of biology in planetary evolution: Cyanobacterial primary production in low-oxygen Proterozoic oceans. Environ. Microbiol. 2016, 18, 325–340. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Elvitigala, T.; Welsh, E.; Stöckel, J.; Liberton, M.; Min, H.; Sherman, L.A.; Pakrasi, H.B. Novel metabolic attributes of the genus Cyanothece, comprising a group of unicellular nitrogen-fixing cyanobacteria. MBio 2011, 2. [Google Scholar] [CrossRef]
- Zhang, Y.; Whalen, J.K.; Cai, C.; Shan, K.; Zhou, H. Harmful cyanobacteria-diatom/dinoflagellate blooms and their cyanotoxins in freshwaters: A nonnegligible chronic health and ecological hazard. Water Res. 2023, 233, 119807. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.; Woodhouse, J.N. Defining cyanobacterial species: Diversity and description through genomics. Crit. Rev. Plant Sci. 2020, 39, 101–124. [Google Scholar] [CrossRef]
- Sadvakasova, A.K.; Kossalbayev, B.D.; Bauenova, M.O.; Balouch, H.; Leong, Y.K.; Zayadan, B.K.; Huang, Z.; Alharby, H.F.; Tomo, T.; Chang, J.-S. Microalgae as a key tool in achieving carbon neutrality for bioproduct production. Algal Res. 2023, 72, 103096. [Google Scholar] [CrossRef]
- Brunschwig, C.; Moussavou, W.; Blin, J. Use of bioethanol for biodiesel production. Prog. Energy Combust. Sci. 2012, 38, 283–301. [Google Scholar] [CrossRef]
- Moravvej, Z.; Makarem, M.A.; Rahimpour, M.R. The fourth generation of biofuel. In Second and Third Generation of Feedstocks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 557–597. [Google Scholar]
- Lin, B.B. Resilience in agriculture through crop diversification: Adaptive management for environmental change. BioScience 2011, 61, 183–193. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and challenges of large-scale cultivation of photosynthetic microalgae and cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- Das, M.; Maiti, S.K. Current knowledge on cyanobacterial biobutanol production: Advances, challenges, and prospects. Rev. Environ. Sci. Bio/Technol. 2022, 21, 483–516. [Google Scholar] [CrossRef]
