Tuberculosis Notification in Jordan, 2016–2020
Abstract
1. Introduction
2. Methods
3. Results
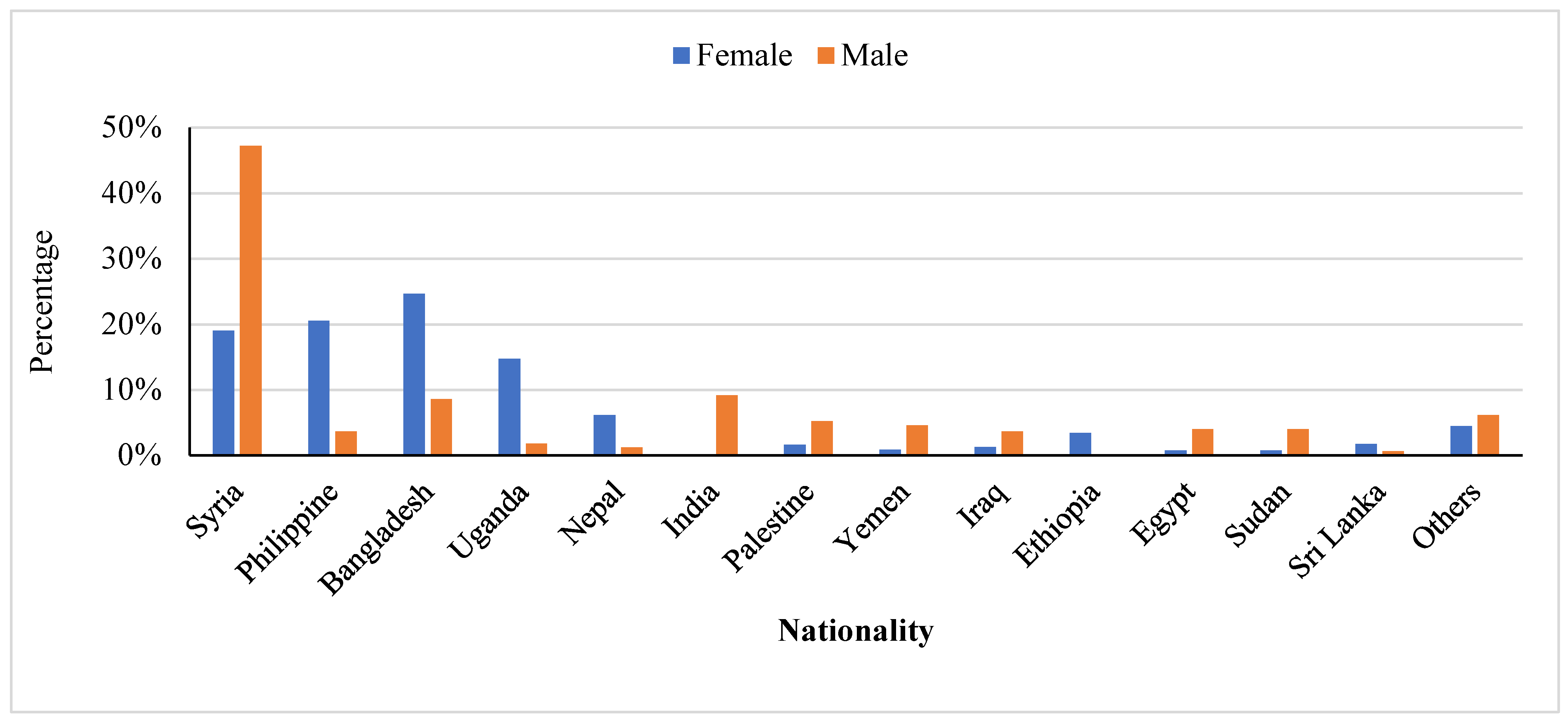
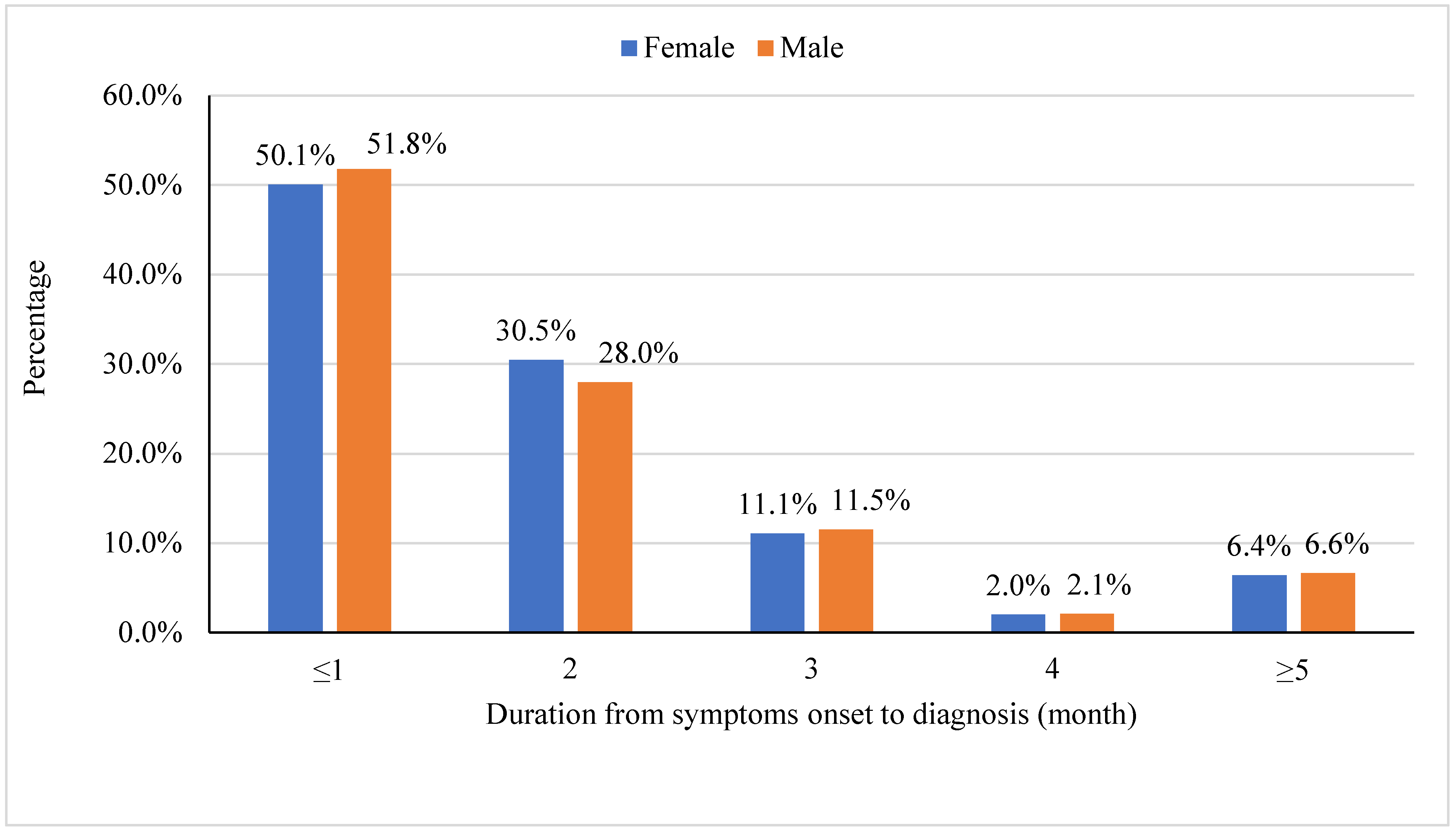
3.1. Patients’ Characteristics
3.2. TB Notification
3.3. The Trend of TB Notification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report: Executive Summary 2020. World Heal Organ. 2020. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 5 October 2021).
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 27 June 2023).
