Thermal Activation of Digested Sewage Sludges for Carbon Dioxide Removal from Biogas
Abstract
:1. Introduction
2. Materials and Methods
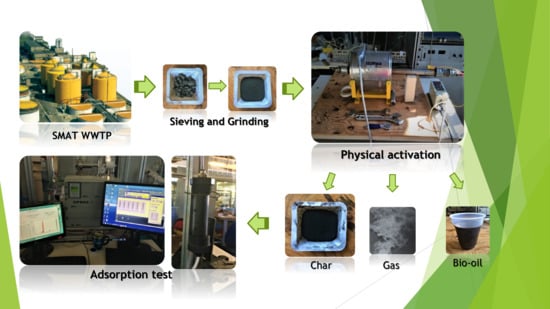
2.1. Sample Preparation
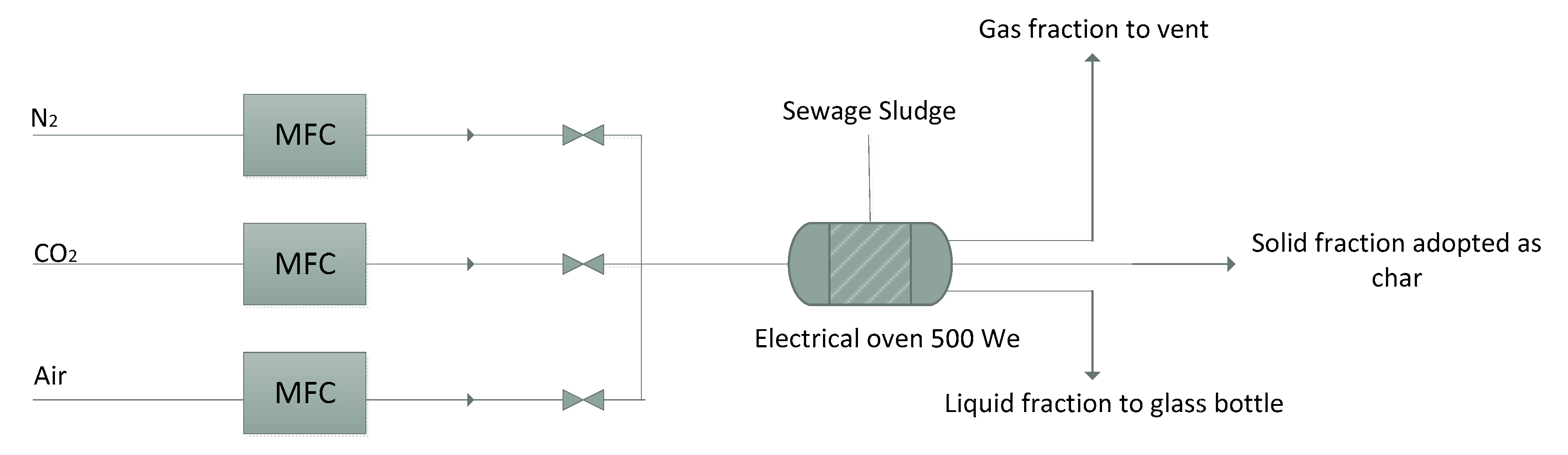
2.2. Physical Activation and Adsorption Test Equipment
3. Results and Discussion
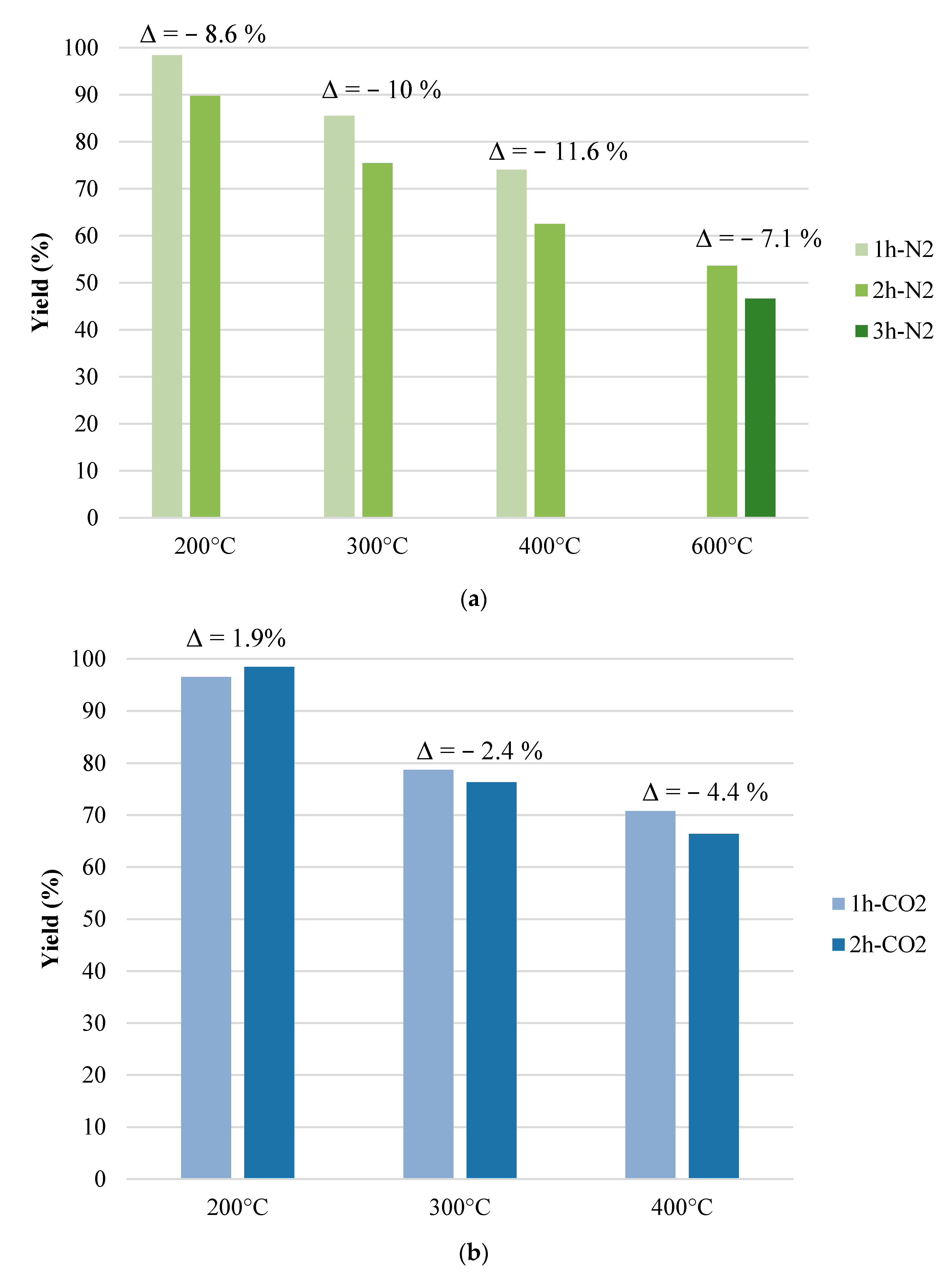
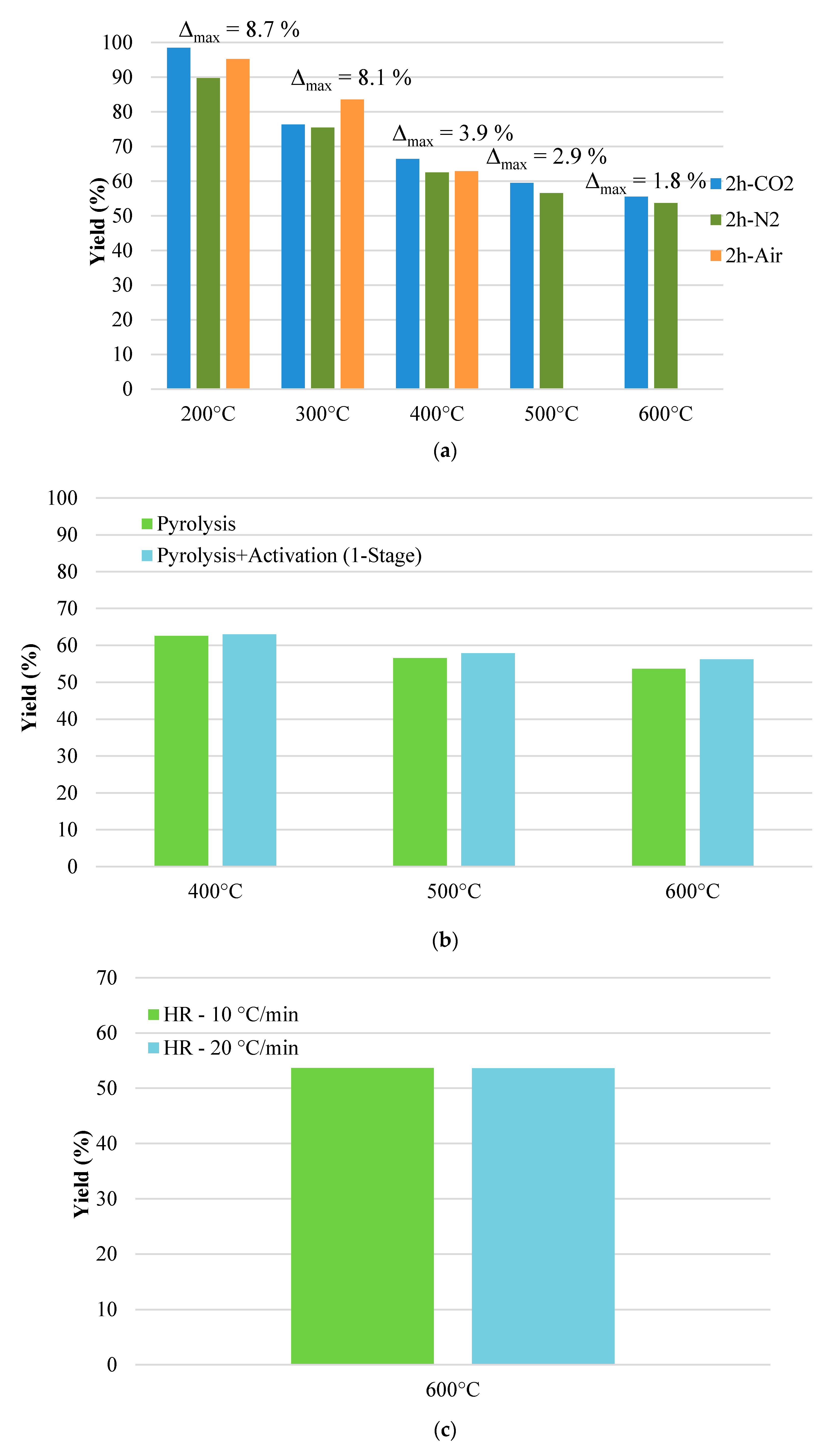
