Circulating miR-122 and miR-139-3p: Association with Lipid, Inflammatory, and Glycemic Profile in Adolescents with Insulin-Resistant and Overweight
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Anamnesis and Clinical Evaluation
2.3. Procedures for Measurement of miRNA Expression
2.4. Hemolysis Control
2.5. RNA Extraction
2.6. Reverse Transcription
2.7. Preamplification
2.8. qPCR
2.9. Target Gene Prediction
2.10. Statistical Analyses
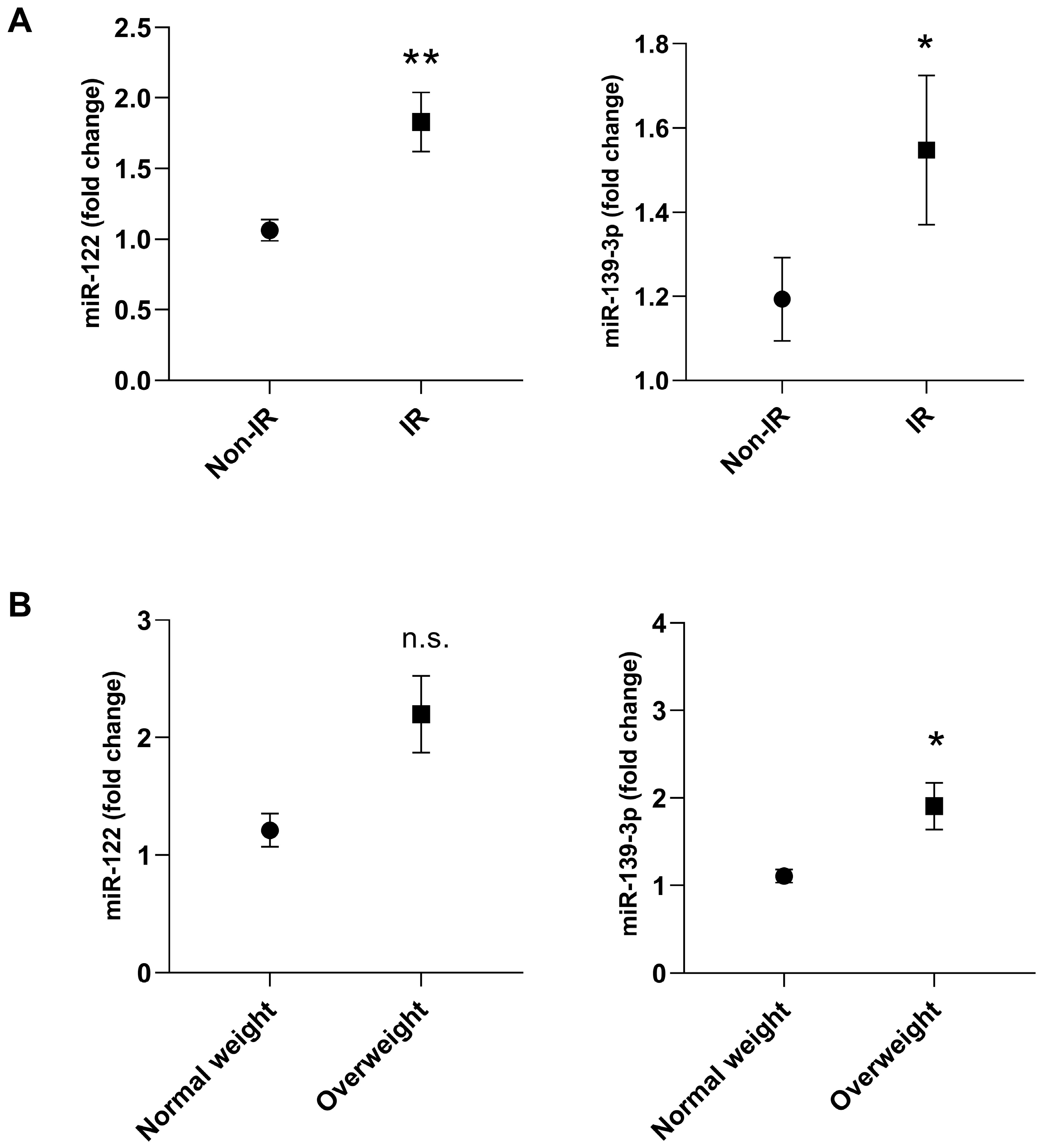
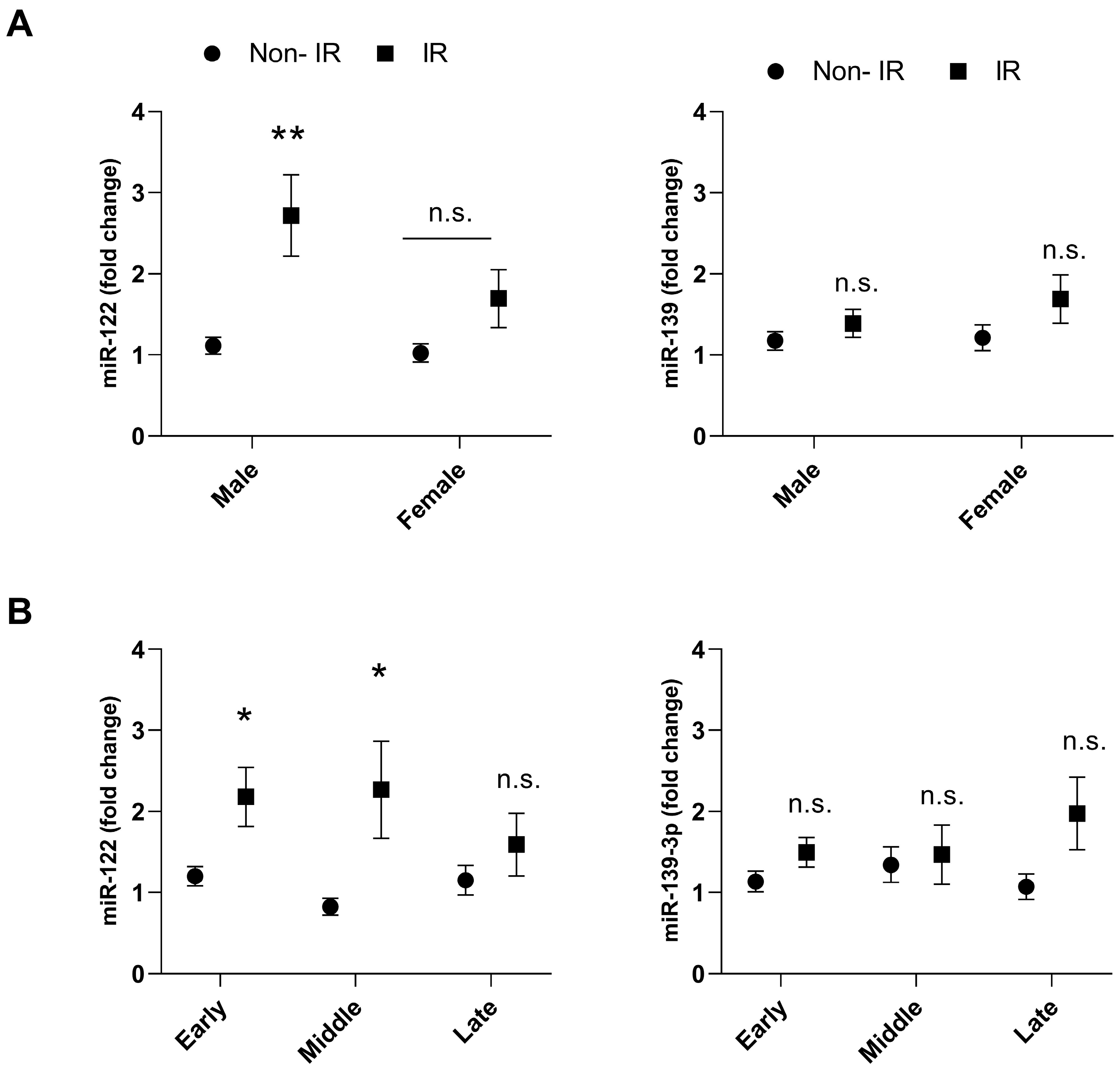
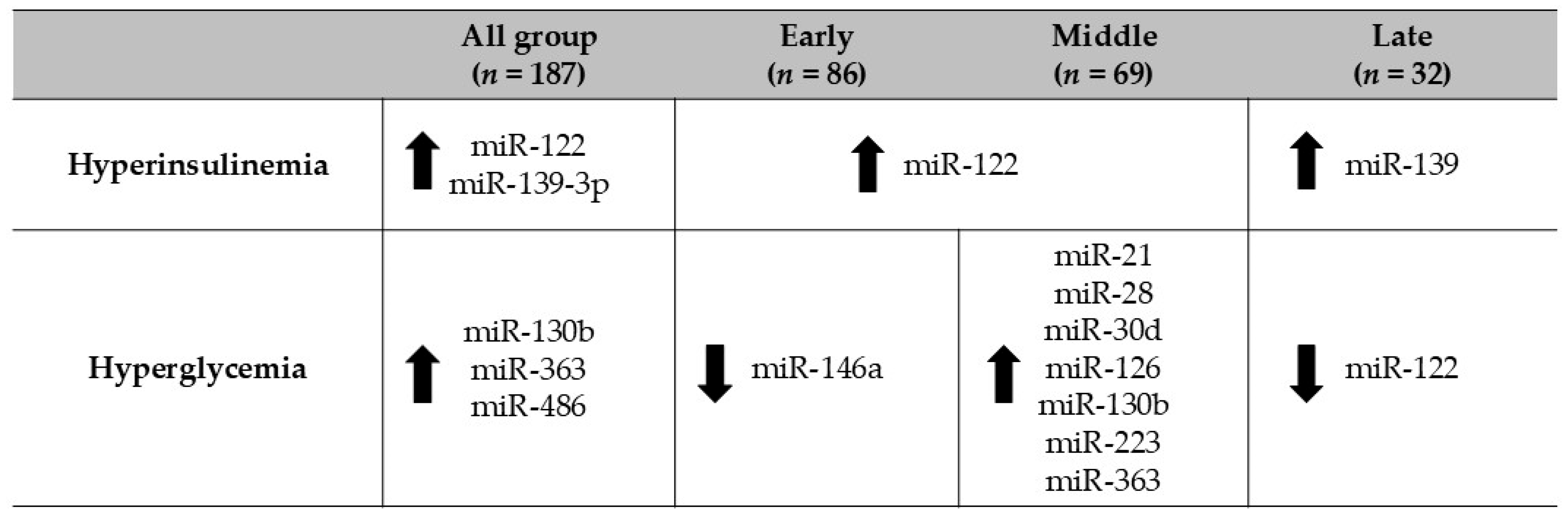
3. Results
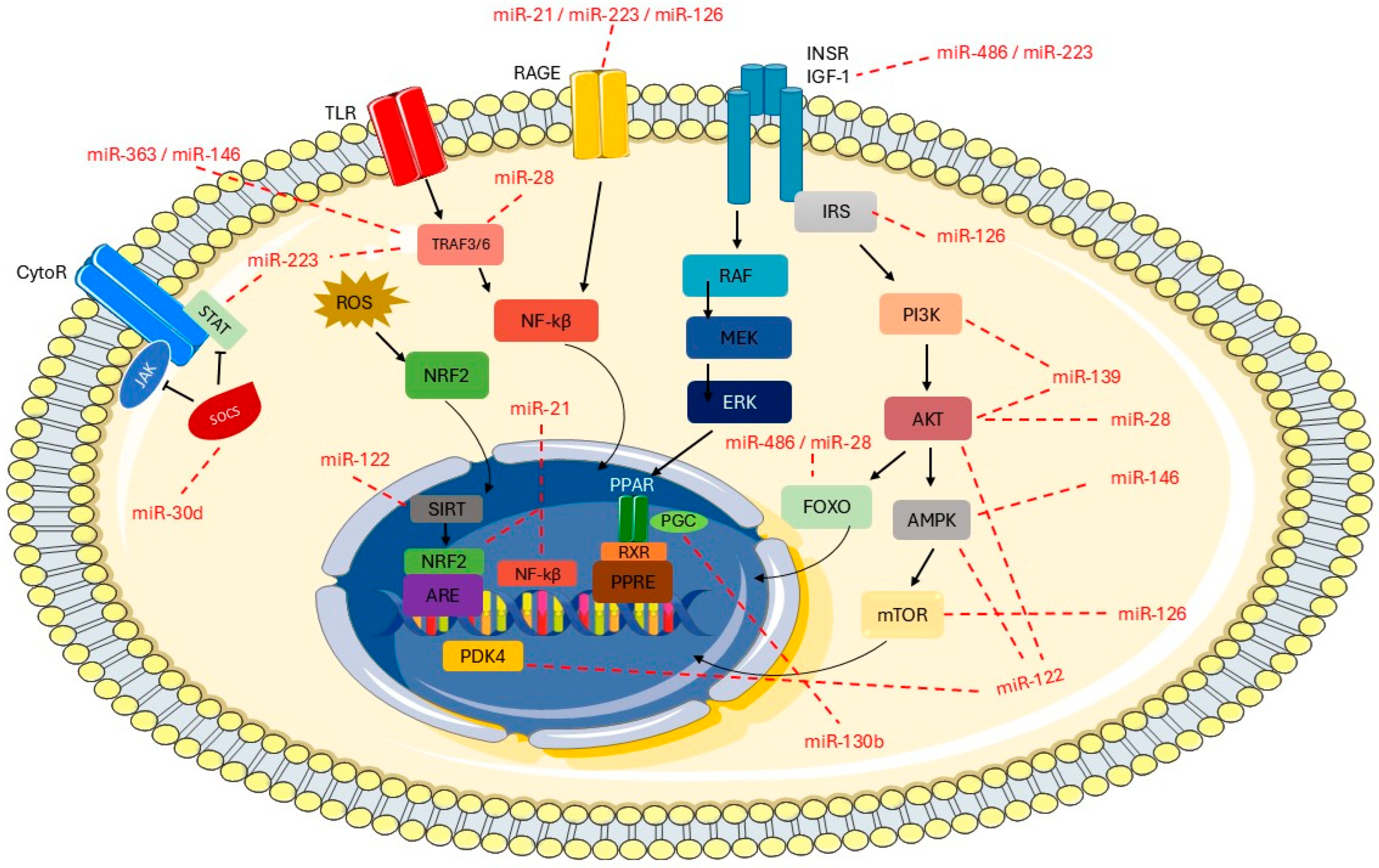
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. The Value of Anthropometric Measures in Nutrition and Metabolism: Comment on Anthropometrically Predicted Visceral Adipose Tissue and Blood-Based Biomarkers: A Cross-Sectional Analysis. Nutr. Metab. Insights 2019, 57, 191. [Google Scholar] [CrossRef]
- Seong, J.; Yun, J.; Ji, K.; Sun, S.; Woo, K.; Kim, K.W. Hypothalamic inflammation and obesity: A mechanistic review. Arch. Pharm. Res. 2019, 42, 121–135. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- McArdle, M.A.; Finucane, O.M.; Connaughton, R.M.; McMorrow, A.M.; Roche, H.M. Mechanisms of obesity-induced inflammation and insulin resistance: Insights into the emerging role of nutritional strategies. Front. Endocrinol. 2013, 4, 52. [Google Scholar] [CrossRef]
- Velloso, L.A. O controle hipotalâmico da fome e da termogênese-Implicações no desenvolvimento da obesidade. Arq. Bras. Endocrinol. Metabol. 2006, 50, 165–176. [Google Scholar] [CrossRef]
- Brandão-Lima, P.N.; de Carvalho, G.B.; Payolla, T.B.; Sarti, F.M.; Fisberg, R.M.; Malcomson, F.C.; Mathers, J.C.; Macedo, M.R. Circulating microRNAs Showed Specific Responses according to Metabolic Syndrome Components and Sex of Adults from a Population-Based Study. Metabolites 2022, 13, 2. [Google Scholar] [CrossRef]
- Lischka, J.; Schanzer, A.; Hojreh, A.; Ba-Ssalamah, A.; de Gier, C.; Valent, I.; Item, C.B.; Greber-Platzer, S.; Zeyda, M. Circulating microRNAs 34a, 122, and 192 are linked to obesity-associated inflammation and metabolic disease in pediatric patients. Int. J. Obes. 2021, 45, 1763–1772. [Google Scholar] [CrossRef]
- Etheridge, A.; Lee, I.; Hood, L.; Galas, D.; Wang, K. Extracellular microRNA: A new source of biomarkers. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 717, 85–90. [Google Scholar] [CrossRef]
- Matin, F.; Jeet, V.; Moya, L.; Selth, L.A.; Chambers, S.; Australian Prostate Cancer BioResource; Clements, J.A.; Batra, J. A Plasma Biomarker Panel of Four MicroRNAs for the Diagnosis of Prostate Cancer. Sci. Rep. 2018, 8, 6653. [Google Scholar] [CrossRef] [PubMed]
