Systemic Sclerosis with Interstitial Lung Disease: Identification of Novel Immunogenetic Markers and Ethnic Specificity in Kazakh Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Considerations
2.3. Sample Collection and Preparation
2.4. Autoantibody Profiling
2.5. Cytokine Quantification
2.6. Genetic Analysis
2.7. Statistical Analysis
3. Results
3.1. The Role of Autoantibodies in Systemic Sclerosis with Pulmonary Involvement
| Markers | Description | Clinical Significance | Form of SSc | Reference |
|---|---|---|---|---|
| Anti-Scl-70 | Anti-topoisomerase I antibody | ATA autoantibodies are strongly associated with the development of SSc-ILD, while ACA are protective for ILD. | diffuse | [29] |
| RNAP-III | Anti-RNA Polymerase III | Increased risk of PAH and skeletal muscle involvement, used to predict disease severity. | Diffuse | [30] |
| U1-snRNP | Anti-U1RNP antibodies | SSc patients with anti-U1RNP antibodies were more likely to develop ILD than SSc patients without anti-U1RNP. As a result, the hypothesis that these patients would experience a faster decline in FVC was raised. | limited | [31] |
| anti-BICD2 | Intracellular protein bicaudal D2 | For patients with SSc, a reduction in FEV1 and the carbon monoxide transfer coefficient has been observed. | - | [25,32] |
| anti-Th/To | Antibodies against Th/To ribonucleoprotein complex | Characterized by low organ damage, favorable ILD outcome, and good survival rates. | limited | [25] |
| anti-U11/U12 RNP | Antibodies against the U11/U12 minor spliceosomal ribonucleoprotein complex | Associated with ILD and fibrotic progression in SSc. | Overlap | [25] |
| CA15-3 | Carbohydrate antigen 15-3 (MUC1) | Elevated levels indicate epithelial injury and fibrosis in SSc-ILD. | - | [26] |
| ICAM-1 | Intercellular Adhesion Molecule 1 | Indicator of endothelial activation and systemic inflammation. | - | [26] |
| Anti-C1q | Anti-C1q autoAbs | Anti-C1q autoAbs were frequently detected in patients with SSc, and their high levels predict the co-occurrence of pulmonary fibrosis or pulmonary arterial hypertension. | limited | [33] |
| KL-6 | Krebs von den Lungen 6 glycoprotein | High KL-6 serum value (>923 U/mL) associated with more severe pulmonary functional impairment (large prospective trial) used to assess the severity of ILD. | - | [7,34] |
| SP-D | Surfactant protein D | Serum SP-D may be considered in several biomarkers for the severity of lung injury in SSc, including GER-associated lung injury. | - | [34] |
| Anti-U3-RNP | Anti-Fibrillarin | More prevalent in males with SSc with Afro-Caribbean ancestry rates between 7 and 11, who have a higher risk of developing pulmonary arterial hypertension (PAH). | Diffuse | [35,36] |
| Anti-PM/Scl | Anti-aminoacyl-tRNA synthetase | Anti-PM/Scl antibodies frequently resemble patients with Antisynthetase syndrome—an overlap myositis, interstitial lung disease and arthritis. | limited SSc, overlap | [27] |
| PIP4K2B | Autoantibodies against anti-phosphatidylinositol-5-phosphate 4-kinase type 2 beta (PIP4K2B) | Autoantibodies against PIP4K2B and AKT3 are increased risk of skin and lung fibrosis in patients with SSc. | - | [25] |
| AKT 3 | AKT serine/threonine kinase 3 (AKT3) | Autoantibodies against PIP4K2B and AKT3 show increased risk of skin and lung fibrosis in patients with SSc. | - | [25] |
| anti-TRIM21/Ro52 | anti-TRIM21/Ro52 | The highest frequency of patients with lung fibrosis and PAH. | limited, diffuse, other autoimmune disease | [27] |
3.2. Cytokines Profile in SSc-ILD
| Marker | Description | Clinical Significance | Reference |
|---|---|---|---|
| IL-7 | Interleukin-7 | Predictor for a decline of diffusion capacity (DLCO) by 20 or 30% in ILD patients. | [37] |
| IL-8 | Interleukin-8 | Serum IL-8 correlated with BAL IL-8 (r = 0.574, p = 0.006). | [37] |
| CCL4 | C-C motif ligand 4 | Significantly increased in patients with SSc presence of fibrosis, regardless of the subtype or stage of the disease and correlated with the severity of pulmonary fibrosis. | [48] |
| IL-6 | Interleukin 6 | Predictive marker of early disease progression in patients with mild ILD, and a marker for mortality in SSc-ILD patients. | [38,49,50,51,52] |
| Tenascin C | Tenascin C | Fibronectin-EDA and tenascin-C may activate Toll receptor 4 (TLR4) and lead to uncontrolled extracellular matrix (ECM) deposition in systemic sclerosis (SSc), which subsequently enhances transforming growth factor beta (TGF-β) signaling, thus promoting fibrotic responses. | [39,40] |
| TGF-β | Transforming Growth Factor-bet | TGF-β stimulates the expression of CUX1 isoforms, and the levels of ET-1, COL1, Wnt1, CTGF, and β-catenin increase after TGF-β treatment in normal and SSc lung fibroblasts. | [46,47] |