- Cookson, S.T.; Abaza, H.; Clarke, K.R.; Burton, A.; Sabrah, N.A.; Rumman, K.A.; Odeh, N.; Naoum, M. Impact of and response to increased tuberculosis prevalence among Syrian refugees compared with Jordanian tuberculosis prevalence: Case study of a tuberculosis public health strategy. Confl. Health 2015, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2014. World Health Organization. 2014. Available online: https://apps.who.int/iris/handle/10665/137094 (accessed on 5 October 2021).
- Floyd, K.; Glaziou, P.; Houben, R.M.G.J.; Sumner, T.; White, R.G.; Raviglione, M. Global tuberculosis targets and milestones set for 2016-2035: Definition and rationale. Int. J. Tuberc. Lung Dis. 2018, 22, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, S. Situational analysis of tuberculosis control programs in the Eastern Mediterranean Region: Gaps and solutions. Int. J. Mycobacteriol. 2016, 5 (Suppl. S1), S14. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Immunization Profile. 2020. Available online: http://www.who.int/immunization/monitoring_surveillance/data/gs_gloprofile.pdf?ua=1 (accessed on 27 June 2023).
- Department of Statistics (DOS) and ICF. Jordan Population and Family and Health Survey 2017–18: Key Indicators; DOS and ICF: Amman, Jordan; Rockville, ML, USA, 2018. [Google Scholar]
- Zwerling, A.; Behr, M.A.; Verma, A.; Brewer, T.F.; Menzies, D.; Pai, M. The BCG world atlas: A database of global BCG vaccination policies and practices. PLoS Med. 2011, 8, e1001012. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, I.; Stagg, H.R.; Cohen, T.; Mangtani, P.; Rodrigues, L.C.; Pimpin, L.; Watson, J.M.; Squire, S.B.; Zumla, A. Controversies and unresolved issues in tuberculosis prevention and control: A low-burden-country perspective. J. Infect. Dis. 2012, 205 (Suppl. 2), S293–S300. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Incidence of Tuberculosis. 2021. Available online: https://data.worldbank.org/indicator/SH.TBS.INCD?locations=JO (accessed on 20 September 2021).
- Syrian Center for Policy Research. Confronting Fragmentation: Impact of Syrian Crisis Report. 2016. Available online: https://www.alnap.org/help-library/confronting-fragmentation-impact-of-syrian-crisis-report (accessed on 27 June 2023).
- United Nations Office for the Coordination of Humanitarian Affairs. Syria Crisis: Bi-Weekly Situation Report, 17 October 2016. Geneva. 2016. Available online: https://www.humanitarianresponse.info/en/operations/whole-of-syria/document/syria-crisis-bi-weekly-situation-report-no15-17-october (accessed on 27 June 2023).
- Kimbrough, W.; Saliba, V.; Dahab, M.; Haskew, C.; Checchi, F. The burden of tuberculosis in crisis-affected populations: A systematic review. Lancet Infect. Dis. 2012, 12, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Heesterbeek, H.; Klinkenberg, D.; Hollingsworth, T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 2020, 395, 10228. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, L.; Fu, H.; Vesga, J.F.; Dowdy, D.; Pretorius, C.; Ahmedov, S.; Nair, S.A.; Mosneaga, A.; Masini, E.; Sahu, S.; et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine 2020, 28, 100603. [Google Scholar] [CrossRef] [PubMed]
- Golandaj, J.A. Insight into the COVID-19 led slow-down in TB notifications in India. Indian J. Tuberc. 2021, 68, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Shewade, H.D. The potential impact of the COVID-19 response related lockdown on TB incidence and mortality in India. Indian J. Tuberc. 2020, 67, S139–S146. [Google Scholar] [CrossRef] [PubMed]
- Loveday, M.; Cox, H.; Evans, D.; Furin, J.; Ndjeka, N.; Osman, M. Naidoo for the National Tb Think Tank ‘Optimising Tb Treatment Outcomes’ Task Team K. Opportunities from a new disease for an old threat: Extending COVID-19 efforts to address tuberculosis in South Africa. S. Afr. Med. J. 2020, 110, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Implementing the WHO Stop TB Strategy: A Handbook for National Tuberculosis Control Programmes. In Case Detection; WHO: Geneva, Switzerland, 2008; Volume 1. Available online: https://www.ncbi.nlm.nih.gov/books/NBK310769/ (accessed on 20 September 2021).