- Ajit, S.K. Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules. Sensors 2012, 12, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Wronska, A.; Kurkowska-Jastrzebska, I.; Santulli, G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol. 2015, 213, 60–83. [Google Scholar] [CrossRef] [PubMed]
- Garavelli, S.; Bruzzaniti, S.; Tagliabue, E.; Prattichizzo, F.; Di Silvestre, D.; Perna, F.; Sala, L.L.; Ceriello, A.; Mozzillo, E.; Fattorusso, V.; et al. Blood co-circulating extracellular micrornas and immune cell subsets associate with type 1 diabetes severity. Int. J. Mol. Sci. 2020, 21, 477. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–435. [Google Scholar] [CrossRef]
- Fichtlscherer, S.; De Rosa, S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Röxe, T.; Müller-Ardogan, M.; et al. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef]
- La Sala, L.; Crestani, M.; Garavelli, S.; de Candia, P.; Pontiroli, A.E. Does microRNA perturbation control the mechanisms linking obesity and diabetes? Implications for cardiovascular risk. Int. J. Mol. Sci. 2021, 22, 143. [Google Scholar] [CrossRef]
- Higuchi, C.; Nakatsuka, A.; Eguchi, J.; Teshigawara, S.; Kanzaki, M.; Katayama, A.; Yamaguchi, S.; Takahashi, N.; Murakami, K.; Ogawa, D.; et al. Identification of circulating miR-101, miR-375, and miR-802 as biomarkers for type 2 diabetes. Metabolism 2015, 64, 489–497. [Google Scholar] [CrossRef]
- Prabu, P.; Rome, S.; Sathishkumar, C.; Aravind, S.; Mahalingam, B.; Shanthirani, C.S.; Gastebois, C.; Villard, A.; Mohan, V.; Balasubramanyam, M.; et al. Circulating miRNAs of ‘Asian Indian phenotype’ identified in subjects with impaired glucose tolerance and patients with type 2 diabetes. PLoS ONE 2015, 10, e0128372. [Google Scholar] [CrossRef]
- Sucharita, S.; Ashwini, V.; Prabhu, J.S.; Avadhany, S.T.; Ayyar, V.; Bantwal, G. The role of circulating microRNA in the regulation of beta cell function and insulin resistance among Indians with type 2 diabetes. Indian J. Endocrinol. Metab. 2018, 22, 770–773. [Google Scholar] [CrossRef] [PubMed]
- De Candia, P.; Spinetti, G.; Specchia, C.; Sangalli, E.; Sala, L.; Uccellatore, A.; Lupini, S.; Genovese, S.; Matarese, G.; Ceriello, A.; et al. A unique plasma microRNA profile defines type 2 diabetes progression. PLoS ONE 2017, 12, e0188980. [Google Scholar]
- Párrizas, M.; Brugnara, L.; Esteban, Y.; González-Franquesa, A.; Canivell, S.; Murillo, S.; Gordillo-Bastidas, E.; Cussó, R.; Cadefau, J.A.; García-Roves, P.M.; et al. Circulating miR-192 and miR-193b are markers of prediabetes and are modulated by an exercise intervention. J. Clin. Endocrinol. Metab. 2015, 100, E407–E415. [Google Scholar] [CrossRef]