| CCL2 (MCP-1) | C-C motif ligand 2 | A positive correlation has been found between CCL2 levels and the severity of pulmonary dysfunction, as measured by DLCO and FVC. | [41,42] |
| CXCL10 | C-X-C motif chemokine ligand 10 | CXCL10 is potentially involved in early SSc-ILD. Fibroblasts treated with serum or BAL fluid from patients with SSc overexpress CXCL10. | [43] |
| CCL18 | C-C motif chemokine ligand 18 | High CCL18 level is an independent predictor of pulmonary function decrease in SSc-ILD (>10% in forced vital capacity). | [33,44,45] |
3.3. Genetic Predisposition of Lung Injury in Systemic Sclerosis in Different Nations
| Genes | Polymorphism | Function | Population | Reference |
|---|---|---|---|---|
| IRF5 | rs10488631, rs12537284, rs4728142 | Associated with lower IRF5 transcript levels, was predictive of longer survival and milder ILD in patients with SSc. | Caucasian | [58] |
| CD247 | rs2056626 | Associated with T cell activation and function. | Caucasian Turkish Chinese | [58,60,62] |
| CD226 | rs763361 | CD226 (also known as T cell Ig and ITIM domain) are involved in the T-cell activation and function. | Iranian | [62] |
| IRF 5 | rs2004640 | Important regulatory factors in controlling innate immune responses. | French | [60] |
| IRF5 | rs4728142 | Lower IRF5 transcript levels are associated with longer survival and milder ILD. | Caucasian Turkish | [60] |
| STAT4 | rs3821236 | Important in predisposition to SSc-ILD. | Caucasian | [58] |
| STAT4 | rs7574865 | Important regulatory factors in controlling innate immune responses and participating in the extracellular matrix synthesis in the pathogenesis of in SSc-ILD. | Russian | [58,59,63] |
| IRAK1 | rs1059702 | Mediator of TLR/IL-1R signaling; contributes to proinflammatory and profibrotic responses in SSc-ILD. | European Caucasian | [60] |
| SPINT2 | - | Serine protease inhibitor; suppresses epithelial-to-mesenchymal transition and fibrosis. Overexpressed in activated myofibroblasts. | Not specified | [64] |
| MFAP5 | - | ECM protein upregulated in myofibroblasts; promotes collagen deposition and tissue remodeling in lung fibrosis. | Not specified | [64] |
| CDKN2C (p18) | CDK inhibitors | The potential role of the cdkn2c (p18), associated with protein expression, is increased in progressing SSc-ILD lung sections. | Not specified | [20] |
| PTGS2 COX-2 | - | Inflammatory mediator gene downregulated via ncRNAs; positively associated with the occurrence of SSc-ILD and abnormal immune cell infiltration. Potential factor for the progression of SSc-ILD to malignancy. | Nor specified | [65] |
| CTLA-4 | 318C/T | Immune checkpoint gene; polymorphism associated with increased susceptibility to SSc. | Not specified | [66] |
| CD38 | - | Markers of plasma cells, co-expressed in fibrotic SSc lungs, indicating B cell and antibody-producing cell involvement. | Not specified | [67] |
| CD138 | - | Markers of plasma cells; co-expressed in fibrotic SSc lungs, indicating B cell and antibody-producing cell involvement. | Not specified | [67] |
| miR-155 | - | miRNAs regulate gene expression; expression levels correlate with lung function and fibrosis progression in SSc-ILD. | Not specified | [68] |
| miR-143 | - | miRNAs regulate gene expression; expression levels correlate with lung function and fibrosis progression in SSc-ILD. | Not specified | [68] |
| MMP7 | - | Matrix metalloproteinase involved in ECM degradation and fibrosis; may modulate GPCR signaling and promote fibrotic remodeling. | Not specified | [61] |
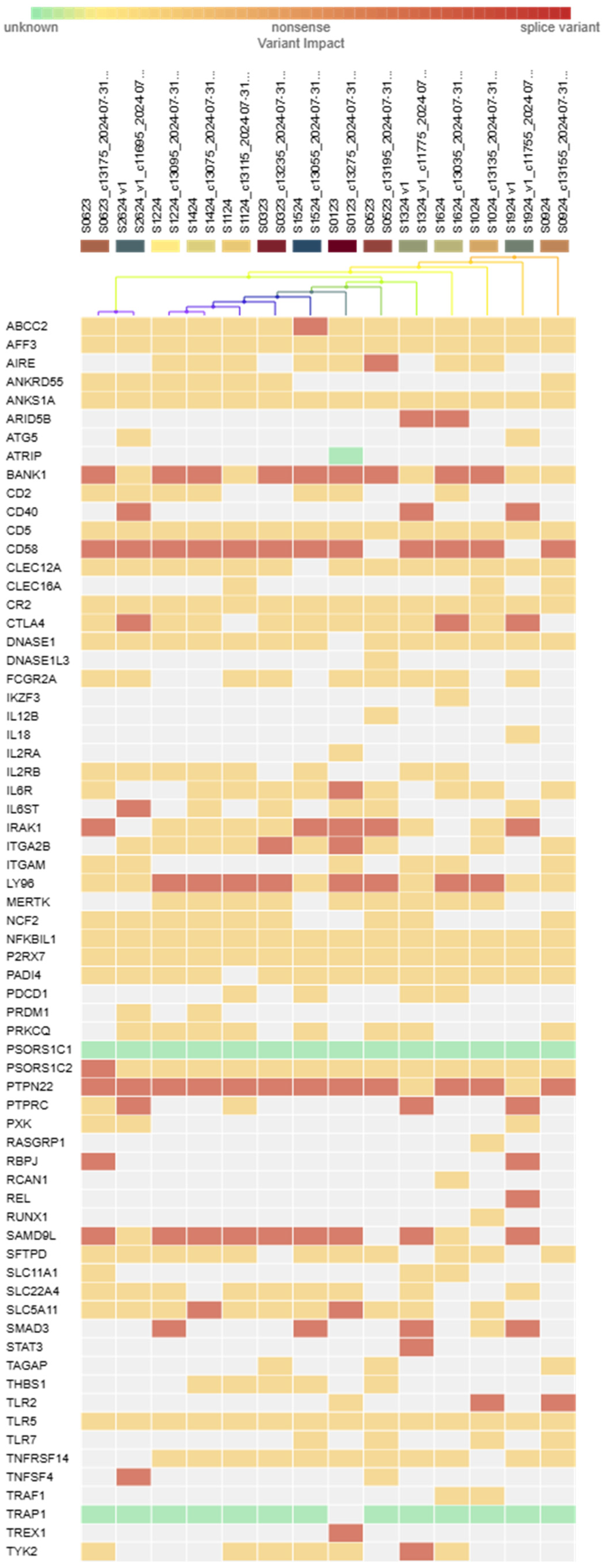
3.4. Immunogenetic Profiling of Kazakh Patients with SSc-ILD
3.5. Autoantibodies and Their Association in the Development of SSc-ILD
3.6. Genetic Variability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACA | anti-centromere antibodies |
| ANF | anti-nuclear factor |
| Anti-C1q | Anti-C1q autoAbs |
| Anti-PM/Scl | Anti-aminoacyl-tRNA synthetase |
| Anti-Scl-70 | Anti-topoisomerase I antibody |
| Anti-U3-RNP | Anti-Fibrillarin |
| ATA | anti-topoisomerase I |
| CCL18 | C-C motif chemokine ligand 18 |
| CCL2 (MCP-1) | C-C motif ligand 2 |
| CCL4 | C-C motif ligand 4 |
| CD226 | Cluster of differentiation 226 |
| CD247 | Cluster of differentiation 247 |
| CTD | connective tissue disease |
| CTGF | Connective tissue growth factor (CTGF) |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| DcSSc | Diffuse cutaneous systemic sclerosis |
| ECM | Extracellular matrix |
| EScSG | The European Scleroderma Study Group activity index |
| GER | Gastroesophageal reflux |
| HRCT | High-resolution computed tomography |
| IL6 | Interleukin-6 |