- Carroll, A.L.; Case, A.E.; Zhang, A.; Atsumi, S. Metabolic engineering tools in model cyanobacteria. Metab. Eng. 2018, 50, 47–56. [Google Scholar] [CrossRef]
- Singhal, A.; Prashant. Biofuels: Perspective for sustainable development and climate change mitigation. In Biotechnology for Biofuels: A Sustainable Green Energy Solution; Springer: Singapore, 2020; pp. 1–22. [Google Scholar]
- Mastropetros, S.G.; Pispas, K.; Zagklis, D.; Ali, S.S.; Kornaros, M. Biopolymers production from microalgae and cyanobacteria cultivated in wastewater: Recent advances. Biotechnol. Adv. 2022, 60, 107999. [Google Scholar] [CrossRef]
- Shah, S.; Kumar, A. Polyhydroxyalkanoates: Advances in the synthesis of sustainable bio-plastics. Eur. J. Environ. Sci. 2020, 10, 76–88. [Google Scholar] [CrossRef]
- Umar, A. The Screening, Fabrication and Production of Microalgae Biocomposites for Carbon Capture and Utilisation. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2019. [Google Scholar]
- Kovacic, Z.; Strand, R.; Völker, T. The Circular Economy in Europe: Critical Perspectives on Policies and Imaginaries; Taylor & Francis: Abingdon, UK, 2020. [Google Scholar]
- Caldwell, G.S.; In-na, P.; Hart, R.; Sharp, E.; Stefanova, A.; Pickersgill, M.; Walker, M.; Unthank, M.; Perry, J.; Lee, J.G. Immobilising microalgae and cyanobacteria as biocomposites: New opportunities to intensify algae biotechnology and bioprocessing. Energies 2021, 14, 2566. [Google Scholar] [CrossRef]
