The Prevalence of Various Autoimmune Comorbidities in Patients with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Methods
2.1. Data Source
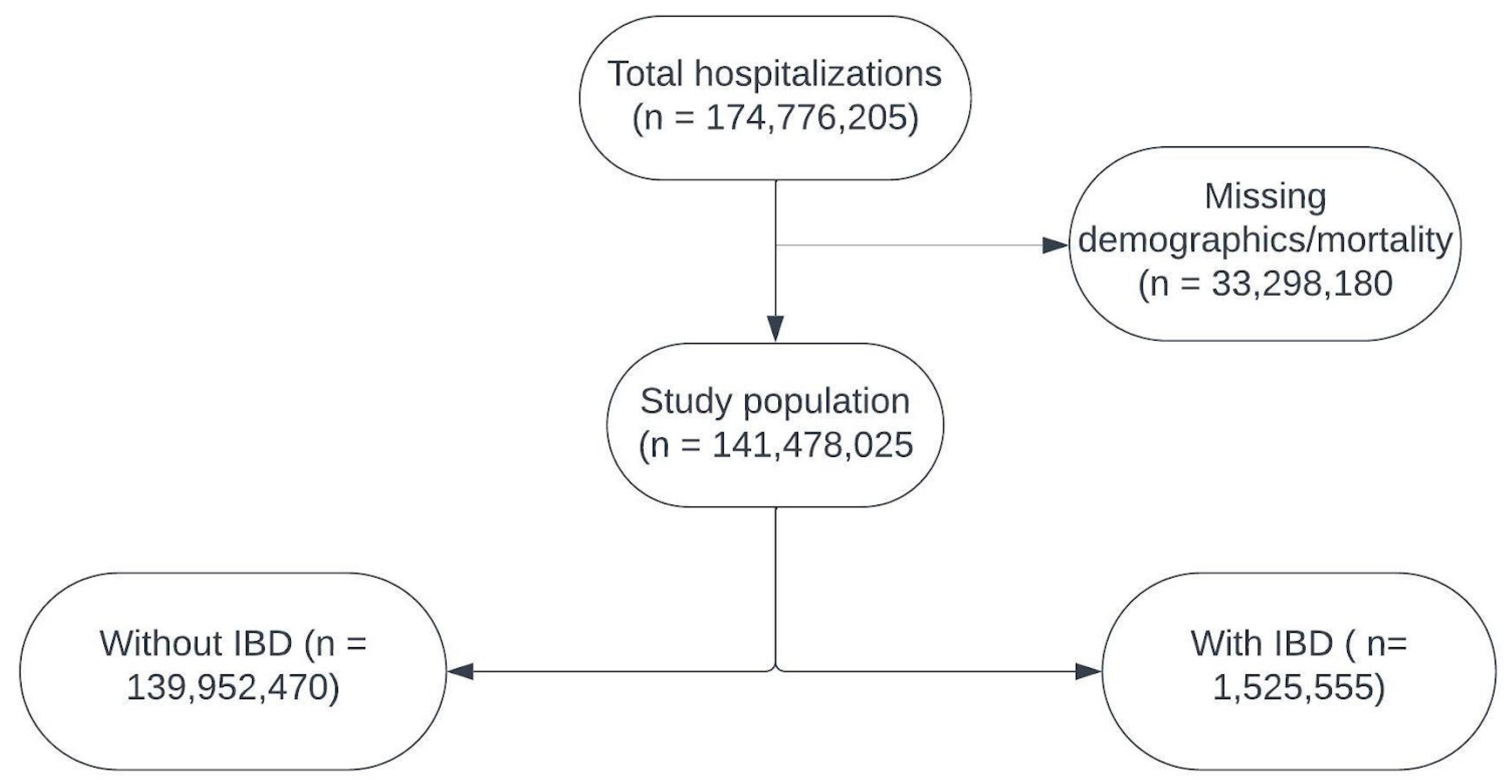
2.2. Study Population
2.3. Study Variables
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Autoimmune Comorbidities and IBD
3.2.1. Autoimmune Cutaneous Disorders
3.2.2. Autoimmune Muscular Disorders
3.2.3. Autoimmune Hematological Disorders
3.2.4. Autoimmune Gastrointestinal and Hepatic Disorders
3.2.5. Autoimmune Neurologic Disorders
3.2.6. Autoimmune Endocrine Disorders
3.2.7. Autoimmune Connective Tissue Disorders
3.2.8. Autoimmune Vasculitis
3.3. Factors Associated with In-Hospital Mortality Among Patients with IBD
4. Discussion
4.1. Autoimmune Cutaneous Disorders
4.2. Autoimmune Muscular Disorders
4.3. Autoimmune Hematological Disorders
4.4. Autoimmune Gastrointestinal and Hepatic Disorders
4.5. Autoimmune Neurologic Disorders
4.6. Autoimmune Endocrine Disorders
4.7. Autoimmune Connective Tissue Disorders
4.8. Autoimmune Vasculitis
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical Aspects and Pathophysiology of Inflammatory Bowel Disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Kaplan, G.G. Environmental risk factors for inflammatory bowel disease. Gastroenterol. Hepatol. 2010, 6, 339–346. [Google Scholar]
- Tlaskalová-Hogenová, H.; Stěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrncir, T.; Kverka, M.; Zakostelska, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef]
- Freuer, D.; Linseisen, J.; Meisinger, C. Association Between Inflammatory Bowel Disease and Both Psoriasis and Psoriatic Arthritis: A Bidirectional 2-Sample Mendelian Randomization Study. JAMA Dermatol. 2022, 158, 1262–1268. [Google Scholar] [CrossRef]
- Chua, K.H.; Lian, L.H.; Khor, W.C.; Lee, W.S.; Hilmi, I.; Goh, K.L.; Kee, B.P. Association between genetic polymorphisms in interferon regulatory factor 5 (IRF5) gene and Malaysian patients with Crohn’s disease. J. Dig. Dis. 2015, 16, 205–216. [Google Scholar] [CrossRef]
- Li, P.; Lv, H.; Yang, H.; Qian, J.M. IRF5, but not TLR4, DEFB1, or VDR, is associated with the risk of ulcerative colitis in a Han Chinese population. Scand. J. Gastroenterol. 