Extending the Color Retention of an Electrochromic Device by Immobilizing Color Switching and Ion-Storage Complementary Layers
Abstract
:1. Introduction
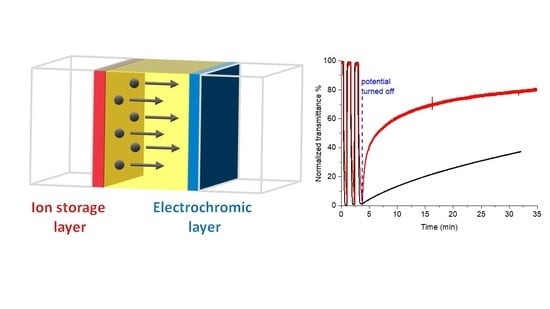
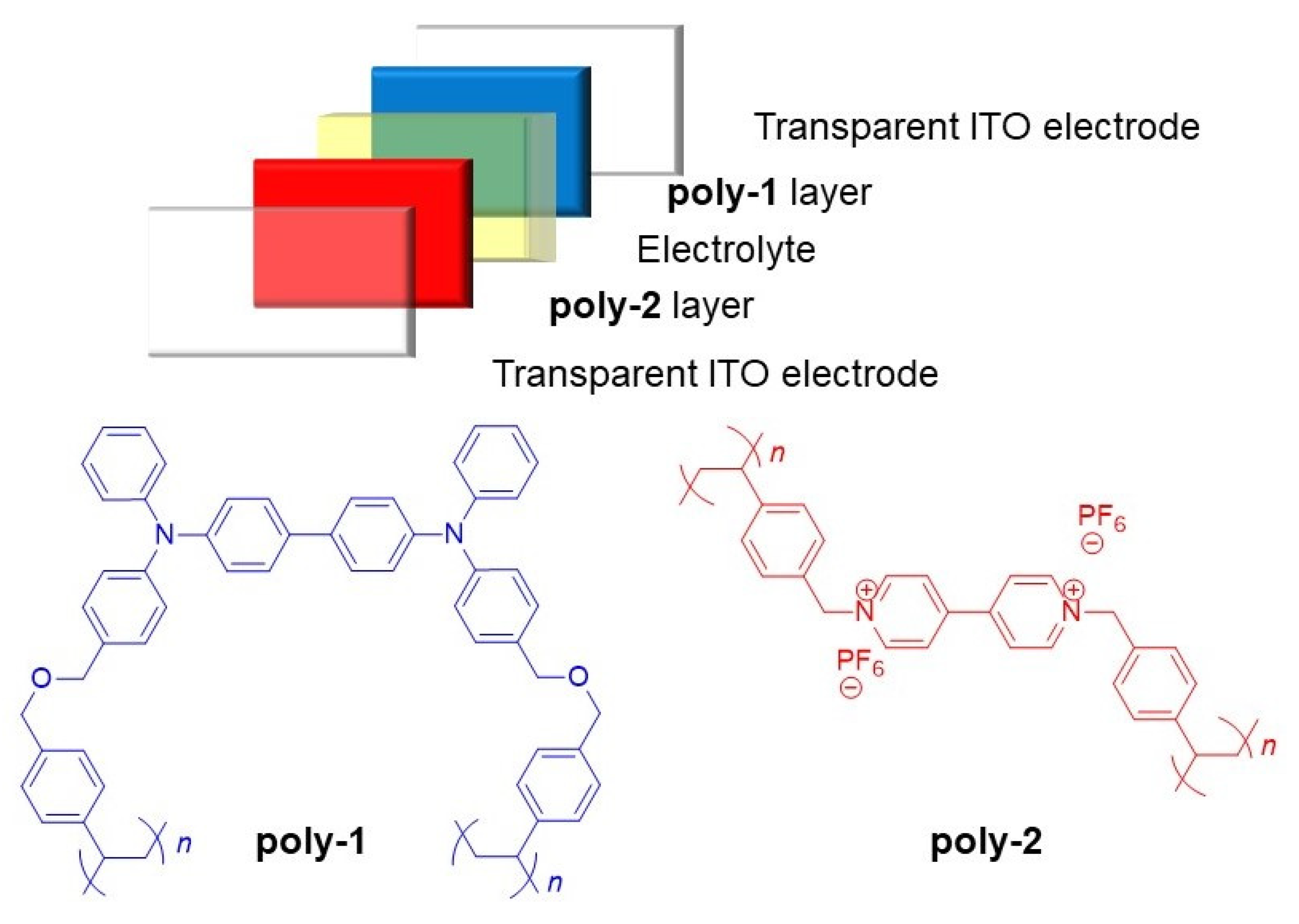
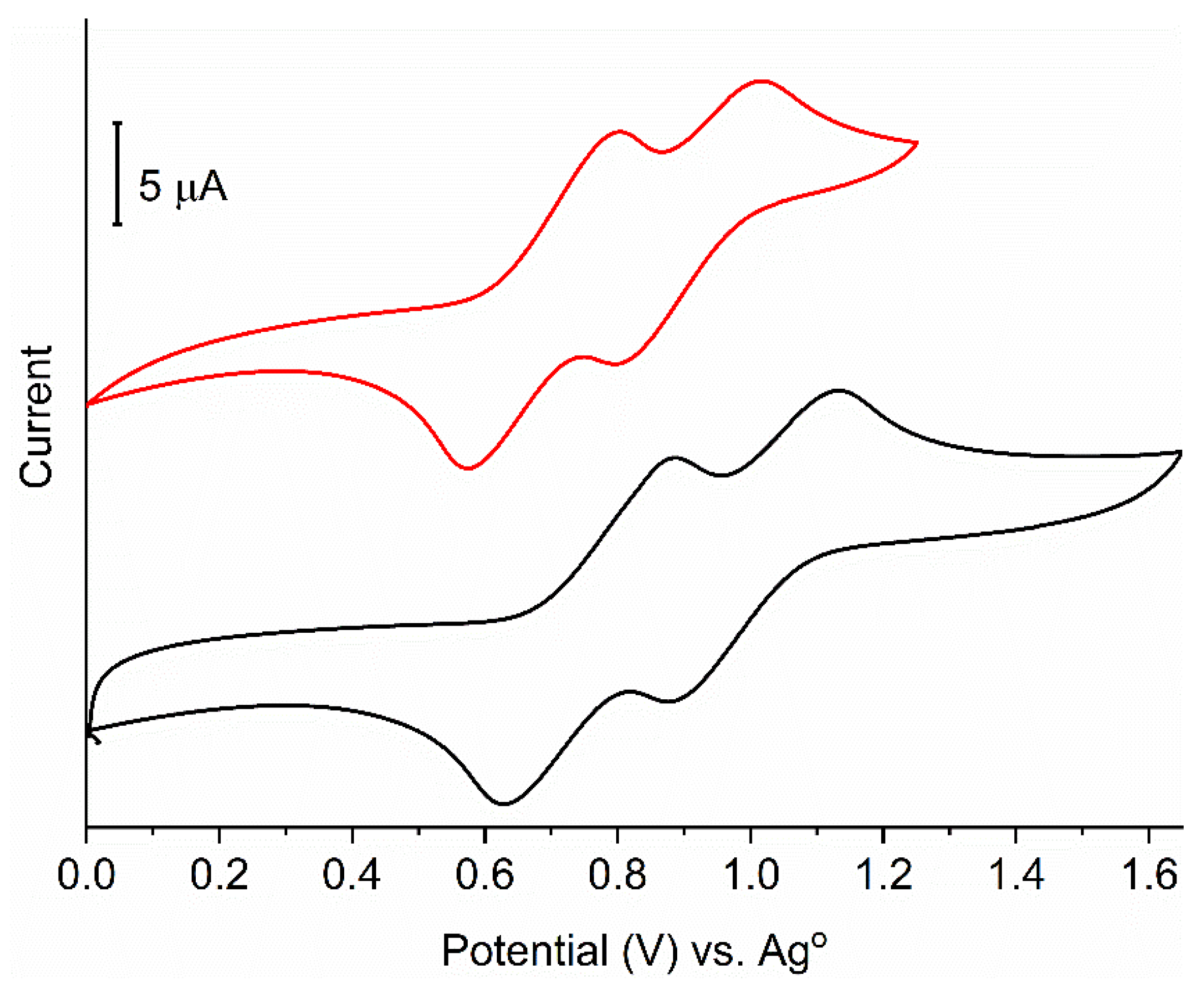
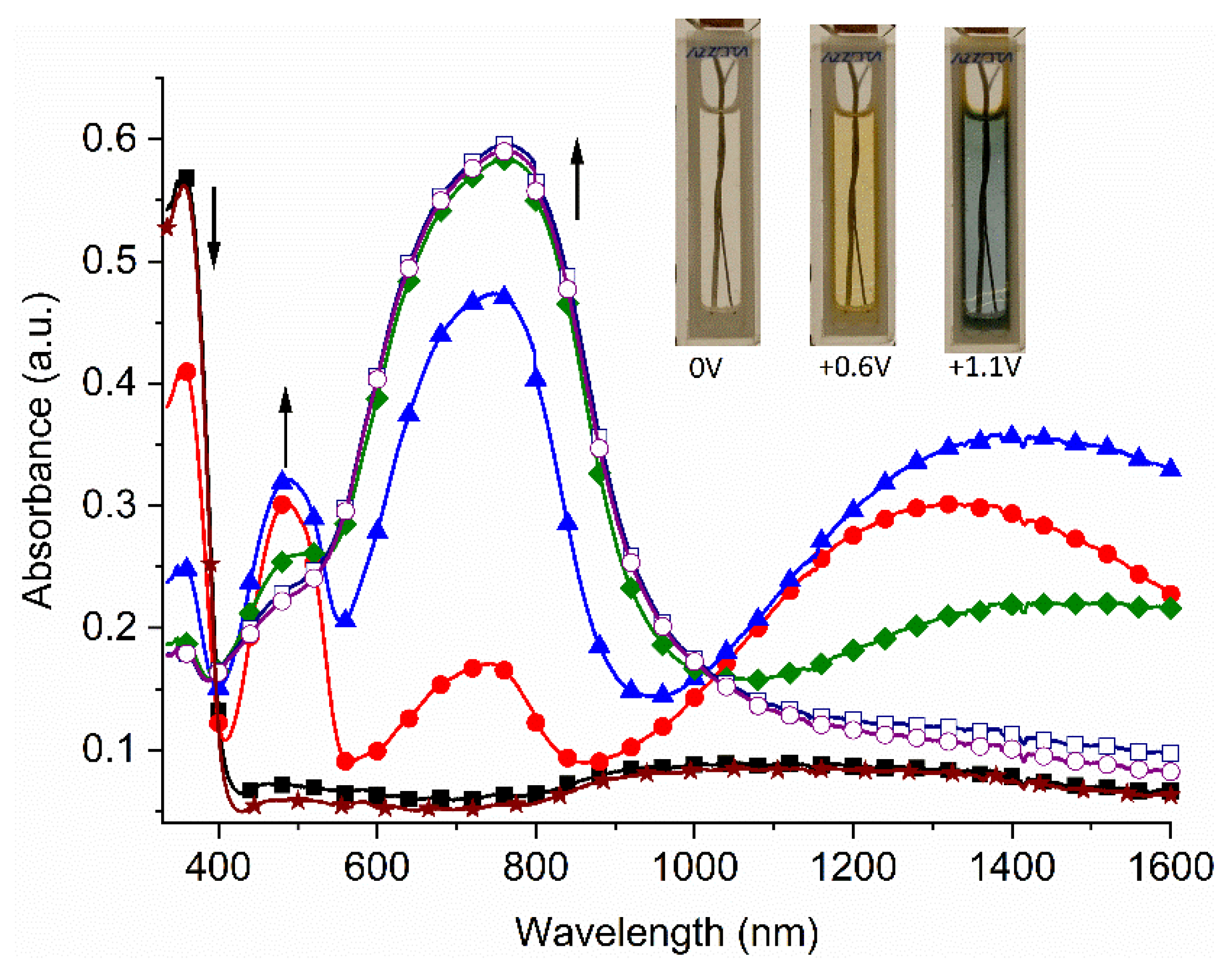
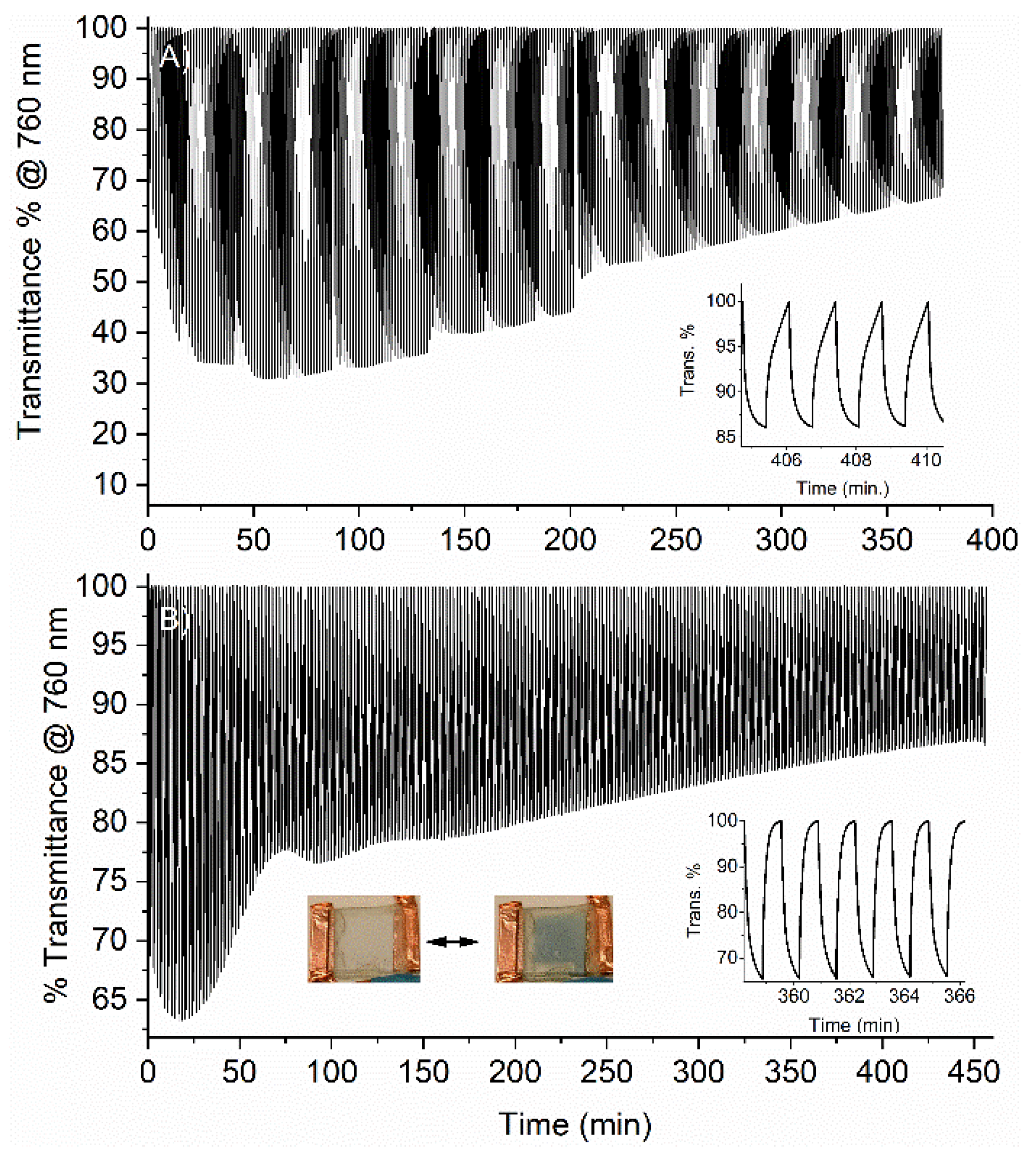
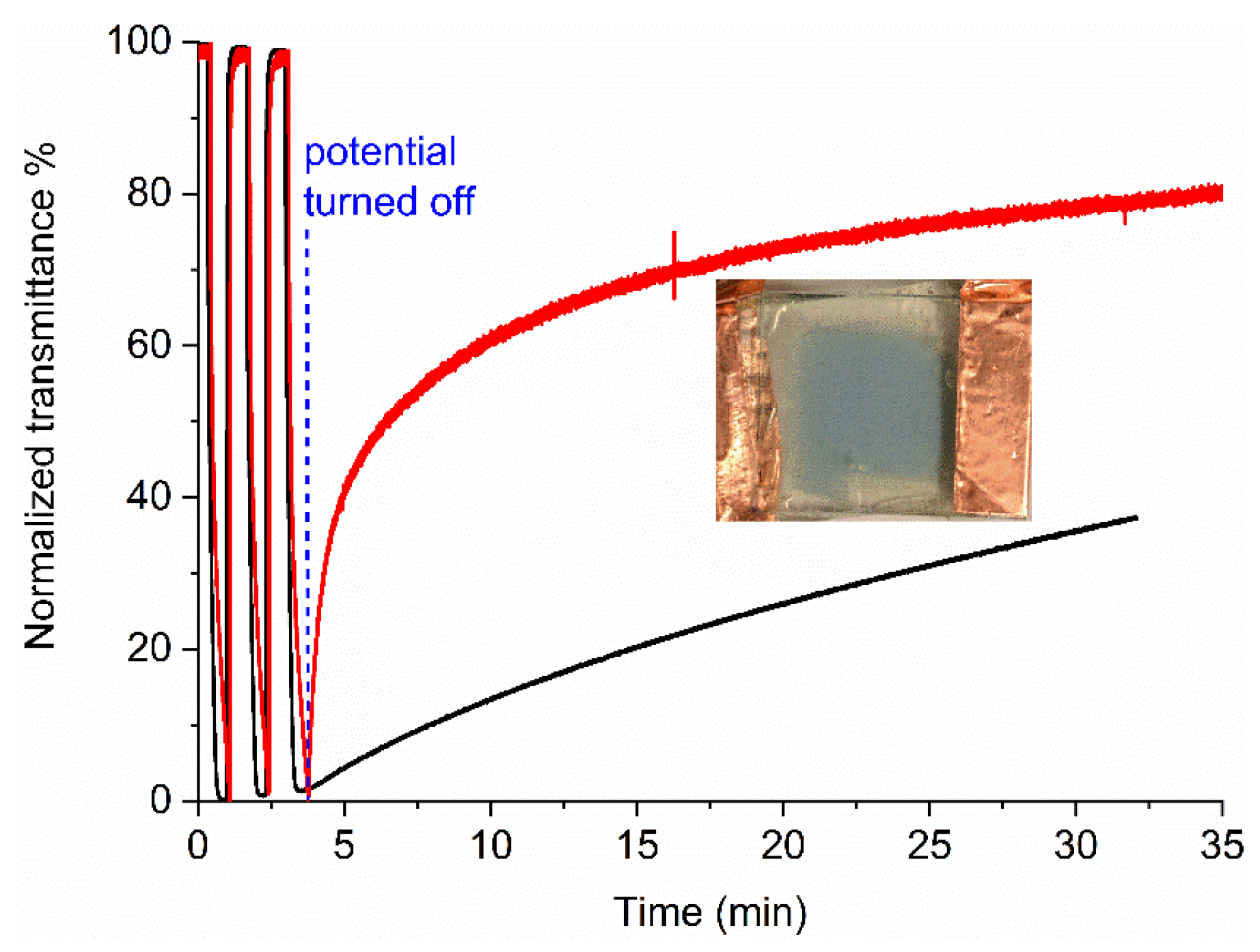
2. Results and Discussion
3. Materials and Methods
3.1. Electrochemistry
3.2. Spectroelectrochemistry
3.3. Polymerization
3.4. Film Thickness Measurements
3.5. Device Fabrication
3.6. Synthesis (See the Reaction Scheme in the Supporting Information for the Corresponding Numbering of the Compounds)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yen, H.-J.; Liou, G.-S. Recent Advances in Triphenylamine-Based Electrochromic Derivatives and Polymers. Polym. Chem. 2018, 9, 3001–3018. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liao, W.-K.; Liou, G.-S. A Comparative Study of Redox-Active, Ambipolar Electrochromic Triphenylamine-Based Polyimides Prepared by Electrochemical Polymerization and Conventional Polycondensation Methods. Polym. Chem. 2018, 9, 236–248. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chen, Y.-Z. Electroactive and Ambipolar Electrochromic Polyimides from Arylene Diimides with Triphenylamine N-Substituents. Dye Pigment 2017, 144, 173–183. [Google Scholar] [CrossRef]
- Huang, Q.; Evmenenko, G.A.; Dutta, P.; Lee, P.; Armstrong, N.R.; Marks, T.J. Covalently Bound Hole-Injecting Nanostructures. Systematics of Molecular Architecture, Thickness, Saturation, and Electron-Blocking Characteristics on Organic Light-Emitting Diode Luminance, Turn-on Voltage, and Quantum Efficiency. J. Am. Chem. Soc. 2005, 127, 10227–10242. [Google Scholar] [CrossRef]
- Huang, Q.; Evmenenko, G.; Dutta, P.; Marks, T.J. Molecularly “Engineered” Anode Adsorbates for Probing OLED Interfacial Structure-Charge Injection/Luminance Relationships: Large, Structure-Dependent Effects. J. Am. Chem. Soc. 2003, 125, 14704–14705. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; You, J.; Zhang, Y.; Li, X. Solution-Processed Thermally Stable Amorphous Films of Small Molecular Hole Injection/Transport Bi-Functional Materials and Their Application in High Efficiency OLEDs. J. Mater. Chem. C 2015, 3, 11377–11384. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Gong, Y.; Chen, S.; Shen, X.Y.; Lam, J.W.Y.; Lu, P.; Lu, Y.; Wang, Z.; Hu, R.; Xie, N.; et al. Efficient Solid Emitters with Aggregation-Induced Emission and Intramolecular Charge Transfer Characteristics: Molecular Design, Synthesis, Photophysical Behaviors, and OLED Application. Chem. Mater. 2012, 24, 1518–1528. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Lu, P.; Chen, S.; Lam, J.W.Y.; Wang, Z.; Liu, Y.; Kwok, H.S.; Ma, Y.; Tang, B.Z. Changing the Behavior of Chromophores from Aggregation-Caused Quenching to Aggregation-Induced Emission: Development of Highly Efficient Light Emitters in the Solid State. Adv. Mater. 2010, 22, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kuwano, S.; Sawaki, Y.; Fujikawa, H.; Noda, K.; Taga, Y.; Takagi, K. New 9-Fluorene-Type Trispirocyclic Compounds for Thermally Stable Hole Transport Materials in OLEDs. J. Mater. Chem. 2005, 15, 2393–2398. [Google Scholar] [CrossRef]
- Bacher, E.; Bayerl, M.; Rudati, P.; Reckefuss, N.; Müller, C.D.; Meerholz, K.; Nuyken, O. Synthesis and Characterization of Photo-Cross-Linkable Hole-Conducting Polymers. Macromolecules 2005, 38, 1640–1647. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, L.; Zhang, F.; You, J.; Xiao, Y.; Li, D.; Wang, S.; Meng, Q.; Li, X. Stable Perovskite Solar Cells based on Hydrophobic Triphenylamine Hole-Transport Materials. Energy Technol. 2017, 5, 312–320. [Google Scholar] [CrossRef]
- Song, Y.; Lv, S.; Liu, X.; Li, X.; Wang, S.; Wei, H.; Li, D.; Xiao, Y.; Meng, Q. Energy Level Tuning of TPB-Based Hole-Transporting Materials for Highly Efficient Perovskite Solar Cells. Chem. Commun. 2014, 50, 15239–15242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, T.; Zhang, Y.; Wang, S.; Li, X.; Xiao, Y.; Hou, X.; Liu, Z.; Shi, W.; Dennis, T.J.S. Organic Single-Crystalline Donor–Acceptor Heterojunctions with Ambipolar Band-Like Charge Transport for Photovoltaics. Adv. Mater. Interfaces 2018, 5, 1800336. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Design and Preparation of Triphenylamine-Based Polymeric Materials towards Emergent Optoelectronic Applications. Prog. Polym. Sci. 2019, 89, 250–287. [Google Scholar] [CrossRef]
