An Outbreak of Pulmonary Tularemia in Slovenia in Summer 2024
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbiological Testing in Humans
2.2. Microbiological Testing in Veterinary Samples
3. Results
3.1. Notified Tularemia Cases
3.2. Tularemia in Animals
4. Discussion
- -
- during mechanical farm work, the doors and windows of tractors should be closed at all times;
- -
- farmers should protect themselves by wearing a surgical mask when handling hay indoors (especially if rodent excreta are present), while cleaning woodsheds and during other farm work where dust is intense;
- -
- insect repellents should be used when engaging in outdoor activities.
- -
- only safe drinking water from controlled water supply systems should be consumed;
- -
- the access of rodents and insects to indoor spaces should be prevented;
- -
- disinsection and deratization should be carried out regularly, especially if rodent excreta are present;
- -
- raw meat and other foods of animal origin should be properly cooked before consumption;
- -
- cross-contamination of foods (either with dirty hands, kitchen utensils, work surfaces or contaminated raw meat), and especially contamination of already cleaned and ready-made foods, should be avoided;
- -
- direct contact with wild animals should be avoided, and touching wild animals—especially if they appear to be ill—is strongly advised against. Gloves should be used while handling dead wild animals. Animal carcasses should be sealed in a polyvinyl bag and properly disposed of, using gloves at all times. In the case of a large number of dead animals or the carcasses of larger wild animals, informing the veterinary hygiene service is warranted.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hestvik, G.; Warns-Petit, E.; Smith, L.A.; Fox, N.J.; Uhlhorn, H.; Artois, M.; Hannant, D.; Hutchings, M.R.; Mattsson, R.; Yon, L.; et al. The status of tularemia in Europe in a one-health context: A review. Epidemiol. Infect. 2015, 143, 2137–2160. [Google Scholar] [CrossRef]
- Molins, C.R.; Delorey, M.J.; Yockey, B.M.; Young, J.W.; Belisle, J.T.; Schriefer, M.E.; Petersen, J.M. Virulence difference between the prototypic Schu S4 strain (A1a) and Francisella tularensis A1a, A1b, A2 and type B strains in a murine model of infection. BMC Infect. Dis. 2014, 14, 67. [Google Scholar] [CrossRef]
- Lopes de Carvalho, I.; Núncio, M.S.; David de Morais, J. Tularémia. Acta Med. Port. 2009, 3, 281–290. [Google Scholar]
- Gyuranecz, M.; Szeredi, L.; Makrai, L. Tularemia of European brown hare (Lepus europaeus): A pathological, histopathological, and immunohistochemical study. Vet. Pathol. 2010, 47, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, A.; Shulze, C.; Kutzer, P.; Probst, C.; Hlinak, A.; Ochs, A.; Grunow, R. Tularaemia seroprevalence of captured and wild animals in Germany: The fox (Vulpes vulpes) as a biological indicator. Epidemiol. Infect. 2013, 141, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Gyuranecz, M. Tularemia. In Infectious Diseases of Wild Mammals and Birds in Europe; Gavier-Widén, D., Duff, J.P., Meredith, A., Eds.; Wiley-Blackwell: London, UK, 2012; pp. 303–309. [Google Scholar]