2013, 48, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, K.; Allanore, Y.; Guedj, M.; Pierlot, C.; Bombardieri, S.; Balsa, A.; Westhovens, R.; Barrera, P.; Alves, H.; Teixeira, V.H.; et al. The interferon regulatory factor 5 gene confers susceptibility to rheumatoid arthritis and influences its erosive phenotype. Ann. Rheum. Dis. 2011, 70, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, N.; Burton, J.P.; Suppiah, P.; Reid, G.; Stebbings, S. The role of the microbiome in rheumatic diseases. Curr. Rheumatol. Rep. 2013, 15, 314. [Google Scholar] [CrossRef]
- Cohen, R.; Robinson, D.; Paramore, C.; Fraeman, K.; Renahan, K.; Bala, M. Autoimmune disease concomitance among inflammatory bowel disease patients in the United States, 2001–2002. Inflamm Bowel Dis. 2008, 14, 738–743. [Google Scholar] [CrossRef]
- Chen, Y.J.; Juan, C.K.; Chang, Y.T.; Wu, C.Y.; Ho, H.J.; Tseng, H.C. Association between inflammatory bowel disease and bullous pemphigoid: A population-based case-control study. Sci. Rep. 2020, 10, 12727. [Google Scholar] [CrossRef]
- Zeng, R.; Wang, J.; Jiang, R.; Yang, J.; Zheng, C.; Wu, H.; Zhuo, Z.; Yang, Q.; Li, J.; Leung, F.W.; et al. Investigating Causality and Shared Genetic Architecture between Neurodegenerative Disorders and Inflammatory Bowel Disease. Aging Dis. 2023, 14, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Wajda, A.; Blanchard, J.F. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: A population-based study. Gastroenterology 2005, 129, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Li, X.; Sundquist, K.; Sundquist, J. Familial association of inflammatory bowel diseases with other autoimmune and related diseases. Am. J. Gastroenterol. 2010, 105, 139–147. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Galanko, J.A.; Porter, C.Q.; Sandler, R.S. Association of paediatric inflammatory bowel disease with other immune-mediated diseases. Arch. Dis. Child. 2011, 96, 1042–1046. [Google Scholar] [CrossRef]
- Weng, X.; Liu, L.; Barcellos, L.F.; Allison, J.E.; Herrinton, L.J. Clustering of inflammatory bowel disease with immune mediated diseases among members of a Northern California-managed care organization. Am. J. Gastroenterol. 2007, 102, 1429–1435. [Google Scholar] [CrossRef]
- Wilson, J.C.; Furlano, R.I.; Jick, S.S.; Meier, C.R. Inflammatory Bowel Disease and the Risk of Autoimmune Diseases. J. Crohns Colitis. 2016, 10, 186–193. [Google Scholar] [CrossRef]
- NIS Database Documentation [Internet]. Available online: https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp (accessed on 13 June 2025).