- Singh, S.; Tiwari, B.S. Biosynthesis of high-value amino acids by synthetic biology. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 257–294. [Google Scholar]
- Guzmán-Lagunes, F.; Wongsirichot, P.; Winterburn, J.; Guerrero Sanchez, C.; Montiel, C. Polyhydroxyalkanoates Production: A Challenge for the Plastic Industry. Ind. Eng. Chem. Res. 2023, 62, 18133–18158. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Zhang, B.; Gong, F.; Feng, J.; Huang, H.; Vanka, S.; Fan, R.; Cao, Q.; Shen, M. Renewable formate from sunlight, biomass and carbon dioxide in a photoelectrochemical cell. Nat. Commun. 2023, 14, 1013. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current developments on polyhydroxyalkanoates synthesis by using halophiles as a promising cell factory. Microb. Cell Factories 2020, 19, 86. [Google Scholar] [CrossRef] [PubMed]
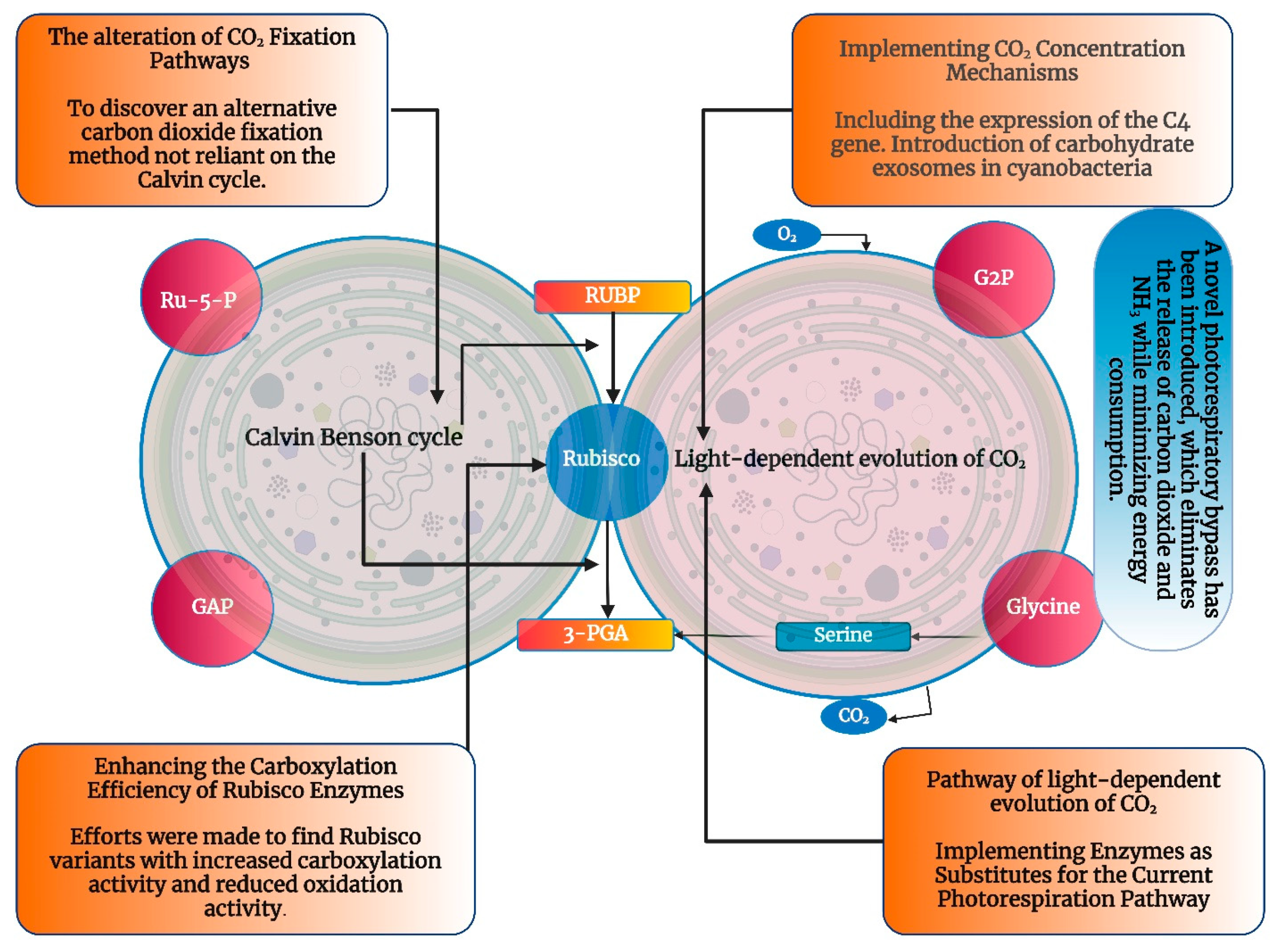
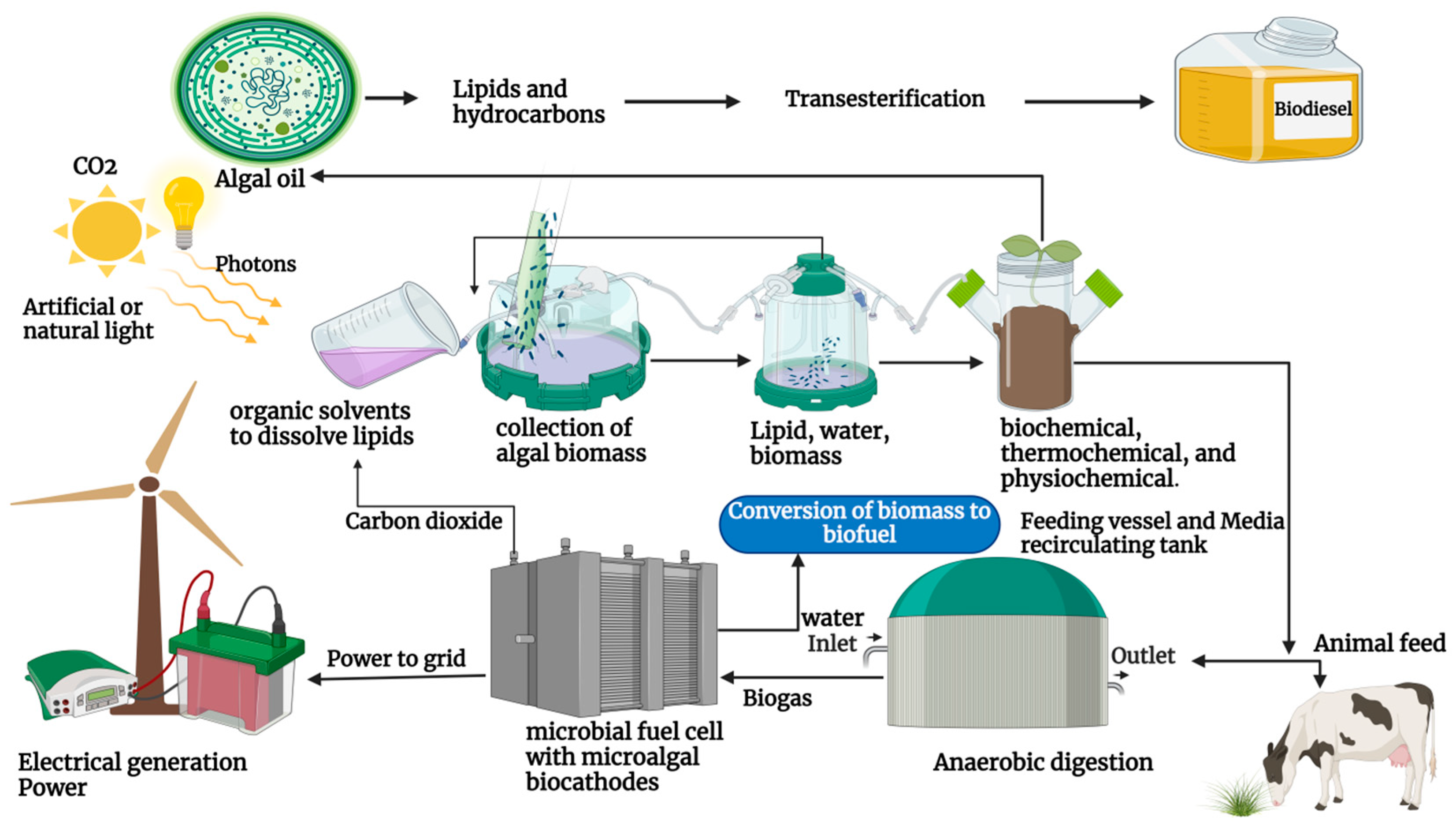
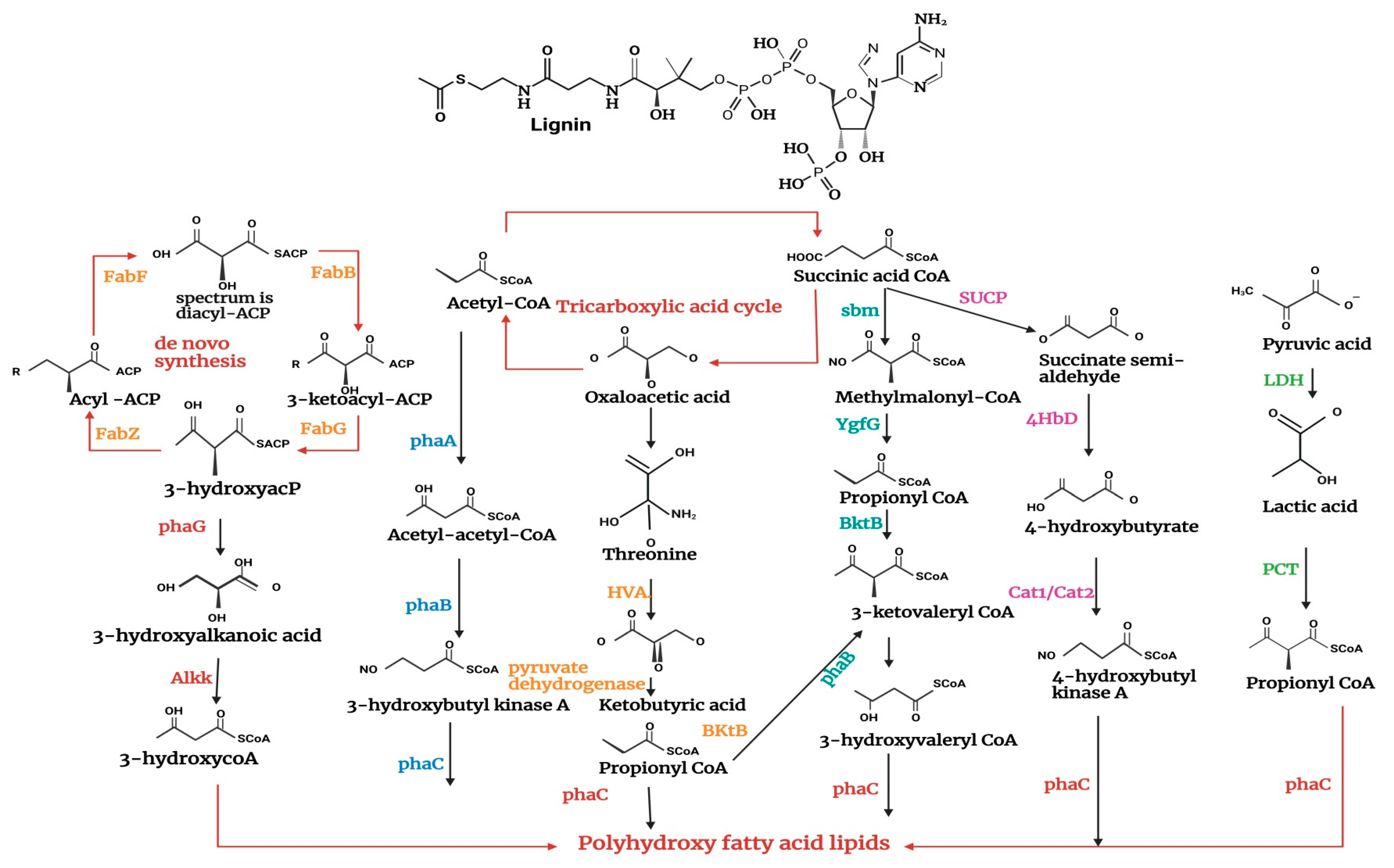

| Common Synonyms | Strains | Initiate Enzymes | Ring-Cleavage Substrates |
|---|---|---|---|
| aniline | P. pseudoalcaligenes JS45 | NR | o-Aminopheno |
| 2-nitrochlorobenzene | P. putida OCNB-1 | NR | 1-Chloro-2,3-dihydroxybenzene |
| 4-Chloro-1-nitrobenzene | P. putida ZWL73 | NR | (4-CAP) |
| 2-hydroxynitrobenzene | P. putida B2, | One-component Monooxygenases | pyrocatechol |
| 3-Nitrophenol (m-) | P. putida B2 | NR | 1,2,4-Benzenetriol |
| p-nitrophenol | Pseudomonas sp. WBC-3 | One-component Monooxygenases | benzene-1,4-diol |
| 2-4-DNP | R. erythropolis HL24-1 R. erythropolis HL24-2 | – | (anionic ς-complex) |
| 2-6-DNP | C. necator JMP134 | – | 3-Nitroquinolin-4-ol |
| Picric acid | Nocardioides sp. CB22-2 R. erythropolis HLPM-1 | – | (anionic ς-complex) |
| nitrofungin | Burkholderia sp. strain SJ98 Burkholderia sp. RKJ 800 Arthrobacter sp. SJCon | One-component Monooxygenases | 2-Chlorohydroquinone C6H5ClO2 |