- Cui, X.; You, L.; Zhu, L.; Wang, X.; Zhou, Y.; Li, Y.; Wen, J.; Xia, Y.; Wang, X.; Ji, C.; et al. Change in circulating microRNA profile of obese children indicates future risk of adult diabetes. Metabolism 2018, 78, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G.; Russo, P.; Marena, P.; Lauria, F.; Venezia, A.; Ahrens, W.; De Henauw, S.; De Luca, P.; Foraita, R.; Günther, K.; et al. Circulating microRNAs are associated with early childhood obesity: Results of the I. Family Study. Genes Nutr. 2019, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Doumatey, A.P.; He, W.J.; Gaye, A.; Lei, L.; Zhou, J.; Gibbons, G.H.; Adeyemo, A.; Rotimi, C.R. Circulating MiR-374a-5p is a potential modulator of the inflammatory process in obesity. Sci. Rep. 2018, 8, 7680. [Google Scholar]
- Hijmans, J.G.; Diehl, K.J.; Bammert, T.D.; Kavlich, P.J.; Lincenberg, G.M.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Influence of Overweight and Obesity on Circulating Inflammation-Related microRNA. MicroRNA 2018, 7, 148–154. [Google Scholar] [CrossRef]
- Wang, R.; Cao, Y.; Hong, J.; Shi, J.; Gu, W.; Zhang, Y.; Wang, W. Elevated Circulating MicroRNA-122 Is Associated with Obesity and Insulin Resistance in Young Adults. Eur. Soc. Endocrinol. 2015, 172, 291–300. [Google Scholar] [CrossRef]
- Corona-Meraz, F.I.; Vázquez-Del Mercado, M.; Ortega, F.J.; Ruiz-Quezada, S.L.; Guzmán-Ornelas, M.O.; Navarro-Hernández, R.E. Ageing influences the relationship of circulating miR-33a and miR-33b levels with insulin resistance and adiposity. Diabetes Vasc. Dis. Res. 2019, 16, 244–253. [Google Scholar]
- Badawy, H.K.; Abo-Elmatty, D.M.; Mesbah, N.M. Association between serum microRNA-605 and microRNA-623 expression and essential hypertension in Egyptian patients. Meta Gene 2018, 16, 62–65. [Google Scholar]
- Shi, J.; Ren, Y.; Liu, Y.; Cheng, Y.; Liu, Y. Circulating miR-3135b and miR-107 are potential biomarkers for severe hypertension. J. Hum. Hypertens. 2021, 35, 343–350. [Google Scholar] [CrossRef]
- Al-Rawaf, H.A. Circulating microRNAs and adipokines as markers of metabolic syndrome in adolescents with obesity. Clin. Nutr. 2019, 38, 2231–2238. [Google Scholar] [CrossRef]
- de Carvalho, G.; Payolla, T.; Brandão-Lima, P.; Sarti, S.; Fisberg, R.; Rogero, M. Association between circulating micro-ribonucleic acids and metabolic syndrome in older adults from a population-based study. Clin. Nutr. ESPEN 2023, 58, 320–325. [Google Scholar] [CrossRef]
