Techno-Economic Analysis of Large Scale Production of Poly(oxymethylene) Dimethyl Ether Fuels from Methanol in Water-Tolerant Processes
Abstract
:1. Introduction
2. Methodology
2.1. Description of Processes
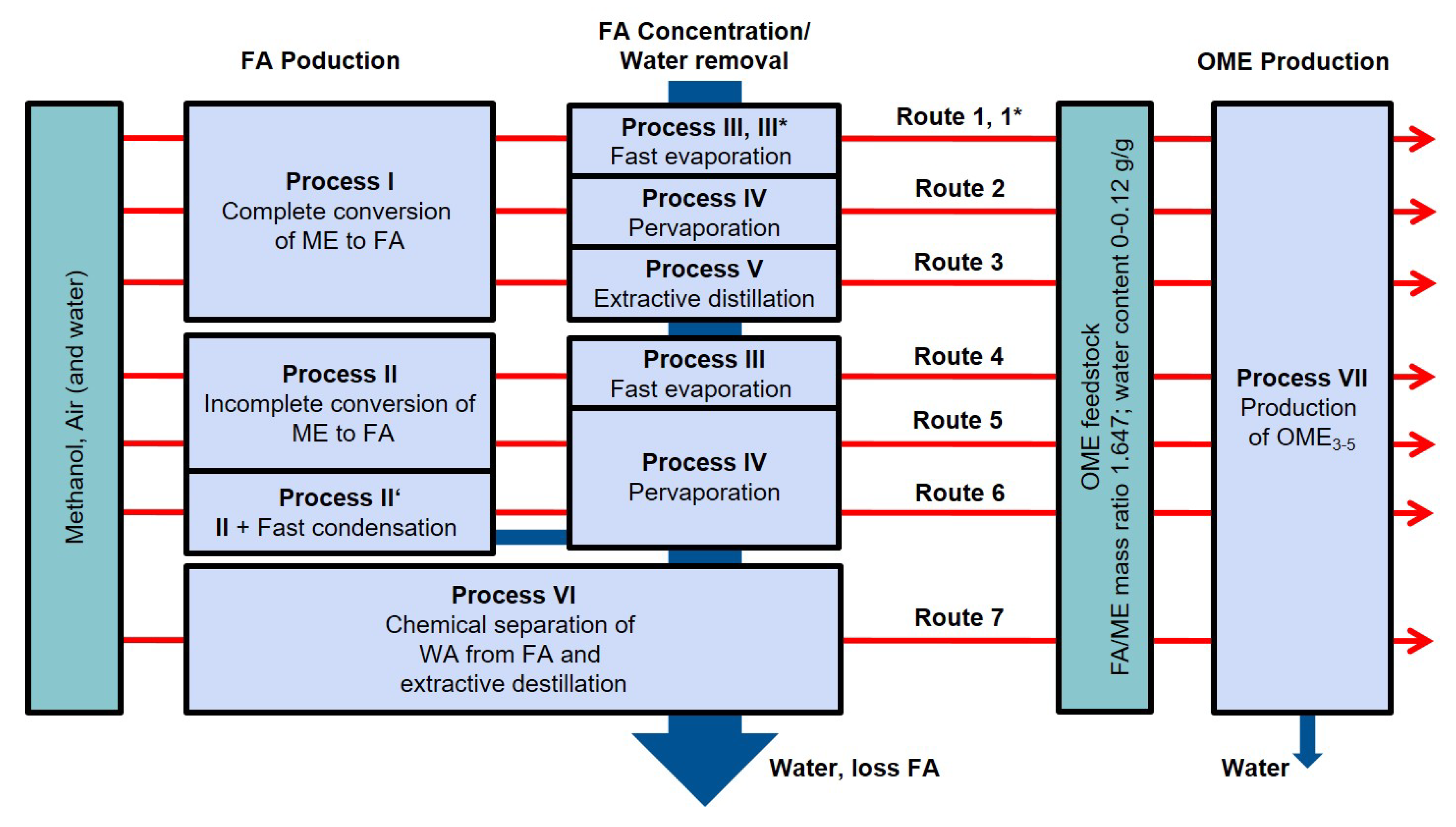
2.1.1. Overview
2.1.2. Formaldehyde Production
2.1.3. Formaldehyde Concentration
2.1.4. Ome Production
2.1.5. Detailed Descriptions
2.2. Process Simulation
2.2.1. Property Model
2.2.2. Unit Modeling and Design
- Distillation and absorption columns: All columns are simulated using the equilibrium stage model, assuming physical equilibrium and chemical equilibrium considering Reactions (6)–(9) on all stages. For distillation columns, the condenser (=total condenser) and reboiler are modeled with one additional stage, respectively. The pressure drop along the column is neglected. If not given in the reference, the stage numbers are chosen so that 1.2 times the minimum required reboiler heat duty is used. The feed stage is found using the built-in feed stage optimization of Aspen Plus. The remaining two degrees of freedom per column were fixed by the specification of top and bottom product concentrations as given in the original source; the corresponding concentrations are highlighted in the respective stream tables as bold (cf. Supplementary Materials). The extractive distillation columns in processes V and VI were not modeled with an equilibrium stage model because of missing property data (for extraction agents). Instead, the minimum reflux ratio is calculated based on Underwood’s Equation [50] and is used to estimate the approximate heat duties, cf. Supplementary Materials. For these extractive distillation columns, height and diameter are adopted from the original work. For all other columns, height and diameter are estimated with the Aspen Process Economic Analyzer, both packed and trayed columns are sized with the default option as DTW Tower.
