Diagnosis of Congenital and Acquired Generalized Lipodystrophies—Similarities and Differences
Abstract
1. Introduction
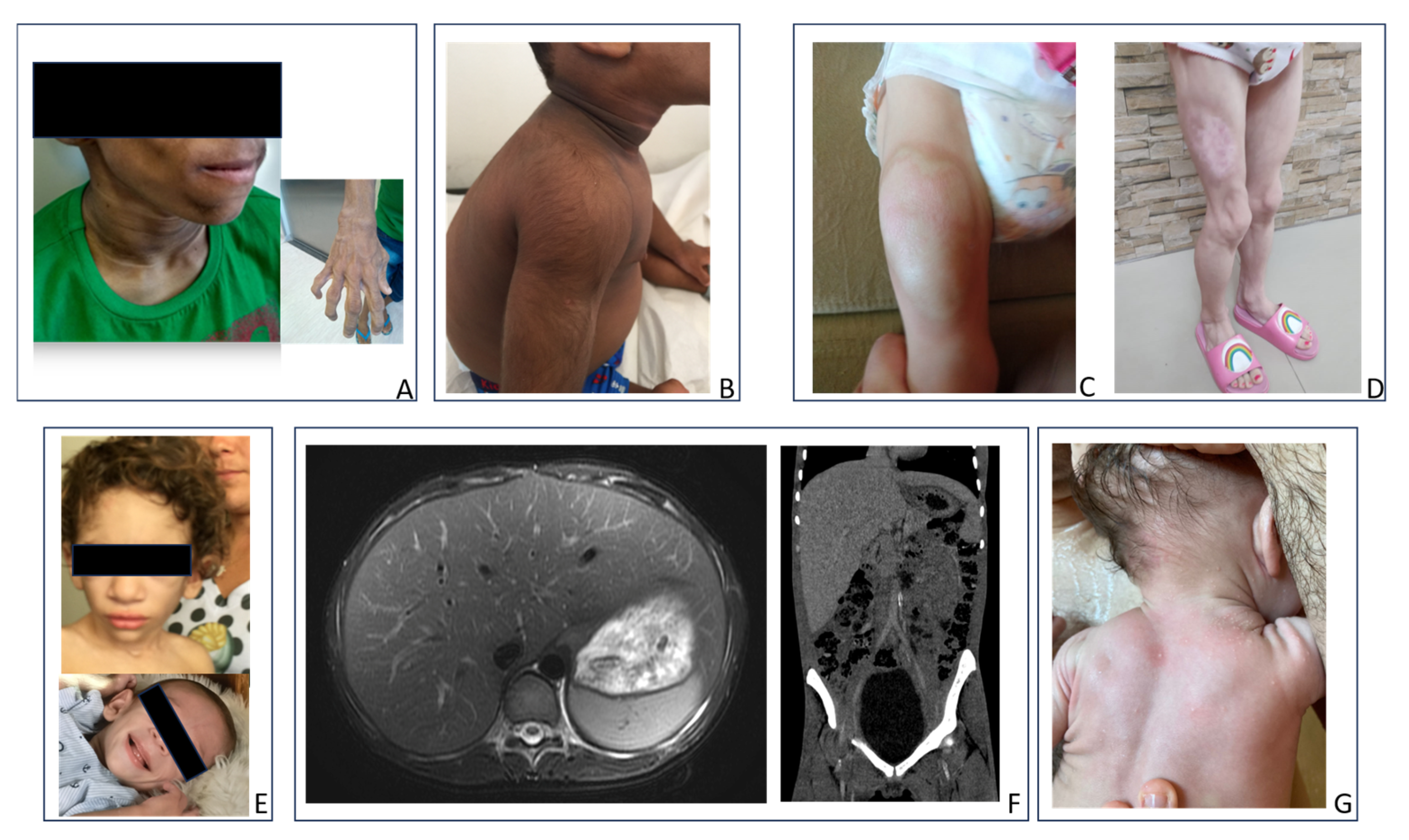
2. Cases Description
3. Similar Findings in Congenital and Acquired Generalized Lipodystrophies
3.1. Clinical History
3.2. Physical Examination
3.3. Laboratory
3.4. Imaging Exams
3.5. Metreleptin Treatment
4. Differences Between Congenital and Acquired Generalized Lipodystrophies
4.1. Clinical History
4.2. Physical Examination
4.3. Laboratory
4.4. Imaging Exams
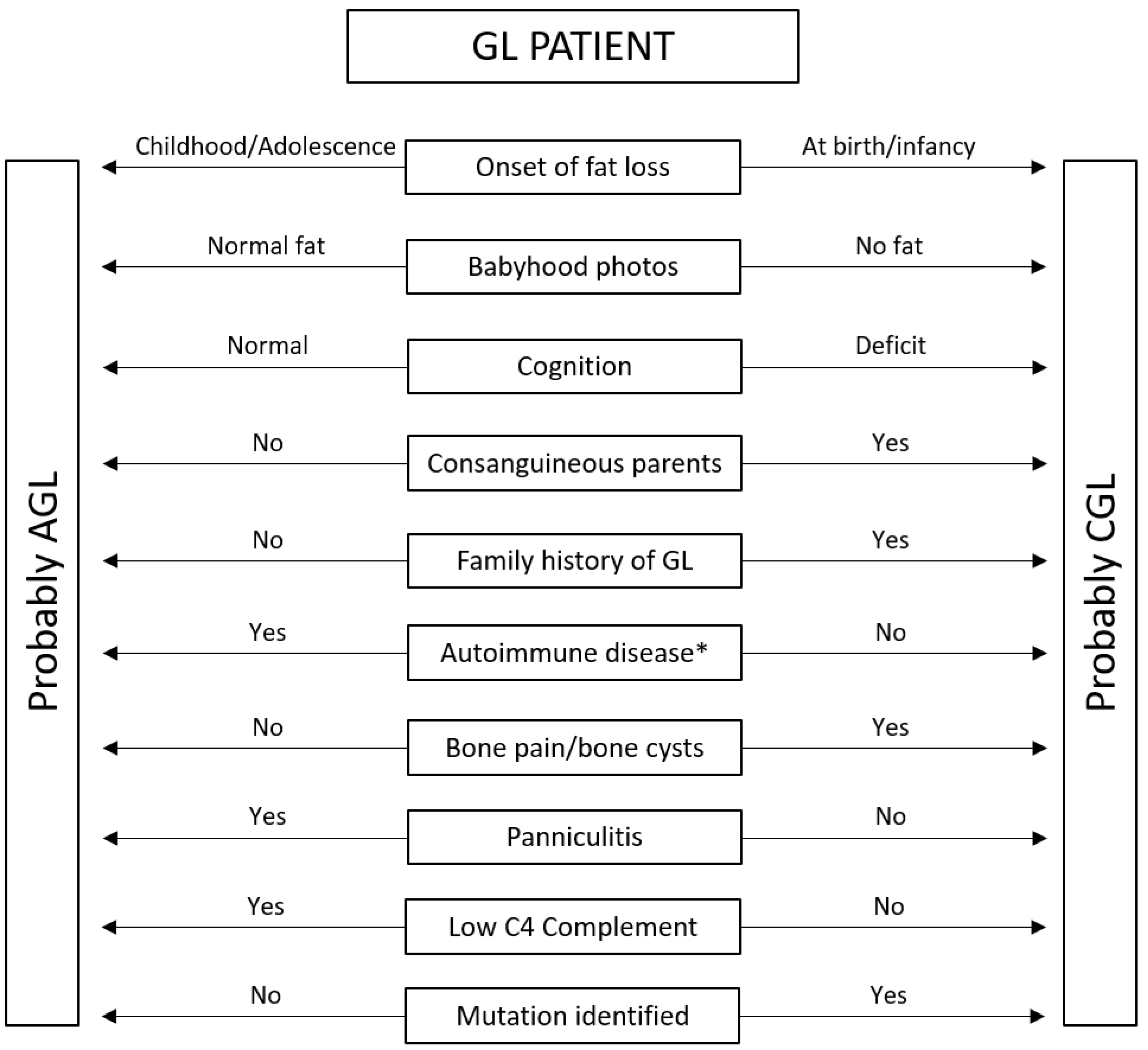
5. Back to Cases—Clinical Practice Algorithm
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CGL | Congenital Generalized Lipodystrophy |
| AGL | Acquired Generalized Lipodystrophy |
| GL | Generalized Lipodystrophy |
References
- Patni, N.; Garg, A. Lipodystrophy for the Diabetologist-What to Look For. Curr. Diab. Rep. 2022, 22, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.G.; Nobrega, L.H.C.; De Lima, N.N.; Do Nascimento Santos, M.G.; Baracho, M.F.P.; Jeronimo, S.M.B. Clinical and Laboratory Data of a Large Series of Patients with Congenital Generalized Lipodystrophy. Diabetol. Metab. Syndr. 2016, 8, 23. [Google Scholar] [CrossRef]
- Berardinelli, W. An Undiagnosed Endocrinometabolic Syndrome: Report of 2 Cases. J. Clin. Endocrinol. Metab. 1954, 14, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Seip, M. Lipodystrophy and Gigantism with Associated Endocrine Manifestations. A New Diencephalic Syndrome? Acta Paediatr. 1959, 48, 555–574. [Google Scholar] [CrossRef]
- Lawrence, R.D. Lipodystrophy and Hepatomegaly, with Diabetes, Lipaemia, and Other Metabolic Disturbances; a Case Throwing New Light on the Action of Insulin. Lancet 1946, 247, 724–731. [Google Scholar] [CrossRef]
- Ziegler, L.H. Lipodystrophies: Report of seven cases. Brain 1928, 51, 147–167. [Google Scholar] [CrossRef]
- Hussain, I.; Garg, A. Lipodystrophy Syndromes. Endocrinol. Metab. Clin. N. Am. 2016, 45, 783–797. [Google Scholar] [CrossRef]
- Patni, N.; Chard, C.; Araújo-Vilar, D.; Phillips, H.; Magee, D.A.; Akinci, B. Diagnosis, Treatment and Management of Lipodystrophy: The Physician Perspective on the Patient Journey. Orphanet. J. Rare Dis. 2024, 19, 263. [Google Scholar] [CrossRef]