- Redling, D.; Bialak, S.; El ghormli, L.; Chernausek, S.D.; Jones, K.; Tryggestad, J.B. Circulating MicroRNAs as Predictors of Beta Cell Function in Youth-onset Type 2 Diabetes: The TODAY Study. J. Clin. Endocrinol. Metab. 2024, 109, 3027–3035. [Google Scholar] [CrossRef]
- Jeong, H.R.; Hwang, I.T. MicroRNAs as novel biomarkers for the diagnosis and treatment of pediatric diseases. Clin. Exp. Pediatr. 2024, 67, 119–125. [Google Scholar] [CrossRef]
- Jeong, H.R.; Hwang, I.T. The role of MicroRNAs as fine-tuners in the onset of puberty: A comprehensive review. Ann. Pediatr. Endocrinol. Metab. 2024, 29, 211–219. [Google Scholar] [CrossRef]
- Fisberg, R.M.; Sales, C.H.; Fontanell Mde, M.; Pereira, J.L.; Alves, M.C.G.P.; Escuder, M.M.L.; César, C.L.G.; Goldbaum, M. Health Survey of São Paulo with Focus on Nutrition: Rationale, Design, and Procedures. Nutrients 2018, 10, 169. [Google Scholar] [CrossRef]
- Alves, M.C.G.P.; Escuder, M.M.L.; Goldbaum, M.; Barros, M.B.A.; Fisberg, R.M.; Cesar, C.L.G. Sampling plan in health surveys, city of São Paulo, Brazil, 2015. Rev. Saúde Pública 2018, 52, 81. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-de-Almeida, C.A.; de Mello, E.D. Different criteria for the definition of insulin resistance and its relation with Dyslipidemia in overweight and obese children and adolescents. Pediatr. Gastroenterol. Hepatol. Nutr. 2018, 21, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Geloneze, B.; Vasques, A.C.; Stabe, C.F.; Pareja, J.C.; Rosado, L.E.; Queiroz, E.C.; Tambascia, M.A. Índices HOMA1-IR e HOMA2-IR para identificação de resistência à insulina e síndrome metabólica-Estudo Brasileiro de Síndrome Metabólica (BRAMS). Arq. Bras. Endocrinol. Metabol. 2009, 53, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Streiner, D.L.; Norman, G.R. Correction for multiple testing: Is there a resolution? Chest 2011, 1, 16–18. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a Metabolic Syndrome Phenotype in Adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.S.; Pollack, H.A. Epidemic Increase in Childhood Overweight. JAMA 2001, 286, 2845–2848. [Google Scholar] [CrossRef]
- Casagrande, S.S.; Lawrence, J.M. Cardiovascular disease risk factors and their associations with inflammation among US adolescents: NHANES, 2015 to March 2020. BMJ Open Diabetes Res. Care 2024, 12, e004148. [Google Scholar] [CrossRef] [PubMed]
- Dali-Youcef, N.; Mecili, M.; Ricci, R.; Andrès, E. Metabolic inflammation: Connecting obesity and insulin resistance. Ann. Med. 2013, 45, 242–253. [Google Scholar] [CrossRef]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-Reactive Protein Levels in Overweight and Obese Adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef]