- Reactors: Reactor product compositions, conversion and selectivity are adopted from the original source. The heat duty is calculated via the energy balance:Reactor cost is based on and the temperature level; no further design was done.
- Heat exchangers, condensers, evaporators: Heat is generally provided via a steam network operating at the pressure stages given in Table 2. The water condensate leaves the heat exchanger at the same temperature as the steam entered. Cooling is done preferably by producing steam at the maximum pressure level. Low temperature (LT) cooling is done using the media listed in Table 2. Cooling demand at temperatures above 40 °C is realized via cooling water, which enters the heat exchanger at 25 °C and leaves it at a maximum of 60 °C. Cooling below 40 °C requires different cooling mediums at an increased cost. Ammonia and salt solutions were only used for condensation at a constant temperature. Using the mean logarithmic temperature difference and the respective heat transfer coefficient for the heat exchanger type (cf. Supplementary Materials) the area of the heat exchanger is determined.The steam level and cooling media are chosen so that a minimum temperature difference of 10 K [50] throughout the heat exchanger is not undercut. Heat integration along the process chain from ME to OME is realized via the steam network. Heat below 160 °C is not considered for steam generation, therefore, not considered for heat integration unless stated otherwise in the process description. Note that it is likely possible to use part of this excess heat to supply district heat, but this depends on the boundary conditions of the plant and is not further considered here.
- Thin film evaporators (TFEs) and other kinetic separators: Fast evaporation of aqueous and methanolic FA solutions results in a reactive phase equilibrium that does not reach the chemical equilibrium. These units are modeled as open evaporation with multiple stages considering the vapor-liquid equilibrium and the reaction kinetics of reactions (6)–(9) [47] on every stage. Details on the model are given in our previous work [29].
- Pervaporation: The primary energy demand for pervaporation results from the permeate’s phase change and is calculated from its heat of vaporization. The membrane surface area is calculated based on the correlation given by Schmitz et al. [34] from the given output stream’s specification.
2.3. Process Economics
3. Results and Discussion
3.1. Material Balances
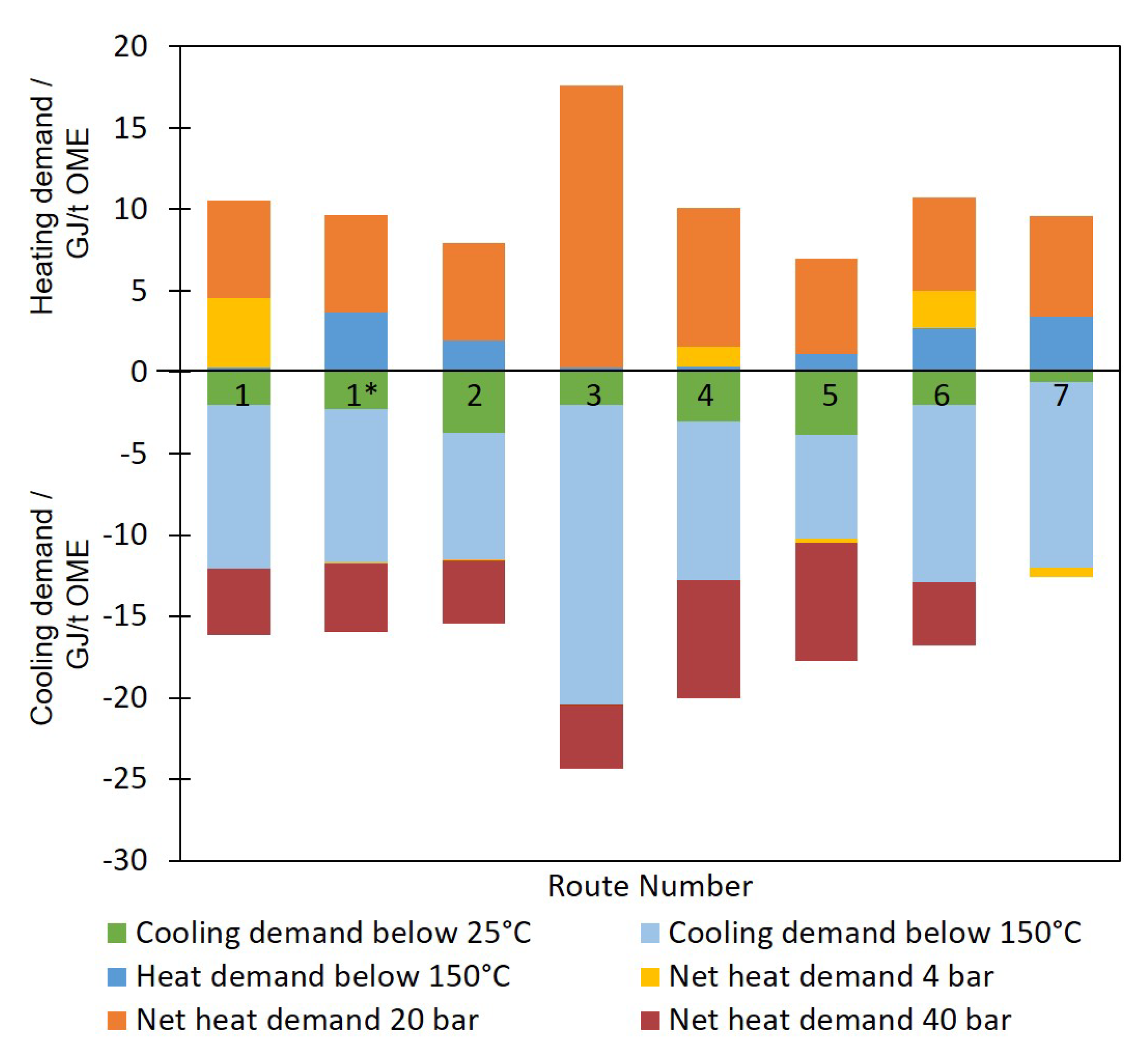
3.2. Energy Balance