- Jarosz, T.; Gebka, K.; Stolarczyk, A.; Domagala, W. Transparent to Black Electrochromism—The “Holy Grail” of Organic Optoelectronics. Polymers 2019, 11, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santra, D.C.; Nad, S.; Malik, S. Electrochemical Polymerization of Triphenylamine End-Capped Dendron: Electrochromic and Electrofluorochromic Switching Behaviors. J. Electroanal. Chem. 2018, 823, 203–212. [Google Scholar] [CrossRef]
- Ji, L.; Dai, Y.; Yan, S.; Lv, X.; Su, C.; Xu, L.; Lv, Y.; Ouyang, M.; Chen, Z.; Zhang, C. A Fast Electrochromic Polymer Based on TEMPO Substituted Polytriphenylamine. Sci. Rep. 2016, 6, 30068. [Google Scholar] [CrossRef]
- Moon, H.C.; Kim, C.-H.; Lodge, T.P.; Frisbie, C.D. Multicolored, Low-Power, Flexible Electrochromic Devices Based on Ion Gels. ACS Appl. Mater. Interfaces 2016, 8, 6252–6260. [Google Scholar] [CrossRef]
- Gélinas, B.; Das, D.; Rochefort, D. Air-Stable, Self-Bleaching Electrochromic Device Based on Viologen- and Ferrocene-Containing Triflimide Redox Ionic Liquids. ACS Appl. Mater. Interfaces 2017, 9, 28726–28736. [Google Scholar] [CrossRef]
- Ho, K.-C.; Lu, H.-C.; Yu, H.-F. Chapter 12 Viologens-based Electrochromic Materials and Devices. In Electrochromic Smart Materials: Fabrication and Applications; Xu, J.W., Chua, M.H., Shah, K.W., Eds.; The Royal Society of Chemistry: London, UK, 2019; pp. 372–405. [Google Scholar]
- Granqvist, C.-G. Out of a Niche. Nat. Mater. 2006, 5, 89–90. [Google Scholar] [CrossRef]
- Heusing, S.; Aegerter, M.A. Sol-Gel Coatings for Electrochromic Devices. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–49. [Google Scholar]
- Eh, A.L.-S.; Tan, A.W.M.; Cheng, X.; Magdassi, S.; Lee, P.S. Recent Advances in Flexible Electrochromic Devices: Prerequisites, Challenges, and Prospects. Energy Technol. 2018, 6, 33–45. [Google Scholar] [CrossRef]