- Yildirim Simsir, I.; Tuysuz, B.; Ozbek, M.N.; Tanrikulu, S.; Celik Guler, M.; Karhan, A.N.; Denkboy Ongen, Y.; Gunes, N.; Soyaltin, U.E.; Altay, C.; et al. Clinical Features of Generalized Lipodystrophy in Turkey: A Cohort Analysis. Diabetes Obes. Metab. 2023, 25, 1950–1963. [Google Scholar] [CrossRef]
- Seip, M.; Trygstad, O. Generalized Lipodystrophy, Congenital and Acquired (Lipoatrophy). Acta Paediatr. 1996, 413, 2–28. [Google Scholar] [CrossRef]
- Akinci, B.; Onay, H.; Demir, T.; Ozen, S.; Kayserili, H.; Akinci, G.; Nur, B.; Tuysuz, B.; Nuri Ozbek, M.; Gungor, A.; et al. Natural History of Congenital Generalized Lipodystrophy: A Nationwide Study from Turkey. J. Clin. Endocrinol. Metab. 2016, 101, 2759–2767. [Google Scholar] [CrossRef]
- Sorkina, E.; Chichkova, V. Generalized Lipoatrophy Syndromes. Presse Medicale 2021, 50, 104075. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, Y.; Oral, E.A.; Bloomgarden, Z.T.; Brown, R.J.; Chan, J.L.; Einhorn, D.; Garber, A.J.; Garg, A.; Timothy Garvey, W.; Grunberger, G.; et al. The Clinical Approach to the Detection of Lipodystrophy-An Aace Consensus Statement. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2013, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Akinci, B.; Al Yaarubi, S.; Bismuth, E.; Cappa, M.; Deeb, A.; Kamrath, C.; Musso, C.; Patni, N.; Prodam, F.; et al. The Clinical Approach to Child and Adolescent Patients with Lipodystrophy: A Series of International Case Discussions. Front. Endocrinol. 2025, 16, 1597053. [Google Scholar] [CrossRef] [PubMed]
- Garg, A. Acquired and Inherited Lipodystrophies. N. Engl. J. Med. 2004, 350, 1220–1234. [Google Scholar] [CrossRef]
- Araújo-Vilar, D.; Santini, F. Diagnosis and Treatment of Lipodystrophy: A Step-by-Step Approach. J. Endocrinol. Investig. 2019, 42, 61–73. [Google Scholar] [CrossRef]
- Fernandez-Pombo, A.; Prado-Moraña, T.; Diaz-Lopez, E.J.; Sanchez-Iglesias, S.; Castro, A.I.; Cobelo-Gomez, S.; Araujo-Vilar, D. Clinical Characterisation and Comorbidities of Acquired Generalised Lipodystrophy: A 14-Year Follow-Up Study. J. Clin. Med. 2023, 12, 7344. [Google Scholar] [CrossRef]
- Deeb, A.; Hassan Beck, R.; Fatima, U.; Yoosuf, H. Acquired Generalized Lipodystrophy with Extensive Autoimmune Involvement: A Case Report and Review of the Literature. Horm. Res. Paediatr. 2025, 1–19. [Google Scholar] [CrossRef]