- Devaraj, S.; Singh, U.; Jialal, I. Human C-reactive protein and the metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 182–189. [Google Scholar] [CrossRef]
- den Engelsen, C.; Koekkoek, P.S.; Gorter, K.J.; van den Donk, M.; Salomé, P.L.; Rutten, G.E. High-sensitivity C-reactive protein to detect metabolic syndrome in a centrally obese population: A cross-sectional analysis. Cardiovasc. Diabetol. 2012, 11, 25. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R.; Howard, G.; Mykkänen, L.; Tracy, R.P.; Haffner, S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome: The insulin resistance atherosclerosis study (IRAS). Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef]
- Vu, J.D.; Vu, J.B.; Pio, J.R.; Malik, S.; Franklin, S.S.; Chen, R.S.; Wong, N.D. Impact of C-reactive protein on the likelihood of peripheral arterial disease in United States adults with the metabolic syndrome, diabetes mellitus, and preexisting cardiovascular disease. Am. J. Cardiol. 2005, 96, 655–658. [Google Scholar] [CrossRef]
- Freeman, D.J.; Norrie, J.; Caslake, M.J.; Gaw, A.; Ford, I.; Lowe, G.D.O.; O’Reilly, D.S.; Packard, C.J.; Sattar, N. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland coronary prevention study. Diabetes 2002, 51, 1596–1600. [Google Scholar] [CrossRef]
- Juhan-Vague, I.; Alessi, M.C.; Mavri, A.; Morange, P.E. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J. Thromb. Haemost. 2003, 1, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Alessi, M.C.; Juhan-Vague, I. PAI-1 and the metabolic syndrome: Links, causes, and consequences. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Qamhieh, H.T.; Wing, R.R.; Jeffery, R.W.; Stinson, V.L.; Kuller, L.H.; Wu, K.K. Impact of weight loss on plasminogen activator inhibitor (PAI-1), factor VII, and other hemostatic factors in moderately overweight adults. Arterioscler. Thromb. 1993, 13, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Murthy, V.; Pacold, M.; Danielson, K.; Tanriverdi, K.; Larson, M.G.; Hanspers, K.; Pico, A.; Mick, E.; Rei, J.; et al. Extracellular RNAs are associated with insulin resistance and metabolic phenotypes. Diabetes Care 2017, 40, 546–553. [Google Scholar] [CrossRef]
- Mohany, K.M.; Al Rugaie, O.; Al-Wutayd, O.; Al-Nafeesah, A. Investigation of the levels of circulating miR-29a, miR-122, sestrin 2 and inflammatory markers in obese children with/without type 2 diabetes: A case control study. BMC Endocr. Disord. 2021, 21, 152. [Google Scholar] [CrossRef]