- Liu, H.-S.; Pan, B.-C.; Huang, D.-C.; Kung, Y.-R.; Leu, C.-M.; Liou, G.-S. Highly Transparent to Truly Black Electrochromic Devices Based on an Ambipolar System of Polyamides and Viologen. NPG Asia Mater. 2017, 9, e388. [Google Scholar] [CrossRef]
- Wu, J.-T.; Lin, H.-T.; Liou, G.-S. Synthesis and Characterization of Novel Triarylamine Derivatives with Dimethylamino Substituents for Application in Optoelectronic Devices. ACS Appl. Mater. Interfaces 2019, 11, 14902–14908. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhao, W.; Wang, L.; Li, F.; Wang, W.; Liu, S.; Huang, W.; Zhao, Q. Soluble Triarylamine Functionalized Symmetric Viologen for all-solid-state electrochromic supercapacitors. Sci. China Chem. 2020, 63, 1632–1644. [Google Scholar] [CrossRef]
- Cospito, S.; De Simone, B.C.; Beneduci, A.; Imbardelli, D.; Chidichimo, G. Novel Electrochromic Gel with High Optical Contrast in the Visible and Near-Infrared. Mater. Chem. Phys. 2013, 140, 431–434. [Google Scholar] [CrossRef]
- Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. Viologen-Based Electrochromic Materials and Devices. J. Mater. Chem. C 2019, 7, 4622–4637. [Google Scholar] [CrossRef]
- Ding, J.; Zheng, C.; Wang, L.; Lu, C.; Zhang, B.; Chen, Y.; Li, M.; Zhai, G.; Zhuang, X. Viologen-Inspired Functional Materials: Synthetic Strategies and Applications. J. Mater. Chem. A 2019, 7, 23337–23360. [Google Scholar] [CrossRef]
- Hua, C.; Chan, B.; Rawal, A.; Tuna, F.; Collison, D.; Hook, J.M.; D’Alessandro, D.M. Redox Tunable Viologen-Based Porous Organic Polymers. J. Mater. Chem. C 2016, 4, 2535–2544. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Yang, S.; Zheng, J.; Xu, C. Electrochromic Device Based on Adsorbable Viologens. Acta Chim. Sin. 2014, 72, 456–460. [Google Scholar] [CrossRef] [Green Version]
- Yen, H.-J.; Tsai, C.-L.; Chen, S.-H.; Liou, G.-S. Electrochromism and Nonvolatile Memory Device Derived from Triphenylamine-Based Polyimides with Pendant Viologen Units. Macromol. Rapid Commun. 2017, 38, 1600715. [Google Scholar] [CrossRef]