- Maurin, M.; Pelloux, I.; Brion, J.P.; Del Banõ, J.N.; Picard, A. Human tularemia in France, 2006–2010. Clin. Infect. Dis. 2011, 53, e133–e141. [Google Scholar] [CrossRef]
- Mörner, T.; Addison, E. Tularemia. In Infectious Diseases of Wild Mammals, 3rd ed.; Williams, E.S., Barker, I.K., Eds.; Iowa State University Press: Ames, IA, USA, 2001; pp. 303–312. [Google Scholar]
- Nelson, C.A.; Winberg, J.; Bostic, T.D.; Davis, K.M.; Fleck-Derderian, S. Systematic Review: Clinical Features, Antimicrobial Treatment, and Outcomes of Human Tularemia, 1993–2023. Clin. Infect. Dis. 2024, 78 (Suppl. 1), S15–S28. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.E.; Schneitler, S.; Zange, S.; Linxweiler, M.; Simon, A.; Thurner, L.; Becker, S.L. Clinical haracteristics of and diagnostic approaches to human Francisella tularensis infection: A retrospective, monocentric case study from Germany. Ticks Tick Borne Dis. 2025, 16, 102492. [Google Scholar] [CrossRef]
- Plymoth, M.; Lundqvist, R.; Nystedt, A.; Sjöstedt, A.; Gustafsson, T.N. Targeting Tularemia: Clinical, Laboratory, and Treatment Outcomes From an 11-year Retrospective Observational Cohort in Northern Sweden. Clin. Infect. Dis. 2024, 78, 1222–1231. [Google Scholar] [CrossRef]
- Antonello, R.M.; Giacomelli, A.; Riccardi, N. Tularemia for clinicians: An up-to-date review on epidemiology, diagnosis, prevention and treatment. Eur. J. Intern. Med. 2025, 35, 25–32. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- Versage, J.L.; Severin, D.D.; Chu, M.C.; Petersen, J.M. Development of a multitarget real-time TaqMan PCR assay for enhanced detection of Francisella tularensis in complex specimens. J. Clin. Microbiol. 2003, 41, 5492–5499. [Google Scholar] [CrossRef]
- Fulop, M.; Leslie, D.; Titball, R. A rapid, highly sensitive method for the detection of Francisella tularensis in clinical samples using the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1996, 54, 364–366. [Google Scholar] [CrossRef]
- Rojko, T.; Korva, M.; Lotrič-Furlan, S.; Strle, F.; Avšič-Županc, T. Cluster of ulceroglandular tularemia cases in Slovenia. Ticks Tick Borne Dis. 2016, 7, 1193–1197. [Google Scholar] [CrossRef]
- Glinšek Biškup, U.; Kogoj, R.; Korva, M.; Knap, N.; Cerar, T.; Knapič, T.; Petrovec, M.; Avšič-Županc, T. Characterization of Tularemia Cases in Slovenia with Multiple-Locus Variable-Number Tandem Repeat Analysis. Vector Borne Zoonotic Dis. 2021, 21, 351–357. [Google Scholar] [CrossRef]