- Vatier, C.; Vigouroux, C.; Mosbah, H. Primary Disease of Adipose Tissue: When to Think about and How to Evaluate It in Clinical Practice? Ann. Endocrinol. 2024, 85, 190–194. [Google Scholar] [CrossRef]
- Llamas-Velasco, M.; Daudén, E.; Martínez-Peñas, G.; García-Diez, A. Late-Onset Acquired Generalized Lipodystrophy with Muscle Involvement. Actas Dermo-Sifiliogr. 2012, 103, 729–732. [Google Scholar] [CrossRef]
- Triaille, C.; Corvillo, F.; Mertens, A.; Hoffman, I.; Roskams, T.; López-Trascasa, M.; De Somer, L.; Casteels, K. Perilipin-1 Autoantibodies Are a Robust Marker of Acquired Lipodystrophy and May Precede Clinical Detection. Pediatr. Allergy Immunol. 2025, 36, e70026. [Google Scholar] [CrossRef] [PubMed]
- Mainieri, F.; Chiarelli, F. Lipodystrophies in Children. Horm. Res. Paediatr. 2022, 95, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pombo, A.; Sánchez-Iglesias, S.; Castro-Pais, A.I.; Ginzo-Villamayor, M.J.; Cobelo-Gómez, S.; Prado-Moraña, T.; Díaz-López, E.J.; Casanueva, F.F.; Loidi, L.; Araújo-Vilar, D. Natural History and Comorbidities of Generalised and Partial Lipodystrophy Syndromes in Spain. Front. Endocrinol. 2023, 14, 1250203. [Google Scholar] [CrossRef] [PubMed]
- Pliszka, M.; Szablewski, L. Severe Insulin Resistance Syndromes: Clinical Spectrum and Management. Int. J. Mol. Sci. 2025, 26, 5669. [Google Scholar] [CrossRef] [PubMed]
- Özen, S.; Akıncı, B.; Oral, E.A. Current Diagnosis, Treatment and Clinical Challenges in the Management of Lipodystrophy Syndromes in Children and Young People. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 17–28. [Google Scholar] [CrossRef]
- Cortés, V.A.; Fernández-Galilea, M. Lipodystrophies: Adipose Tissue Disorders with Severe Metabolic Implications. J. Physiol. Biochem. 2015, 71, 471–478. [Google Scholar] [CrossRef]
- İşlek, A.; Sayar, E.; Yılmaz, A.; Duman, Ö.; Artan, R. A Very Rare Cause of Acute Pancreatitis: Berardinelli-Seip Congenital Lipodystrophy. Turk J. Gastroenterol. 2014, 25, 216–219. [Google Scholar] [CrossRef]
- Brahmanandam, L.; Vivekanand, B.; Mythili, A.; Subrahmanyam, K.A.V. Congenital Generalized Lipodystrophy 3 & 4 Presenting as Pancreatitis. J. Evid. Based Med. Healthc. 2019, 6, 2679–2683. [Google Scholar] [CrossRef]
- Cunningham, J.; Nadal, R.; Broome, C. Acquired Generalized Lipodystrophy Following Immune Thrombocytopenia. Am. J. Med. 2017, 130, e445–e446. [Google Scholar] [CrossRef]
