Thermal Activation of Digested Sewage Sludges for Carbon Dioxide Removal from Biogas
Abstract
1. Introduction
2. Materials and Methods
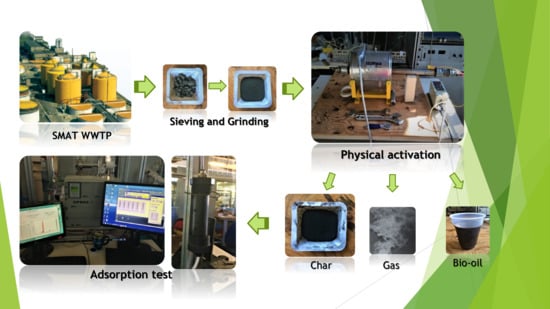
2.1. Sample Preparation
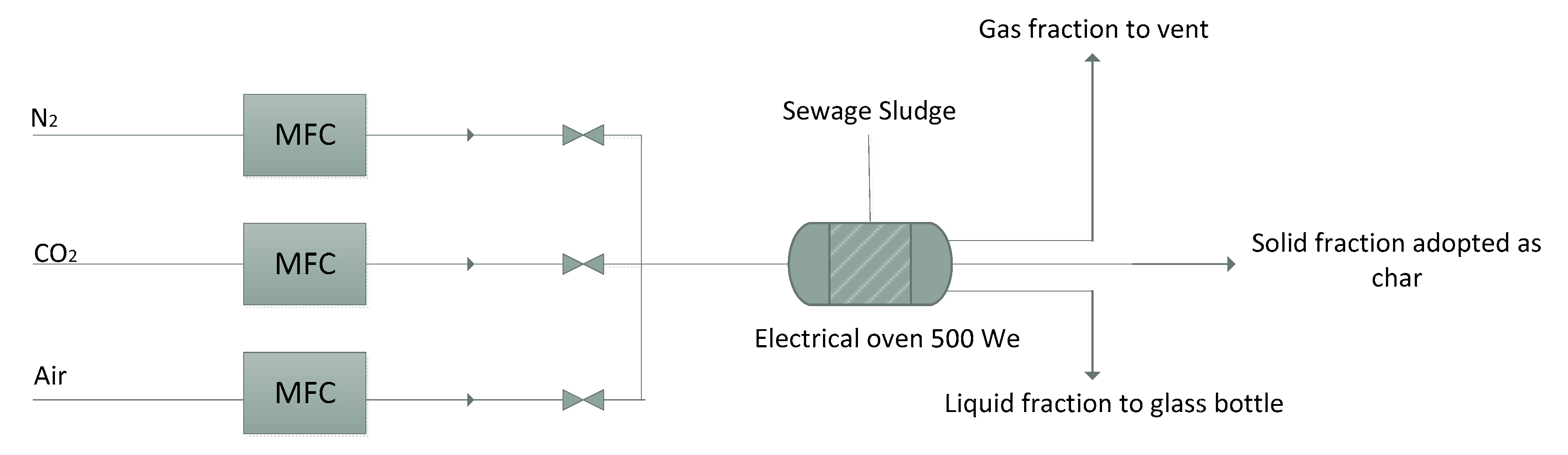
2.2. Physical Activation and Adsorption Test Equipment