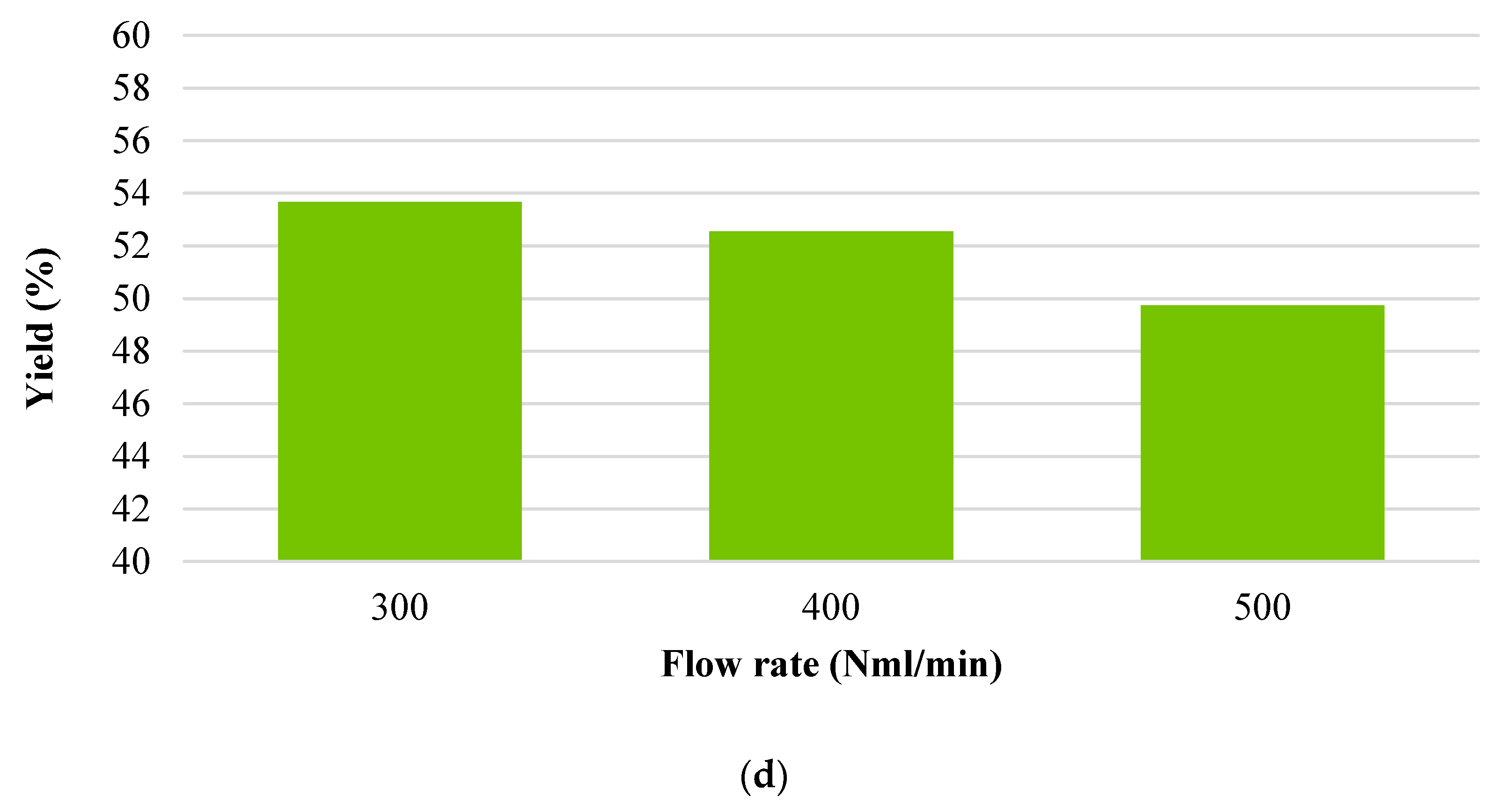
3.1. Char Yield
- -
- Temperature (200–600 °C);
- -
- Residence time (1–2 h);
- -
- Flow rate (300 Nml·min−1);
- -
- Heating rate (10 °C·min−1);
- -
- Flowing agent (N2, CO2, air).