- Giambelluca, D.; Di Martino, E.; Salvaggio, G. The “Kissing Sign” of Liver and Spleen. Abdom. Radiol. 2019, 44, 2323–2324. [Google Scholar] [CrossRef]
- Sahhar, N.N.; Ahmad, A.T.; Ammari, F.L.; Ajlouni, K.M. Dysregulation of Growth Hormone in Acquired Generalized Lipodystrophy. Saudi. Med. J. 2004, 25, 1766–1767. [Google Scholar]
- Meral, R.; Ryan, B.J.; Malandrino, N.; Jalal, A.; Neidert, A.H.; Muniyappa, R.; Akıncı, B.; Horowitz, J.F.; Brown, R.J.; Oral, E.A. “Fat Shadows” From DXA for the Qualitative Assessment of Lipodystrophy: When a Picture Is Worth a Thousand Numbers. Diabetes Care 2018, 41, 2255–2258. [Google Scholar] [CrossRef]
- Brush, M.; Auh, S.; Cochran, E.; Tuska, R.; Koh, C.; Kleiner, D.E.; Lightbourne, M.; Brown, R.J. Effects of Metreleptin in Patients with Generalized Lipodystrophy Before vs After the Onset of Severe Metabolic Disease. J. Clin. Endocrinol. Metab. 2025, 110, e1051–e1061. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.A.; Simha, V.; Ruiz, E.; Andewelt, A.; Premkumar, A.; Snell, P.; Wagner, A.J.; DePaoli, A.M.; Reitman, M.L.; Taylor, S.I.; et al. Leptin-Replacement Therapy for Lipodystrophy. N. Engl. J. Med. 2002, 346, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Diker-Cohen, T.; Cochran, E.; Gorden, P.; Brown, R.J. Partial and Generalized Lipodystrophy: Comparison of Baseline Characteristics and Response to Metreleptin. J. Clin. Endocrinol. Metab. 2015, 100, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Lebastchi, J.; Ajluni, N.; Neidert, A.; Oral, E.A. A Report of Three Cases with Acquired Generalized Lipodystrophy with Distinct Autoimmune Conditions Treated with Metreleptin. J. Clin. Endocrinol. Metab. 2015, 100, 3967–3970. [Google Scholar] [CrossRef]
- Misra, A.; Garg, A. Clinical Features and Metabolic Derangements in Acquired Generalized Lipodystrophy: Case Reports and Review of the Literature. Medicine 2003, 82, 129–146. [Google Scholar] [CrossRef]
- Brown, R.J.; Araujo-Vilar, D.; Cheung, P.T.; Dunger, D.; Garg, A.; Jack, M.; Mungai, L.; Oral, E.A.; Patni, N.; Rother, K.I.; et al. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 4500–4511. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, S.; Fernández-Pombo, A.; Cobelo-Gómez, S.; Hermida-Ameijeiras, Á.; Alarcón-Martínez, H.; Domingo-Jiménez, R.; Riquelme, A.I.R.; Requena, J.R.; Araújo-Vilar, D. Celia’s Encephalopathy (Bscl2-Gene-Related): Current Understanding. J. Clin. Med. 2021, 10, 1435. [Google Scholar] [CrossRef]