3. Results and Discussion
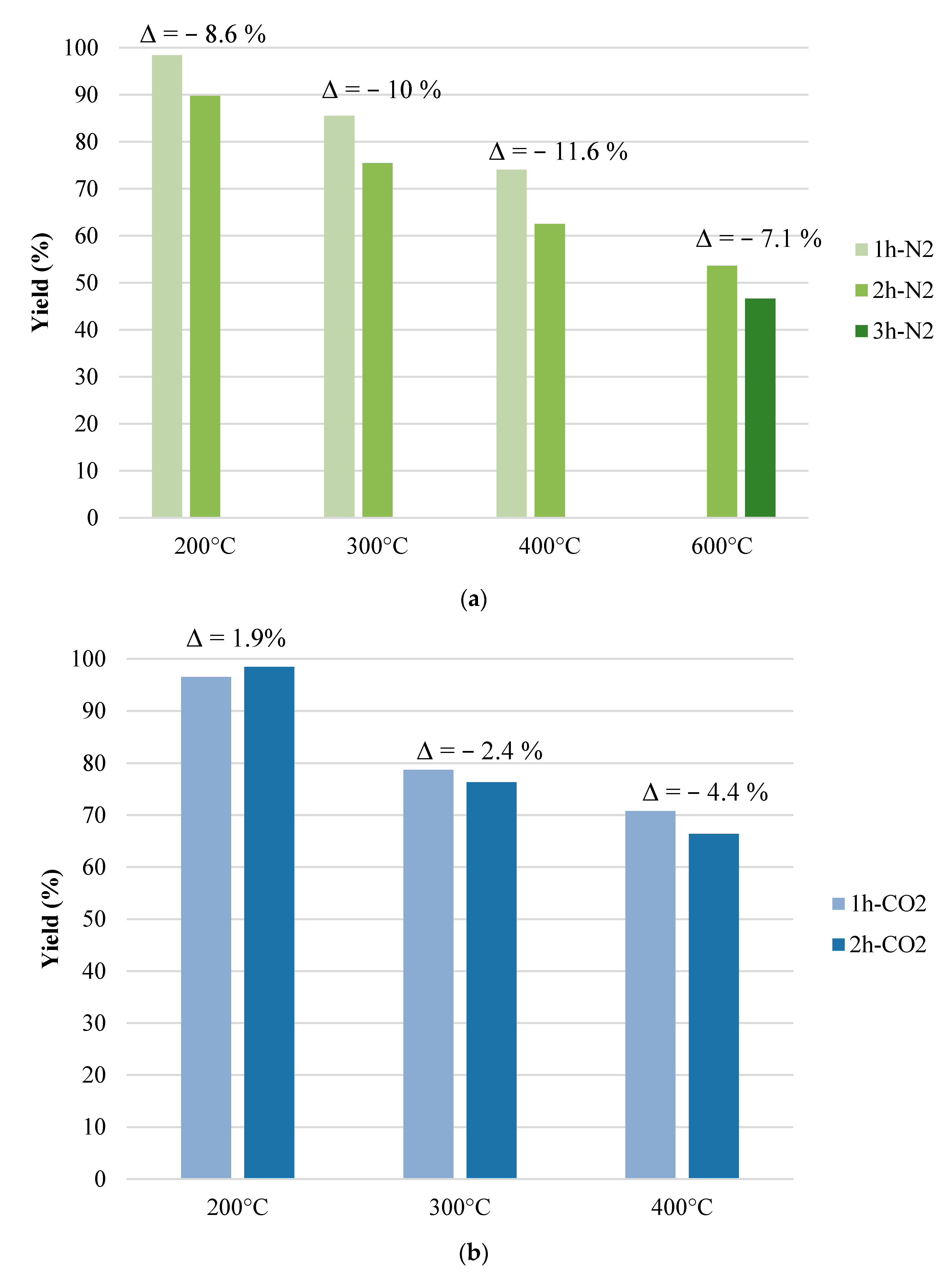
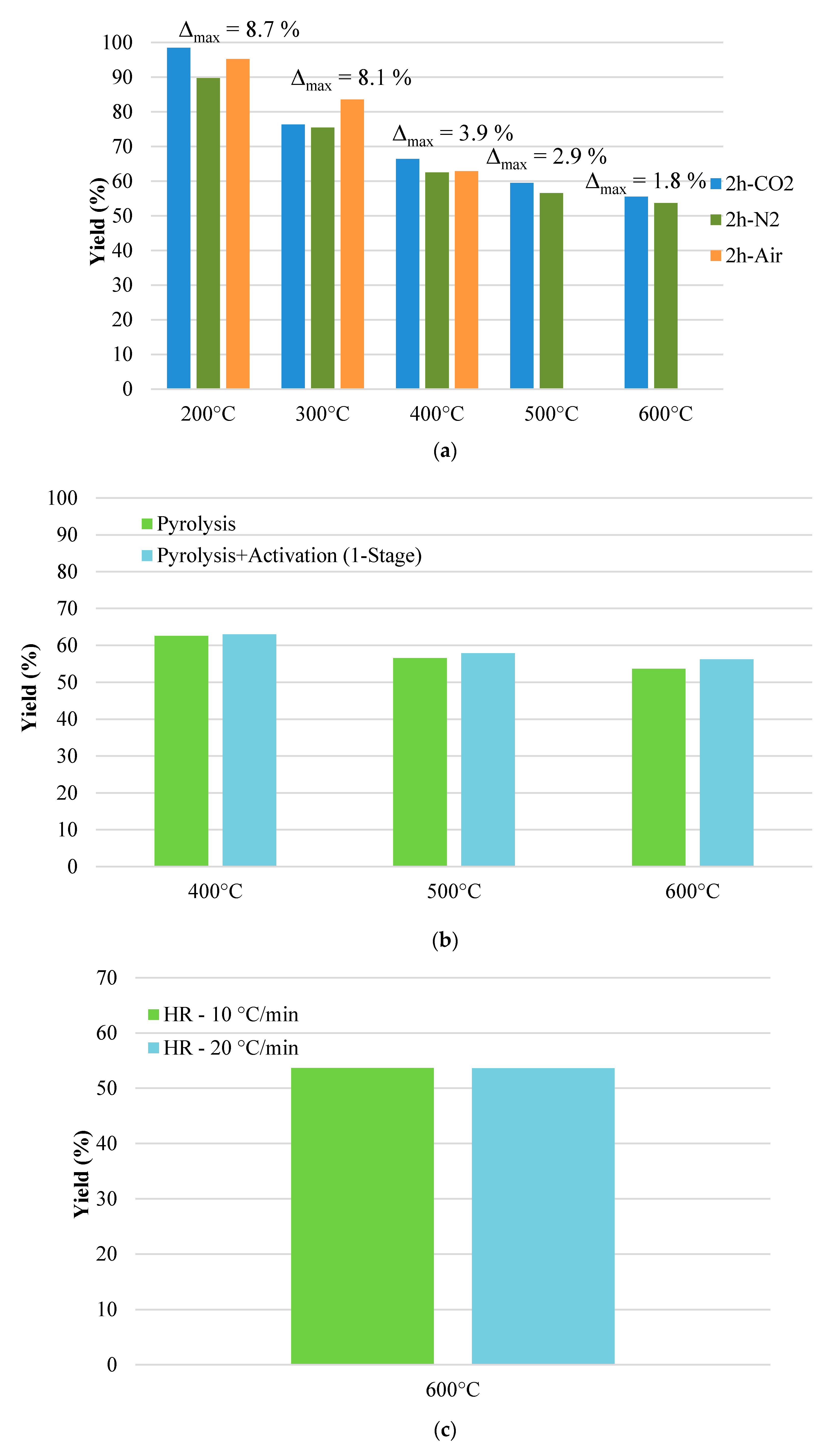
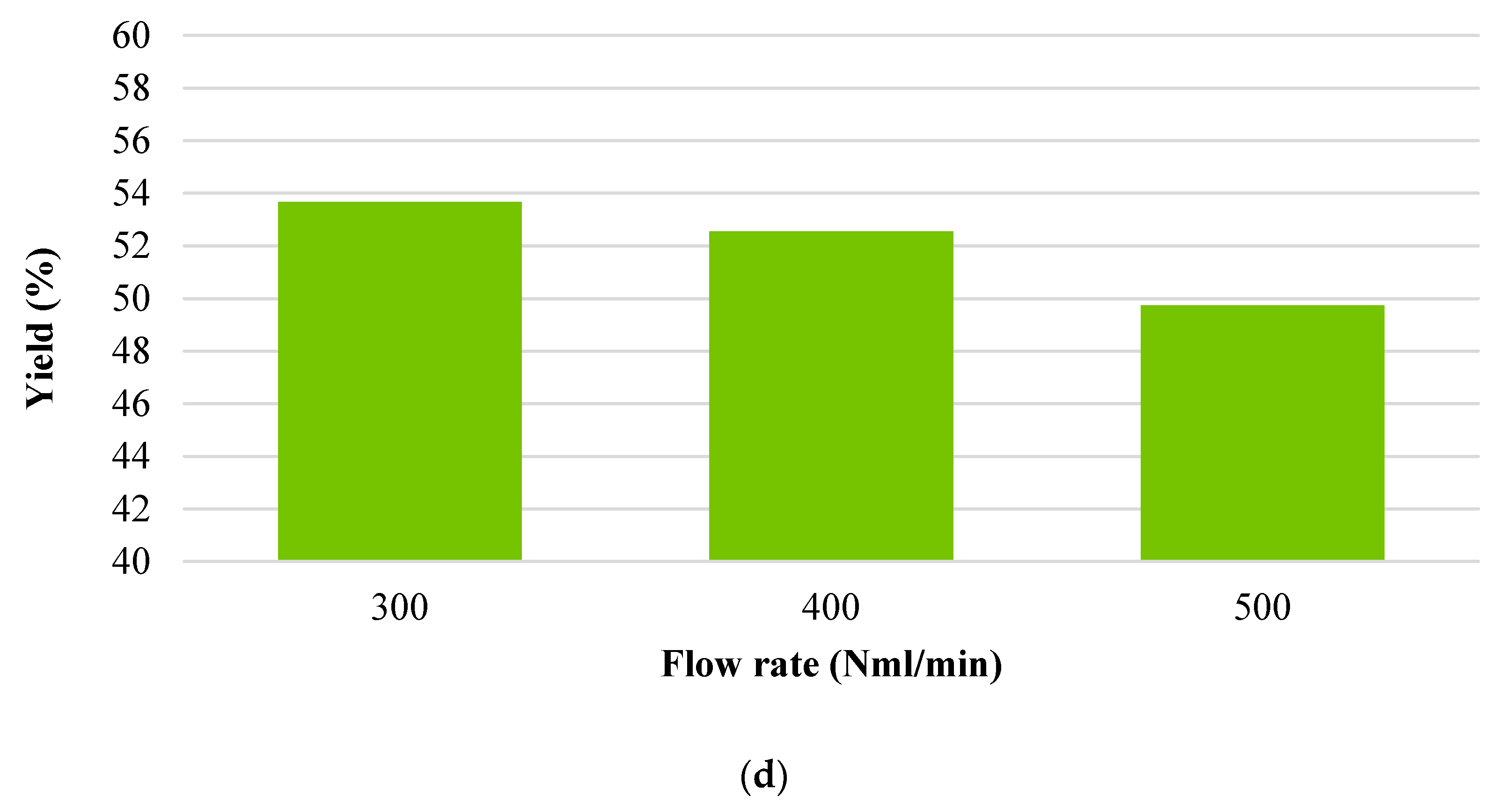
3.1. Char Yield
- -
- Temperature (200–600 °C);
- -
- Residence time (1–2 h);
- -
- Flow rate (300 Nml·min−1);
- -
- Heating rate (10 °C·min−1);
- -
- Flowing agent (N2, CO2, air).