| 4C2NP | Exiguobacterium sp. PMA | NR | 2-Amino-1-hydroxybenzene |
| 2C5NP | C. pinatubonensis JMP134 | NR | 2-Aminohydroquinone hydrobromide |
| 2,6-Dihalo-4-nitrophenol | Cupriavidus sp. CNP-8 | 2-dioxygenase | 6-Quinolinol |
| 2-nitro-benzoic acid | P. fluorescens KU-7 | NR | 3-hydroxyanthranilic acid |
| 3-nitro-benzoic acid | Pseudomonas sp. JS51 Comamonas sp. JS46 | ADO, 2-aminoethanethiol dioxygenase; | 3,4-Dihydroxybenzoic acid |
| 4-nitro-benzoic acid | C. acidovoransNBA-10 Pseudomonas sp. 4NT Pseudomonas putida TW3 Ralstonia sp. SJ98 | NR | 3,4-Dihydroxybenzoic acid |
| 2C4NP | Acinetobacter sp.RKJ12 | (MMO) | pyrocatechol |
| Plastic Type of (Bacteria) | Strain Species | Efficiency of Degradation | Time | Initiation of the Strain Top of Form | Refs. |
|---|---|---|---|---|---|
| Polyethylene | Streptomyces spp. Streptomyces caatingaensis” Streptomyces cacaoi Streptomyces cadmiisoli Streptomyces caelestis Streptomyces caeni Streptomyces caeruleatus Streptomyces caespitosus | 27.5% | 6 weeks | Nile Delta Lagoons | [291] |
| Alcanivorax borkumensis | 3.6% ± 0.36% | 3 months | Seawater samples from northern Corsica (Gulf of Calvi, Mediterranean) | [292] | |
| Kocuria palustris M16 | 1% ± 0.063% | 1 month | – | [293] | |
| P. aeruginosa, P. fluorescens, P. putida, P cepacia, P stutzeri, P. maltophilia, and P putrefaciens. | 3.765% | 2 months | Scrapyard in the north of Ibadan | [294] | |
| Bacillus sp. B. cereus B. clausii B. polyfermenticus SCD B. pumilus | 16.7% | 2 months | Waste Site in Incheon, South Korea | [295] | |
| Brevibacillus sp. | LDPE: 54.21% ± 7% HDPE: 46.4% ± 5% | 4 months | An abandoned dump in Karnataka, India | [296] | |
| Bacillus anthracis | 34.76% ± 4.04% | 4 months | Dandora Dumpsite landfill | [297] | |