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef]
- Bezzio, C.; Della Corte, C.; Vernero, M.; Di Luna, I.; Manes, G.; Saibeni, S. Inflammatory bowel disease and immune-mediated inflammatory diseases: Looking at the less frequent associations. Ther. Adv. Gastroenterol. 2022, 15, 17562848221115312. [Google Scholar] [CrossRef]
- Halling, M.L.; Kjeldsen, J.; Knudsen, T.; Nielsen, J.; Hansen, L.K. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J. Gastroenterol. 2017, 23, 6137–6146. [Google Scholar] [CrossRef]
- Ricart, E.; Panaccione, R.; Loftus, E.V.; Tremaine, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. Autoimmune disorders and extraintestinal manifestations in first-degree familial and sporadic inflammatory bowel disease: A case-control study. Inflamm. Bowel Dis. 2004, 10, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.W.; Barrett, J.C.; Parkes, M.; Satsangi, J. New IBD genetics: Common pathways with other diseases. Gut 2011, 60, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, F.; Tekin, H.G.; Burisch, J.; Wu, J.J.; Thyssen, J.P.; Egeberg, A. Global Prevalence and Bidirectional Association Between Psoriasis and Inflammatory Bowel Disease-A Systematic Review and Meta-analysis. J. Crohns Colitis 2020, 14, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Heelan, K.; Mahar, A.L.; Walsh, S.; Shear, N.H. Pemphigus and associated comorbidities: A cross-sectional study. Clin. Exp. Dermatol. 2015, 40, 593–599. [Google Scholar] [CrossRef]
- Sharif, K.; Ben-Shabat, N.; Mahagna, M.; Shani, U.; Watad, A.; Cohen, A.D.; Amital, H. Inflammatory Bowel Diseases Are Associated with Polymyositis and Dermatomyositis—A Retrospective Cohort Analysis. Medicina 2022, 58, 1727. [Google Scholar] [CrossRef]
- Mayne, C.G.; Williams, C.B. Induced and Natural Regulatory T Cells in the Development of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 1772–1788. [Google Scholar] [CrossRef]
- Uzzan, M.; Galicier, L.; Gornet, J.M.; Oksenhendler, E.; Fieschi, C.; Allez, M.; Bouhnik, Y.; Kirchgesner, J.; Boutboul, D.; Treton, X.; et al. Autoimmune cytopenias associated with inflammatory bowel diseases: Insights from a multicenter retrospective cohort. Dig. Liver Dis. 2017, 49, 397–404. [Google Scholar] [CrossRef]
- Berger, M.; Abdalla, M.; DeCross, A. Hemolytic Anemia in Inflammatory Bowel Disease: Our “Gut” Tells Us to Blame the Drug. Crohns Colitis 360 2021, 3, otab070. [Google Scholar] [CrossRef]
- Waljee, A.K.; Noureldin, M.; Berinstein, J.A.; Cohen-Mekelburg, S.A.; Wallace, B.I.; Cushing, K.C.; Hanauer, D.A.; Keeney-Bonthrone, T.P.; Nallamothu, B.; Higgins, P.D. Mapping the relationships between inflammatory bowel disease and comorbid diagnoses to identify disease associations. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1341. [Google Scholar] [CrossRef]
- Casella, G.; D’Incà, R.; Oliva, L.; Daperno, M.; Saladino, V.; Zoli, G.; Annese, V.; Fries, W.; Cortellezzi, C. Prevalence of celiac disease in inflammatory bowel diseases: An IG-IBD multicentre study. Dig. Liver Dis. 2010, 42, 175–178. [Google Scholar] [CrossRef]