| IL-7 | Interleukin-7 |
| IL-8 | Interleukin-8 |
| ILD | Interstitial lung disease |
| IRAK1 | Interleukin-1 receptor-associated kinase 1 |
| IRF5 | Interferon regulatory factor 5 |
| KL-6 | Krebs von den Lungen 6 glycoprotein |
| LP | likely pathogenic variant |
| MCTD | Mixed connective tissue disease |
| MMP-12 | Matrix metalloproteinase-12 |
| NLRP1 | NLR family, pyrin domain containing 1 |
| PAH | Pulmonary arterial hypertension |
| PFT | Pulmonary function tests |
| PIP4K2B | Autoantibodies against anti-phosphatidylinositol-5-phosphate 4-kinase type 2 beta (PIP4K2B) |
| PLCL2 | Phospholipase C-like 2 |
| PM/DM | Polymyositis/dermatomyositis |
| RNAP-III | Anti-RNA Polymerase III |
| RSS | Rodnan skin score |
| SP-D | Surfactant protein D |
| SSc | Systemic sclerosis |
| SSc-ILD | Systemic sclerosis-related interstitial lung disease |
| STAT4 | Signal transducer and activator of transcription 4 |
| TGF | Transforming growth factor |
| TNFAIP3 | Tumor necrosis factor a -1 induced protein 3 |
| U1-RNP | Anti-U1RNP antibodies |
| VUS | Variant of uncertain significance |
References
- Cavazzana, I.; Vojinovic, T.; Airo’, P.; Fredi, M.; Ceribelli, A.; Pedretti, E.; Lazzaroni, M.G.; Garrafa, E.; Franceschini, F. Systemic sclerosis-specific antibodies: Novel and classical biomarkers. Clin. Rev. Allergy Immunol. 2023, 64, 412–430. [Google Scholar] [CrossRef]
- Baigenzhin, A.; Pak, A.; Zaripova, L.; Zarkumova, Z.; Chuvakova, E. Respiratory support for patients with chronic respiratory failure: The necessity of a long-term homecare ventilation program. J. Clin. Med. Kaz. 2024, 21, 56–60. [Google Scholar] [CrossRef]
- Nihtyanova, S.I.; Sari, A.; Harvey, J.C.; Leslie, A.; Derrett-Smith, E.C.; Fonseca, C.; Ong, V.H.; Denton, C.P. Using autoantibodies and cutaneous subset to develop outcome-based disease classification in systemic sclerosis. Arthritis Rheumatol. 2020, 72, 465–476. [Google Scholar] [CrossRef]
- Cozzani, E.; Muracchioli, A.; Murdaca, G.; Beccalli, M.; Caprioli, S.; Zentilin, P.; Ameri, P.; Grosso, M.; Russo, R.; Carmisciano, L.; et al. Correlation between skin and affected organs in 52 sclerodermic patients followed in a diseases management team: Development of a risk prediction model of organ-specific complications. Front. Immunol. 2021, 12, 588753. [Google Scholar] [CrossRef]
- Hoyles, R.K.; Khan, K.; Xu, S.; Howat, S.L.; Lindahl, G.E.; Leoni, P.; du Bois, R.M.; Wells, A.U.; Black, C.M.; Abraham, D.J.; et al. Fibroblast-specific perturbation of transforming growth factor beta signaling provides insight into potential pathogenic mechanisms of scleroderma-associated lung fibrosis: Exaggerated response to alveolar epithelial injury in a novel mouse model. Arthritis Rheum. 2008, 58, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Agrawal, R.; Lee, J.; Almagor, O.; Nelson, R.; Varga, J.; Cuttica, M.J.; Dematte, J.D.; Chang, R.W.; Hinchcliff, M.E. Esophageal dilatation and interstitial lung disease in systemic sclerosis: A cross-sectional study. Semin. Arthritis Rheum. 2016, 46, 109–114. [Google Scholar] [CrossRef]
- Christmann, R.B.; Wells, A.U.; Capelozzi, V.L.; Silver, R.M. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: Clinical, radiologic, histopathologic, and treatment evidence. Semin. Arthritis Rheum. 2010, 40, 241–249. [Google Scholar] [CrossRef]
- Kreuter, M.; Bonella, F.; Blank, N.; Riemekasten, G.; Müller-Ladner, U.; Henes, J.; Siegert, E.; Günther, C.; Kötter, I.; Pfeiffer, C.; et al. Anti-acid therapy in SSc-associated interstitial lung disease: Long-term outcomes from the German Network for Systemic Sclerosis. Rheumatology 2023, 62, 3067–3074. [Google Scholar] [CrossRef] [PubMed]
- Carlo-Stella, N.; Belloli, L.; Barbera, R.; Gambaro, C.; Rando, G.; Malesci, A.; Marasini, B. Gastroesophageal reflux and lung disease in systemic sclerosis. Am. J. Respir. Crit. Care Med. 2009, 179, 1167. [Google Scholar] [CrossRef]
- Marie, I.; Dominique, S.; Levesque, H.; Ducrotté, P.; Denis, P.; Hellot, M.F.; Courtois, H. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis Rheum. 2001, 45, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; Tashkin, D.P. Treatment of systemic sclerosis-related interstitial lung disease: A review of existing and emerging therapies. Ann. Am. Thorac. Soc. 2016, 13, 2045–2056. [Google Scholar] [CrossRef]
- Jang, H.J.; Woo, A.; Kim, S.Y.; Yong, S.H.; Park, Y.; Chung, K.; Lee, S.H.; Leem, A.Y.; Lee, S.H.; Kim, E.Y.; et al. Characteristics and risk factors of mortality in patients with systemic sclerosis-associated interstitial lung disease. Ann. Med. 2023, 55, 663–671. [Google Scholar] [CrossRef]
- Steen, V.D. Autoantibodies in systemic sclerosis. Semin. Arthritis Rheum. 2005, 35, 35–42. [Google Scholar] [CrossRef]