- Hudelson, P. Gender differentials in tuberculosis: The role of socio-economic and cultural factors. Tuber. Lung Dis. 1996, 77, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Thorson, A.; Diwan, V.K. Gender inequalities in tuberculosis: Aspects of infection, notification rates, and compliance. Curr. Opin. Pulm. Med. 2001, 7, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Allotey, P.; Gyapong, M. Gender in tuberculosis research. Int. J. Tuberc. Lung Dis. 2008, 12, 831–836. [Google Scholar] [PubMed]
- Holmes, C.B.; Hausler, H.; Nunn, P. A review of sex differences in the epidemiology of tuberculosis. Int. J. Tuberc. Lung Dis. 1998, 2, 96–104. [Google Scholar] [PubMed]
- Perumal, R.; Naidoo, K.; Padayatchi, N. TB epidemiology: Where are the young women? Know your tuberculosis epidemic, know your response. BMC Public Health 2018, 18, 417. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Hu, M.; Guo, W.; Hu, W.; Li, X.; Wang, S.; Shangguan, Y.; Zhang, Y.; Yang, S.; Xu, K. Prevalence trends of latent tuberculosis infection at the global, regional, and country levels from 1990–2019. Int. J. Infect. Dis. 2022, 122, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Hosten, E.; Mehta, M.; Andre, E.; Abu Rumman, K.; Van der Linden, D. Tuberculosis contact-tracing among Syrian refugee populations: Lessons from Jordan. Confl. Health 2018, 12, 25. [Google Scholar] [CrossRef] [PubMed]

| Variable | Gender | Total N = 1711 | p-Value | |||
|---|---|---|---|---|---|---|
| Women (n = 989) | Men (n = 722) | |||||
| n | % | n | % | |||
| Age (year) | <0.001 | |||||
| 0–9 | 40 | 4.0 | 52 | 7.2 | 92 | |
| 10–19 | 61 | 6.2 | 53 | 7.3 | 114 | |
| 20–29 | 325 | 32.9 | 137 | 19.0 | 462 | |
| 30–39 | 302 | 30.5 | 139 | 19.3 | 441 | |
| 40–49 | 125 | 12.6 | 118 | 16.3 | 243 | |
| 50–59 | 64 | 6.5 | 104 | 14.4 | 168 | |
| 60–69 | 37 | 3.7 | 62 | 8.6 | 99 | |
| ≥70 | 35 | 3.5 | 57 | 7.9 | 92 | |
| Marital Status | 0.038 | |||||
| Divorced | 6 | 0.6 | 1 | 0.1 | 7 | |
| Married | 678 | 68.6 | 464 | 64.3 | 1142 | |
| Single | 296 | 29.9 | 254 | 35.2 | 550 | |
| Widow | 9 | 0.9 | 3 | 0.4 | 12 | |
| Nationality | <0.001 | |||||
| Non-Jordanian | 556 | 56.2 | 326 | 45.2 | 882 | |
| Jordanian | 433 | 43.8 | 396 | 54.8 | 829 | |
| Site of TB | <0.001 | |||||
| Extra-pulmonary | 390 | 39.4 | 192 | 26.6 | 582 | |
| TB Pulmonary | 599 | 60.6 | 530 | 73.4 | 1129 | |
| Variable | Nationality | |||||
|---|---|---|---|---|---|---|