- Zeinali, F.; Aghaei Zarch, S.M.; Jahan-Mihan, A.; Kalantar, S.M.; Vahidi Mehrjardi, M.Y.; Fallahzadeh, H.; Hosseinzadeh, M.; Rahmanian, M.; Mozaffari-Khosravi, H. Circulating microRNA-122, microRNA-126-3p, and microRNA-146a are associated with inflammation in patients with pre-diabetes and type 2 diabetes mellitus: A case control study. PLoS ONE 2021, 16, e0251697. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Siitonen, N.; Pulkkinen, L.; Lindström, J.; Kolehmainen, M.; Eriksson, J.G.; Venojärvi, M.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Tuomilehto, J.; Uusitupa, M. Association of ADIPOQ gene variants with body weight, type 2 diabetes, and serum adiponectin concentrations: The Finnish Diabetes Prevention Study. BMC Med. Genet. 2011, 12, 5. [Google Scholar] [CrossRef]
- Gupta, V.; Mishra, S.; Mishra, S.; Kumar, S.; Gupta, V. Association of Leptin: Adiponectin ratio and metabolic risk markers in postmenopausal women. Immunol. Lett. 2018, 196, 63–67. [Google Scholar] [CrossRef]
- Hasseine, L.K.; Hinault, C.; Lebrun, P.; Gautier, N.; Paul-Bellon, R.; Van Obberghen, E. miR-139 impacts FoxO1 action by decreasing FoxO1 protein in mouse hepatocytes. Biochem. Biophys. Res. Commun. 2009, 390, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Deiuliis, J.A.; Syed, R.; Duggineni, D.; Rutsky, J.; Rengasamy, P.; Zhang, J.; Huang, K.; Needleman, B.; Mikami, D.; Perry, K.; et al. Visceral Adipose MicroRNA 223 Is Upregulated in Human and Murine Obesity and Modulates the Inflammatory Phenotype of Macrophages. PLoS ONE 2016, 11, e0165962. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.; Liu, Y.; Song, Y.; Lai, L.; Han, Q.; Cao, X.; Wang, Q. Inducible MicroRNA-223 Down-Regulation Promotes TLR-Triggered IL-6 and IL-1b Production in Macrophages by Targeting STAT3. PLoS ONE 2012, 7, e42971. [Google Scholar]
- Madhyastha, R.; Madhyastha, H.; Nurrahmah, Q.I.; Purbasari, B.; Maruyama, M.; Nakajima, Y. MicroRNA 21 Elicits a Pro-inflammatory Response in Macrophages, with Exosomes Functioning as Delivery Vehicles. Inflammation 2021, 44, 1274–1287. [Google Scholar] [CrossRef]
- Nara, K.; Kawashima, N.; Noda, S.; Fujii, M.; Hashimoto, K.; Tazawa, K.; Okiji, T. Anti-inflammatory roles of microRNA 21 in lipopolysaccharide-stimulated human dental pulp cells. J. Cell. Physiol. 2019, 234, 21331–21341. [Google Scholar] [CrossRef]
- Ling, H.-Y.; Hu, B.; Hu, X.-B.; Zhong, J.; Feng, S.-D.; Qin, L.; Liu, G.; Wen, G.B.; Liao, D.F. MiRNA-21 Reverses High Glucose and High Insulin-Induced Insulin Resistance in 3T3-L1 Adipocytes through Targeting Phosphatase and Tensin Homologue. Exp. Clin. Endocrinol. Diabetes 2012, 120, 553–559. [Google Scholar] [CrossRef]