- Fischer, A.; Swigris, J.J.; Groshong, S.D.; Cool, C.D.; Sahin, H.; Lynch, D.A.; Curran-Everett, D.; Gillis, J.Z.; Meehan, R.T.; Brown, K.K. Clinically significant interstitial lung disease in limited scleroderma: Histopathology, clinical features, and survival. Chest 2008, 134, 601–605. [Google Scholar] [CrossRef]
- Goldin, J.G.; Lynch, D.A.; Strollo, D.C.; Suh, R.D.; Schraufnagel, D.E.; Clements, P.J.; Elashoff, R.M.; Furst, D.E.; Vasunilashorn, S.; McNitt-Gray, M.F.; et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 2008, 134, 358–367. [Google Scholar] [CrossRef]
- Desai, S.R.; Veeraraghavan, S.; Hansell, D.M.; Nikolakopolou, A.; Goh, N.S.; Nicholson, A.G.; Colby, T.V.; Denton, C.P.; Black, C.M.; du Bois, R.M.; et al. CT features of lung disease in patients with systemic sclerosis: Comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004, 232, 560–567. [Google Scholar] [CrossRef]
- Fischer, A.; du Bois, R. Interstitial lung disease in connective tissue disorders. Lancet 2012, 380, 689–698. [Google Scholar] [CrossRef]
- Tyndall, A.J.; Bannert, B.; Vonk, M.; Airò, P.; Cozzi, F.; Carreira, P.E.; Bancel, D.F.; Allanore, Y.; Müller-Ladner, U.; Distler, O.; et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann. Rheum. Dis. 2010, 69, 1809–1815. [Google Scholar] [CrossRef]
- Rubio-Rivas, M.; Royo, C.; Simeón, C.P.; Corbella, X.; Fonollosa, V. Mortality and survival in systemic sclerosis: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2014, 44, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Al-Adwi, Y.; Westra, J.; van Goor, H.; van Kempen, L.C.; Osman, M.; Gan, C.T.; Timens, W.; Mulder, D.J. Transcriptomic analyses of lung tissues reveal key genes associated with progression of systemic sclerosis-interstitial lung disease (SSc-ILD). J. Autoimmun. 2024, 148, 103297. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, N. Relationship between idiopathic interstitial pneumonias (IIPs) and connective tissue disease-related interstitial lung disease (CTD-ILD): A narrative review. Respir. Investig. 2024, 62, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Schurawitzki, H.; Stiglbauer, R.; Graninger, W.; Herold, C.; Pölzleitner, D.; Burghuber, O.C.; Tscholakoff, D. Interstitial lung disease in progressive systemic sclerosis: High-resolution CT versus radiography. Radiology 1990, 176, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.J.W.; Renzoni, E.A. Genetic predictors of systemic sclerosis-associated interstitial lung disease: A review of recent literature. Eur. J. Hum. Genet. 2018, 26, 765–777. [Google Scholar] [CrossRef]
- Garra, W.; Levy, Y. Prevalence of anti-synthetase antibodies among systemic sclerosis patients. Eur. J. Intern. Med. 2023, 117, 98–102. [Google Scholar] [CrossRef]
- Geroldinger-Simić, M.; Bayati, S.; Pohjanen, E.; Sepp, N.; Nilsson, P.; Pin, E. Autoantibodies against PIP4K2B and AKT3 are associated with skin and lung fibrosis in patients with systemic sclerosis. Int. J. Mol. Sci. 2023, 24, 5629. [Google Scholar] [CrossRef]
- Jee, A.S.; Stewart, I.; Youssef, P.; Adelstein, S.; Lai, D.; Hua, S.; Stevens, W.; Proudman, S.; Ngian, G.S.; Glaspole, I.N.; et al. A Composite Serum Biomarker Index for the Diagnosis of Systemic Sclerosis-Associated Interstitial Lung Disease: A Multicenter, Observational Cohort Study. Arthritis Rheumatol. 2023, 75, 1424–1433. [Google Scholar] [CrossRef]
- Nayebirad, S.; Mohamadi, A.; Yousefi-Koma, H.; Javadi, M.; Farahmand, K.; Atef-Yekta, R.; Tamartash, Z.; Jameie, M.; Mohammadzadegan, A.M.; Kavosi, H. Association of anti-Ro52 autoantibody with interstitial lung disease in autoimmune diseases: A systematic review and meta-analysis. BMJ Open Respir. Res. 2023, 10, e002076. [Google Scholar] [CrossRef] [PubMed]
- Selva-O’Callaghan, A.; Simeon-Aznar, C.P. The scleromyositis phenotype. Lessons from a multicentre international cohort of anti-PM/Scl-positive patients. Rheumatology 2021, 60, 4956–4957. [Google Scholar] [CrossRef] [PubMed]
- Cerro-Chiang, G.; Ayres, M.; Rivas, A.; Romero, T.; Parker, S.J.; Mastali, M.; Elashoff, D.; Chen, P.; Van Eyk, J.E.; Wolters, P.J.; et al. Protein biomarkers of disease progression in patients with systemic sclerosis associated interstitial lung disease. Sci. Rep. 2023, 13, 8645. [Google Scholar] [CrossRef]
- Kelly, A.; Derk, C. Anti-RNA Polymerase III antibodies in systemic sclerosis. Open J. Rheumatol. Autoimmun. Dis. 2015, 5, 81–86. [Google Scholar] [CrossRef]
- Chevalier, K.; Chassagnon, G.; Leonard-Louis, S.; Cohen, P.; Dunogue, B.; Regent, A.; Thoreau, B.; Mouthon, L.; Chaigne, B. Anti-U1RNP antibodies are associated with a distinct clinical phenotype and a worse survival in patients with systemic sclerosis. J. Autoimmun. 2024, 146, 103220. [Google Scholar] [CrossRef]