- Ceccarini, G.; Magno, S.; Gilio, D.; Pelosini, C.; Santini, F. Autoimmunity in Lipodystrophy Syndromes. Presse Medicale 2021, 50, 104073. [Google Scholar] [CrossRef]
- Knebel, B.; Müller-Wieland, D.; Kotzka, J. Lipodystrophies—Disorders of the Fatty Tissue. Int. J. Mol. Sci. 2020, 21, 8778. [Google Scholar] [CrossRef]
- Ribeiro, A.; Brandão, J.R.; Cleto, E.; Santos, M.; Borges, T.; Santos Silva, E. Fatty Liver and Autoimmune Hepatitis: Two Forms of Liver Involvement in Lipodystrophies. GE Port J. Gastroenterol. 2019, 26, 362–369. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, M.; Cao, H.; Zheng, J.; Ruan, Y.; Zhao, X. Acquired Generalized Lipodystrophy in a Juvenile Dermatomyositis Patient. Int. J. Rheum. Dis. 2024, 27, 15101. [Google Scholar] [CrossRef]
- Kumar, R.; Pilania, R.K.; Bhatia, A.; Dayal, D. Acquired Generalised Lipodystrophy and Type 1 Diabetes Mellitus in a Child: A Rare and Implacable Association. BMJ Case Rep. 2018, 2018, bcr-2018-225553. [Google Scholar] [CrossRef] [PubMed]
- Sorkina, E.; Frolova, E.; Rusinova, D.; Polyakova, S.; Roslavtseva, E.; Vasilyev, E.; Petrov, V.; Tiulpakov, A. Progressive Generalized Lipodystrophy as a Manifestation of Autoimmune Polyglandular Syndrome Type 1. J. Clin. Endocrinol. Metab. 2016, 101, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Cavdar, U.; Akinci, B.; Foss de Freitas, M.C.; Conjeevaram, H.; Fox, D.; Fontana, R.; Tayeh, M.; Frame, D.; Innis, J.; Walkovich, K.; et al. Untangling the Heterogeneity of Acquired Generalized Lipodystrophy. J. Endocr. Soc. 2021, 5, A39–A40. [Google Scholar] [CrossRef]
- Foss-Freitas, M.C.; Akinci, B.; Luo, Y.; Stratton, A.; Oral, E.A. Diagnostic Strategies and Clinical Management of Lipodystrophy. Expert Rev. Endocrinol. Metab. 2020, 15, 95–114. [Google Scholar] [CrossRef]
- Mathiesen, P.; Hegaard, H.; Herlin, T.; Zak, M.; Pedersen, F.K.; Nielsen, S. Long-Term Outcome in Patients with Juvenile Dermatomyositis: A Cross-Sectional Follow-up Study. Scand. J. Rheumatol. 2012, 41, 50–58. [Google Scholar] [CrossRef]
