Imaging the Morphological Structure of Silk Fibroin Constructs through Fluorescence Energy Transfer and Confocal Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silk Fibroin Degumming
2.2. Preparation of the Solution and Fluorescent Fibroin Films
2.3. Preparation of Silk Fibroin Mats by Electrospinning
2.4. Characterization
3. Results
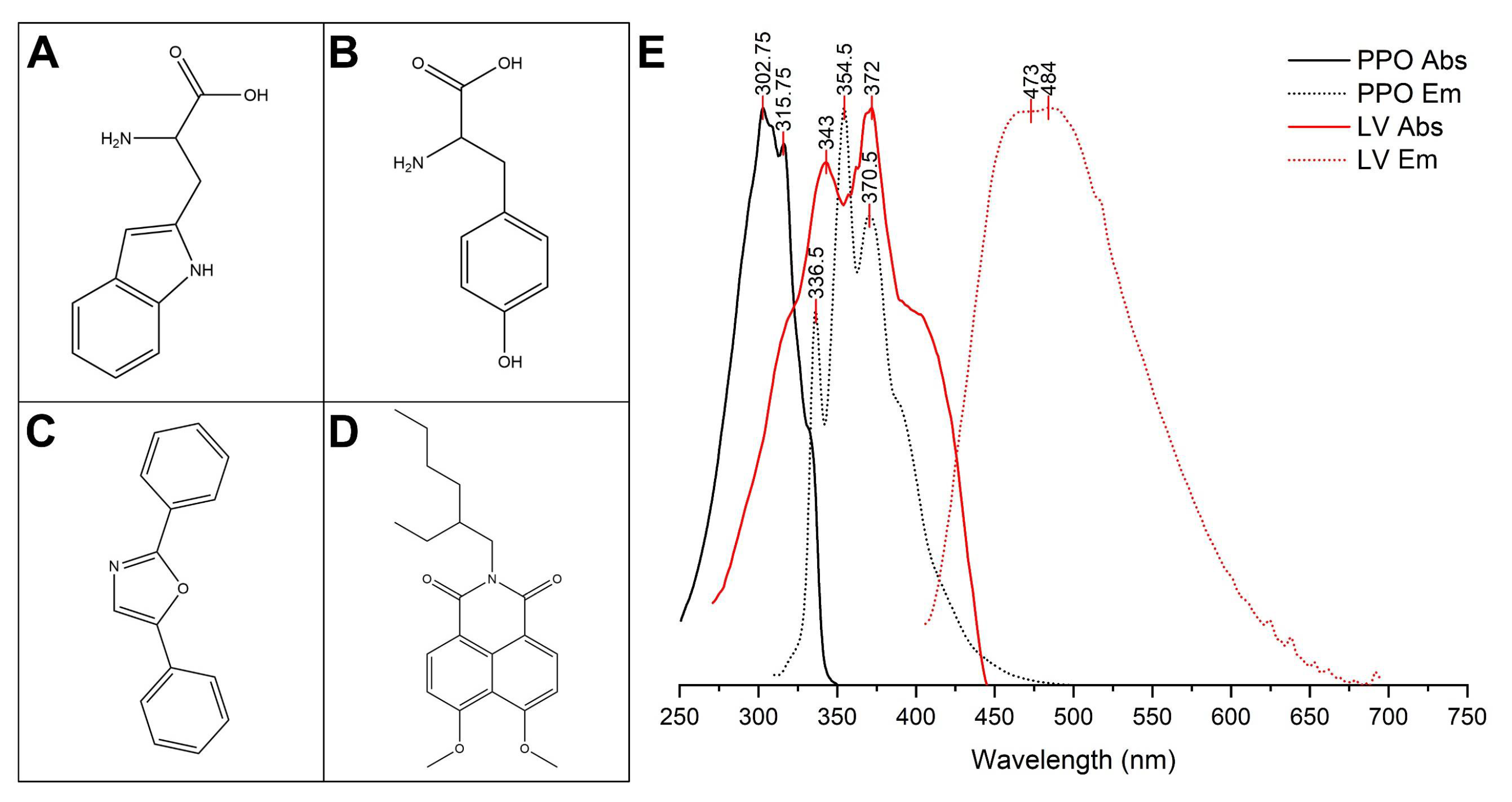
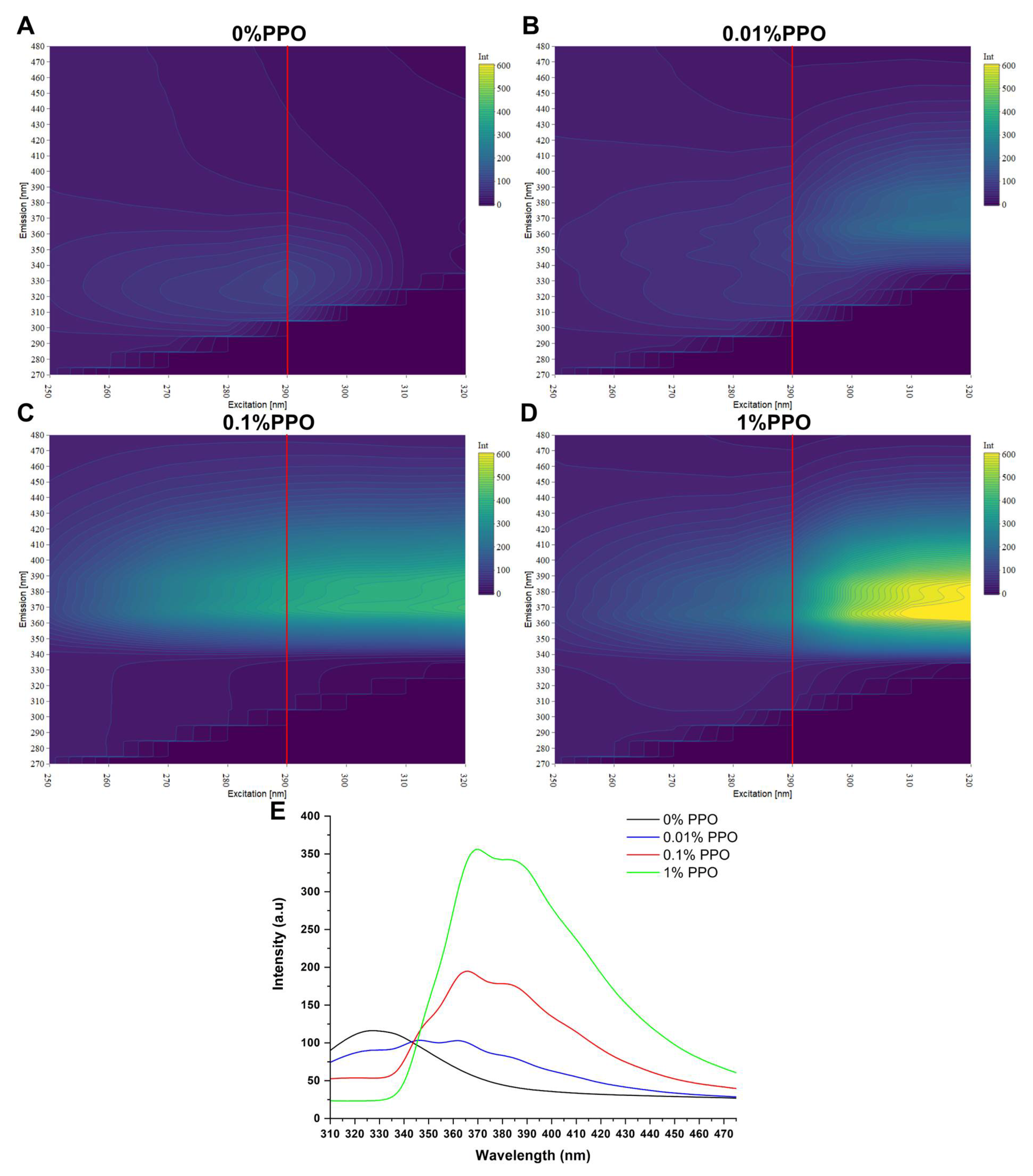
3.1. Emission Spectra
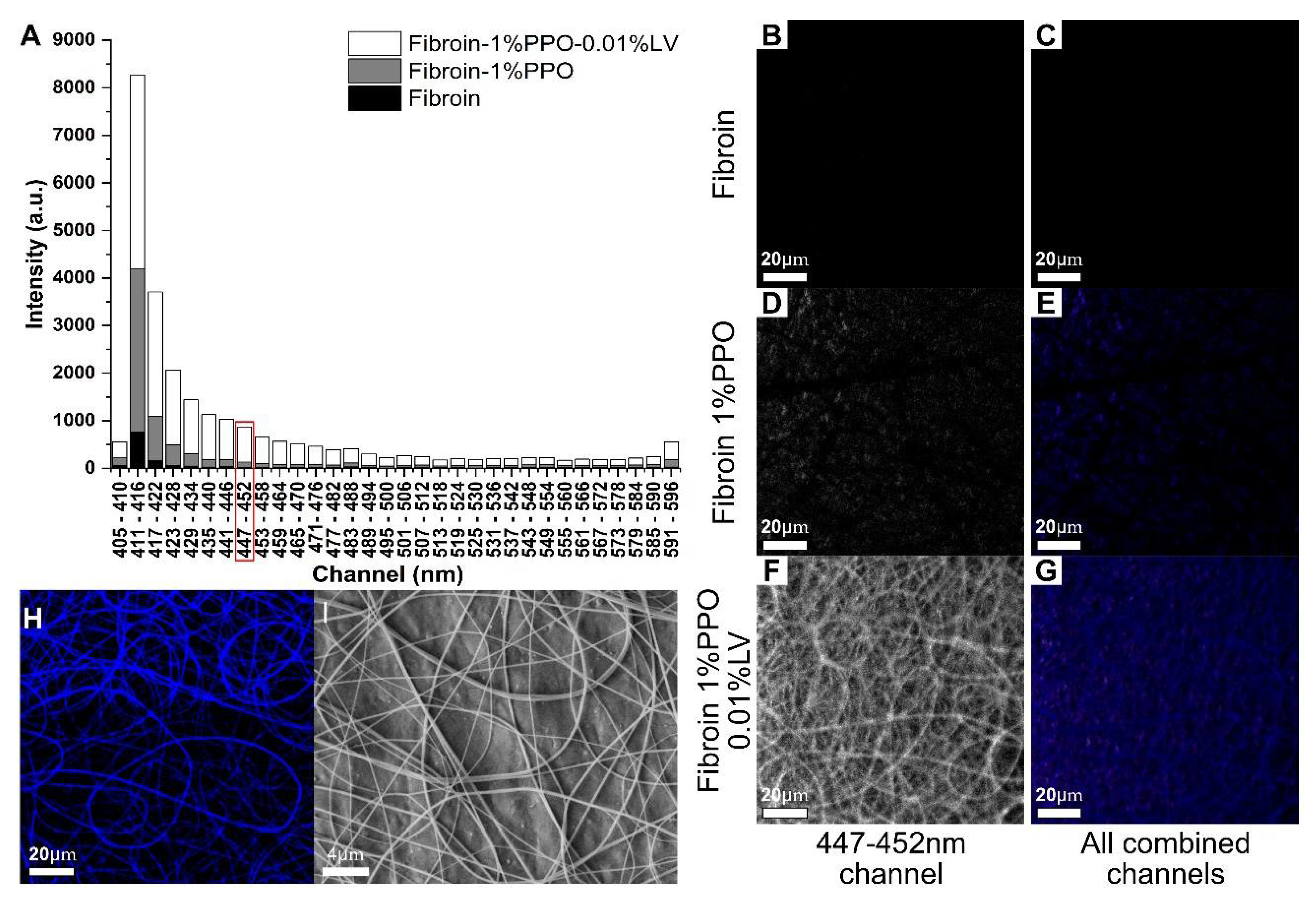
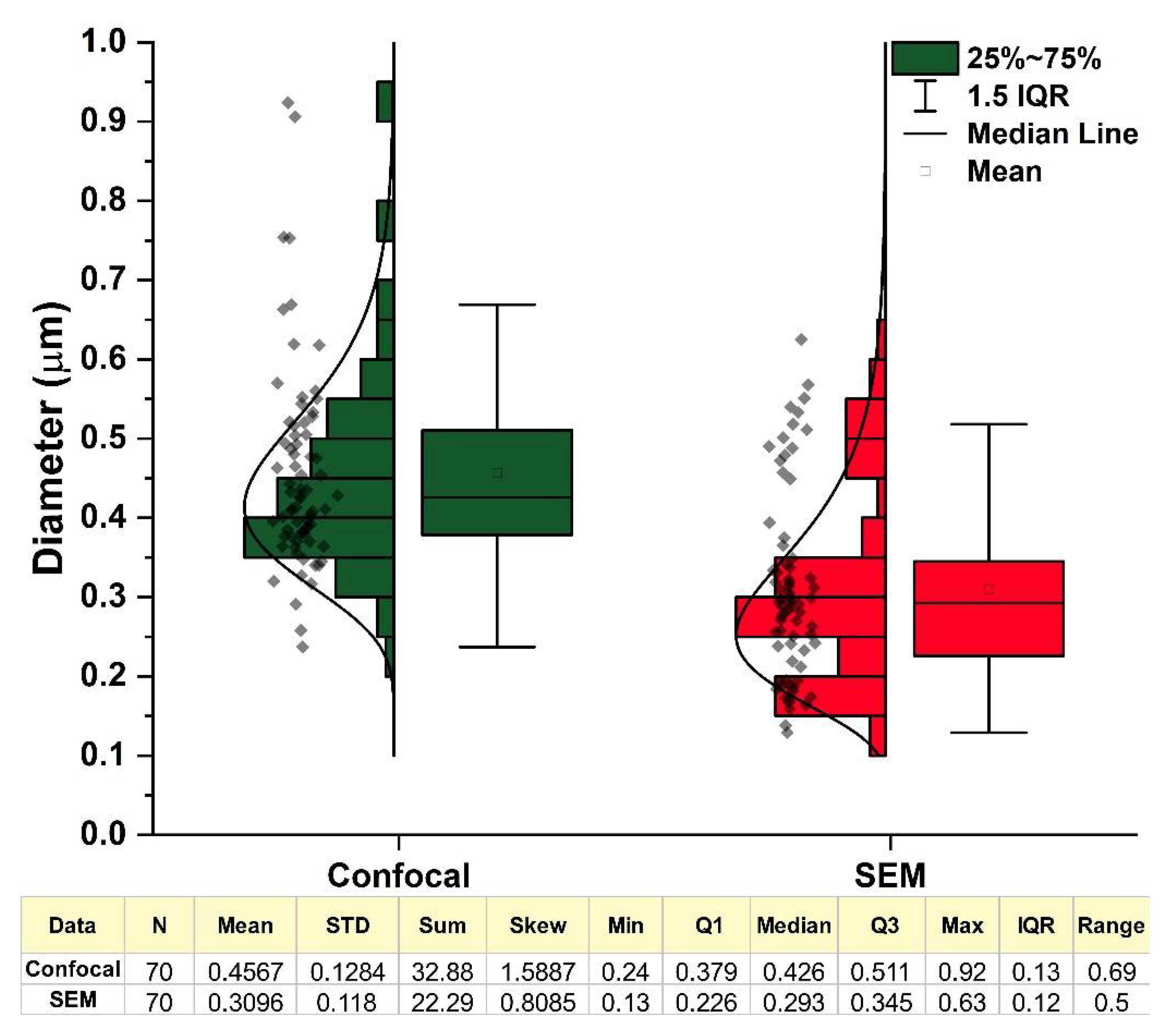
3.2. Confocal Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.J.; Ganbat, D.; Aramwit, P.; Bucciarelli, A.; Chen, J.; Migliaresi, C.; Motta, A. Processing keratin from camel hair and cashmere with ionic liquids. Express Polym. Lett. 2019, 13. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Burgo, L.S.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Bucciarelli, A.; Greco, G.; Corridori, I.; Pugno, N.M.; Motta, A. A Design of Experiment Rational Optimization of the Degumming Process and Its Impact on the Silk Fibroin Properties. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Hwi Cho, H.; Young Been, S.; Youp Kim, W.; Min Choi, J.; Hee Choi, J.; Ui Song, C.; Eun Song, J.; Bucciarelli, A.; Khang, G. Comparative Study on the Effect of the Different Harvesting Sources of Demineralized Bone Particles on the Bone Regeneration of a Composite Gellan Gum Scaffold for Bone Tissue Engineering Applications. ACS Appl. Bio Mater. 2021. [Google Scholar] [CrossRef]
- Been, S.; Choi, J.; Cho, H.; Jeon, G.; Song, J.E.; Bucciarelli, A.; Khang, G. Preparation and characterization of a soluble eggshell membrane/agarose composite scaffold with possible applications in cartilage regeneration. J. Tissue Eng. Regen. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, A.; Muthukumar, T.; Kim, J.S.; Kim, W.K.; Quaranta, A.; Maniglio, D.; Khang, G.; Motta, A. Preparation and Statistical Characterization of Tunable Porous Sponge Scaffolds using UV Cross-linking of Methacrylate-Modified Silk Fibroin. ACS Biomater. Sci. Eng. 2019, 5, 6374–6388. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, A.; Chiera, S.; Quaranta, A.; Yadavalli, V.K.; Motta, A.; Maniglio, D. A Thermal-Reflow-Based Low-Temperature, High-Pressure Sintering of Lyophilized Silk Fibroin for the Fast Fabrication of Biosubstrates. Adv. Funct. Mater. 2019, 29, 1901134. [Google Scholar] [CrossRef]
- Cho, H.; Bucciarelli, A.; Kim, W.; Jeong, Y.; Kim, N.; Jung, J.; Yoon, S.; Khang, G. Natural Sources and Applications of Demineralized Bone Matrix in the Field of Bone and Cartilage Tissue Engineering. In Bioinspired Biomaterials. Advances in Experimental Medicine and Biology; Chun, H.J., Reis, R.L., Motta, A., Khang, G., Eds.; Springer: Singapore, 2020; pp. 3–14. [Google Scholar]
- Zhu, B.; Wang, H.; Leow, W.R.; Cai, Y.; Loh, X.J.; Han, M.Y.; Chen, X. Silk Fibroin for Flexible Electronic Devices. Adv. Mater. 2016, 28, 4250–4265. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, A.; Pal, R.K.; Maniglio, D.; Quaranta, A.; Mulloni, V.; Motta, A.; Yadavalli, V.K. Fabrication of Nanoscale Patternable Films of Silk Fibroin Using Benign Solvents. Macromol. Mater. Eng. 2017, 302, 1700110. [Google Scholar] [CrossRef]