- Jandali, B.; Salazar, G.A.; Hudson, M.; Fritzler, M.J.; Lyons, M.A.; Estrada-Y-Martin, R.M.; Charles, J.; Terracina, K.A.; Mayes, M.D.; Assassi, S. The Effect of Anti-Scl-70 Antibody Determination Method on Its Predictive Significance for Interstitial Lung Disease Progression in Systemic Sclerosis. ACR Open Rheumatol. 2022, 4, 345–351. [Google Scholar] [CrossRef]
- Liaskos, C.; Rentouli, S.; Simopoulou, T.; Gkoutzourelas, A.; Norman, G.L.; Brotis, A.; Alexiou, I.; Katsiari, C.; Bogdanos, D.P.; Sakkas, L.I. Anti-C1q autoantibodies are frequently detected in patients with systemic sclerosis associated with pulmonary fibrosis. Br. J. Dermatol. 2019, 181, 138–146. [Google Scholar] [CrossRef]
- Elhai, M.; Hoffmann-Vold, A.M.; Avouac, J.; Pezet, S.; Cauvet, A.; Leblond, A.; Fretheim, H.; Garen, T.; Kuwana, M.; Molberg, Ø.; et al. Performance of candidate serum biomarkers for systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol. 2019, 71, 972–982. [Google Scholar] [CrossRef]
- Sosnovskaya, A.V.; Fomin, V.V.; Popova, E.N.; Lebedeva, M.V.; Moiseev, S.V.; Svistunov, A.A.; Mukhin, N.A. Clinical value of surfactant protein D as a biomarker of pulmonary fibrosis in patients with scleroderma systematica in relation to the presence of gastroesophageal reflux. Ter. Arkh. 2015, 87, 42–47. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.D.; Shah, A.A.; Mayes, M.D.; Domsic, R.T.; Medsger, T.A., Jr.; Steen, V.D.; Varga, J.; Carns, M.; Ramos, P.S.; Silver, R.M.; et al. Clinical and serological features of systemic sclerosis in a multicenter African American cohort: Analysis of the genome research in African American scleroderma patients clinical database. Medicine 2017, 96, e8980. [Google Scholar] [CrossRef]
- Fritzler, M.J.; Bentow, C.; Beretta, L.; Palterer, B.; Perurena-Prieto, J.; Sanz-Martínez, M.T.; Guillen-Del-Castillo, A.; Marín, A.; Fonollosa-Pla, V.; Callejas-Moraga, E.; et al. Anti-U11/U12 antibodies as a rare but important biomarker in patients with systemic sclerosis: A narrative review. Diagnostics 2023, 13, 1257. [Google Scholar] [CrossRef]
- Becker, M.O.; Radic, M.; Schmidt, K.; Huscher, D.; Riedlinger, A.; Michelfelder, M.; Meisel, C.; Ewert, R.; Burmester, G.R.; Riemekasten, G. Serum cytokines and their predictive value in pulmonary involvement of systemic sclerosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2019, 36, 274–284. [Google Scholar] [CrossRef]
- De Lauretis, A.; Sestini, P.; Pantelidis, P.; Hoyles, R.; Hansell, D.M.; Goh, N.S.; Zappala, C.J.; Visca, D.; Maher, T.M.; Denton, C.P.; et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J. Rheumatol. 2013, 40, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Brissett, M.; Veraldi, K.L.; Pilewski, J.M.; Medsger, T.A., Jr.; Feghali-Bostwick, C.A. Localized expression of tenascin in systemic sclerosis-associated pulmonary fibrosis and its regulation by insulin-like growth factor binding protein 3. Arthritis Rheum. 2012, 64, 272–280. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Wang, W.; Morales-Nebreda, L.; Feng, G.; Wu, M.; Zhou, X.; Lafyatis, R.; Lee, J.; Hinchcliff, M.; Feghali-Bostwick, C.; et al. Tenascin-C drives persistence of organ fibrosis. Nat. Commun. 2016, 7, 11703. [Google Scholar] [CrossRef]
- Carulli, M.T.; Handler, C.; Coghlan, J.G.; Black, C.M.; Denton, C.P. Can CCL2 serum levels be used in risk stratification or to monitor treatment response in systemic sclerosis? Ann. Rheum. Dis. 2008, 67, 105–109. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferri, C.; Fallahi, P.; Ferrari, S.M.; Giuggioli, D.; Colaci, M.; Manfredi, A.; Frascerra, S.; Franzoni, F.; Galetta, F.; et al. CXCL10 (alpha) and CCL2 (beta) chemokines in systemic sclerosis—A longitudinal study. Rheumatology 2008, 47, 45–49. [Google Scholar] [CrossRef]
- Al-Adwi, Y.; Atzeni, I.M.; Doornbos-van der Meer, B.; van der Leij, M.J.; Varkevisser, R.D.M.; Kroesen, B.J.; Stel, A.; Timens, W.; Gan, C.T.; van Goor, H.; et al. High serum C-X-C motif chemokine ligand 10 (CXCL10) levels may be associated with new onset interstitial lung disease in patients with systemic sclerosis: Evidence from observational, clinical, transcriptomic and in vitro studies. EBioMedicine 2023, 98, 104883. [Google Scholar] [CrossRef]
- Giacomelli, R.; Afeltra, A.; Alunno, A.; Bartoloni-Bocci, E.; Berardicurti, O.; Bombardieri, M.; Bortoluzzi, A.; Caporali, R.; Caso, F.; Cervera, R.; et al. Guidelines for biomarkers in autoimmune rheumatic diseases—Evidence based analysis. Autoimmun. Rev. 2019, 18, 93–106. [Google Scholar] [CrossRef]