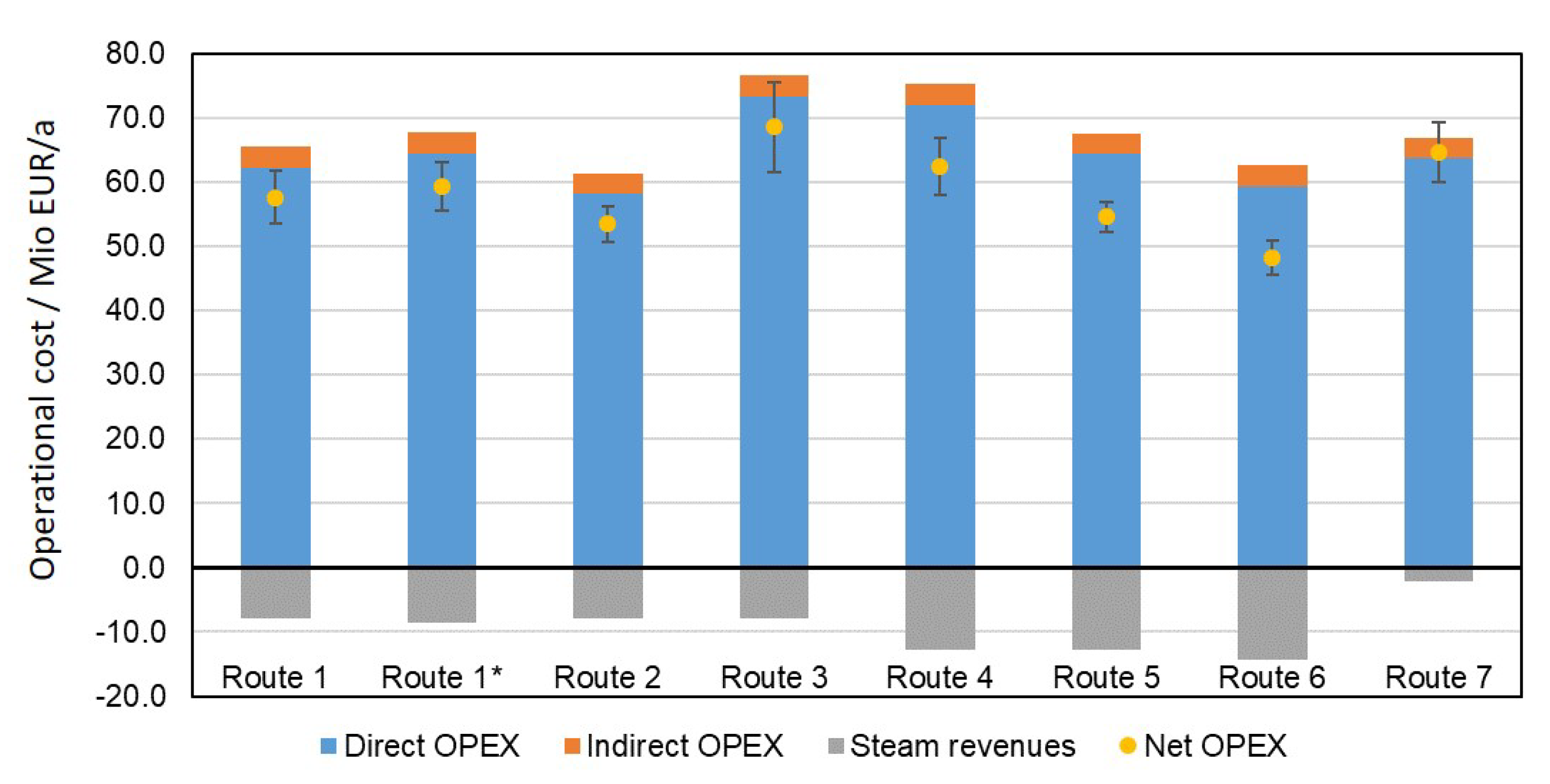
3.3. Economics
3.3.1. Base Case
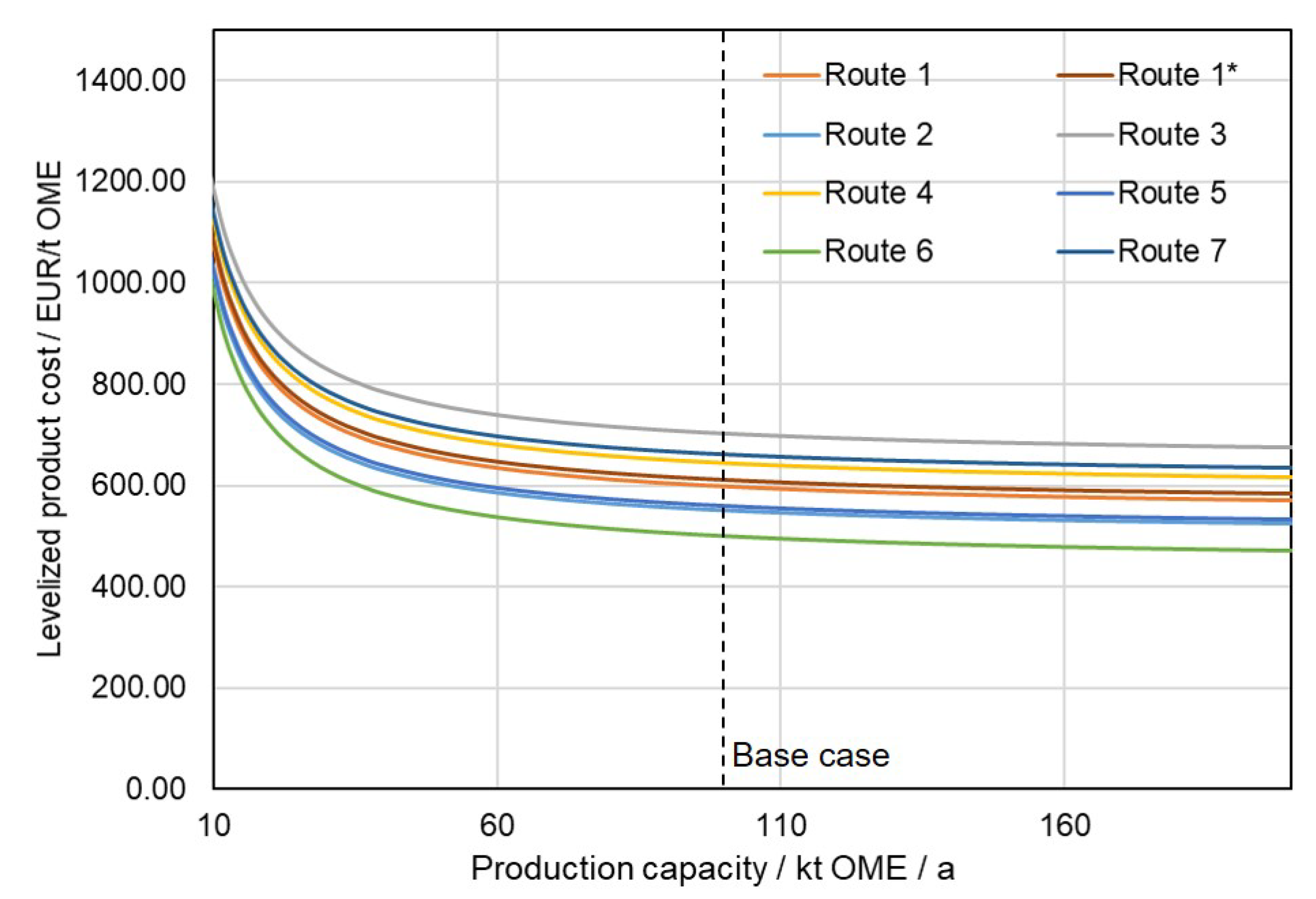
3.3.2. Sensitivitiy Studies: Manufacturing Cost for OME
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | |
| C | Column |
| CAPEX | Capital Expenditure |
| CEPCI | Chemical Engineering Plant Cost Index |
| DE | Diesel equivalent |
| DME | Dimethylether |
| E | Various unit operations with phase change |
| EUR | Euro |
| FA | Formaldehyde |
| HFn | Poly(oxymethylene) hemiformal |
| HX | Heat exchanger |
| LPC | Levelized product cost |
| MAL | Methylal |
| ME | Methanol |
| MGn | Poly(oxymethylene) glycol |
| OMEn | Poly(oxymethylene) dimethyl ether of chain length n |
| OP | Operating point |
| OPEX | Operational Expenditure |
| RR | Reflux Ratio |
| TFE | Thin Film Evaporator |
| TRI | Trioxane |
| TRL | Technology Readiness Level |
| USD | US Dollar |
| VLE | Vapor-liquid equilibrium |
| WA | Water |
| WACC | Weighted Average Cost of Capital |
| Variables | |
| A | Surface |
| c | Cost |
| Isobaric heat capacity | |
| Enthalpy of vaporization | |
| Reaction enthalpy | |
| H | Enthalpy |
| Specific enthalpy of component i | |
| Standard enthalpy of formation of component i | |
| K | Number of components |
| k | Heat transfer coefficient |
| Masss flow rate | |
| N | Number of stages |
| n | Chain length |
| p | pressure |
| Heat duty of unit i | |
| Total heat duty | |
| R | Universal gas constant |
| T | Temperature |
| Critical temperature | |
| Mass fraction of component i in k | |
References
- Burger, J.; Siegert, M.; Ströfer, E.; Hasse, H. Poly(oxymethylene) dimethyl ethers as components of tailored diesel fuel: Properties, synthesis and purification concepts. Fuel 2010, 89, 3315–3319. [Google Scholar] [CrossRef]
- Lumpp, B.; Rothe, D.; Pastötter, C.; Lämmermann, R.; Jacob, E. Oxymethylene ethers as diesel fuel additives of the future. MTZ Worldw. eMagazine 2011, 72, 34–38. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Zhang, Z.; Han, Y.; Tan, Y. Low-Temperature Oxidation of Dimethyl Ether to Polyoxymethylene Dimethyl Ethers over CNT-Supported Rhenium Catalyst. Catalysts 2016, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Baranowski, C.J.; Bahmanpour, A.M.; Kröcher, O. Catalytic synthesis of polyoxymethylene dimethyl ethers (OME): A review. Appl. Catal. B Environ. 2017, 217, 407–420. [Google Scholar] [CrossRef]
- Härtl, M.; Seidenspinner, P.; Jacob, E.; Wachtmeister, G. Oxygenate screening on a heavy-duty diesel engine and emission characteristics of highly oxygenated oxymethylene ether fuel OME1. Fuel 2015, 153, 328–335. [Google Scholar] [CrossRef]