- Weng, D.; Shi, Y.; Zheng, J.; Xu, C. High Performance Black-to-Transmissive Electrochromic Device with Panchromatic Absorption Based on TiO2-Supported Viologen and Triphenylamine Derivatives. Org. Electron. 2016, 34, 139–145. [Google Scholar] [CrossRef]
- Li, M.; Wei, Y.; Zheng, J.; Zhu, D.; Xu, C. Highly Contrasted and Stable Electrochromic Device Based on Well-Matched Viologen and Triphenylamine. Org. Electron. 2014, 15, 428–434. [Google Scholar] [CrossRef]
- Ah, C.S.; Song, J.; Cho, S.M.; Kim, T.-Y.; Ryu, H.; Cheon, S.; Kim, J.Y.; Hwang, C.-S.; Kim, Y.-H. Optical and Electrical Properties of Electrochromic Devices Depending on Electrolyte Concentrations and Cell Gaps. Bull. Korean Chem. Soc. 2016, 37, 1812–1819. [Google Scholar] [CrossRef]
- Rauh, R.D. Electrochromic Windows: An Overview. Electrochim. Acta 1999, 44, 3165–3176. [Google Scholar] [CrossRef]
- Zuniga, C.A.; Barlow, S.; Marder, S.R. Approaches to Solution-Processed Multilayer Organic Light-Emitting Diodes Based on Cross-Linking. Chem. Mater. 2010, 23, 658–681. [Google Scholar] [CrossRef]
- Kim, S.; Jin, X.; Tanaka, A.; Nagamura, T. High Contrast and Dynamic Electrochromic Display with Thin Solid Hyper-Branched Polymer Film. Mol. Crystal Liq. Cryst. 2011, 539, 5–10. [Google Scholar] [CrossRef]
- Kao, S.-Y.; Lu, H.-C.; Kung, C.-W.; Chen, H.-W.; Chang, T.-H.; Ho, K.-C. Thermally Cured Dual Functional Viologen-Based All-in-One Electrochromic Devices with Panchromatic Modulation. ACS Appl. Mater. Interfaces 2016, 8, 4175–4184. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Ahn, J.; Kim, J.; Yoon, S.; Kim, Y.S.; Cho, K.Y. Improved Electrochromic Device Performance from Silver Grid on Flexible Transparent Conducting Electrode Prepared by Electrohydrodynamic Jet Printing. J. Mater. Chem. C 2017, 5, 566022. [Google Scholar] [CrossRef]
- Lu, H.-C.; Kao, S.-Y.; Chang, T.-H.; Kung, C.-W.; Ho, K.-C. An Electrochromic Device Based on Prussian Blue, Self-immobilized Vinyl Benzyl Viologen, and Ferrocene. Sol. Energy Mater. Sol. Cells 2016, 147, 75–84. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Skene, W.G. Visible-to-NIR Electrochromic Device Prepared from a Thermally Polymerizable Electroactive Organic Monomer. ACS Appl. Mater. Interfaces 2017, 9, 21524–21531. [Google Scholar] [CrossRef]