- Guiot, J.; Moermans, C.; Henket, M.; Corhay, J.L.; Louis, R. Blood biomarkers in idiopathic pulmonary fibrosis. Lung 2017, 195, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Fragiadaki, M.; Shi-Wen, X.; Ponticos, M.; Khan, K.; Denton, C.; Garcia, P.; Bou-Gharios, G.; Yamakawa, A.; Morimoto, C.; et al. Transforming growth factor-β-induced CUX1 isoforms are associated with fibrosis in systemic sclerosis lung fibroblasts. Biochem. Biophys. Rep. 2016, 7, 246–252. [Google Scholar] [CrossRef][Green Version]
- Muruganandam, M.; Ariza-Hutchinson, A.; Patel, R.A.; Sibbitt, W.L., Jr. Biomarkers in the pathogenesis, diagnosis, and treatment of systemic sclerosis. J. Inflamm. Res. 2023, 16, 4633–4660. [Google Scholar] [CrossRef] [PubMed]
- Codullo, V.; Baldwin, H.M.; Singh, M.D.; Fraser, A.R.; Wilson, C.; Gilmour, A.; Hueber, A.J.; Bonino, C.; McInnes, I.B.; Montecucco, C.; et al. An investigation of the inflammatory cytokine and chemokine network in systemic sclerosis. Ann. Rheum. Dis. 2011, 70, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; O’Reilly, S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin. Exp. Immunol. 2019, 195, 310–321. [Google Scholar] [CrossRef]
- Renaud, L.; da Silveira, W.A.; Takamura, N.; Hardiman, G.; Feghali-Bostwick, C. Prominence of IL6, IGF, TLR, and bioenergetics pathway perturbation in lung tissues of scleroderma patients with pulmonary fibrosis. Front. Immunol. 2020, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Numajiri, H.; Yoshizaki, A.; Fukasawa, T.; Ebata, S.; Nakamura, K.; Yamashita, T.; Saigusa, R.; Miura, S.; Hirabayashi, M.; Yoshizaki, A.; et al. Rapid alteration of serum interleukin-6 levels may predict the reactivity of i.v. cyclophosphamide pulse therapy in systemic sclerosis-associated interstitial lung disease. J. Dermatol. 2018, 45, 1221–1224. [Google Scholar] [CrossRef]
- Le, T.T.; Karmouty-Quintana, H.; Melicoff, E.; Le, T.T.; Weng, T.; Chen, N.Y.; Pedroza, M.; Zhou, Y.; Davies, J.; Philip, K.; et al. Blockade of IL-6 trans signaling attenuates pulmonary fibrosis. J. Immunol. 2014, 193, 3755–3768. [Google Scholar] [CrossRef]
- Kuo, C.F.; Luo, S.F.; Yu, K.H.; See, L.C.; Zhang, W.; Doherty, M. Familial risk of systemic sclerosis and co-aggregation of autoimmune diseases in affected families. Arthritis Res. Ther. 2016, 18, 231. [Google Scholar] [CrossRef]
- Boleto, G.; Campochiaro, C.; Distler, O.; Balanescu, A.; Launay, D.; Bergmann, C.; Airò, P.; Oksel, F.; Gheorghiu, A.M.; Anic, B.; et al. Interstitial lung disease in anti-U1RNP systemic sclerosis patients: A European Scleroderma Trials and Research analysis. J. Scleroderma Relat. Disord. 2025, in press. [Google Scholar] [CrossRef]
- Yen, E.Y.; Singh, D.R.; Singh, R.R. Trends in systemic sclerosis mortality over forty-eight years, 1968–2015: A US population-based study. Arthritis Care Res. 2021, 73, 1502–1510. [Google Scholar] [CrossRef]
- Arnett, F.C.; Howard, R.F.; Tan, F.; Moulds, J.M.; Bias, W.B.; Durban, E.; Cameron, H.D.; Paxton, G.; Hodge, T.J.; Weathers, P.E.; et al. Increased prevalence of systemic sclerosis in a Native American tribe in Oklahoma. Association with an Amerindian HLA haplotype. Arthritis Rheum. 1996, 39, 1362–1370. [Google Scholar] [CrossRef]
- Kuwana, M.; Kaburaki, J.; Arnett, F.C.; Howard, R.F.; Medsger, T.A., Jr.; Wright, T.M. Influence of ethnic background on clinical and serologic features in patients with systemic sclerosis and anti-DNA topoisomerase I antibody. Arthritis Rheum. 1999, 42, 465–474. [Google Scholar] [CrossRef]
- Sharif, R.; Mayes, M.D.; Tan, F.K.; Gorlova, O.Y.; Hummers, L.K.; Shah, A.A.; Furst, D.E.; Khanna, D.; Martin, J.; Bossini-Castillo, L.; et al. IRF5 polymorphism predicts prognosis in patients with systemic sclerosis. Ann. Rheum. Dis. 2012, 71, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Krylov, M.Y.; Ananyeva, L.P.; Koneva, O.A.; Starovoytova, M.N.; Desinova, O.V.; Ovsyannikova, O.B.; Aleksandrova, E.N.; Novikov, A.A.; Guseva, I.A.; Konovalova, N.V.; et al. The influence of STAT4 rs7574865 (G/T) polymorphism on the risk of clinical and immunological phenotypes of systemic sclerosis in a Russian patient population: Results of a pilot study. Ter. Arkh. 2017, 89, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yue, X.; Liu, K.; Zheng, J.; Huang, R.; Zou, J.; Riemekasten, G.; Petersen, F.; Yu, X. The status of pulmonary fibrosis in systemic sclerosis is associated with IRF5, STAT4, IRAK1, and CTGF polymorphisms. Rheumatol. Int. 2017, 37, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Mansouri, R.; Gharibdoost, F.; Aslani, S.; Mostafaei, S.; Kavosi, H.; Poursani, S.; Sobhani, S.; Mahmoudi, M. Association Study of CD226 and CD247 Genes Single Nucleotide Polymorphisms in Iranian Patients with Systemic Sclerosis. Iran. J. Allergy Asthma Immunol. 2017, 16, 471–479. [Google Scholar] [PubMed]