- Pélerin, D.; Gaukel, K.; Härtl, M.; Wachtmeister, G. Simplifying of the Fuel Injection System and lowest Emissions with the alternative Diesel Fuel Oxymethylene Ether. In Proceedings of the 16th Conference: The Working Process of the Internal Combustion Engine, Graz, Austria, 28–29 September 2017. [Google Scholar]
- Pélerin, D.; Gaukel, K.; Härtl, M.; Wachtmeister, G. Recent results of the sootless Diesel fuel oxymethylene ether. In Proceedings of the International Motor Congress, Baden-Baden, Germany, 21–22 February 2017; pp. 439–456. [Google Scholar] [CrossRef]
- Schemme, S.; Samsun, R.C.; Peters, R.; Stolten, D. Power-to-fuel as a key to sustainable transport systems - An analysis of diesel fuels produced from CO2 and renewable electricity. Fuel 2017, 205, 198–221. [Google Scholar] [CrossRef]
- Burre, J.; Bongartz, D.; Deutz, S.; Mebrahtu, C.; Osterthun, O.; Sun, R.; Völker, S.; Bardow, A.; Klankermayer, J.; Palkovits, R.; et al. Comparing pathways for electricity-based production of dimethoxymethane as a sustainable fuel. Energy Environ. Sci. 2021, 14, 3686–3699. [Google Scholar] [CrossRef]
- Mantei, F.; Ali, R.E.; Baensch, C.; Voelker, S.; Haltenort, P.; Burger, J.; Dietrich, R.U.; Von Der Assen, N.; Schaadt, A.; Sauer, J.; et al. Techno-economic assessment and Carbon footprint of processes for the large-scale production of Oxymethylene Dimethyl Ethers from Carbon Dioxide and Hydrogen. Sustain. Energy Fuels 2021, 6, 528–549. [Google Scholar] [CrossRef]
- Burger, J. A Novel Process for the Production of Diesel Fuel Additives by Hierarchical Design. Ph.D. Thesis, Technical University of Kaiserslautern, Kaiserslautern, Germany, 2012. [Google Scholar]
- Boyd, R. Some Physical Properties of Polyoxymethylene Dimethyl Ethers. J. Polym. Sci. 1961, 50, 133–141. [Google Scholar] [CrossRef]
- Schmitz, N.; Burger, J.; Hasse, H. Reaction Kinetics of the Formation of Poly(oxymethylene) Dimethyl Ethers from Formaldehyde and Methanol in Aqueous Solutions. Ind. Eng. Chem. Res. 2015, 54, 12553–12560. [Google Scholar] [CrossRef]
- Burger, J.; Ströfer, E.; Hasse, H. Chemical Equilibrium and Reaction Kinetics of the Heterogeneously Catalyzed Formation of Poly(oxymethylene) Dimethyl Ethers from Methylal and Trioxane. Ind. Eng. Chem. Res. 2012, 51, 12751–12761. [Google Scholar] [CrossRef]
- Burger, J.; Ströfer, E.; Hasse, H. Production process for diesel fuel components poly(oxymethylene) dimethyl ethers from methane-based products by hierarchical optimization with varying model depth. Chem. Eng. Res. Des. 2013, 91, 2648–2662. [Google Scholar] [CrossRef]