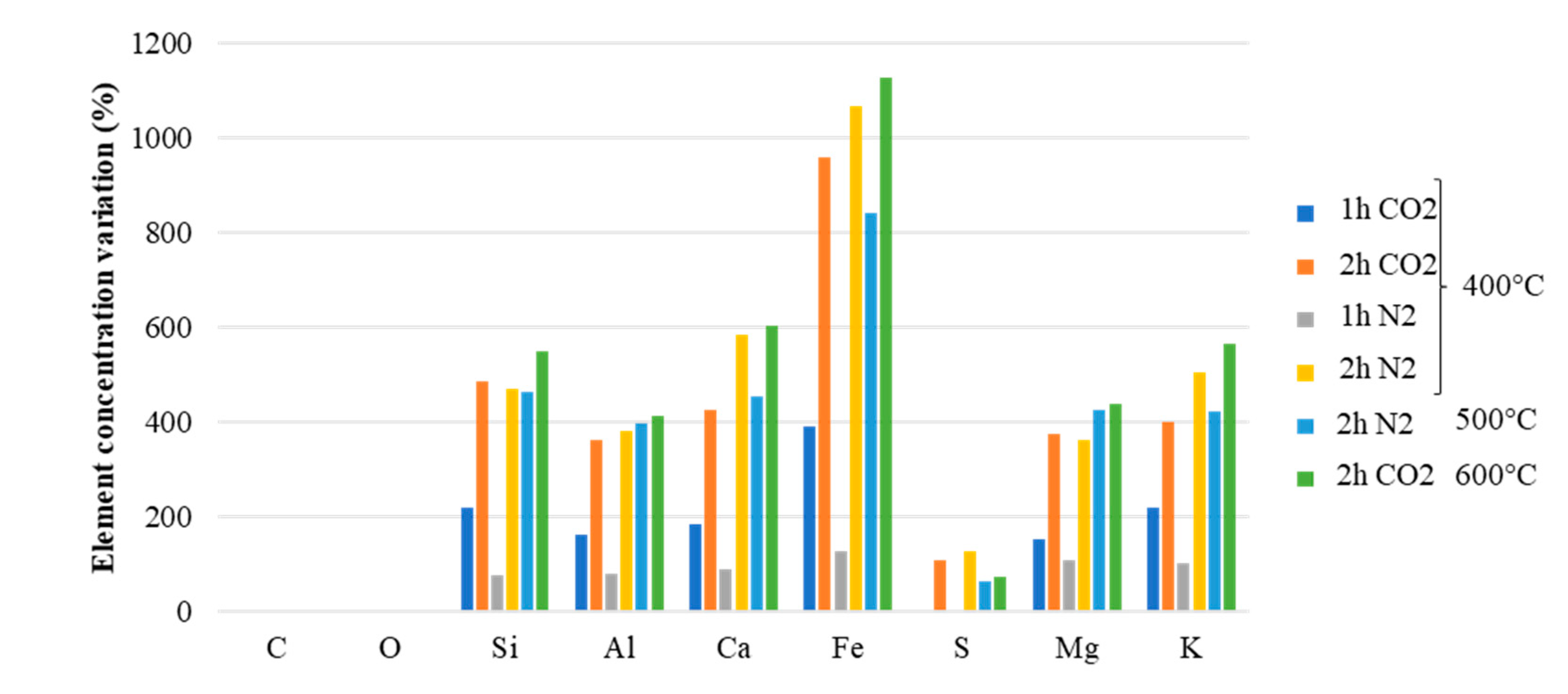
3.2. Microscopic Characteristics
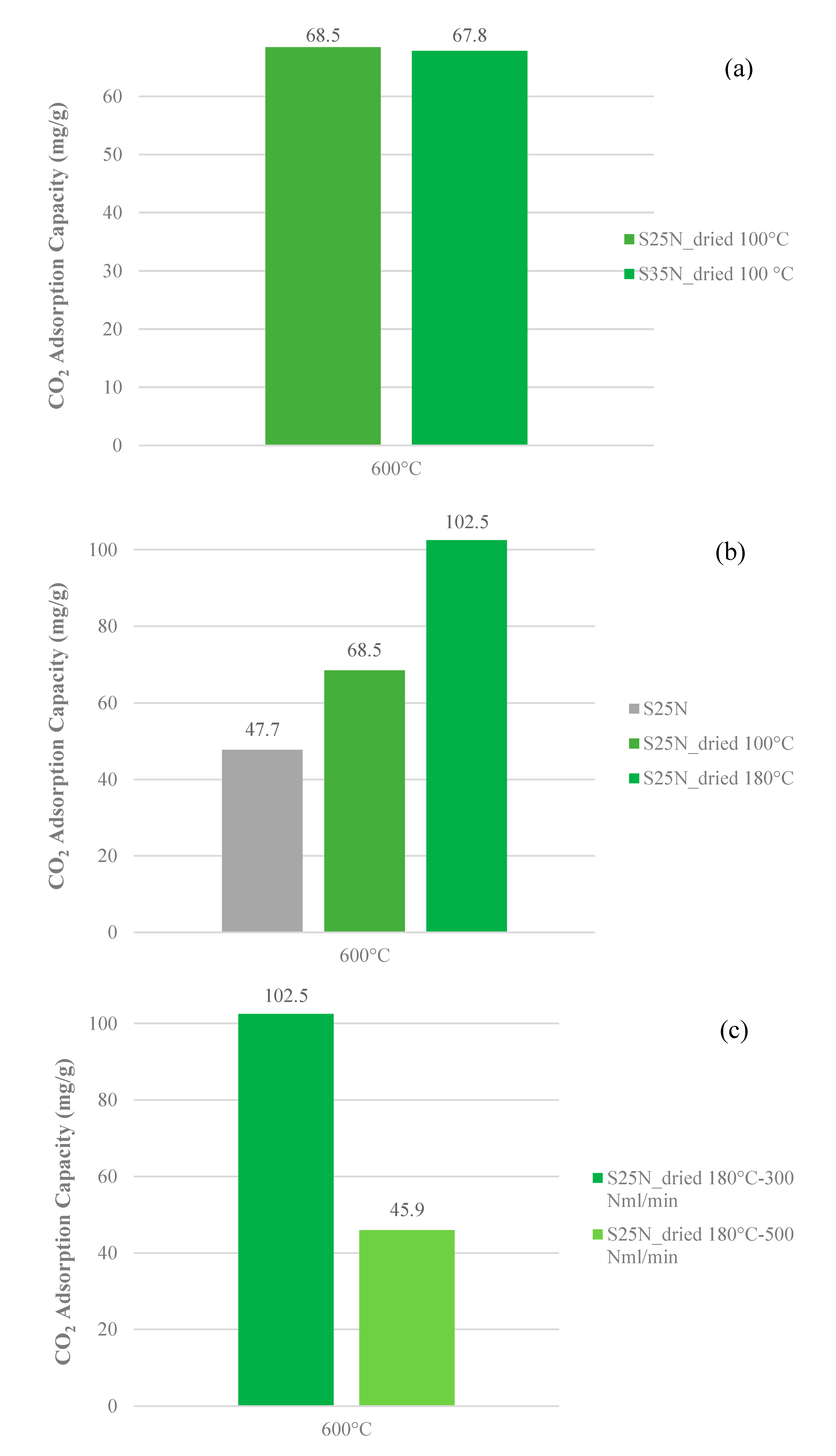
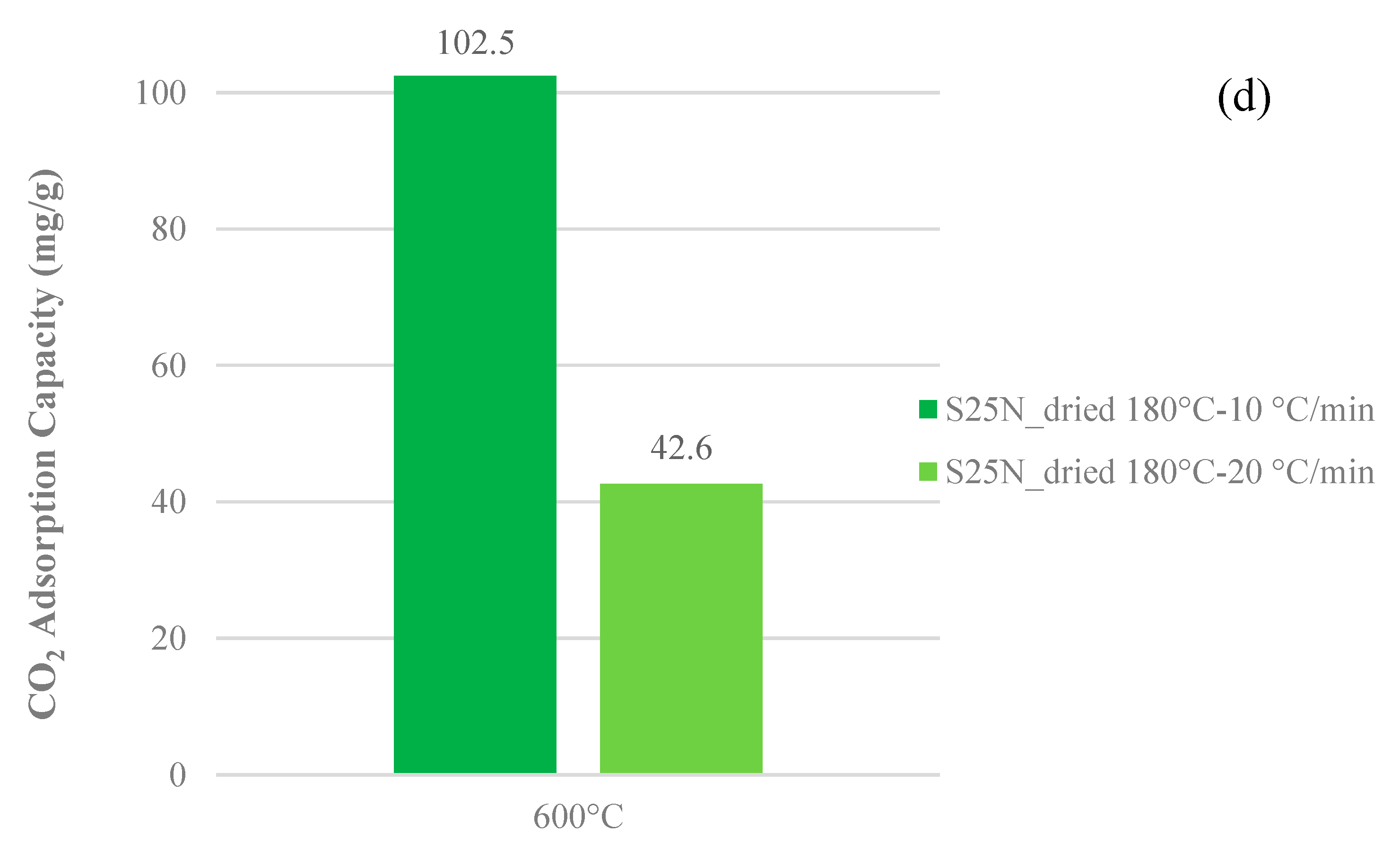
3.3. CO2 Adsorption Capacity
- -
- Biogas flow rate: 153.8 Nml·min−1;
- -
- GHSV (gas hourly space velocity): 131·h−1;
- -
- Cb: CO2 breakthrough concentration limit: 2.5%.
- -
- is the CO2 adsorbed in mg per g of sorbent;
- -
- is the breakthrough time;
- -
- is the simulated biogas flow rate;
- -
- (ppmv) is the total CO2 concentration in the mixture;
- -
- ( is the CO2 molar weight;
- -
- is the molar volume of an ideal gas;
- -
- is the mass of the sorbent;
- -
- is the unit conversion from ppmv to molar concentration.
- -
- It gives a higher amount of the solid fraction produced during the physical activation due to the greater yield value (53.7 vs. 46.6%);
- -
- It results in lower energy costs during the thermal treatment for char production.