- Kravdal, A.; Stubhaug, Ø.O.; Wågø, A.G.; Sætereng, M.S.; Amundsen, D.; Piekuviene, R.; Kristiansen, A. Pulmonary tularaemia: A differential diagnosis to lung cancer. ERJ Open Res. 2020, 6, 00093–02019. [Google Scholar] [CrossRef]
- Kravdal, A.; Stubhaug, Ø.O.; Piekuviene, R.; Sandnes, A. Pulmonary tularaemia. Tidsskr. Nor. Laegeforen. 2021, 141, 11. [Google Scholar]
- Dahlstrand, S.; Ringertz, O.; Zetterberg, B. Airborne tularemia in Sweden. Scand. J. Infect. Dis. 1971, 3, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Castrillón, J.L.; Bachiller-Luque, P.; Martín-Luquero, M.; Mena-Martín, F.J.; Herreros, V. Tularemia epidemic in northwestern Spain: Clinical description and therapeutic response. Clin. Infect. Dis. 2001, 33, 573–576. [Google Scholar] [CrossRef]
- Eliasson, H.; Lindbäck, J.; Nuorti, J.P.; Arneborn, M.; Giesecke, J.; Tegnell, A. The 2000 tularemia outbreak: A case-control study of risk factors in disease-endemic and emergent areas, Sweden. Emerg. Infect. Dis. 2002, 8, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Allue, M.; Sopeña, C.R.; Gallardo, M.T.; Mateos, L.; Vian, E.; Garcia, M.J.; Ramos, J.; Berjon, A.C.; Viña, M.C.; Garcia, M.P.; et al. Tularaemia outbreak in Castilla y Leon, Spain, 2007: An update. Euro Surveill. 2008, 13, 18948. [Google Scholar] [CrossRef]
- Mailles, A.; Vaillant, V. 10 years of surveillance of human tularaemia in France. Euro Surveill. 2014, 19, 20956. [Google Scholar] [CrossRef]
- Faber, M.; Heuner, K.; Jacob, D.; Grunow, R. Tularemia in Germany—A Re-emerging Zoonosis. Front. Cell. Infect. Microbiol. 2018, 8, 40. [Google Scholar] [CrossRef]
- Syrjälä, H.; Kujala, P.; Myllylä, V.; Salminen, A. Airborne transmission of tularemia in farmers. Scand. J. Infect. Dis. 1985, 17, 371–375. [Google Scholar] [CrossRef]
- Feldman, K.A.; Enscore, R.E.; Lathrop, S.L.; Matyas, B.T.; McGuill, M.; Schriefer, M.E.; Stiles-Enos, D.; Dennis, D.T.; Petersen, L.R.; Hayes, E.B. An outbreak of primary pneumonic tularemia on Martha’s Vineyard. N. Engl. J. Med. 2001, 345, 1601–1606. [Google Scholar] [CrossRef]
- Siret, V.; Barataud, D.; Prat, M.; Vaillant, V.; Ansart, S.; Le Coustumier, A.; Vaissaire, J.; Raffi, F.; Garre, M.; Capek, I. An outbreak of airborne tularaemia in France, August 2004. Euro Surveill. 2006, 11, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Farlow, J.; Wagner, D.M.; Dukerich, M.; Stanley, M.; Chu, M.; Kubota, K.; Petersen, J.; Keim, P. Francisella tularensis in the United States. Emerg. Infect. Dis. 2005, 11, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Hauri, A.M.; Hofstetter, I.; Seibold, E.; Kaysser, P.; Eckert, J.; Neubauer, H.; Splettstoesser, W.D. Investigating an airborne tularemia outbreak, Germany. Emerg. Infect. Dis. 2010, 16, 238–243. [Google Scholar] [CrossRef]