- Mahbub, N.; Oyedun, A.O.; Kumar, A.; Oestreich, D.; Arnold, U.; Sauer, J. A life cycle assessment of oxymethylene ether synthesis from biomass-derived syngas as a diesel additive. J. Clean. Prod. 2017, 165, 1249–1262. [Google Scholar] [CrossRef]
- Deutz, S.; Bongartz, D.; Heuser, B.; Kätelhön, A.; Schulze Langenhorst, L.; Omari, A.; Walters, M.; Klankermayer, J.; Leitner, W.; Mitsos, A.; et al. Cleaner production of cleaner fuels: Wind-to-wheel - environmental assessment of CO2-based oxymethylene ether as a drop-in fuel. Energy Environ. Sci. 2018, 11, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2017, 118, 434–504. [Google Scholar] [CrossRef]
- Sternberg, A.; Bardow, A. Power-to-What?—Environmental assessment of energy storage systems. Energy Environ. Sci. 2015, 8, 389–400. [Google Scholar] [CrossRef]
- Schmitz, N.; Ströfer, E.; Burger, J.; Hasse, H. Conceptual Design of a Novel Process for the Production of Poly(oxymethylene) Dimethyl Ethers from Formaldehyde and Methanol. Ind. Eng. Chem. Res. 2017, 56, 11519–11530. [Google Scholar] [CrossRef]
- Xue, Z.; Shang, H.; Xiong, C.; Lu, C.; An, G.; Zhang, Z.; Cui, C.; Xu, M. Synthesis of polyoxymethylene dimethyl ethers catalyzed by sulfonic acid-functionalized mesoporous SBA-15. RSC Adv. 2017, 7, 20300–20308. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhu, H.; Wu, Z.; Qin, Z.; Yan, L.; Du, B.; Fan, W.; Wang, J. High Si/Al ratio HZSM-5 zeolite: An efficient catalyst for the synthesis of polyoxymethylene dimethyl ethers from dimethoxymethane and trioxymethylene. Green Chem. 2015, 17, 2353–2357. [Google Scholar] [CrossRef]
- Held, M.; Tönges, Y.; Pélerin, D.; Härtl, M.; Wachtmeister, G.; Burger, J. On the energetic efficiency of producing polyoxymethylene dimethyl ethers from CO2 using electrical energy. Energy Environ. Sci. 2019, 12, 1019–1034. [Google Scholar] [CrossRef]
- Schmitz, N.; Burger, J.; Ströfer, E.; Hasse, H. From methanol to the oxygenated diesel fuel poly(oxymethylene) dimethyl ether: An assessment of the production costs. Fuel 2016, 185, 67–72. [Google Scholar] [CrossRef]
- Burger, J.; Hasse, H. Processes for the production of OME fuels. In Proceedings of the Internationaler Motorenkongress, Baden-Baden, Germany, 29 August 2020. [Google Scholar] [CrossRef]
- Ouda, M.; Mantei, F.; Hesterwerth, K.; Bargiacchi, E.; Klein, H.; White, R.J. A hybrid description and evaluation of oxymethylene dimethyl ethers synthesis based on the endothermic dehydrogenation of methanol. React. Chem. Eng. 2018, 3, 676–695. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Kumar, A.; Oestreich, D.; Arnold, U.; Sauer, J. The development of the production cost of oxymethylene ethers as diesel additives from biomass. Biofuels Bioprod. Biorefining 2018, 12, 694–710. [Google Scholar] [CrossRef]