- -
- Temperature: 600 °C;
- -
- Dwell time: 2 h;
- -
- Drying temperature: 170–180 °C;
- -
- Physical activation flow rate: 300 Nml·min−1;
- -
- Heating rate: 10 °C·min−1.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| BET C | Brunauer–Emmett–Teller analytical method carbon content (%) |
| Cb | CO2 breakthrough concentration limit |
| CHP | Combined Heat and Power |
| CO2cap | CO2 adsorption capacity (mgCO2·gsorb−1) |
| CO2conc | CO2 concentration (ppmv) |
| Dry_x | biochar samples which received further post-drying treatment at the “x” temperature |
| EDS | Energy Dispersive X-ray Spectrometry |
| GHSV | Gas Hourly Space Velocity (h−1) |
| H IUPAC | hydrogen content (%) International Union of Pure and Applied Chemistry |
| mchar | mass of char (g) |
| mraw | mass of feedstock (g) |
| msorb | mass of sorbent (g) |
| N | nitrogen content (%) |
| N2/CO2/Air/N2-CO2 | Respectively, nitrogen used only, carbon dioxide used only, air used only, nitrogen for the transitory phase and carbon dioxide for the stationary phase (one-stage method) |
| P | phosphorous content (%) |
| PID | proportional–integral–derivative controller |
| Q | simulated biogas flow rate (l min−1) |
| S | sulfur content (%) |
| SBET | active surface evaluated with BET method (m2/g) |
| SOFC | Solid Oxide Fuel Cell |
| SS | sewage sludge |
| Stot.pores | total surface of pores (m2/g) |
| St-plot ext | active surface evaluated with t-plot method (m2/g) |
| tb | breakthrough time (s) |
| Vpores (d < 1.308 nm) | microporous pores (cm3/g) |
| Vpores (d < 44.9 nm) | mesoporous pores (cm3/g) |
| vwc | variable water content |
| WWTP | WasteWater Treatment Plant |
| ychar | Char yield (%) |
References
- Hadi, P.; Xu, M.; Ning, C.; Lin, C.S.K.; McKay, G. A critical review on preparation, characterization and utilization of sludge-derived activated carbons for wastewater treatment. Chem. Eng. J. 2015, 260, 895–906. [Google Scholar] [CrossRef]
- Papurello, D.; Boschetti, A.; Silvestri, S.; Khomenko, I.; Biasioli, F. Real-time monitoring of removal of trace compounds with PTR-MS: Biochar experimental investigation. Renew. Energy 2018, 125, 344–355. [Google Scholar] [CrossRef]
- Santarelli, M.; Briesemeister, L.; Gandiglio, M.; Herrmann, S.; Kuczynski, P.; Kupecki, J.; Lanzini, A.; Llovell, F.; Papurello, D.; Spliethoff, H.; et al. Carbon recovery and re-utilization (CRR) from the exhaust of a solid oxide fuel cell (SOFC): Analysis through a proof-of-concept. J. CO2 Util. 2017, 18, 206–221. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Sarmah, A.K.; Dubey, B. Downstream augmentation of hydrothermal carbonization with anaerobic digestion for integrated biogas and hydrochar production from the organic fraction of municipal solid waste: A circular economy concept. Sci. Total Environ. 2020, 706, 135907. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.V.; Salvador, R.; De Francisco, A.C.; Piekarski, C.M. Mapping of research lines on circular economy practices in agriculture: From waste to energy. Renew. Sustain. Energy Rev. 2020, 131, 109958. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Sengupta, S.; Gupta, A.; Kumar, S.S.; Vijay, V.; Kumar, V.; Vijay, V.K.; Pant, D. Valorization of agricultural waste for biogas based circular economy in India: A research outlook. Bioresour. Technol. 2020, 304, 123036. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Samolada, M.; Zabaniotou, A. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014, 34, 411–420. [Google Scholar] [CrossRef]
- Prabhakar, A.K.; Mohan, B.C.; Tay, T.S.; Lee, S.S.-C.; Teo, S.L.-M.; Wang, C.-H. Incinerated Sewage Sludge Bottom Ash- Chemical processing, Leaching patterns and Toxicity testing. J. Hazard. Mater. 2020, 402, 123350. [Google Scholar] [CrossRef]
- Gent, S.; Twedt, M.; Gerometta, C.; Almberg, E. Theoretical and Applied Aspects of Biomass Torrefaction; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Bolognesi, S.; Bernardi, G.; Callegari, A.; Dondi, D.; Capodaglio, A.G. Biochar production from sewage sludge and microalgae mixtures: Properties, sustainability and possible role in circular economy. Biomass-Con. Bioref. 2019, 1–11. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Nam, H.; Sajib, S.K. Effect of bio-char on methane generation from glucose and aqueous phase of algae liquefaction using mixed anaerobic cultures. Biomass Bioenergy 2018, 108, 479–486. [Google Scholar] [CrossRef]
- Biochar, a potential hydroponic growth substrate, enhances the nutritional status and growth of leafy vegetables -ScienceDirect, (n.d.). Available online: https://www.sciencedirect.com/science/article/pii/S0959652617307886?casa_token=WaP93qJSXUUAAAAA:t4Knu-YZwwoBIuWmoeB2AwS16y3TRDjy-bVxgNqnVZ3Mdr_95Ug0jEzPrkrshQIvOX9tZucoYA (accessed on 18 October 2020).