- Sun, Y.; Chien, S.-C.; Yip, H.-L.; Zhang, Y.; Chen, K.-S.; Zeigler, D.F.; Chen, F.-C.; Lin, B.; Jen, A.K.Y. Chemically Doped and Cross-linked Hole-Transporting Materials as an Efficient Anode Buffer Layer for Polymer Solar Cells. Chem. Mater. 2011, 23, 5006–5015. [Google Scholar] [CrossRef]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-Type Coupling Reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Sale, D.; Braddock, D.C.; Armstrong, A.; Brennan, C.; Davies, R.P. Mechanistic Studies on the Copper-Catalyzed N-Arylation of Alkylamines Promoted by Organic Soluble Ionic Bases. ACS Catal. 2016, 6, 3965–3974. [Google Scholar] [CrossRef] [Green Version]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Janoschka, T.; Morgenstern, S.; Hiller, H.; Friebe, C.; Wolkersdorfer, K.; Haupler, B.; Hager, M.D.; Schubert, U.S. Synthesis and Characterization of TEMPO- and Viologen-Polymers for Water-Based Redox-Flow Batteries. Polym. Chem. 2015, 6, 7801–7811. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.S.Y.; Scherman, O.A. Dynamic Interfacial Adhesion through Cucurbit[n]uril Molecular Recognition. Angew. Chem. Int. Ed. 2018, 57, 8854–8858. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-J.; Liu, M.S.; Zhang, Y.; Niu, Y.; Huang, F.; Ka, J.-W.; Yip, H.-L.; Tian, Y.; Jen, A.K.Y. Thermally Cross-Linkable Hole-Transporting Materials on Conducting Polymer: Synthesis, Characterization, and Applications for Polymer Light-Emitting Devices. Chem. Mater. 2008, 20, 413–422. [Google Scholar] [CrossRef]
- Niu, Y.H.; Munro, A.M.; Cheng, Y.J.; Tian, Y.Q.; Liu, M.S.; Zhao, J.L.; Bardecker, J.A.; Jen-La Plante, I.; Ginger, D.S.; Jen, A.K.Y. Improved Performance from Multilayer Quantum Dot Light-Emitting Diodes via Thermal Annealing of the Quantum Dot Layer. Adv. Mater. 2007, 19, 3371–3376. [Google Scholar] [CrossRef]
- Sun, Y.; Gong, X.; Hsu, B.B.Y.; Yip, H.-L.; Jen, A.K.-Y.; Heeger, A.J. Solution-Processed Cross-Linkable Hole Selective Layer for Polymer Solar Cells in the Inverted Structure. Appl. Phys. Lett. 2010, 97, 193310. [Google Scholar] [CrossRef]