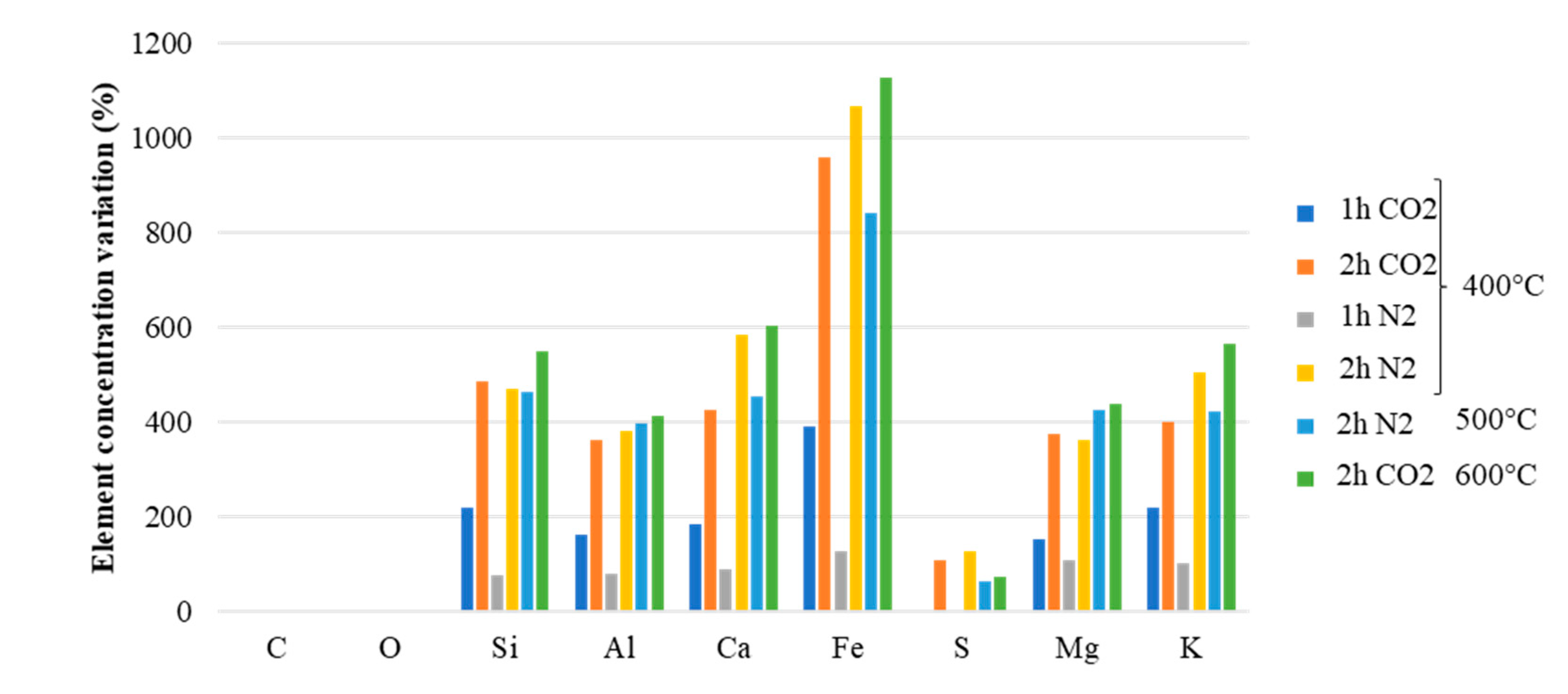
3.2. Microscopic Characteristics
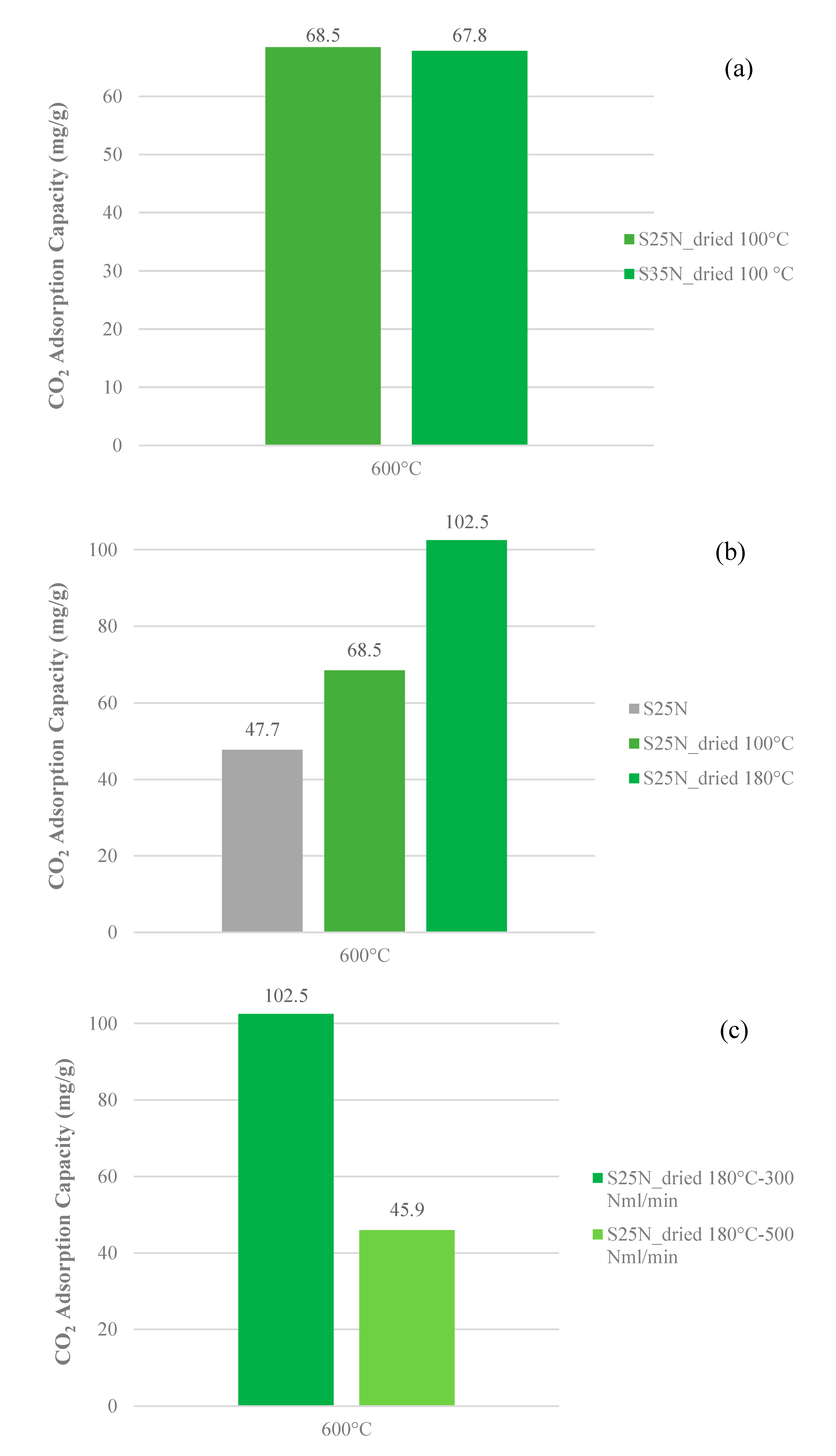
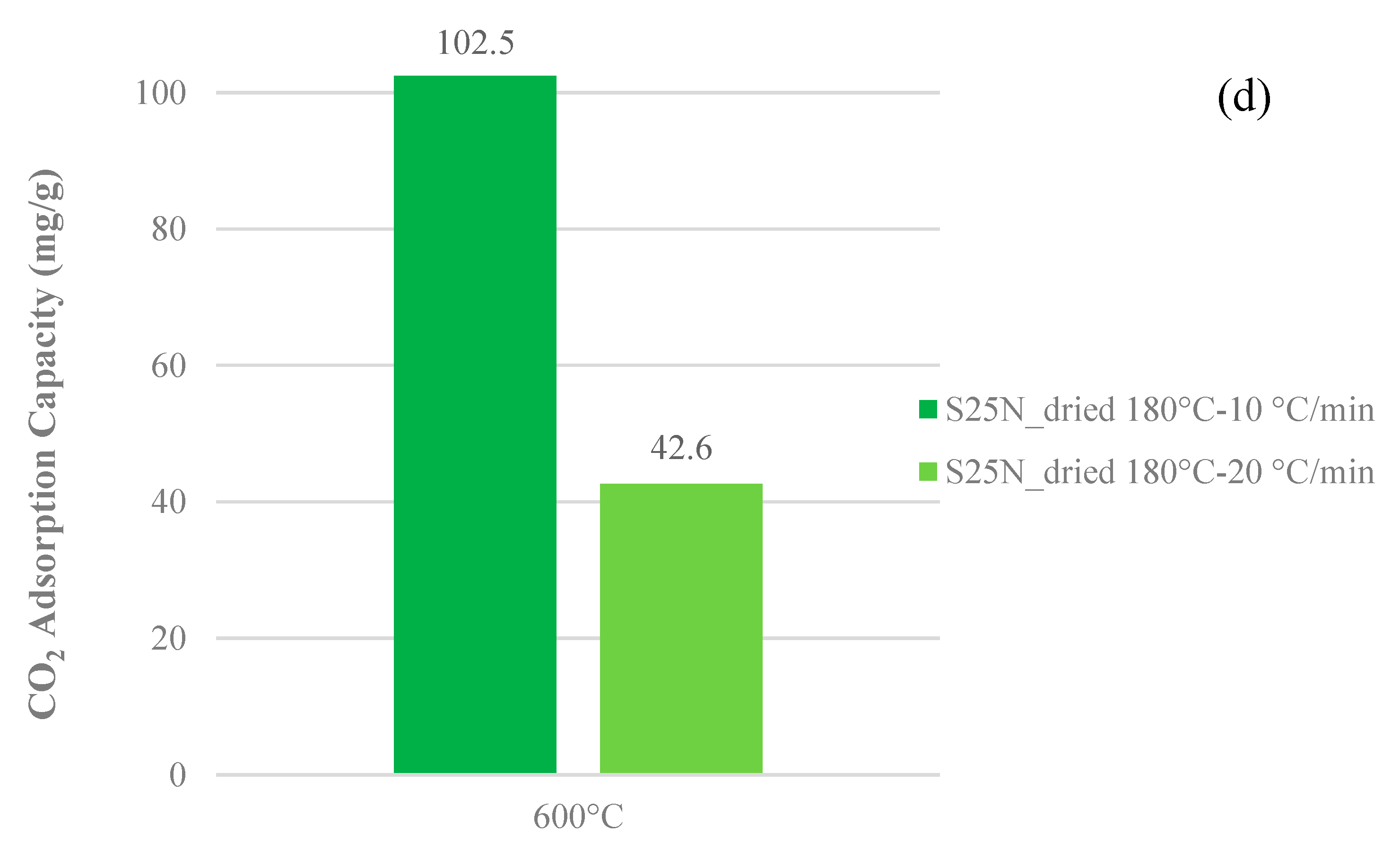
3.3. CO2 Adsorption Capacity
- -
- Biogas flow rate: 153.8 Nml·min−1;
- -
- GHSV (gas hourly space velocity): 131·h−1;
- -
- Cb: CO2 breakthrough concentration limit: 2.5%.
- -
- is the CO2 adsorbed in mg per g of sorbent;
- -
- is the breakthrough time;
- -
- is the simulated biogas flow rate;
- -
- (ppmv) is the total CO2 concentration in the mixture;
- -
- ( is the CO2 molar weight;
- -
- is the molar volume of an ideal gas;
- -
- is the mass of the sorbent;
- -
- is the unit conversion from ppmv to molar concentration.
- -
- It gives a higher amount of the solid fraction produced during the physical activation due to the greater yield value (53.7 vs. 46.6%);
- -
- It results in lower energy costs during the thermal treatment for char production.