- Schemme, S.; Breuer, J.L.; Köller, M.; Meschede, S.; Walman, F.; Samsun, R.C.; Peters, R.; Stolten, D. H2-based synthetic fuels: A techno-economic comparison of alcohol, ether and hydrocarbon production. Int. J. Hydrog. Energy 2020, 45, 5395–5414. [Google Scholar] [CrossRef]
- Tönges, Y. Advances in the Production of Poly(oxymethylene) Dimethyl Ethers and Methanolic Formaldehyde Solution. Ph.D. Thesis, Technical University of Munich, Munich, Germany, 2022. [Google Scholar]
- Towler, G.; Sinnott, R.K. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill: New York, NY, USA, 2003; Volume 369, pp. 1–1006. [Google Scholar]
- Reuss, G.; Disteldorf, W.; Gamer, A.O.; Hilt, A. Formaldehyde. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Eek Vancells, L. Method for Producing Stabilized Solutions of Formaldehyde with Methanol. U.S. Patent EP0434783B1, 17 August 1994. [Google Scholar]
- Schmitz, N.; Breitkreuz, C.F.; Ströfer, E.; Burger, J.; Hasse, H. Separation of water from mixtures containing formaldehyde, water, methanol, methylal, and poly(oxymethylene) dimethyl ethers by pervaporation. J. Membr. Sci. 2018, 564, 806–812. [Google Scholar] [CrossRef]
- Dams, A.; Fried, A.; Hammann, F. Verfahren zur Gewinnung konzentrierter wäßriger Formaldehydlösungen durch Pervaporation. U.S. Patent DE4337231A1, 25 August 1993. [Google Scholar]
- Morishita, H.; Masamoto, J.; Hata, T. Process for Producing High Purity Formaldehyde. U.S. Patent 4,962,235A, 9 October 1990. [Google Scholar]
- Kloepper, D.L.; Stevenson, L.G. Method for Making Alcoholformaldehyde Product. U.S. Patent 3,214,891A, 2 November 1965. [Google Scholar]
- Masamoto, J.; Ohtake, J.; Kawamura, M. Process for Producing Formaldehyde and Dervatives Thereof. U.S. Patent 4,967,014, 30 October 1989. [Google Scholar]
- Millar, G.J.; Collins, M. Industrial Production of Formaldehyde Using Polycrystalline Silver Catalyst. Ind. Eng. Chem. Res. 2017, 56, 9247–9265. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.F. Formaldehyde; Reinhold Publishing Corporation: Washington, DC, USA, 1964; pp. 2–408. [Google Scholar]
- Ott, M. Reaktionskinetik und Destillation formaldehydhaltiger Mischungen (Berichte aus der Verfahrenstechnik). Ph.D. Thesis, Technical University of Kaiserslautern, Kaiserslautern, Germany, 2004. [Google Scholar]