- Xu, Y.; Wang, W.; Tian, Y.; Liu, J.; Yang, R. Polymorphisms in STAT4 and IRF5 Increase the Risk of Systemic Sclerosis: A Meta-Analysis. Int. J. Dermatol. 2016, 55, 408–416. [Google Scholar] [CrossRef]
- Valenzi, E.; Bulik, M.; Tabib, T.; Morse, C.; Sembrat, J.; Trejo Bittar, H.; Rojas, M.; Lafyatis, R. Single-Cell Analysis Reveals Fibroblast Heterogeneity and Myofibroblasts in Systemic Sclerosis-Associated Interstitial Lung Disease. Ann. Rheum. Dis. 2019, 78, 1379–1387. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, C. The Downregulation of PTGS2 Mediated by ncRNAs is Tightly Correlated with Systemic Sclerosis-Interstitial Lung Disease. Front. Genet. 2022, 12, 795034. [Google Scholar] [CrossRef]
- Song, G.G.; Lee, Y.H. The CTLA-4 and MCP-1 Polymorphisms and Susceptibility to Systemic Sclerosis: A Meta-Analysis. Immunol. Investig. 2013, 42, 481–492. [Google Scholar] [CrossRef]
- Jia, G.; Ramalingam, T.R.; Van Heiden, J.; Gao, X.; DePianto, D.; Morshead, K.B.; Modrusan, Z.; Ramamoorthi, N.; Wolters, P.; Lin, C.; et al. An Interleukin 6 Responsive Plasma Cell Signature Is Associated with Disease Progression in Systemic Sclerosis Interstitial Lung Disease. iScience 2023, 26, 108133. [Google Scholar] [CrossRef]
- Christmann, R.B.; Wooten, A.; Sampaio-Barros, P.; Borges, C.L.; Carvalho, C.R.; Kairalla, R.A.; Feghali-Bostwick, C.; Ziemek, J.; Mei, Y.; Goummih, S.; et al. miR-155 in the Progression of Lung Fibrosis in Systemic Sclerosis. Arthritis Res. Ther. 2016, 18, 155. [Google Scholar] [CrossRef]
- Tesi, B.; Davidsson, J.; Voss, M.; Rahikkala, E.; Holmes, T.D.; Chiang, S.C.C.; Komulainen-Ebrahim, J.; Gorcenco, S.; Rundberg Nilsson, A.; Ripperger, T.; et al. Gain-of-Function SAMD9L Mutations Cause a Syndrome of Cytopenia, Immunodeficiency, MDS, and Neurological Symptoms. Blood 2017, 129, 2266–2279. [Google Scholar] [CrossRef]
- Gilmore, T.D.; Gerondakis, S. The c-Rel Transcription Factor in Development and Disease. Genes Cancer 2011, 2, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.M.; Rowe, G.; O’Brien, K.; Kim, G.; Luu, M. A diffuse, pustular eruption in a neonate: Recognizing SAMD9L-associated autoinflammatory disease (SAAD). Pediatr Dermatol. 2024, 41, 112–114. [Google Scholar] [CrossRef]
- Vreca, M.; Andjelkovic, M.; Tosic, N.; Zekovic, A.; Damjanov, N.; Pavlovic, S.; Spasovski, V. Impact of Alterations in X-Linked IRAK1 Gene and miR-146a on Susceptibility and Clinical Manifestations in Patients with Systemic Sclerosis. Immunol. Lett. 2018, 204, 1–8. [Google Scholar] [CrossRef]
- Liao, Z.; Zheng, R.; Shao, G. Mechanisms and application strategies of miRNA-146a regulating inflammation and fibrosis at molecular and cellular levels. Int. J. Mol. Med. 2023, 51, 7. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Jiang, L.; Jiang, X.; Zhou, X.; Xu, N. The rs878081 Polymorphism of AIRE Gene Increases the Risk of Rheumatoid Arthritis in a Chinese Han Population: A Case-Control Study. Braz. J. Med. Biol. Res. 2018, 51, e7944. [Google Scholar] [CrossRef]
- García-Lozano, J.R.; Torres-Agrela, B.; Montes-Cano, M.A.; Ortiz-Fernández, L.; Conde-Jaldón, M.; Teruel, M.; García, A.; Núñez-Roldán, A.; Martín, J.; González-Escribano, M.F. Association of the AIRE Gene with Susceptibility to Rheumatoid Arthritis in a European Population: A Case Control Study. Arthritis Res. Ther. 2013, 15, R11. [Google Scholar] [CrossRef]
- Grieves, J.L.; Fye, J.M.; Harvey, S.; Grayson, J.M.; Hollis, T.; Perrino, F.W. Exonuclease TREX1 Degrades Double-Stranded DNA to Prevent Spontaneous Lupus-Like Inflammatory Disease. Proc. Natl. Acad. Sci. USA 2015, 112, 5117–5122. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C.; Choi, S.J.; Ji, D.D.; Song, G.G. Associations between the Functional CD40 rs4810485 G/T Polymorphism and Susceptibility to Rheumatoid Arthritis and Systemic Lupus Erythematosus: A Meta-Analysis. Lupus 2015, 24, 1177–1183. [Google Scholar] [CrossRef]
- Hajialilo, M.; Noorabadi, P.; Tahsini Tekantapeh, S.; Malek Mahdavi, A. Endothelin-1, α-Klotho, 25(OH) Vit D Levels and Severity of Disease in Scleroderma Patients. Rheumatol. Int. 2017, 37, 1651–1657. [Google Scholar] [CrossRef]
- Abraham, D.J.; Vancheeswaran, R.; Dashwood, M.R.; Rajkumar, V.S.; Pantelides, P.; Xu, S.W.; du Bois, R.M.; Black, C.M. Increased Levels of Endothelin-1 and Differential Endothelin Type A and B Receptor Expression in Scleroderma-Associated Fibrotic Lung Disease. Am. J. Pathol. 1997, 151, 831–841. [Google Scholar] [PubMed]