- Broomé, U.; Glaumann, H.; Hellers, G.; Nilsson, B.; Sörstad, J.; Hultcrantz, R. Liver disease in ulcerative colitis: An epidemiological and follow up study in the county of Stockholm. Gut 1994, 35, 84–89. [Google Scholar] [CrossRef]
- Teufel, A.; Weinmann, A.; Kahaly, G.J.; Centner, C.; Piendl, A.; Wörns, M.; Lohse, A.W.; Galle, P.R.; Kanzler, S. Concurrent autoimmune diseases in patients with autoimmune hepatitis. J. Clin. Gastroenterol. 2010, 44, 208–213. [Google Scholar] [CrossRef]
- Yang, Y.; Musco, H.; Simpson-Yap, S.; Zhu, Z.; Wang, Y.; Lin, X.; Zhang, J.; Taylor, B.; Gratten, J.; Zhou, Y. Investigating the shared genetic architecture between multiple sclerosis and inflammatory bowel diseases. Nat. Commun. 2021, 12, 5641. [Google Scholar] [CrossRef]
- Wang, X.; Wan, J.; Wang, M.; Zhang, Y.; Wu, K.; Yang, F. Multiple sclerosis and inflammatory bowel disease: A systematic review and meta-analysis. Ann. Clin. Transl. Neurol. 2022, 9, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Yang, T.M.; Ou, R.W.; Wei, Q.Q.; Shang, H.F. Genome-wide genetic links between amyotrophic lateral sclerosis and autoimmune diseases. BMC Med. 2021, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Gong, J.; Tan, Y.; Liu, D. Epidemiologic Association between Inflammatory Bowel Diseases and Type 1 Diabetes Mellitus: A Meta-Analysis. J. Gastrointest. Liver Dis. 2020, 29, 407–413. [Google Scholar] [CrossRef]
- Brearley, K.D.; Spiers, A.S. Autoimmune disease of the thyroid and colon, with a report of a case of chronic ulcerative colitis in association with Hashimoto’s disease and penicillin allergy. Med. J. Aust. 1962, 49, 789–795. [Google Scholar] [CrossRef]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. Available online: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.1007165/full (accessed on 12 June 2025). [CrossRef]
- de Almeida Martins, C.; Caon, A.E.R.; Facanali, C.B.G.; Sobrado, C.W.; Nahas, S.C.; Pereira, R.M.R.; Margalit-Yehuda, R.; Kopylov, U.; Queiroz, N.S.F. Coexistence of Takayasu’s Arteritis in Patients with Inflammatory Bowel Diseases. Gastroenterol. Res. Pract. 2021, 2021, 8831867. [Google Scholar] [CrossRef]
- Bekele, D.I.; Warrington, K.J.; Koster, M.J. Giant cell arteritis associated with inflammatory bowel disease: A case-series and review of the literature. Rheumatol. Int. 2021, 41, 487–492. [Google Scholar] [CrossRef]
| Absence of IBD n (%) | Presence of IBD n (%) | p-Value | |
|---|---|---|---|
| Age Categories | <0.001 | ||
| 18–44 | 39,180,126 (28.0) | 518,360 (34.0) | |
| 45–65 | 39,511,457 (28.2) | 486,845 (32.0) | |
| >65 | 61,260,887 (44.0) | 520,350 (34.0) | |
| Gender | <0.001 | ||
| Male | 59,448,781 (42.0) | 665,530 (44.0) | |
| Female | 80,503,690 (58.0) | 860,025 (56.0) | |
| Race | <0.001 | ||
| White | 93,732,609 (66.8) | 1,202,635 (78.8) | |
| African American | 21,460,831 (15.3) | 171,125 (11.2) | |
| Hispanic | 15,778,430 (11.3) | 93,165 (6.1) | |
| Asian/Pacific islander | 3,930,613 (3.0) | 18,390 (1.2) | |
| Native American | 873,360 (0.6) | 5,675 (0.4) | |