- Song-Tao, T.; Wang, F.; Shao, M.; Wang, Y.; Hua-Qing, Z. MicroRNA-126 suppresses inflammation in endothelial cells under hyperglycemic condition by targeting HMGB1. Vasc. Pharm. 2017, 88, 48–55. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Kong, Y. Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Invest. Ophthalmol. Vis. Sci. 2019, 60, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Thandavarayan, R.A.; Joladarashi, D.; Jeyabal, P.; Krishnamurthy, S.; Bhimaraj, A.; Youker, K.A.; Krishnamurthy, P. MicroRNA-126 overexpression rescues diabetes-induced impairment in efferocytosis of apoptotic cardiomyocytes. Sci. Rep. 2016, 6, 36207. [Google Scholar]
- Guo, B.; Gu, J.; Zhuang, T.; Zhang, J.; Fan, C.; Li, Y.; Zhao, M.; Chen, R.; Wang, R.; Kong, Y.; et al. MicroRNA-126: From biology to therapeutics. Biomed. Pharmacoth. 2025, 185, 117953. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-P.; Bi, Y.-J.; Liu, D.-M.; Wang, L.-Y. Hsa-miR-375 promotes the progression of inflammatory bowel disease by upregulating TLR4. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7543–7549. [Google Scholar] [CrossRef] [PubMed]
- Li, X. miR-375, a microRNA related to diabetes. Gene 2014, 533, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Villagrán-Silva, F.; Loren, P.; Sandoval, C.; Lanas, F.; Salazar, L.A. Circulating microRNAs as Potential Biomarkers of Overweight and Obesity in Adults: A Narrative Review. Genes 2025, 16, 349. [Google Scholar] [CrossRef]
- Bielska, A.; Niemira, M.; Kretowski, A. Recent Highlights of Research on miRNAs as Early Potential Biomarkers for Cardiovascular Complications of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3153. [Google Scholar] [CrossRef]
- Pointner, A.; Krammer, U.D.B.; Tomeva, E.; Magnet, U.; Hippe, B.; Jacob, U.; Haslberger, A.G. Lifestyle-Driven Variations in Nutrimiromic MicroRNA Expression Patterns across and beyond Genders. Life 2024, 14, 390. [Google Scholar] [CrossRef]

| MiRNA | Nomenclature of Access miRBase | Sequence |
|---|---|---|
| miR-15a | hsa-miR-15a-5p | UAGCAGCACAUAAUGGUUUGUG |
| miR-16 | hsa-miR-16-5p | UAGCAGCACGUAAAUAUUGGCG |
| miR-21 | hsa-miR-21-5p | UAGCUUAUCAGACUGAUGUUGA |
| miR-28 | hsa-miR-28-3p | CACUAGAUUGUGAGCUCCUGGA |
| miR-30 | hsa-miR-30a-5p | UGUAAACAUCCUCGACUGGAAG |
| miR-30d | hsa-mir-30d-5p | UGUAAACAUCCCCGACUGGAAG |
| miR-122 | hsa-miR-122-5p | UGGAGUGUGACAAUGGUGUUUG |
| miR-126 | hsa-miR-126-3p | UCGUACCGUGAGUAAUAAUGCG |
| miR-130 | hsa-miR-130b-3p | CAGUGCAAUGAUGAAAGGGCAU |