- Mann, H.J.; Pohl, W.; Simon, K.; Weigert, W. Production of free flowing paraformaldehyde. U.S. Patent 3,595,926A, 27 July 1971. [Google Scholar]
- Ströfer, E.; Gruetzner, T.; Hasse, H.; Lang, N.; Ott, M. Verfahren zur Erzeugung Hochkonzentrierter formaldehydlösungen. U.S. Patent WO2004078678A3, 16 September 2004. [Google Scholar]
- Ströfer, E.; Sohn, M.; Hasse, H.; Schilling, K. Highly Concentrated Formaldehyde Solution, Production and Reaction Thereof. U.S. Patent 7,193,115,B2, 20 March 2007. [Google Scholar]
- Ferre, A.; Voggenreiter, J.; Tönges, Y.; Burger, J. Demonstrationsanlage für die Synthese von OME-Kraftstoffen. MTZ—Mot. Z. 2021, 82, 28–33. [Google Scholar] [CrossRef]
- Kuhnert, C.; Albert, M.; Breyer, S.; Hahnenstein, I.; Hasse, H.; Maurer, G. Phase Equilibrium in Formaldehyde Containing Multicomponent Mixtures: Experimental Results for Fluid Phase Equilibria of (Formaldehyde + (Water or Methanol) + Methylal)) and (Formaldehyde + Water + Methanol + Methylal) and Comparison with Predictions. Ind. Eng. Chem. Res. 2006, 45, 5155–5164. [Google Scholar] [CrossRef]
- Ott, M.; Fischer, H.H.; Maiwald, M.; Albert, K.; Hasse, H. Kinetics of oligomerization reactions in formaldehyde solutions: NMR experiments up to 373K and thermodynamically consistent model. Chem. Eng. Process. Process. Intensif. 2005, 44, 653–660. [Google Scholar] [CrossRef]
- Brigham University. Thermophysical Properties Laboratory Project 801; Department of Chemical Engineering, Brigham University: Pravo, UT, USA, 2009. [Google Scholar]
- Schmitz, N. Production of Poly(oxymethylene) Dimethyl Ethers from Formaldehyde and Methanol. Ph.D. Thesis, Technical University of Kaiserslautern, Kaiserslautern, Germany, 2018. [Google Scholar]
- Smith, R. Chemical Process Design and Integration; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; p. 712. [Google Scholar]
- Christensen, P.; Dysert, L.R. Cost Estimate Classification system-as applied in engineering, procurement, and construction for the process industries—TCM Framework: 7.3—Cost Estimating and Budgeting. In AACE International Recommended Practice No. 18R-97; AACE: Morgantown, WV, USA, 2005; pp. 1–9. [Google Scholar]
- Schemme, S.; Meschede, S.; Köller, M.; Samsun, R.C.; Peters, R.; Stolten, D. Property Data Estimation for Hemiformals, Methylene Glycols and Polyoxymethylene Dimethyl Ethers and Process Optimization in Formaldehyde Synthesis. Energies 2020, 13, 3401. [Google Scholar] [CrossRef]



| Unit | Cost Determining Factors |
|---|---|
| Distillation columns, absorbers | Column height and diameter, Column internals: Trays/Packing |
| Reactors | Heat duty, Catalyst mass |