| Other | 4,176,628 (3.0) | 34,415 (2.3) | |
| Insurance | <0.001 | ||
| Medicare | 66,862,465 (49.3) | 655,570 (44.2) | |
| Medicaid | 25,763,816 (18.9) | 224,660 (15.3) | |
| Private | 37,174,100 (27.6) | 546,910 (36.9) | |
| Uninsured | 5,650,175 (4.2) | 53,760 (3.6) | |
| Income | <0.001 | ||
| Lowest quartile | 42,924,734 (30.0) | 375,405 (25.0) | |
| Second quartile | 36,708,956 (26.0) | 390,265 (26.0) | |
| Third quartile | 32,967,139 (24.0) | 392,715 (26.0) | |
| Highest quartile | 27,351,642 (20.0) | 367,170 (24.0) | |
| Elixhauser comorbidities | <0.001 | ||
| 0 | 20,353,951 (14.5) | 183.270 (12) | |
| 1 | 17,719,254 (12.7) | 255,670 (16.8) | |
| 2 | 20,065,959 (14.3) | 269,820 (17.7) | |
| 3 or more | 81,813,307 (58.5) | 816,795 (53.5) |
| Autoimmune Skin Conditions | Absence of IBD n (%) | Presence of IBD n (%) | p-Value | aOR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Bullous pemphigoid | 47,220 (0.03) | 435 (0.03) | 0.14 | ||
| Pemphigus vulgaris | 13,980 (0.01) | 195 (0.01) | 0.14 | ||
| Psoriasis | 767,620 (0.5) | 20,375 (1.3) | <0.001 | 2.35 (2.28–2.43) | <0.001 |
| Autoimmune muscle disease | |||||
| Polymyositis | 64,950 (0.05) | 600 (0.04) | 0.08 | ||
| Myasthenia gravis | 207,225 (0.15) | 2245 (0.1) | 0.90 | ||
| Autoimmune hematological disease | |||||
| Autoimmune hemolytic anemias | 71,930 (0.05) | 990 (0.06) | 0.003 | 1.30 (1.12–1.51) | 0.001 |
| Idiopathic thrombocytopenic purpura | 276,325 (0.2) | 3420 (0.2) | 0.003 | 1.10 (1.02–1.20) | 0.01 |
| Pernicious anemia | 76,735 (0.05) | 1935 (0.1) | <0.001 | 2.47 (2.23–2.73) | <0.001 |
| Autoimmune gastrointestinal and hepatic disorders | |||||
| Celiac disease | 180,245 (0.1) | 7150 (0.5) | <0.001 | 3.08 (2.92–3.26) | <0.001 |
| Primary biliary cirrhosis | 55,755 (0.04) | 2335 (0.15) | <0.001 | 3.82 (3.47–4.21) | <0.001 |
| Autoimmune hepatitis | 100,885 (0.07) | 4595 (0.3) | <0.001 | 4.01 (3.73–4.31) | <0.001 |
| Primary sclerosing cholangitis | 7435 (0.01) | 7345 (4.8) | <0.001 | 80.26 (74.04–87.00) | <0.001 |
| Autoimmune neurologic disorders | |||||
| Amyotrophic lateral sclerosis | 70,360 (0.05) | 600 (0.04) | 0.09 | ||
| Multiple sclerosis | 719,790 (0.5) | 8670 (0.6) | 0.0001 | 0.96 (0.91–1.01) | 0.08 |
| Guillain-Barre syndrome | 90,780 (0.1) | 1180 (0.1) | 0.01 | 1.13 (0.99–1.29) | 0.06 |
| Autoimmune endocrine disorders | |||||
| Addison disease | 123,200 (0.01) | 4040 (0.3) | <0.001 | 2.68 (2.47–2.90) | <0.001 |
| Graves’ Disease | 186,100 (0.1) | 2995 (0.2) | <0.001 | 1.40 (1.28–1.52) | <0.001 |
| Type-1 diabetes mellitus | 1,692,589 (1.2) | 12,590 (1.0) | <0.001 | 0.52 (0.50–0.54) | <0.001 |
| Autoimmune connective tissue disorder | |||||
| Ankylosing spondylitis | 80,205 (0.06) | 8050 (0.5) | <0.001 | 8.88 (8.42–9.38) | <0.001 |
| Bechet’s disease | 13,675 (0.01) | 830 (0.05) | <0.001 | 4.29 (3.64–5.05) | <0.001 |
| Rheumatoid arthritis | 2,658,864 (2.0) | 55,935 (4.0) | <0.001 | 2.07 (2.03–2.12) | <0.001 |
| Sarcoidosis | 384,720 (0.3) | 4605 (0.3) | 0.0067 | 1.12 (1.04–1.19) | 0.002 |
| Sjogren syndrome | 256,890 (0.2) | 4955 (0.3) | <0.001 | 1.64 (1.53–1.76) | <0.001 |