- Kassim, M.A.; Meng, T.K. Carbon dioxide (CO2) biofixation by microalgae and its potential for biorefinery and biofuel production. Sci. Total Environ. 2017, 584–585, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. CO2 adsorption on biochar from co-torrefaction of sewage sludge and leucaena wood using microwave heating. Energy Procedia 2019, 158, 4435–4440. [Google Scholar] [CrossRef]
- Florent, M.; Policicchio, A.; Niewiadomski, S.; Bandosz, T.J. Exploring the options for the improvement of H2S adsorption on sludge derived adsorbents: Building the composite with porous carbons. J. Clean. Prod. 2020, 249, 119412. [Google Scholar] [CrossRef]
- Xu, G.; Yang, X.; Spinosa, L. Development of sludge-based adsorbents: Preparation, characterization, utilization and its feasibility assessment. J. Environ. Manag. 2015, 151, 221–232. [Google Scholar] [CrossRef]
- Tang, S.; Tian, S.; Zheng, C.; Zhang, Z. Effect of Calcium Hydroxide on the Pyrolysis Behavior of Sewage Sludge: Reaction Characteristics and Kinetics. Energy Fuels 2017, 31, 5079–5087. [Google Scholar] [CrossRef]
- Trinh, T.N.; Jensen, P.A.; Dam-Johansen, K.; Knudsen, N.O.; Sørensen, H.R. Influence of the Pyrolysis Temperature on Sewage Sludge Product Distribution, Bio-Oil, and Char Properties. Energy Fuels 2013, 27, 1419–1427. [Google Scholar] [CrossRef]
- Shang, G.; Li, Q.; Liu, L.; Chen, P.; Huang, X. Adsorption of hydrogen sulfide by biochars derived from pyrolysis of different agricultural/forestry wastes. J. Air Waste Manag. Assoc. 2015, 66, 8–16. [Google Scholar] [CrossRef]
- Lashaki, M.J.; Fayaz, M.; Wang, H.; Hashisho, Z.; Philips, J.H.; Anderson, J.E.; Nichols, M. Effect of Adsorption and Regeneration Temperature on Irreversible Adsorption of Organic Vapors on Beaded Activated Carbon. Environ. Sci. Technol. 2012, 46, 4083–4090. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Papurello, D.; Tomasi, L.; Silvestri, S.; Belcari, I.; Santarelli, M.; Smeacetto, F.; Biasioli, F. Biogas trace compound removal with ashes using proton transfer reaction time-of-flight mass spectrometry as innovative detection tool. Fuel Process. Technol. 2016, 145, 62–75. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; Mohedano, A.F.; Rodríguez, J.J. Activated carbons from sewage sludge. Desalination 2011, 277, 377–382. [Google Scholar] [CrossRef]
- Girgis, B.S.; Khalil, L.B.; Tawfik, T.A. Porosity Development in Carbons Derived from Olive Oil Mill Residue Under Steam Pyrolysis. J. Porous Mater. 2002, 9, 105–113. [Google Scholar] [CrossRef]
- Smith, K.; Fowler, G.; Pullket, S.; Graham, N. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications. Water Res. 2009, 43, 2569–2594. [Google Scholar] [CrossRef]
- Ros, A.; Lillo-Ródenas, M.; Fuente, E.; Montes-Morán, M.; Martín, M.; Linares-Solano, A. High surface area materials prepared from sewage sludge-based precursors. Chemosphere 2006, 65, 132–140. [Google Scholar] [CrossRef]
- Papurello, D.; Silvestri, S.; Lanzini, A. Biogas cleaning: Trace compounds removal with model validation. Sep. Purif. Technol. 2019, 210, 80–92. [Google Scholar] [CrossRef]
- Gabelman, A. Adsorption Basics: Part 1. Chem. Eng. Prog. 2017, 113, 48–53. [Google Scholar]
- Papurello, D.; Tomasi, L.; Silvestri, S. Proton transfer reaction mass spectrometry for the gas cleaning using commercial and waste-derived materials: Focus on the siloxane removal for SOFC applications. Int. J. Mass Spectrom. 2018, 430, 69–79. [Google Scholar] [CrossRef]
- Papurello, D.; Tomasi, L.; Silvestri, S.; Santarelli, M. Evaluation of the Wheeler-Jonas parameters for biogas trace compounds removal with activated carbons. Fuel Process. Technol. 2016, 152, 93–101. [Google Scholar] [CrossRef]
- Cheng, F.; Luo, H.; Hu, L.; Yu, B.; Luo, Z.; De Cortalezzi, M.F. Sludge carbonization and activation: From hazardous waste to functional materials for water treatment. J. Environ. Chem. Eng. 2016, 4, 4574–4586. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Cao, Y.; Pawłowski, A. Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: Brief overview and energy efficiency assessment. Renew. Sustain. Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- Linghu, W.; Shen, R. Thermal behaviour of sewage sludge in pyrolysis process. Mater. Res. Innov. 2014, 18, S4–S50. [Google Scholar] [CrossRef]
- Ortiz, F.G.; Aguilera, P.; Ollero, P. Biogas desulfurization by adsorption on thermally treated sewage-sludge. Sep. Purif. Technol. 2014, 123, 200–213. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Magdziarz, A.; Werle, S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag. 2014, 34, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- De Andrés, J.M.; Orjales, L.; Narros, A.; Fuente, M.D.M.D.L.; Rodríguez, M.E. Carbon dioxide adsorption in chemically activated carbon from sewage sludge. J. Air Waste Manag. Assoc. 2013, 63, 557–564. [Google Scholar] [CrossRef]