| Heat exchangers, evaporators, condensers, Thin-film-evaporators, Off-gas-burners | Heat exchange area |
| Membrane units | Membrane surface area |
| Steam Network Pressure Levels | |||
|---|---|---|---|
| TSteam | / °C | / °C | |
| (consumption) | (production) | ||
| Steam 40 bar | 290 | 280 | 300 |
| Steam 20 bar | 220 | 210 | 230 |
| Steam 4 bar | 150 | 140 | 160 |
| LT Cooling Media | |||
| Cooling Medium | / °C | / °C | / °C |
| Cooling water | 40 | 25 | 60 |
| Chilled water | 20 | 10 | 60 |
| Ammonia | 10 | 0 | 0 |
| Salt solutions | −5 | −15 | −15 |
| Salt solutions | −10 | −20 | −20 |
| Pressure Level | Cost/Credit/EUR/t |
|---|---|
| Steam 40 bar | 23.5 |
| Steam 20 bar | 23.1 |
| Steam 4 bar | 22.8 |
| Cooling Medium | Cost/EUR/kWh |
| Cooling water | 0.005 |
| Chilled water | 0.0075 |
| Ammonia | 0.015 |
| Salt solutions | 0.02 |
| Salt solutions | 0.025 |
| Route | 1 | 1* | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|---|
| Carbon yield/mol/mol | 0.935 | 0.881 | 0.927 | 0.925 | 0.817 | 0.812 | 0.928 | 0.926 | 0.05 |
| Heat demand/GJ/t OME | 6.18 | 5.05 | 3.36 | 13.36 | 2.51 | −1.11 | 6.57 | 8.70 | 4.05 |
| CAPEX/Mio EUR | 32.39 | 28.32 | 24.66 | 27.80 | 31.83 | 23.34 | 28.66 | 27.18 | 2.92 |
| OPEX/Mio EUR/a | 57.66 | 59.32 | 53.46 | 68.63 | 62.40 | 54.57 | 48.23 | 64.63 | 6.15 |
| LPC/EUR/t OME | 597.26 | 611.3 | 550.31 | 704.13 | 644.41 | 560.43 | 500.69 | 662.98 | 61.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tönges, Y.; Dieterich, V.; Fendt, S.; Spliethoff, H.; Burger, J. Techno-Economic Analysis of Large Scale Production of Poly(oxymethylene) Dimethyl Ether Fuels from Methanol in Water-Tolerant Processes. Fuels 2023, 4, 1-18. https://doi.org/10.3390/fuels4010001
Tönges Y, Dieterich V, Fendt S, Spliethoff H, Burger J. Techno-Economic Analysis of Large Scale Production of Poly(oxymethylene) Dimethyl Ether Fuels from Methanol in Water-Tolerant Processes. Fuels. 2023; 4(1):1-18. https://doi.org/10.3390/fuels4010001
Chicago/Turabian StyleTönges, Yannic, Vincent Dieterich, Sebastian Fendt, Hartmut Spliethoff, and Jakob Burger. 2023. "Techno-Economic Analysis of Large Scale Production of Poly(oxymethylene) Dimethyl Ether Fuels from Methanol in Water-Tolerant Processes" Fuels 4, no. 1: 1-18. https://doi.org/10.3390/fuels4010001
APA StyleTönges, Y., Dieterich, V., Fendt, S., Spliethoff, H., & Burger, J. (2023). Techno-Economic Analysis of Large Scale Production of Poly(oxymethylene) Dimethyl Ether Fuels from Methanol in Water-Tolerant Processes. Fuels, 4(1), 1-18. https://doi.org/10.3390/fuels4010001