| Systemic lupus erythematosus | 836,735 (0.6) | 15,650 (1.0) | <0.001 | 1.49 (1.43–1.55) | <0.001 |
| Polymyalgia rheumatica | 354,010 (0.3) | 4545 (0.3) | <0.001 | 1.31 (1.22–1.40) | <0.001 |
| Discoid lupus erythematosus | 62,540 (0.04) | 1110 (0.07) | <0.001 | 1.58 (1.38–1.80) | <0.001 |
| Autoimmune vasculitis | |||||
| Takayasu arteritis | 5645 (0.004) | 215 (0.01) | <0.001 | 3.15 (2.28–4.34) | <0.001 |
| Thrombotic microangiopathy | 31,880 (0.02) | 395 (0.03) | 0.2651 | ||
| Polyarteritis nodosa | 16,040 (0.01) | 380 (0.02) | <0.001 | 2.13 (1.69–2.69) | <0.001 |
| Giant cell arteritis | 82,095 (0.06) | 1080 (0.01) | 0.0061 | 1.36 (1.19–1.56) | <0.001 |
| Wegener’s granulomatosis | 54,060 (0.04) | 790 (0.05) | 0.0007 | 1.32 (1.11–1.57) | 0.001 |
| Age Categories | Adjusted Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| 18–44 | |||
| 45–65 | 2.47 | 2.19–2.80 | <0.001 |
| >65 | 5.05 | 4.41–5.78 | <0.001 |
| Gender | |||
| Male | |||
| Female | 0.85 | 0.81–0.90 | <0.001 |
| Insurance | |||
| Medicare | |||
| Medicaid | 1.12 | 0.99–1.27 | 0.07 |
| Private | 1.05 | 0.96–1.15 | 0.30 |
| Uninsured | 1.20 | 0.96–1.51 | 0.12 |
| Elixhauser comorbidities | |||
| 0 | |||
| 1 | 3.17 | 1.90–5.27 | <0.001 |
| 2 | 7.22 | 4.41–11.81 | <0.001 |
| 3 or more | 32.74 | 20.23–53 | <0.001 |
| Autoimmune comorbidities | |||
| Psoriasis vulgaris | 0.53 | 0.38–0.74 | <0.001 |
| Polymyositis | 2.56 | 1.18–5.54 | 0.017 |
| Autoimmune hemolytic anemia | 2.60 | 1.32–5.12 | 0.006 |
| Idiopathic thrombocytopenic purpura | 2.19 | 1.54–3.10 | <0.001 |
| Amyotrophic lateral sclerosis | 1.97 | 0.85–4.55 | 0.111 |
| Hypothyroidism | 0.70 | 0.64–0.76 | <0.001 |
| Systemic sclerosis | 1.69 | 0.98–2.92 | 0.059 |
| Polymyalgia rheumatica | 0.72 | 0.47–1.09 | 0.116 |
| Thrombotic microangiopathy | 4.94 | 2.22–10.97 | <0.001 |
| Giant cell arteritis | 1.37 | 0.71–2.66 | 0.346 |
| Wegners granulomatosis | 1.57 | 0.68–3.66 | 0.293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, B.; Kalra, S.; Khanna, T.; Kohli, I.; Kumar, V.; Sohal, A.; Sejpal, D. The Prevalence of Various Autoimmune Comorbidities in Patients with Inflammatory Bowel Disease. Epidemiologia 2025, 6, 52. https://doi.org/10.3390/epidemiologia6030052
Singh B, Kalra S, Khanna T, Kohli I, Kumar V, Sohal A, Sejpal D. The Prevalence of Various Autoimmune Comorbidities in Patients with Inflammatory Bowel Disease. Epidemiologia. 2025; 6(3):52. https://doi.org/10.3390/epidemiologia6030052
Chicago/Turabian StyleSingh, Bipneet, Shivam Kalra, Tejasvini Khanna, Isha Kohli, Vikash Kumar, Aalam Sohal, and Divyesh Sejpal. 2025. "The Prevalence of Various Autoimmune Comorbidities in Patients with Inflammatory Bowel Disease" Epidemiologia 6, no. 3: 52. https://doi.org/10.3390/epidemiologia6030052
APA StyleSingh, B., Kalra, S., Khanna, T., Kohli, I., Kumar, V., Sohal, A., & Sejpal, D. (2025). The Prevalence of Various Autoimmune Comorbidities in Patients with Inflammatory Bowel Disease. Epidemiologia, 6(3), 52. https://doi.org/10.3390/epidemiologia6030052