- Bucciarelli, A.; Mulloni, V.; Maniglio, D.; Pal, R.K.; Yadavalli, V.K.; Motta, A.; Quaranta, A. A comparative study of the refractive index of silk protein thin films towards biomaterial based optical devices. Opt. Mater. 2018, 78, 407–414. [Google Scholar] [CrossRef]
- Lee, O.J.; Sultan, M.T.; Hong, H.; Lee, Y.J.; Lee, J.S.; Lee, H.; Kim, S.H.; Park, C.H. Recent Advances in Fluorescent Silk Fibroin. Front. Mater. 2020, 7, 50. [Google Scholar] [CrossRef]
- Georgakoudi, I.; Tsai, I.; Greiner, C.; Wong, C.; Defelice, J.; Kaplan, D. Intrinsic fluorescence changes associated with the conformational state of silk fibroin in biomaterial matrices. Opt. Express 2007, 15, 1043–1053. [Google Scholar] [CrossRef]
- Amirikia, M.; Shariatzadeh, S.M.A.; Jorsaraei, S.G.A.; Mehranjani, M.S. Auto-fluorescence of a silk fibroin-based scaffold and its interference with fluorophores in labeled cells. Eur. Biophys. J. 2018, 47, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Paddock, S.W. Principles and practices of laser scanning confocal microscopy. Appl. Biochem. Biotechnol. Part B Mol. Biotechnol. 2000, 16, 127–149. [Google Scholar] [CrossRef]
- Hussain, S.A. An Introduction to Fluorescence Resonance Energy Transfer (FRET). Energy 2009, 132, 4. [Google Scholar]
- Preus, S.; Wilhelmsson, L.M. Advances in Quantitative FRET-Based Methods for Studying Nucleic Acids. ChemBioChem 2012, 13, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Sekar, R.B.; Periasamy, A. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J. Cell Biol. 2003, 160, 629–633. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Huang, X.X.; Kong, D.M.; Shen, H.X.; Liu, Y. Ultrasensitive, high temperature and ionic strength variation-tolerant Cu2+ fluorescent sensor based on reconstructed Cu2+-dependent DNAzyme/substrate complex. Biosens. Bioelectron. 2013, 42, 225–228. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, S.; Lu, W.; Gao, B.; Xu, L.; Sun, X.; Jiang, D.; Xu, H.-J.; Ma, M.; Cao, F. A novel fluorescence “turn off-on” nano-sensor for detecting Cu2+ and Cysteine in living cells. J. Photochem. Photobiol. A Chem. 2018, 362, 14–20. [Google Scholar] [CrossRef]
- Danos, L.; Parel, T.; Markvart, T.; Barrioz, V.; Brooks, W.S.M.; Irvine, S.J.C. Increased efficiencies on CdTe solar cells via luminescence down-shifting with excitation energy transfer between dyes. Sol. Energy Mater. Sol. Cells 2012, 98, 486–490. [Google Scholar] [CrossRef]
- Quaranta, A.; Carturan, S.; Marchi, T.; Cinausero, M.; Scian, C.; Kravchuk, V.L.; Degerlier, M.; Gramegna, F.; Poggi, M.; Maggioni, G. Doping of Polysiloxane Rubbers for the Production of Organic Scintillators. In Optical Materials; Elsevier B.V.: Amsterdam, The Netherlands, 2010; Volume 32, pp. 1317–1320. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R foundation: Vienna, Austria, 2019. [Google Scholar]
- Plotly Technologies Inc. Collaborative Data Science. Available online: https://plot.ly (accessed on 30 March 2021).