- Appelt, S.; Faber, M.; Köppen, K.; Jacob, D.; Grunow, R.; Heuner, K. Francisella tularensis Subspecies holarctica and Tularemia in Germany. Microorganisms 2020, 8, 1448. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Patil, R.D.; Singh, B.; Chakraborty, S.; Chandran, D.; Dhama, K.; Gopinath, D.; Jairath, G.; Rialch, A.; Mal, G.; et al. Tularemia—A re-emerging disease with growing concern. Vet. Q. 2023, 43, 1–16. [Google Scholar] [CrossRef]
- Roth, K.; Chelikam, N.; Rathore, H.; Chittivelu, S. An Uncommon Presentation of Pulmonary Tularemia: A Case Report and Literature Review. Cureus 2022, 14, e30379. [Google Scholar] [CrossRef] [PubMed]
- Dryselius, R.; Hjertqvist, M.; Mäkitalo, S.; Lindblom, A.; Lilja, T.; Eklöf, D.; Lindström, A. Large outbreak of tularaemia, central Sweden, July to September 2019. Euro Surveill. 2019, 24, 1900603. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Gyuranecz, M. Tularaemia: Clinical aspects in Europe. Lancet Infect. Dis. 2016, 16, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Preventing Tularemia. Available online: https://www.cdc.gov/tularemia/prevention/index.html (accessed on 11 April 2025).
- Occupational Safety and Health Administration. Tularemia. Control and Prevention. Available online: https://www.osha.gov/tularemia/control-prevention (accessed on 11 April 2025).
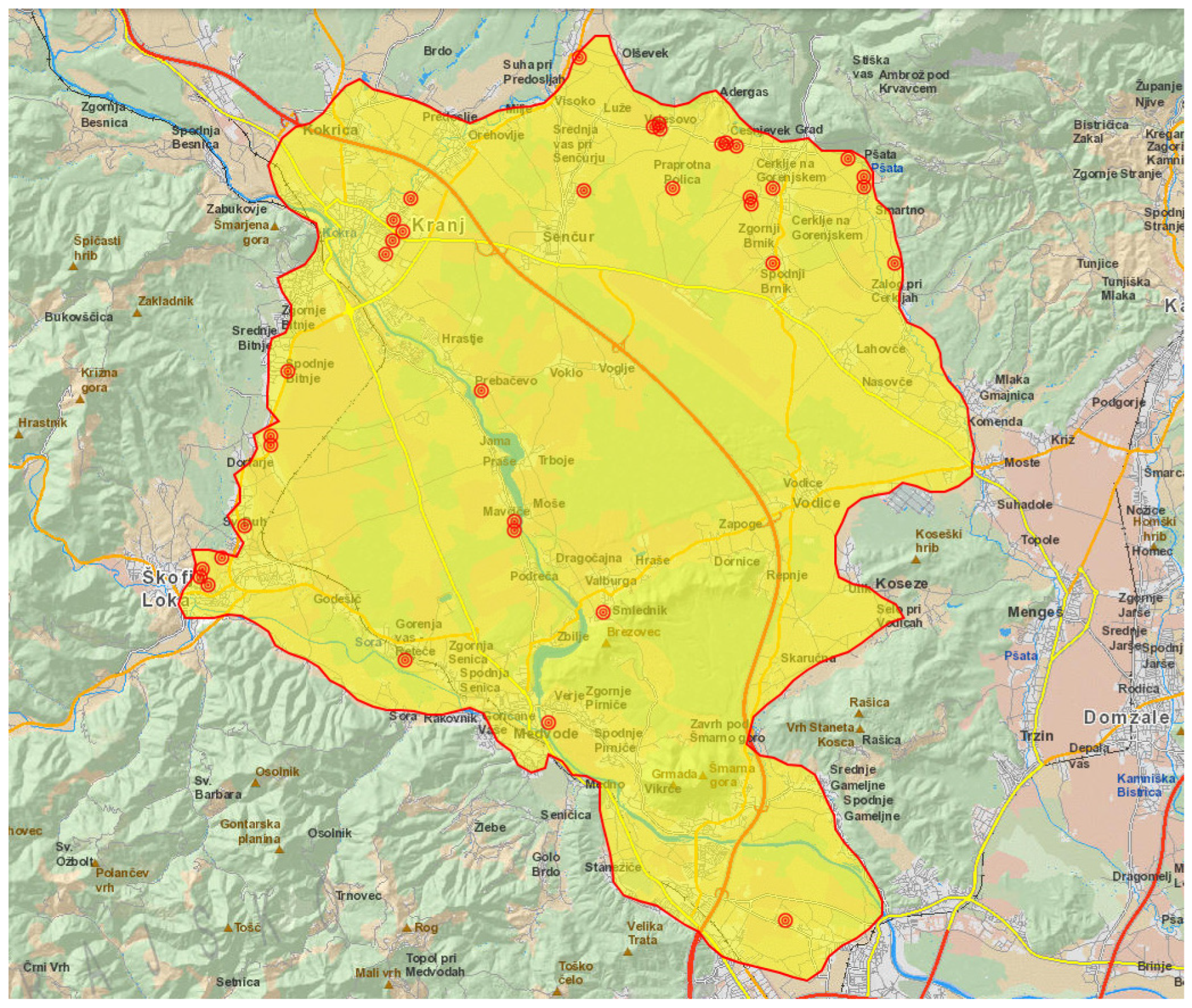
| No. | Age/ Sex | Onset of Illness | Time Elapsed from Symptoms to Testing (in Days) | Exposure | Contact with Animals | Clinical Form | Hospitali-sation | IgM (second) | IgG (second) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 56/M | May | 12 | mowing hay | Yes | febrille illness | Yes | 64 | 256 |
| 2. | 53/M | May | 14 | mowing hay, farming | Yes | febrille illness | No | neg (>256) | 256 (>256) |
| 3. | 57/M | May | 73 | nature walks | No | pulmonary | No | 256 | >1024 |
| 4. | 63/F | May | 39 | contact with domestic animals | Yes | pulmonary | Yes | 256 | >256 |
| 5. | 29/M | June | 14 | mowing hay, farming | Yes | pulmonary | Yes | >256 | >256 |
| 6. | 46/M | June | 20 | not identified | No | pulmonary | No | >1024 | >1024 |
| 7. | 56/M | June | 16 | mowing hay, farming | Yes | pulmonary | No | >256 | >256 |
| 8. | 59/M | June | 12 | mowing hay, farming | Yes | pulmonary | Yes | >256 | >256 |
| 9. | 52/M | June | 33 | not identified | No | pulmonary | No | >1024 | >1024 |
| 10. | 67/M | June | 35 | mowing hay | Yes | pulmonary | No | 1024 | >1024 |
| 11. | 71/M | June | 30 | mowing hay, gardening | No | pulmonary | No | 1024 | >1024 |
| 12. | 74/M | June | 59 | gardening | Yes | ulceroglan | Yes | 1024 * | >1024 |
| 13. | 82/M | June | 37 | mowing hay, gardening | No | pulmonary | Yes | >1024 | >1024 |
| 14. | 62/M | June | 85 | mowing hay, farming | Yes | pulmonary | No | 256 | >256 |
| 15. | 59/M | June | 97 | farming | Yes | pulmonary | No | 256 | >1024 |
| 16. | 66/M | July | 13 | beekeeping | Yes | pulmonary | Yes | 1024 | 256 |
| 17. | 62/M | July | 16 | gardening | Yes | pulmonary | No | 1024 | 256 |
| 18. | 67/M | July | 18 | gardening | No | pulmonary | Yes | >1024 | >1024 |