- Esfandiari, N.H.; Rubenfire, M.; Neidert, A.H.; Hench, R.; Eldin, A.J.; Meral, R.; Oral, E.A. Diagnosis of Acquired Generalized Lipodystrophy in a Single Patient with T-Cell Lymphoma and No Exposure to Metreleptin. Clin. Diabetes Endocrinol. 2019, 5, 4. [Google Scholar] [CrossRef]
- Brown, R.J.; Chan, J.L.; Jaffe, E.S.; Cochran, E.; Depaoli, A.M.; Gautier, J.F.; Goujard, C.; Vigouroux, C.; Gorden, P. Lymphoma in Acquired Generalized Lipodystrophy. Leuk. Lymphoma 2016, 57, 45–50. [Google Scholar] [CrossRef]
- Kennedy, R.; Macrohon, C.R.L.; David, M.L.G.V.; Lee, M.; Salama, A.K.S.; Shariff, A. Acquired Generalized Lipodystrophy as an Adverse Event of Combined Immune Checkpoint Inhibitor Therapy. JCEM Case Rep. 2025, 3, luaf023. [Google Scholar] [CrossRef]
- Corvillo, F.; Aparicio, V.; López-Lera, A.; Garrido, S.; Araújo-Vilar, D.; de Miguel, M.P.; López-Trascasa, M. Autoantibodies Against Perilipin 1 as a Cause of Acquired Generalized Lipodystrophy. Front. Immunol. 2018, 9, 2142. [Google Scholar] [CrossRef] [PubMed]
- Mandel-Brehm, C.; Vazquez, S.E.; Liverman, C.; Cheng, M.; Quandt, Z.; Kung, A.F.; Parent, A.; Miao, B.; Disse, E.; Cugnet-Anceau, C.; et al. Autoantibodies to Perilipin-1 Define a Subset of Acquired Generalized Lipodystrophy. Diabetes 2023, 72, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kozusko, K.; Tsang, V.H.M.; Bottomley, W.; Cho, Y.H.; Gandotra, S.; Mimmack, M.; Lim, K.; Isaac, I.; Patel, S.; Saudek, V.; et al. Clinical and Molecular Characterization of a Novel PLIN1 Frameshift Mutation Identified in Patients with Familial Partial Lipodystrophy. Diabetes 2015, 64, 299–310. [Google Scholar] [CrossRef]
- Bhatia, R.; Chennupathi, P.; Rosenstein, E.D.; Advani, S. Spontaneous Remission of Acquired Generalized Lipodystrophy Presenting in the Postpartum Period. JCEM Case Rep. 2024, 2, luae009. [Google Scholar] [CrossRef]
- Hill, M.; Weissman, A.S.; Hirshburg, J.; McBride, J.D.; Lawrence, H. An Unusual Case of Acquired Generalized Lipodystrophy (Panniculitis Variety). Pediatr. Dermatol. 2024, 41, 1152–1155. [Google Scholar] [CrossRef]
- Fourman, L.T.; Lima, J.G.; Simha, V.; Cappa, M.; Alyaarubi, S.; Montenegro, R.; Akinci, B.; Santini, F. A Rapid Action Plan to Improve Diagnosis and Management of Lipodystrophy Syndromes. Front. Endocrinol. 2024, 15, 1383318. [Google Scholar] [CrossRef]