- -
- Temperature: 600 °C;
- -
- Dwell time: 2 h;
- -
- Drying temperature: 170–180 °C;
- -
- Physical activation flow rate: 300 Nml·min−1;
- -
- Heating rate: 10 °C·min−1.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| BET C | Brunauer–Emmett–Teller analytical method carbon content (%) |
| Cb | CO2 breakthrough concentration limit |
| CHP | Combined Heat and Power |
| CO2cap | CO2 adsorption capacity (mgCO2·gsorb−1) |
| CO2conc | CO2 concentration (ppmv) |
| Dry_x | biochar samples which received further post-drying treatment at the “x” temperature |
| EDS | Energy Dispersive X-ray Spectrometry |
| GHSV | Gas Hourly Space Velocity (h−1) |
| H IUPAC | hydrogen content (%) International Union of Pure and Applied Chemistry |
| mchar | mass of char (g) |
| mraw | mass of feedstock (g) |
| msorb | mass of sorbent (g) |
| N | nitrogen content (%) |
| N2/CO2/Air/N2-CO2 | Respectively, nitrogen used only, carbon dioxide used only, air used only, nitrogen for the transitory phase and carbon dioxide for the stationary phase (one-stage method) |
| P | phosphorous content (%) |
| PID | proportional–integral–derivative controller |
| Q | simulated biogas flow rate (l min−1) |
| S | sulfur content (%) |
| SBET | active surface evaluated with BET method (m2/g) |
| SOFC | Solid Oxide Fuel Cell |
| SS | sewage sludge |
| Stot.pores | total surface of pores (m2/g) |
| St-plot ext | active surface evaluated with t-plot method (m2/g) |
| tb | breakthrough time (s) |
| Vpores (d < 1.308 nm) | microporous pores (cm3/g) |
| Vpores (d < 44.9 nm) | mesoporous pores (cm3/g) |
| vwc | variable water content |
| WWTP | WasteWater Treatment Plant |
| ychar | Char yield (%) |
References
- Hadi, P.; Xu, M.; Ning, C.; Lin, C.S.K.; McKay, G. A critical review on preparation, characterization and utilization of sludge-derived activated carbons for wastewater treatment. Chem. Eng. J. 2015, 260, 895–906. [Google Scholar] [CrossRef]
- Papurello, D.; Boschetti, A.; Silvestri, S.; Khomenko, I.; Biasioli, F. Real-time monitoring of removal of trace compounds with PTR-MS: Biochar experimental investigation. Renew. Energy 2018, 125, 344–355. [Google Scholar] [CrossRef]
- Santarelli, M.; Briesemeister, L.; Gandiglio, M.; Herrmann, S.; Kuczynski, P.; Kupecki, J.; Lanzini, A.; Llovell, F.; Papurello, D.; Spliethoff, H.; et al. Carbon recovery and re-utilization (CRR) from the exhaust of a solid oxide fuel cell (SOFC): Analysis through a proof-of-concept. J. CO2 Util. 2017, 18, 206–221. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Sarmah, A.K.; Dubey, B. Downstream augmentation of hydrothermal carbonization with anaerobic digestion for integrated biogas and hydrochar production from the organic fraction of municipal solid waste: A circular economy concept. Sci. Total Environ. 2020, 706, 135907. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.V.; Salvador, R.; De Francisco, A.C.; Piekarski, C.M. Mapping of research lines on circular economy practices in agriculture: From waste to energy. Renew. Sustain. Energy Rev. 2020, 131, 109958. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Sengupta, S.; Gupta, A.; Kumar, S.S.; Vijay, V.; Kumar, V.; Vijay, V.K.; Pant, D. Valorization of agricultural waste for biogas based circular economy in India: A research outlook. Bioresour. Technol. 2020, 304, 123036. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Samolada, M.; Zabaniotou, A. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014, 34, 411–420. [Google Scholar] [CrossRef]
- Prabhakar, A.K.; Mohan, B.C.; Tay, T.S.; Lee, S.S.-C.; Teo, S.L.-M.; Wang, C.-H. Incinerated Sewage Sludge Bottom Ash- Chemical processing, Leaching patterns and Toxicity testing. J. Hazard. Mater. 2020, 402, 123350. [Google Scholar] [CrossRef]
- Gent, S.; Twedt, M.; Gerometta, C.; Almberg, E. Theoretical and Applied Aspects of Biomass Torrefaction; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Bolognesi, S.; Bernardi, G.; Callegari, A.; Dondi, D.; Capodaglio, A.G. Biochar production from sewage sludge and microalgae mixtures: Properties, sustainability and possible role in circular economy. Biomass-Con. Bioref. 2019, 1–11. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Nam, H.; Sajib, S.K. Effect of bio-char on methane generation from glucose and aqueous phase of algae liquefaction using mixed anaerobic cultures. Biomass Bioenergy 2018, 108, 479–486. [Google Scholar] [CrossRef]
- Biochar, a potential hydroponic growth substrate, enhances the nutritional status and growth of leafy vegetables -ScienceDirect, (n.d.). Available online: https://www.sciencedirect.com/science/article/pii/S0959652617307886?casa_token=WaP93qJSXUUAAAAA:t4Knu-YZwwoBIuWmoeB2AwS16y3TRDjy-bVxgNqnVZ3Mdr_95Ug0jEzPrkrshQIvOX9tZucoYA (accessed on 18 October 2020).