- Schneider, C.A.; Rasband, W.S.; Eliceri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.D.; Cheng, Y.; Teng, C.P.; Khin, Y.W.; Loh, X.J.; Tee, S.Y.; Low, M.; Ye, E.; Yu, H.D.; Zhang, Y.W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Wang, H.-Y.Y.; Zhang, Y.-Q.Q. Effect of regeneration of liquid silk fibroin on its structure and characterization. Soft Matter 2013, 9, 138–145. [Google Scholar] [CrossRef]
- Min, K.; Kim, S.; Kim, C.G.; Kim, S. Colored and fluorescent nanofibrous silk as a physically transient chemosensor and vitamin deliverer. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Myung, S.J.; Kim, H.-S.; Kim, Y.; Chen, P.; Jin, H.-J. Fluorescent silk fibroin nanoparticles prepared using a reverse microemulsion. Macromol. Res. 2008, 16, 604–608. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.; Park, Y.H.; Hudson, S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int. J. Biol. Macromol. 2001, 29, 91–97. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.Y.; Lee, K.G.; Park, Y.H. The role of formic acid in solution stability and crystallization of silk protein polymer. Int. J. Biol. Macromol. 2003, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Meng, Z.; Toh, G.W.; Zhen, Y.; Diao, Y.; Xu, H.; Liu, X.Y. Engineering of fluorescent emission of silk fibroin composite materials by material assembly. Small 2015, 11, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Ming, J.; Pan, F.; Ning, X. Fabrication of silk fibroin fluorescent nanofibers via electrospinning. Polymers 2019, 11, 986. [Google Scholar] [CrossRef] [Green Version]
- Khalid, A.; Bai, D.; Abraham, A.; Jadhav, A.; Linklater, D.; Matusica, A.; Nguyen, D.; Murdoch, B.J.; Zakhartchouk, N.; Dekiwadia, C.; et al. Electrospun nanodiamond-silk fibroin membranes: A multifunctional platform for biosensing and wound healing applications. ACS Appl. Mater. Interfaces 2020, 12, 48408–48419. [Google Scholar] [CrossRef] [PubMed]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core–Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Lee, O.J.; Kim, S.W.; Ki, C.S.; Chao, J.R.; Yoo, H.; Yoon, S.-I.; Lee, J.E.; Park, Y.R.; Kweon, H.Y.; et al. Novel fabrication of fluorescent silk utilized in biotechnological and medical applications. Biomaterials 2015, 70, 48–56. [Google Scholar] [CrossRef]
- Hu, F.; Lin, N.; Liu, X.Y. Interplay between Light and Functionalized Silk Fibroin and Applications. Iscience 2020, 23, 101035. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, A.; Reddy Chandraiahgari, C.; Adami, A.; Mulloni, V.; Lorenzelli, L. Precise dot inkjet printing thought multifactorial statistical optimization of the piezoelectric actuator waveform. Flex. Print. Electron. 2020, 5, 045002. [Google Scholar] [CrossRef]
- Bucciarelli, A.; Adami, A.; Chandaiahgari, C.R.; Lorenzelli, L. Multivariable Optimization of Inkjet Printing Process of Ag Nanoparticle Ink on Kapton. In Proceedings of the 2020 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 16–19 August 2020; pp. 1–4. [Google Scholar]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucciarelli, A.; Quaranta, A.; Maniglio, D. Imaging the Morphological Structure of Silk Fibroin Constructs through Fluorescence Energy Transfer and Confocal Microscopy. Electron. Mater. 2021, 2, 186-197. https://doi.org/10.3390/electronicmat2020015
Bucciarelli A, Quaranta A, Maniglio D. Imaging the Morphological Structure of Silk Fibroin Constructs through Fluorescence Energy Transfer and Confocal Microscopy. Electronic Materials. 2021; 2(2):186-197. https://doi.org/10.3390/electronicmat2020015
Chicago/Turabian StyleBucciarelli, Alessio, Alberto Quaranta, and Devid Maniglio. 2021. "Imaging the Morphological Structure of Silk Fibroin Constructs through Fluorescence Energy Transfer and Confocal Microscopy" Electronic Materials 2, no. 2: 186-197. https://doi.org/10.3390/electronicmat2020015
APA StyleBucciarelli, A., Quaranta, A., & Maniglio, D. (2021). Imaging the Morphological Structure of Silk Fibroin Constructs through Fluorescence Energy Transfer and Confocal Microscopy. Electronic Materials, 2(2), 186-197. https://doi.org/10.3390/electronicmat2020015