| Jordanians | Syrians | Other Nationalities | ||||
| n | % | n | % | n | % | |
| Gender | ||||||
| Women | 433 | 52.2 | 106 | 40.8 | 450 | 72.3 |
| Men | 396 | 47.8 | 154 | 59.2 | 172 | 27.7 |
| Age (year) | ||||||
| 0–9 | 54 | 6.5 | 25 | 9.6 | 13 | 2.1 |
| 10–19 | 70 | 8.4 | 24 | 9.2 | 20 | 3.2 |
| 20–29 | 160 | 19.3 | 25 | 9.6 | 277 | 44.5 |
| 30–39 | 170 | 20.5 | 61 | 23.5 | 210 | 33.8 |
| 40–49 | 122 | 14.7 | 44 | 16.9 | 77 | 12.4 |
| 50–59 | 107 | 12.9 | 44 | 16.9 | 17 | 2.7 |
| 60–69 | 76 | 9.2 | 19 | 7.3 | 4 | 0.6 |
| ≥70 | 70 | 8.4 | 18 | 6.9 | 4 | 0.6 |
| Marital Status | ||||||
| Divorced | 3 | 0.4 | 1 | 0.4 | 3 | 0.5 |
| Married | 547 | 66.0 | 171 | 65.8 | 424 | 68.2 |
| Single | 270 | 32.6 | 86 | 33.1 | 194 | 31.2 |
| Widow | 9 | 1.1 | 2 | 0.8 | 1 | 0.2 |
| Year at diagnosis | ||||||
| 2016 | 183 | 22.1 | 56 | 21.5 | 71 | 11.4 |
| 2017 | 206 | 24.8 | 74 | 28.5 | 47 | 7.6 |
| 2018 | 153 | 18.5 | 62 | 23.8 | 167 | 26.8 |
| 2019 | 179 | 21.6 | 35 | 13.5 | 235 | 37.8 |
| 2020 | 108 | 13.0 | 33 | 12.7 | 102 | 16.4 |
| Site of TB | ||||||
| Extra-pulmonary | 429 | 51.7 | 53 | 20.4 | 100 | 16.1 |
| Pulmonary TB | 400 | 48.3 | 207 | 79.6 | 522 | 83.9 |
| Notification Rate per 100,000 Population | |||
|---|---|---|---|
| Age | Women | Men | Total |
| 0–9 | 0.66 | 0.82 | 0.76 |
| 10–19 | 1.2 | 0.96 | 1.08 |
| 20–29 | 7.54 | 2.58 | 4.8 |
| 30–39 | 8.76 | 3.44 | 5.88 |
| 40–49 | 4.9 | 3.88 | 4.34 |
| 50–59 | 4.22 | 6.12 | 5.22 |
| 60–69 | 4.6 | 7.46 | 6.04 |
| 70+ | 6.12 | 9.56 | 7.88 |
| Total | 4.08 | 2.64 | 3.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khader, Y.; Abaza, H.; Satyanarayana, S.; Abu Rumman, A.S.; Alyousfi, M.N. Tuberculosis Notification in Jordan, 2016–2020. Epidemiologia 2023, 4, 276-285. https://doi.org/10.3390/epidemiologia4030028
Khader Y, Abaza H, Satyanarayana S, Abu Rumman AS, Alyousfi MN. Tuberculosis Notification in Jordan, 2016–2020. Epidemiologia. 2023; 4(3):276-285. https://doi.org/10.3390/epidemiologia4030028
Chicago/Turabian StyleKhader, Yousef, Hiba Abaza, Srinath Satyanarayana, Ahmad Saleh Abu Rumman, and Mohamad Nihad Alyousfi. 2023. "Tuberculosis Notification in Jordan, 2016–2020" Epidemiologia 4, no. 3: 276-285. https://doi.org/10.3390/epidemiologia4030028
APA StyleKhader, Y., Abaza, H., Satyanarayana, S., Abu Rumman, A. S., & Alyousfi, M. N. (2023). Tuberculosis Notification in Jordan, 2016–2020. Epidemiologia, 4(3), 276-285. https://doi.org/10.3390/epidemiologia4030028