- Kassim, M.A.; Meng, T.K. Carbon dioxide (CO2) biofixation by microalgae and its potential for biorefinery and biofuel production. Sci. Total Environ. 2017, 584–585, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. CO2 adsorption on biochar from co-torrefaction of sewage sludge and leucaena wood using microwave heating. Energy Procedia 2019, 158, 4435–4440. [Google Scholar] [CrossRef]
- Florent, M.; Policicchio, A.; Niewiadomski, S.; Bandosz, T.J. Exploring the options for the improvement of H2S adsorption on sludge derived adsorbents: Building the composite with porous carbons. J. Clean. Prod. 2020, 249, 119412. [Google Scholar] [CrossRef]
- Xu, G.; Yang, X.; Spinosa, L. Development of sludge-based adsorbents: Preparation, characterization, utilization and its feasibility assessment. J. Environ. Manag. 2015, 151, 221–232. [Google Scholar] [CrossRef]
- Tang, S.; Tian, S.; Zheng, C.; Zhang, Z. Effect of Calcium Hydroxide on the Pyrolysis Behavior of Sewage Sludge: Reaction Characteristics and Kinetics. Energy Fuels 2017, 31, 5079–5087. [Google Scholar] [CrossRef]
- Trinh, T.N.; Jensen, P.A.; Dam-Johansen, K.; Knudsen, N.O.; Sørensen, H.R. Influence of the Pyrolysis Temperature on Sewage Sludge Product Distribution, Bio-Oil, and Char Properties. Energy Fuels 2013, 27, 1419–1427. [Google Scholar] [CrossRef]
- Shang, G.; Li, Q.; Liu, L.; Chen, P.; Huang, X. Adsorption of hydrogen sulfide by biochars derived from pyrolysis of different agricultural/forestry wastes. J. Air Waste Manag. Assoc. 2015, 66, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Lashaki, M.J.; Fayaz, M.; Wang, H.; Hashisho, Z.; Philips, J.H.; Anderson, J.E.; Nichols, M. Effect of Adsorption and Regeneration Temperature on Irreversible Adsorption of Organic Vapors on Beaded Activated Carbon. Environ. Sci. Technol. 2012, 46, 4083–4090. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Papurello, D.; Tomasi, L.; Silvestri, S.; Belcari, I.; Santarelli, M.; Smeacetto, F.; Biasioli, F. Biogas trace compound removal with ashes using proton transfer reaction time-of-flight mass spectrometry as innovative detection tool. Fuel Process. Technol. 2016, 145, 62–75. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; Mohedano, A.F.; Rodríguez, J.J. Activated carbons from sewage sludge. Desalination 2011, 277, 377–382. [Google Scholar] [CrossRef]
- Girgis, B.S.; Khalil, L.B.; Tawfik, T.A. Porosity Development in Carbons Derived from Olive Oil Mill Residue Under Steam Pyrolysis. J. Porous Mater. 2002, 9, 105–113. [Google Scholar] [CrossRef]
- Smith, K.; Fowler, G.; Pullket, S.; Graham, N. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications. Water Res. 2009, 43, 2569–2594. [Google Scholar] [CrossRef]
- Ros, A.; Lillo-Ródenas, M.; Fuente, E.; Montes-Morán, M.; Martín, M.; Linares-Solano, A. High surface area materials prepared from sewage sludge-based precursors. Chemosphere 2006, 65, 132–140. [Google Scholar] [CrossRef]
- Papurello, D.; Silvestri, S.; Lanzini, A. Biogas cleaning: Trace compounds removal with model validation. Sep. Purif. Technol. 2019, 210, 80–92. [Google Scholar] [CrossRef]
- Gabelman, A. Adsorption Basics: Part 1. Chem. Eng. Prog. 2017, 113, 48–53. [Google Scholar]
- Papurello, D.; Tomasi, L.; Silvestri, S. Proton transfer reaction mass spectrometry for the gas cleaning using commercial and waste-derived materials: Focus on the siloxane removal for SOFC applications. Int. J. Mass Spectrom. 2018, 430, 69–79. [Google Scholar] [CrossRef]
- Papurello, D.; Tomasi, L.; Silvestri, S.; Santarelli, M. Evaluation of the Wheeler-Jonas parameters for biogas trace compounds removal with activated carbons. Fuel Process. Technol. 2016, 152, 93–101. [Google Scholar] [CrossRef]
- Cheng, F.; Luo, H.; Hu, L.; Yu, B.; Luo, Z.; De Cortalezzi, M.F. Sludge carbonization and activation: From hazardous waste to functional materials for water treatment. J. Environ. Chem. Eng. 2016, 4, 4574–4586. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Cao, Y.; Pawłowski, A. Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: Brief overview and energy efficiency assessment. Renew. Sustain. Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- Linghu, W.; Shen, R. Thermal behaviour of sewage sludge in pyrolysis process. Mater. Res. Innov. 2014, 18, S4–S50. [Google Scholar] [CrossRef]