- Mayo, F.R. The dimerization of styrene. J. Am. Chem. Soc. 1968, 90, 1289–1295. [Google Scholar] [CrossRef]
- Abraham, S.; Mangalath, S.; Sasikumar, D.; Joseph, J. Transmissive-to-Black Electrochromic Devices Based on Cross-Linkable Tetraphenylethene-Diphenylamine Derivatives. Chem. Mater. 2017, 29, 9877–9881. [Google Scholar] [CrossRef]
- Monthéard, J.-P.; Camps, M.; Chatzopoulos, M.; Pham, Q.-T. A New Convenient Synthesis of Ortho-, Meta- and Para-Chloromethylstyrenes. Makromol. Chem. Phys. 1985, 186, 2513–2518. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Zhang, J.; Sato, T.; Moriyama, S.; Higuchi, M. Black-to-Transmissive Electrochromism with Visible-to-Near-Infrared Switching of a Co (II)-Based Metallo-Supramolecular Polymer for Smart Window and Digital Signage Applications. ACS Appl. Mater. Interfaces 2015, 7, 18266–18272. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.T.; Nelson, R.F.; Fritsch, J.M.; Marcoux, L.S.; Leedy, D.W.; Adams, R.N. Anodic Oxidation Pathways of Aromatic Amines. Electrochemical and Electron Paramagnetic Resonance Studies. J. Am. Chem. Soc. 1966, 88, 3498–3503. [Google Scholar] [CrossRef]
- Rybakiewicz, R.; Zagorska, M.; Pron, A. Triphenylamine-Based Electroactive Compounds: Synthesis, Properties and Application to Organic Electronics. Chem. Pap. 2017, 71, 243–268. [Google Scholar] [CrossRef]
- Szeghalmi, A.V.; Erdmann, M.; Engel, V.; Schmitt, M.; Amthor, S.; Kriegisch, V.; Nöll, G.; Stahl, R.; Lambert, C.; Leusser, D.; et al. How Delocalized Is N,N,N′,N′-Tetraphenylphenylenediamine Radical Cation? An Experimental and Theoretical Study on the Electronic and Molecular Structure. J. Am. Chem. Soc. 2004, 126, 7834–7845. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-H.; Wang, H.-M.; Liao, S.-H. Redox-Stable and Visible/Near-Infrared Electrochromic Aramids with Main-Chain Triphenylamine and Pendent 3,6-di-tert-Butylcarbazole Units. Polym. Chem. 2014, 5, 2473–2483. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Yao, C.; Tuner, G.; Skene, W.G. Electrochemical and Solvent Mediated Visible-to-Near Infrared Spectroscopic Switching of Benzoselenodiazole Fluorophores. Chem. Eur. J. 2020. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Skene, W.G. Engaging the Reversible Bonds of an Immobilized Styreno-Thiophene Film. Cryst. Growth Des. 2020, 20, 5688–5697. [Google Scholar] [CrossRef]
- Bao, L.Q.; Phuong, H.; Thuy, C.T.T.; Lee, J.W.; Kim, J.H.; Thogiti, S. Synthesis and Investigation of Triphenylamine-Based Double Branched Organic Dyes for p-Type Dye-Sensitized Solar Cells. Mol. Cryst. Liq. Cryst. 2017, 653, 109–117. [Google Scholar] [CrossRef]
- Shin, W.S.; Kim, S.C.; Lee, E.-J.; Park, S.-M.; Jin, S.-H.; Lee, J.W.; Gal, Y.-S. Introduction of Interlayer via Oxetane Based Crosslinking Method for Blue Polymer Light-Emitting Diode Applications. Mol. Cryst. Liq. Cryst. 2007, 471, 335–343. [Google Scholar] [CrossRef]
- Liu, X.; Neoh, K.G.; Zhao, L.; Kang, E.T. Surface Functionalization of Glass and Polymeric Substrates via Graft Copolymerization of Viologen in an Aqueous Medium. Langmuir 2002, 18, 2914–2921. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wałęsa-Chorab, M.; Skene, W.G. Extending the Color Retention of an Electrochromic Device by Immobilizing Color Switching and Ion-Storage Complementary Layers. Electron. Mater. 2020, 1, 40-53. https://doi.org/10.3390/electronicmat1010005
Wałęsa-Chorab M, Skene WG. Extending the Color Retention of an Electrochromic Device by Immobilizing Color Switching and Ion-Storage Complementary Layers. Electronic Materials. 2020; 1(1):40-53. https://doi.org/10.3390/electronicmat1010005
Chicago/Turabian StyleWałęsa-Chorab, Monika, and William G. Skene. 2020. "Extending the Color Retention of an Electrochromic Device by Immobilizing Color Switching and Ion-Storage Complementary Layers" Electronic Materials 1, no. 1: 40-53. https://doi.org/10.3390/electronicmat1010005
APA StyleWałęsa-Chorab, M., & Skene, W. G. (2020). Extending the Color Retention of an Electrochromic Device by Immobilizing Color Switching and Ion-Storage Complementary Layers. Electronic Materials, 1(1), 40-53. https://doi.org/10.3390/electronicmat1010005