- Papurello, D.; Gandiglio, M.; Kafashan, J.; Lanzini, A. Biogas Purification: A Comparison of Adsorption Performance in D4 Siloxane Removal Between Commercial Activated Carbons and Waste Wood-Derived Char Using Isotherm Equations. Processes 2019, 7, 774. [Google Scholar] [CrossRef]
- Cabrera-Codony, A.; Santos-Clotas, E.; Ania, C.O.; Martín, M.J. Competitive siloxane adsorption in multicomponent gas streams for biogas upgrading. Chem. Eng. J. 2018, 344, 565–573. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Xia, H.; Zhang, L.; Srinivasakannan, C.; Guo, S. Textural characteristics of activated carbon by single step CO2 activation from coconut shells. J. Taiwan Inst. Chem. Eng. 2010, 41, 367–372. [Google Scholar] [CrossRef]
- Tay, J.; Chen, X.; Jeyaseelan, S.; Graham, N. Optimising the preparation of activated carbon from digested sewage sludge and coconut husk. Chemosphere 2001, 44, 45–51. [Google Scholar] [CrossRef]
- Juárez, M.F.-D.; Mostbauer, P.; Knapp, A.; Müller, W.; Tertsch, S.; Bockreis, A.; Insam, H. Biogas purification with biomass ash. Waste Manag. 2018, 71, 224–232. [Google Scholar] [CrossRef]
- Lombardi, L.; Costa, G.; Spagnuolo, R. Accelerated carbonation of wood combustion ash for CO2 removal from gaseous streams and storage in solid form. Environ. Sci. Pollut. Res. 2018, 25, 35855–35865. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Hamedeyazdan, Y.J.A.S. Floating Drug Delivery Systems for Eradication of Helicobacter pylori in Treatment of Peptic Ulcer Disease. Trends in Helicobacter pylori Infection 2014, i, 13. [Google Scholar] [CrossRef]
- González, A.; Plaza, M.; Rubiera, F.; Pevida, C. Sustainable biomass-based carbon adsorbents for post-combustion CO2 capture. Chem. Eng. J. 2013, 230, 456–465. [Google Scholar] [CrossRef]
- Hornung, A.; Neumann, J.; Daschner, R. Sustainable Utilization Of Municipal, (n.d.); IWWG: Padua, Italy, 2018; pp. 21–23. [Google Scholar]
- Papurello, D.; Bressan, M.; Bona, D.; Flaim, G.; Cerasino, L.; Silvestri, S. Simulated Sofc Exhausts and Their Fixation on Chlorella Vulgaris: Study on Affecting Parameters. Detritus 2019, 5, 99–104. [Google Scholar] [CrossRef]
- Bona, D.; Papurello, D.; Flaim, G.; Cerasino, L.; Biasioli, F.; Silvestri, S. Management of Digestate and Exhausts from Solid Oxide Fuel Cells Produced in the Dry Anaerobic Digestion Pilot Plant: Microalgae Cultivation Approach. Waste Biomass-Valorization 2020, 1–16. [Google Scholar] [CrossRef]

| C (%) | 35 ± 0.8 | As (mg/kg) | 5.6 ± 0.28 |
| H (%) | 4.8 ± 0.3 | Cd (mg/kg) | 1.55 ± 0.08 |
| N (%) | 4.7 ± 0.1 | Cr (mg/kg) | 224 ± 5.8 |
| S (%) | <2 | Hg (mg/kg) | 0.89 ± 0.03 |
| P (%) | 2.9 ± 0.08 | Ni (mg/kg) | 147 ± 1.28 |
| SBET (m2/g) | 0.33 ± 0.016 | Pb (mg/kg) | 77 ± 3.4 |
| St-plot ext (m2/g) | 0.377 ± 0.015 | K (mg/kg) | 1801 ± 41 |
| Vpores (d < 1.308 nm) (cm3/g) | 0.00002 ± 1·10−6 | Cu (mg/kg) | 388 ± 4.2 |
| Vpores (d < 44.9 nm) (cm3/g) | 0.00121 ± 6·10−5 | Se (mg/kg) | 3.15 ± 0.34 |
| Stot.pores (m2/g) | 0.111 ± 4.3·10−3 | Zn (mg/kg) | 1109 ± 13 |
| Sample Label | SBET (m2/g) | St-Plot ext (m2/g) | Vpores (d < 1.308 nm) (cm3/g) | Vpores (d < 44.9 nm) (cm3/g) | Stot.pores (m2/g) |
|---|---|---|---|---|---|
| S0 | 0.32 | 0.38 | 0.00002 | 0.0012 | 0.11 |
| S1 | 2.65 | 3.27 | 0.00038 | 0.0139 | 2.06 |
| S2 | 3.29 | 3.82 | 0.00024 | 0.0191 | 3.48 |
| S23N | 2.32 | 2.75 | 0.00015 | 0.0146 | 2.4 |
| S24N | 4.32 | 4.7 | 0.00074 | 0.0226 | 3.41 |
| Sample Label | Adsorption Capacity vwc (mgCO2·g−1) | Adsorption Capacity Dry (mgCO2·g−1) |
|---|---|---|
| S0 | 4.0 | 9.9 |
| S11 | 2.4 | 4.6 |
| S12 | 3.7 | 11.1 |
| S1 | 4.4 | 20.1 |
| S21 | 4.9 | 6.3 |
| S22 | 7.7 | 9.4 |
| S2 | 16.1 | 26.2 |
| S23 | 19.6 | 49.1 |
| S24 | 35.0 | 35.5 |
| S11N | 5.2 | 6.4 |
| S12N | 9.9 | 11.9 |
| S13N | 10.6 | 17.2 |
| S21N | 5.1 | 8.9 |
| S22N | 10.9 | 13.4 |
| S23N | 27.7 | 43.5 |
| S24N | 36.5 | 62.3 |
| S25N | 47.7 | 68.5 |
| SA1 | - | 5.7 |
| SA2 | - | 13.0 |
| SA3 | - | 15.5 |
| SB1 | - | 18.4 |
| SB2 | - | 45.7 |
| SB3 | - | 42.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinnirello, M.; Papurello, D.; Santarelli, M.; Fiorilli, S. Thermal Activation of Digested Sewage Sludges for Carbon Dioxide Removal from Biogas. Fuels 2020, 1, 30-46. https://doi.org/10.3390/fuels1010004
Tinnirello M, Papurello D, Santarelli M, Fiorilli S. Thermal Activation of Digested Sewage Sludges for Carbon Dioxide Removal from Biogas. Fuels. 2020; 1(1):30-46. https://doi.org/10.3390/fuels1010004
Chicago/Turabian StyleTinnirello, Mirko, Davide Papurello, Massimo Santarelli, and Sonia Fiorilli. 2020. "Thermal Activation of Digested Sewage Sludges for Carbon Dioxide Removal from Biogas" Fuels 1, no. 1: 30-46. https://doi.org/10.3390/fuels1010004
APA StyleTinnirello, M., Papurello, D., Santarelli, M., & Fiorilli, S. (2020). Thermal Activation of Digested Sewage Sludges for Carbon Dioxide Removal from Biogas. Fuels, 1(1), 30-46. https://doi.org/10.3390/fuels1010004