- Garg, A. Lipodystrophies: Genetic and Acquired Body Fat Disorders. J. Clin. Endocrinol. Metab. 2011, 96, 3313–3325. [Google Scholar] [CrossRef]
- Savage, D.B.; Semple, R.K.; Clatworthy, M.R.; Lyons, P.A.; Morgan, B.P.; Cochran, E.K.; Gorden, P.; Raymond-Barker, P.; Murgatroyd, P.R.; Adams, C.; et al. Complement Abnormalities in Acquired Lipodystrophy Revisited. J. Clin. Endocrinol. Metab. 2009, 94, 10–16. [Google Scholar] [CrossRef]
- Eren, E.; Özkan, T.B.; Papatya Çakir, E.D.; Saǧlam, H.; Tarim, Ö. Acquired Generalized Lipodystrophy Associated with Autoimmune Hepatitis and Low Serum C4 Level. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 39–42. [Google Scholar] [CrossRef]
- Gregory, J.M.; Arkader, A.; Bothari, A.; Dormans, J.P. Case Report: Unicameral Bone Cysts in a Young Patient with Acquired Generalized Lipodystrophy. Clin. Orthop. Relat. Res. 2010, 468, 1440–1446. [Google Scholar] [CrossRef]
- Westvik, J. Radiological Features in Generalized Lipodystrophy. Acta Paediatr. 1996, 85, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Haque, W.A.; Shimomura, I.; Matsuzawa, Y.; Garg, A. Serum Adiponectin and Leptin Levels in Patients with Lipodystrophies. J. Clin. Endocrinol. Metab. 2002, 87, 2395. [Google Scholar] [CrossRef] [PubMed]
- Ponte, C.M.M.; Fernandes, V.O.; Gurgel, M.H.C.; Vasconcelos, I.; Karbage, L.; Liberato, C.B.R.; Negrato, C.A.; Gomes, M.B.; Montenegro, A.; Montenegro Junior, R.M. Early Commitment of Cardiovascular Autonomic Modulation in Brazilian Patients with Congenital Generalized Lipodystrophy. BMC Cardiovasc. Disord. 2018, 18, 6. [Google Scholar] [CrossRef]
- Hussain, I.; Patni, N.; Garg, A. Lipodystrophies, Dyslipidaemias and Atherosclerotic Cardiovascular Disease. Pathology 2019, 51, 202–212. [Google Scholar] [CrossRef]
- Feijó, B.M.X.C.R.R.; Mendonça, R.M.; Egito, E.S.T.; Lima, D.N.; Campos, J.T.A.D.M.; Lima, J.G. Coronary Arterial Calcification in Patients with Congenital Generalised Lipodystrophy: A Case Series. Clin. Endocrinol. 2022, 97, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Akinci, G.; Alyaarubi, S.; Patni, N.; Alhashmi, N.; Al-Shidhani, A.; Prodam, F.; Gagne, N.; Babalola, F.; Al Senani, A.; Muniraj, K.; et al. Metabolic and Other Morbid Complications in Congenital Generalized Lipodystrophy Type 4. Am. J. Med. Genet. A 2024, 194, e63533. [Google Scholar] [CrossRef]