- Ortiz, F.G.; Aguilera, P.; Ollero, P. Biogas desulfurization by adsorption on thermally treated sewage-sludge. Sep. Purif. Technol. 2014, 123, 200–213. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Magdziarz, A.; Werle, S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag. 2014, 34, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- De Andrés, J.M.; Orjales, L.; Narros, A.; Fuente, M.D.M.D.L.; Rodríguez, M.E. Carbon dioxide adsorption in chemically activated carbon from sewage sludge. J. Air Waste Manag. Assoc. 2013, 63, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Papurello, D.; Gandiglio, M.; Kafashan, J.; Lanzini, A. Biogas Purification: A Comparison of Adsorption Performance in D4 Siloxane Removal Between Commercial Activated Carbons and Waste Wood-Derived Char Using Isotherm Equations. Processes 2019, 7, 774. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Codony, A.; Santos-Clotas, E.; Ania, C.O.; Martín, M.J. Competitive siloxane adsorption in multicomponent gas streams for biogas upgrading. Chem. Eng. J. 2018, 344, 565–573. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Xia, H.; Zhang, L.; Srinivasakannan, C.; Guo, S. Textural characteristics of activated carbon by single step CO2 activation from coconut shells. J. Taiwan Inst. Chem. Eng. 2010, 41, 367–372. [Google Scholar] [CrossRef]
- Tay, J.; Chen, X.; Jeyaseelan, S.; Graham, N. Optimising the preparation of activated carbon from digested sewage sludge and coconut husk. Chemosphere 2001, 44, 45–51. [Google Scholar] [CrossRef]
- Juárez, M.F.-D.; Mostbauer, P.; Knapp, A.; Müller, W.; Tertsch, S.; Bockreis, A.; Insam, H. Biogas purification with biomass ash. Waste Manag. 2018, 71, 224–232. [Google Scholar] [CrossRef]
- Lombardi, L.; Costa, G.; Spagnuolo, R. Accelerated carbonation of wood combustion ash for CO2 removal from gaseous streams and storage in solid form. Environ. Sci. Pollut. Res. 2018, 25, 35855–35865. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Hamedeyazdan, Y.J.A.S. Floating Drug Delivery Systems for Eradication of Helicobacter pylori in Treatment of Peptic Ulcer Disease. Trends in Helicobacter pylori Infection 2014, i, 13. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Plaza, M.; Rubiera, F.; Pevida, C. Sustainable biomass-based carbon adsorbents for post-combustion CO2 capture. Chem. Eng. J. 2013, 230, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Hornung, A.; Neumann, J.; Daschner, R. Sustainable Utilization Of Municipal, (n.d.); IWWG: Padua, Italy, 2018; pp. 21–23. [Google Scholar]
- Papurello, D.; Bressan, M.; Bona, D.; Flaim, G.; Cerasino, L.; Silvestri, S. Simulated Sofc Exhausts and Their Fixation on Chlorella Vulgaris: Study on Affecting Parameters. Detritus 2019, 5, 99–104. [Google Scholar] [CrossRef]
- Bona, D.; Papurello, D.; Flaim, G.; Cerasino, L.; Biasioli, F.; Silvestri, S. Management of Digestate and Exhausts from Solid Oxide Fuel Cells Produced in the Dry Anaerobic Digestion Pilot Plant: Microalgae Cultivation Approach. Waste Biomass-Valorization 2020, 1–16. [Google Scholar] [CrossRef]

| C (%) | 35 ± 0.8 | As (mg/kg) | 5.6 ± 0.28 |
| H (%) | 4.8 ± 0.3 | Cd (mg/kg) | 1.55 ± 0.08 |
| N (%) | 4.7 ± 0.1 | Cr (mg/kg) | 224 ± 5.8 |
| S (%) | <2 | Hg (mg/kg) | 0.89 ± 0.03 |
| P (%) | 2.9 ± 0.08 | Ni (mg/kg) | 147 ± 1.28 |
| SBET (m2/g) | 0.33 ± 0.016 | Pb (mg/kg) | 77 ± 3.4 |
| St-plot ext (m2/g) | 0.377 ± 0.015 | K (mg/kg) | 1801 ± 41 |
| Vpores (d < 1.308 nm) (cm3/g) | 0.00002 ± 1·10−6 | Cu (mg/kg) | 388 ± 4.2 |
| Vpores (d < 44.9 nm) (cm3/g) | 0.00121 ± 6·10−5 | Se (mg/kg) | 3.15 ± 0.34 |
| Stot.pores (m2/g) | 0.111 ± 4.3·10−3 | Zn (mg/kg) | 1109 ± 13 |
| Sample Label | SBET (m2/g) | St-Plot ext (m2/g) | Vpores (d < 1.308 nm) (cm3/g) | Vpores (d < 44.9 nm) (cm3/g) | Stot.pores (m2/g) |
|---|---|---|---|---|---|
| S0 | 0.32 | 0.38 | 0.00002 | 0.0012 | 0.11 |
| S1 | 2.65 | 3.27 | 0.00038 | 0.0139 | 2.06 |
| S2 | 3.29 | 3.82 | 0.00024 | 0.0191 | 3.48 |
| S23N | 2.32 | 2.75 | 0.00015 | 0.0146 | 2.4 |
| S24N | 4.32 | 4.7 | 0.00074 | 0.0226 | 3.41 |
| Sample Label | Adsorption Capacity vwc (mgCO2·g−1) | Adsorption Capacity Dry (mgCO2·g−1) |
|---|---|---|
| S0 | 4.0 | 9.9 |
| S11 | 2.4 | 4.6 |
| S12 | 3.7 | 11.1 |
| S1 | 4.4 | 20.1 |
| S21 | 4.9 | 6.3 |
| S22 | 7.7 | 9.4 |
| S2 | 16.1 | 26.2 |
| S23 | 19.6 | 49.1 |
| S24 | 35.0 | 35.5 |
| S11N | 5.2 | 6.4 |
| S12N | 9.9 | 11.9 |
| S13N | 10.6 | 17.2 |
| S21N | 5.1 | 8.9 |
| S22N | 10.9 | 13.4 |
| S23N | 27.7 | 43.5 |
| S24N | 36.5 | 62.3 |
| S25N | 47.7 | 68.5 |
| SA1 | - | 5.7 |
| SA2 | - | 13.0 |
| SA3 | - | 15.5 |
| SB1 | - | 18.4 |
| SB2 | - | 45.7 |
| SB3 | - | 42.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinnirello, M.; Papurello, D.; Santarelli, M.; Fiorilli, S. Thermal Activation of Digested Sewage Sludges for Carbon Dioxide Removal from Biogas. Fuels 2020, 1, 30-46. https://doi.org/10.3390/fuels1010004
Tinnirello M, Papurello D, Santarelli M, Fiorilli S. Thermal Activation of Digested Sewage Sludges for Carbon Dioxide Removal from Biogas. Fuels. 2020; 1(1):30-46. https://doi.org/10.3390/fuels1010004
Chicago/Turabian StyleTinnirello, Mirko, Davide Papurello, Massimo Santarelli, and Sonia Fiorilli. 2020. "Thermal Activation of Digested Sewage Sludges for Carbon Dioxide Removal from Biogas" Fuels 1, no. 1: 30-46. https://doi.org/10.3390/fuels1010004