| 19. | 62/F | July | 24 | mowing hay | Yes | pulmonary | No | 512 | >1024 |
| 20. | 43/F | July | 4 (HT) | gardening | No | pulmonary | Yes | >256 ** | >256 |
| 21. | 68/F | July | 21 | nature walks | No | pulmonary | No | >1024 | >1024 |
| 22. | 77/M | July | 33 | mowing hay, gardening | Yes | pulmonary | Yes | >1024 | >1024 |
| 23. | 77/M | July | 7 (PCR) | mowing hay, farming | Yes | pulmonary | No | neg # | neg |
| 24. | 80/F | July | 35 | nature walks | Yes | ulceroglan | Yes | neg. (neg.) | 2048 (2048) |
| 25. | 39/M | July | 29 | mowing hay, farming | Yes | pulmonary | Yes | >1024 | >1024 |
| 26. | 42/M | July | 12 | mowing hay | Yes | pulmonary | Yes | neg (>256) | 64 (>256) |
| 27. | 64/M | July | 11 | mowing hay | No | pulmonary | No | >256 | >256 |
| 28. | 43/F | August | 10 | contact with domestic animals | Yes | glandular | No | neg (>256) | 1024 (>256) |
| 29. | 43/M | August | 13 | farming, collecting straw | Yes | oculoglan | No | neg. (neg.) | 256 (8192) |
| 30. | 76/M | August | 21 | gardening, grafting trees | No | pulmonary | Yes | 128 | >256 |
| 31. | 69/F | August | 39 | gardening | No | ulceroglan | No | >256 | >256 |
| 32. | 78/M | August | 21 | farming | Yes | febrille illness | No | 128 | >256 |
| 33. | 69/M | August | 0 (neg) | gardening | No | pulmonary | No | neg (1024) | neg (>1024) |
| 34. | 60/M | September | 16 | farming | Yes | pulmonary | No | neg (>1024) | >256 (>1024) |
| 35. | 57/F | September | 24 | gardening | No | ulceroglan | Yes | >256 | >256 |
| 36. | 59/M | December | 33 | farming | Yes | pulmonary | No | 1024 | >1024 |
| 37. | 52/M | December | 116 | timber work | No | pulmonary | Yes | 1024 | >1024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grmek Košnik, I.; Orožen, K.; Ribnikar, M.; Grilc, E.; Bitežnik, B.; Korva, M.; Zdovc, I.; Avberšek, J.; Vengušt, G.; Sočan, M. An Outbreak of Pulmonary Tularemia in Slovenia in Summer 2024. Epidemiologia 2025, 6, 51. https://doi.org/10.3390/epidemiologia6030051
Grmek Košnik I, Orožen K, Ribnikar M, Grilc E, Bitežnik B, Korva M, Zdovc I, Avberšek J, Vengušt G, Sočan M. An Outbreak of Pulmonary Tularemia in Slovenia in Summer 2024. Epidemiologia. 2025; 6(3):51. https://doi.org/10.3390/epidemiologia6030051
Chicago/Turabian StyleGrmek Košnik, Irena, Kristina Orožen, Monika Ribnikar, Eva Grilc, Barbara Bitežnik, Miša Korva, Irena Zdovc, Jana Avberšek, Gorazd Vengušt, and Maja Sočan. 2025. "An Outbreak of Pulmonary Tularemia in Slovenia in Summer 2024" Epidemiologia 6, no. 3: 51. https://doi.org/10.3390/epidemiologia6030051
APA StyleGrmek Košnik, I., Orožen, K., Ribnikar, M., Grilc, E., Bitežnik, B., Korva, M., Zdovc, I., Avberšek, J., Vengušt, G., & Sočan, M. (2025). An Outbreak of Pulmonary Tularemia in Slovenia in Summer 2024. Epidemiologia, 6(3), 51. https://doi.org/10.3390/epidemiologia6030051