| Brevibacillus borstelensis strain B2, 2 (MG645267) | 22.23% ± 2.20% | 4 months | Dandora Dumpsite landfill | [297] | |
| Polyvinyl chloride (PVC) | Pseudomonas citronellolis | 18.56% ± 0.02% | 1 month | – | [298] |
| Erysipelothrix rhusiopathiae sp. | 11.4% ± 0.6% | 6 months | oceanic | [299] | |
| Bacillus sp. AIIW2 | 0.29% | 3 months | oceanic | [300] | |
| Polypropylene (pp) | Stenotrophomonas panacihumi PA3-2 | 20.9% ± 1.30% (molecular weight 10 300) 16.8% ± 1.70% (molecular weight 19 700) | 3 months | Open-air solid wasteyard in South Korea | [301] |
| Nocardia, Rhodococcus, Amycolata,. Amycolatopsis, Gordona and Pseudoamycolata | 4.4% 6.5% | 1 month | Sediment samples from mangroves in Matang, Peninsular Malaysia | [302] | |
| Bacillus and Pseudomonas | 1.93% ± 0.18% | 1 year | – | [303] | |
| Aneurinibacillus sp. | 53% ± 2% (PP band) 46.2% ± 3% (PP board) | 4 months | Wastewater Treatment Plant and Transfer Station Site | [296] | |
| Pseudomonas azotoformans, P. stutzeri, Bacillus subtilis, B. flexus | 2.6% | 1 year | Earth for dumping plastic waste | [304] | |
| Polystyrene (PS) | Enterobacter sp., | 15.4% | 1 month | transfer station | [305] |
| Pseudomonas sp., Bacillus sp. | 25% | 1 month | Exploring Soil Composition Near Plastic Waste Dump Site | [306] | |
| Pseudomonas aeruginosa | Bacillus subtilis exhibits the highest degradation efficiency. Top of Form | – | terrestrial sample | [307] | |
| Acinetobacter sp. | 12.16% | 2 months | Tribolium castaneum Intestine of a larva | [308] | |
| Pseudomonas aeruginosa | – | – | Tribolium castaneum Intestine of a larva | [309] | |
| Exiguobacterium sp. strain YT2 | 7.5% ± 0.5% | 1 month | Larval intestine | [310] | |
| Pseudomonas aeruginosa strain DSM 50071 | – | – | Zophobas atratus | [311] | |