- Bonhomme, O.; André, B.; Gester, F.; de Seny, D.; Moermans, C.; Struman, I.; Louis, R.; Malaise, M.; Guiot, J. Biomarkers in Systemic Sclerosis-Associated Interstitial Lung Disease: Review of the Literature. Rheumatology 2019, 58, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, J.; Zhao, J. Screening and Identification of Potential Biomarkers and Therapeutic Targets for Systemic Sclerosis-Associated Interstitial Lung Disease. Arch. Rheumatol. 2021, 36, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Park, K.S.; Kim, K.J. Integrative Analysis of Lung Molecular Signatures Reveals Key Drivers of Systemic Sclerosis-Associated Interstitial Lung Disease. Ann. Rheum. Dis. 2022, 81, 108–116. [Google Scholar] [CrossRef]
- Fernandez, I.E.; Eickelberg, O. The Impact of TGF-β on Lung Fibrosis: From Targeting to Biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef]
- Jiménez, S.A.; Castro, S.V.; Piera-Velázquez, S. Role of Growth Factors in the Pathogenesis of Tissue Fibrosis in Systemic Sclerosis. Curr. Rheumatol. Rev. 2010, 6, 283–294. [Google Scholar] [CrossRef]
- Manetti, M.; Guiducci, S.; Romano, E.; Bellando-Randone, S.; Conforti, M.L.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Increased Serum Levels and Tissue Expression of Matrix Metalloproteinase-12 in Patients with Systemic Sclerosis: Correlation with Severity of Skin and Pulmonary Fibrosis and Vascular Damage. Ann. Rheum. Dis. 2012, 71, 1064–1072. [Google Scholar] [CrossRef]
- Tochimoto, A.; Kawaguchi, Y.; Yamanaka, H. Genetic Susceptibility to Interstitial Lung Disease Associated with Systemic Sclerosis. Clin. Med. Insights Circ. Respir. Pulm. Med. 2016, 9, 135–140. [Google Scholar] [CrossRef]
- van Caam, A.; Vonk, M.; van den Hoogen, F.; van Lent, P.; van der Kraan, P. Unraveling SSc Pathophysiology; The Myofibroblast. Front. Immunol. 2018, 9, 2452. [Google Scholar] [CrossRef] [PubMed]
- Nihtyanova, S.I.; Denton, C.P. Pathogenesis of Systemic Sclerosis Associated Interstitial Lung Disease. J. Scleroderma Relat. Disord. 2020, 5, 6–16. [Google Scholar] [CrossRef]
- Iversen, L.V.; Tandrup Nielsen, C.; Jacobsen, S.; Hermansen, M.L.; Diederichsen, L.P.; Friis, T. Bicaudal D2 autoantibodies are highly specific for systemic sclerosis. Scand. J. Rheumatol. 2024, 53, 349–358. [Google Scholar] [CrossRef] [PubMed]
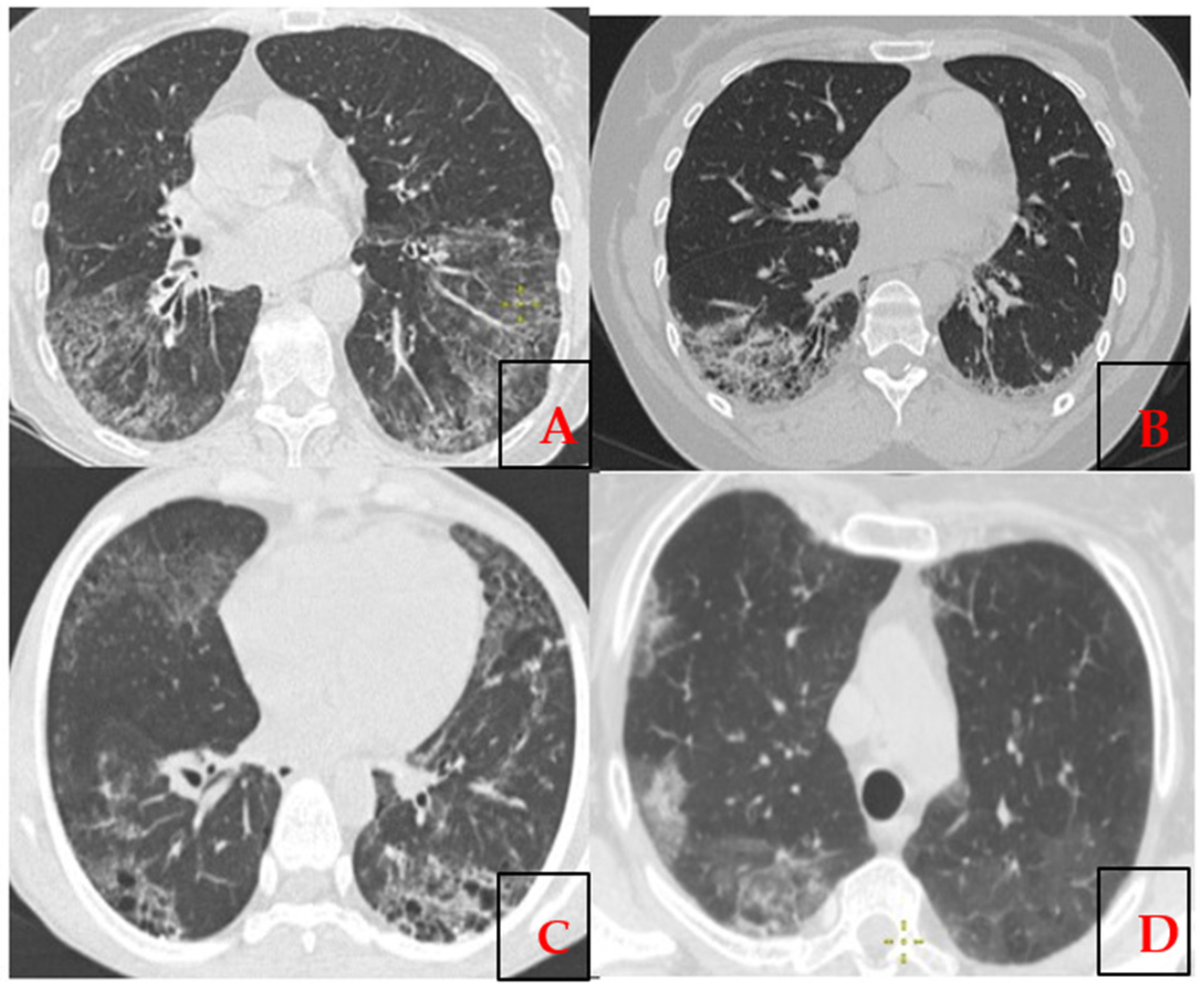

| Clinical Manifestation | Number of Patients (n = 14) |
|---|---|
| Lung involvement: | 14 (100%) |
| PAH + ILD | 4 (28.6%) |
| ILD | 13 (92.9%) |
| Skin involvement | 14 (100%) |
| Joint involvement: | 13 (92.9%) |
| Sclerodactyly—2 out of 13 (15%) | 2 (14.3%) |
| Joint functional impairment (Grade II) | 6 (42.9%) |
| Joint functional impairment (Grade I) | 1 (7.1%) |
| Gastrointestinal (GI) tract involvement | 10 (71.4%) |
| Raynaud’s phenomenon | 12 (85.7%) |
| Cardiosclerosis | 2 (14.3%) |
| Sjögren’s syndrome | 5 (35.7%) |
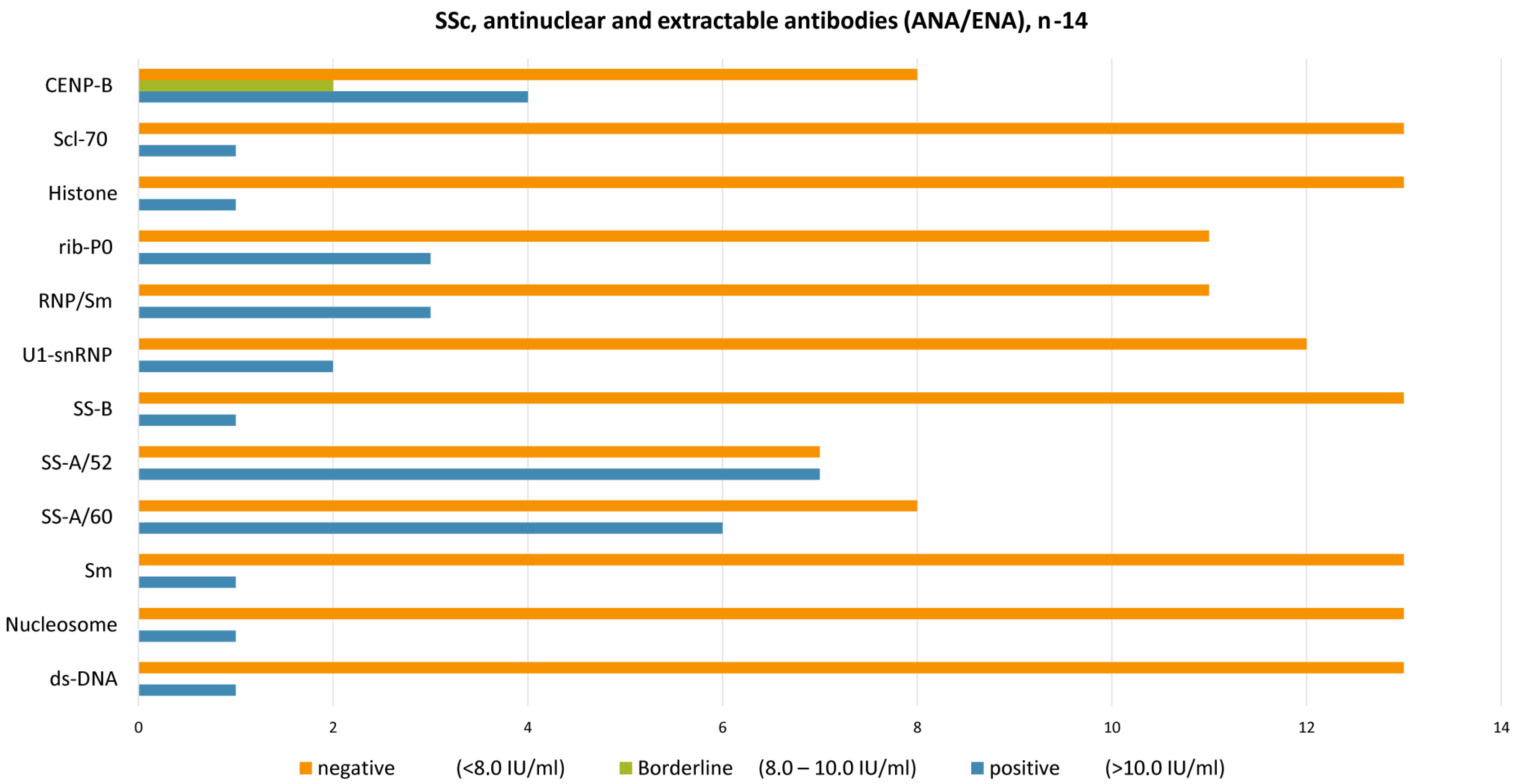
| SSc, Antinuclear and Extractable Antibodies (ANA/ENA), n = 14 | ds-DNA | Nucleosome | Sm | SS-A/60 | SS-A/52 | SS-B | U1-snRNP | RNP/Sm | rib-P0 | Histone | Scl-70 | CENP-B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive (>10.0 IU/mL) | 1 | 1 | 1 | 6 | 7 | 1 | 2 | 3 | 3 | 1 | 1 | 4 |
| Borderline (8.0–10.0 IU/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Negative (<8.0 IU/mL) | 13 | 13 | 13 | 8 | 7 | 13 | 12 | 11 | 11 | 13 | 13 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaripova, L.; Baigenzhin, A.; Zarkumova, Z.; Zhabakova, Z.; Boltanova, A.; Solomadin, M.; Pak, A. Systemic Sclerosis with Interstitial Lung Disease: Identification of Novel Immunogenetic Markers and Ethnic Specificity in Kazakh Patients. Epidemiologia 2025, 6, 41. https://doi.org/10.3390/epidemiologia6030041
Zaripova L, Baigenzhin A, Zarkumova Z, Zhabakova Z, Boltanova A, Solomadin M, Pak A. Systemic Sclerosis with Interstitial Lung Disease: Identification of Novel Immunogenetic Markers and Ethnic Specificity in Kazakh Patients. Epidemiologia. 2025; 6(3):41. https://doi.org/10.3390/epidemiologia6030041
Chicago/Turabian StyleZaripova, Lina, Abay Baigenzhin, Zhanar Zarkumova, Zhanna Zhabakova, Alyona Boltanova, Maxim Solomadin, and Alexey Pak. 2025. "Systemic Sclerosis with Interstitial Lung Disease: Identification of Novel Immunogenetic Markers and Ethnic Specificity in Kazakh Patients" Epidemiologia 6, no. 3: 41. https://doi.org/10.3390/epidemiologia6030041
APA StyleZaripova, L., Baigenzhin, A., Zarkumova, Z., Zhabakova, Z., Boltanova, A., Solomadin, M., & Pak, A. (2025). Systemic Sclerosis with Interstitial Lung Disease: Identification of Novel Immunogenetic Markers and Ethnic Specificity in Kazakh Patients. Epidemiologia, 6(3), 41. https://doi.org/10.3390/epidemiologia6030041