| CGL (Berardinelli–Seip Syndrome) | AGL (Lawrence Syndrome) | |
|---|---|---|
| DIFFERENCES | ||
| Onset of body fat loss | At birth or infancy | Childhood or adolescence |
| Body fat at birth * | Low or very low | Normal |
| Voracious appetite | Early childhood | Later (if present) |
| Cognition | Intellectual disability can occur in CGL type 2 | Normal cognition |
| Physical exam | Hypertrichosis | Panniculitis, joint deformities (if rheumatoid arthritis) |
| Associated diseases | Bony cysts, cardiovascular disease | Panniculitis, autoimmune diseases ** |
| Liver disease | Induced by ectopic fat | Ectopic fat or autoimmune |
| Serum leptin | Always low | It may be normal if the loss of body fat is not yet general |
| C4 complement | Normal | Can be low |
| Body fat (DXA) | Always low | It may not be low if the loss of body fat is not yet general |
| Insulin resistance | Appears early | Appears later, depending on the loss of body fat |
| SIMILARITIES | ||
| Total body fat | Low or very low | |
| Physical exam | Loss of Bichat’s fat pad, Acanthosis nigricans, acrochordons, phlebomegaly, umbilical scar protrusion, hepatomegaly, muscle hypertrophy | |
| Blood tests | Hyperinsulinemia, hyperglycemia, low HDL, hypertriglyceridemia | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, J.G.; Lima, L.N.; Araujo, V.Y.B.; Nobrega, L.H.C.; Campos, J.T.A.d.M. Diagnosis of Congenital and Acquired Generalized Lipodystrophies—Similarities and Differences. Endocrines 2025, 6, 55. https://doi.org/10.3390/endocrines6040055
Lima JG, Lima LN, Araujo VYB, Nobrega LHC, Campos JTAdM. Diagnosis of Congenital and Acquired Generalized Lipodystrophies—Similarities and Differences. Endocrines. 2025; 6(4):55. https://doi.org/10.3390/endocrines6040055
Chicago/Turabian StyleLima, Josivan Gomes, Lucas Nobrega Lima, Vitor Yan Bezerra Araujo, Lucia Helena Coelho Nobrega, and Julliane Tamara Araújo de Melo Campos. 2025. "Diagnosis of Congenital and Acquired Generalized Lipodystrophies—Similarities and Differences" Endocrines 6, no. 4: 55. https://doi.org/10.3390/endocrines6040055
APA StyleLima, J. G., Lima, L. N., Araujo, V. Y. B., Nobrega, L. H. C., & Campos, J. T. A. d. M. (2025). Diagnosis of Congenital and Acquired Generalized Lipodystrophies—Similarities and Differences. Endocrines, 6(4), 55. https://doi.org/10.3390/endocrines6040055