| polyurethane (PUR/PU) | Bacillus subtilis MZA-75, | Enhanced Degradation Potential through Co-cultivation | 1 month | – | [312] |
| Bacillus amyloliquefaciens | 35–45% | 4 weeks | – | [313] | |
| Plastic type of (Fungi) | Strain name | Evaluation of Degradation Efficiency through Weight Loss Analysis | Incubation time | Origin of Microbial Strain | [314,315] |
| PE (HDPE) High-density polyethylene | Aspergillus flavus PEDX3 | 3.6125% ± 1.18% | 1 month | Intestinal contents of the Galleria mellonella | [289] |
| Penicillium oxalicum NS4 (KU559906) | 20.18% | 1 month | Plastic wasteyardnear Mohanpur campus | [316] | |
| 48.53% | 2 months | ||||
| 45.54% | 3 months | ||||
| Penicillium chrysogenum NS10 (KU559907) | 14.05% | 1 month | Plastic wasteyard near Mohanpur campus | [316] | |
| 44.04% | 2 months | ||||
| 56.548% | 3 months | ||||
| Polyvinyl chloride (PVC) | Chaetomium globosum (ATCC 16021) | “Incipient PVC Absorption by Spores and Hyphae” Top of Form | 1 month | Bought standard trunks | [317] |
| Mucor rouxii | “Onset of Degradation in Glycerin and Urea Modified PVC” Top of Form | 1 month | Polymer recycling site | [318] | |
| Phanerocheate chrysosporium | 14% | 1 month | Plastic waste landfill | [319] | |
| PE (LDPE) Polyethylene | Aspergillus oryzae strain A5, 1 (MG779508) | 34.5% ± 4.55% | 4 months | Dandola landfill soil | [297] |
| Zalerion maritimum | 57.4% ± 2.5% | 14 days | – | [320] | |
| Rhizopus oryzae NS5 | 8.6% ± 4% | 1 month | Laboratory isolate accession number KT160362 | [321] | |
| Penicillium oxalicum NS4 (KU559906) | 26.72% | 1 month | Plastic wasteyard near Mohanpur campus | [316] | |
| 28.70% | 1 month | ||||
| 30.60% | 3 months | ||||
| Penicillium chrysogenum NS10 (KU559907) | 30.32% | 1 month | Plastic wasteyard near Mohanpur campus | [316] | |
| 36.73% | 2 months | ||||
| 44.36% | 3 months | ||||
| Polypropylene | Phanerochaete chrysosporium | 5.5% (iPP/PLA/nCaCO3 Nanocomposite) | 1 month | Soil from the Indian campus | [319] |
| Aspergillus niger | 0.68% (PP) | 1 month | – | ||
| 2.40% (PP/PET/thermoplastic starch blend) | 1 month | [322] | |||
| Engyodontium album MTP091 (F2) | 9.50% (UV pretreatment) | I year | Plastic trunk | [323] | |
| Lasiodiplodia theobromae | 1.5% | 3 months | Plant Endophytes inhabiting Psychotria flavida | [324] | |
| PUR (polyurethane) | Aspergillus tubingensis | 80% | 60 days | Waste disposal site located in Islamabad, Pakistan. | [325] |
| Chaetomium globosum | 20–18% | 3 months | – | [326] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, T.; Gu, L.; Hu, Z.; Fahad, S.; Saud, S.; Zhou, R. Advancements in Synthetic Biology for Enhancing Cyanobacterial Capabilities in Sustainable Plastic Production: A Green Horizon Perspective. Fuels 2024, 5, 394-438. https://doi.org/10.3390/fuels5030023
Nawaz T, Gu L, Hu Z, Fahad S, Saud S, Zhou R. Advancements in Synthetic Biology for Enhancing Cyanobacterial Capabilities in Sustainable Plastic Production: A Green Horizon Perspective. Fuels. 2024; 5(3):394-438. https://doi.org/10.3390/fuels5030023
Chicago/Turabian StyleNawaz, Taufiq, Liping Gu, Zhong Hu, Shah Fahad, Shah Saud, and Ruanbao Zhou. 2024. "Advancements in Synthetic Biology for Enhancing Cyanobacterial Capabilities in Sustainable Plastic Production: A Green Horizon Perspective" Fuels 5, no. 3: 394-438. https://doi.org/10.3390/fuels5030023
APA StyleNawaz, T., Gu, L., Hu, Z., Fahad, S., Saud, S., & Zhou, R. (2024). Advancements in Synthetic Biology for Enhancing Cyanobacterial Capabilities in Sustainable Plastic Production: A Green Horizon Perspective. Fuels, 5(3), 394-438. https://doi.org/10.3390/fuels5030023