| miR-139 | hsa-miR-139-3p | UGGAGACGCGGCCCUGUUGGAGU |
| miR-140 | hsa-miR-140-5p | CAGUGGUUUUACCCUAUGGUAG |
| miR-146 | hsa-miR-146a-5p | UGAGAACUGAAUUCCAUGGGUU |
| miR-150 | hsa-miR-150-5p | UCUCCCAACCCUUGUACCAGUG |
| miR-222 | hsa-miR-222-3p | AGCUACAUCUGGCUACUGGGU |
| miR-223 | hsa-miR-223-3p | UGUCAGUUUGUCAAAUACCCCA |
| miR-363 | hsa-miR-363-3p | AAUUGCACGGUAUCCAUCUGUA |
| miR-375 | hsa-miR-375-3p | UUUGUUCGUUCGGCUCGCGUGA |
| miR-376a | hsa-miR-376a-3p | AUCAUAGAGGAAAAUCCACGU |
| miR-532 | hsa-miR-532-5p | CAUGCCUUGAGUGUAGGACCGU |
| miR-let7c | hsa-miR-let-7c-5p | UGAGGUAGUAGGUUGUAUGGUU |
| Overall | |||||||
|---|---|---|---|---|---|---|---|
| (n = 187) | |||||||
| Sex (male/female) | 84/103 | ||||||
| Early/Middle/Late | 83/71/33 | ||||||
| Non-IR/IR | 105 (56%)/82 (44%) | ||||||
| Normal weight/Overweight + Obesity | 121 (67%)/58 (33%) | ||||||
| Variables | Overall | Non-IR (n = 104) | IR (n = 83) | p-Value | Normal Weight (n = 121) | Overweight (n = 58) | p-Value |
| Age (years) | 15.1 (0.2) | 15.3 (0.2) | 14.8 (0.2) | 0.1655 | 15.2 (0.2) | 15.0 (0.3) | 0.5242 |
| Weight (kg) | 58.8 (1.1) | 54.8 (1.4) | 63.7 (1.8) | 0.0003 ** | 53 (1.0) | 72.3 (1.9) | 0.0001 ** |
| Height (m) | 1.6 (0.0) | 1.6 (01) | 1.6 (0.1) | 0.9818 | 1.64 (0.0) | 1.63 (0.0) | 0.5443 |
| BMI (kg/m2) | 21.7 (0.4) | 20.2 (0.4) | 23.6 (0.6) | 0.0001 ** | 19.5 (0.2) | 27.0 (0.6) | 0.0001 ** |
| Waist circumference (cm) | 77.8 (1.1) | 74.2 (1.5) | 82.4 (1.7) | 0.0007 ** | 72.8 (1.9) | 89.3 (1.7) | 0.0001 ** |
| SBP (mmHg) | 112.6 (1.1) | 111.7 (1.6) | 113.7 (1.3) | 0.3021 | 110.8 (1.2) | 116.5 (1.7) | 0.0055 * |
| DBP (mmHg) | 66.5 (0.8) | 65.8 (1.2) | 67.3 (1.0) | 0.2870 | 64.8 (0.9) | 70.5 (1.2) | 0.0001 ** |
| Total cholesterol (mg/dL) | 143.3 (2.8) | 143.8 (3.8) | 142.8 (3.0) | 0.8038 | 141.3 (3.0) | 148.2 (5.0) | 0.1881 |
| HDL-c (mg/dL) | 44.9 (1.0) | 46.0 (1.1) | 43.6 (1.6) | 0.1884 | 46.7 (1.3) | 41.2 (1.7) | 0.0094 * |
| LDL-c (mg/dL) | 80.6 (2.3) | 81.4 (2.9) | 79.5 (2.8) | 0.5619 | 78.3 (2.9) | 84.9 (3.8) | 0.1015 |
| VLDL-c (mg/dL) | 17.8 (0.7) | 16.3 (0.9 | 19.6 (1.0) | 0.0163 * | 16.2 (0.8) | 21.2 (1.1) | 0.0003 ** |
| Non-HDL cholesterol (mg/dL) | 93.0 (35.0) | 97.8 (3.4) | 99.1 (3.2) | 0.7330 | 94.5 (3.1) | 107.1 (4.3) | 0.0148 * |
| Triacylglycerol (mg/dL) | 89.2 (3.3) | 81.9 (4.4) | 98.3 (5.0) | 0.0172 * | 81.5 (3.9) | 106.1 (5.6) | 0.0003 ** |
| Fasting blood glucose (mg/dL) | 89.7 (0.6) | 87.5 (0.7) | 92.4 (0.8) | 0.0000 ** | 89.0 (0.7) | 90.3 (0.9) | 0.2696 |
| Fasting insulin (µUI/mL) | 15.7 (0.9) | 9.2(0.3) | 23.7 (1.9) | 0.0001 ** | 12.8 (1.0) | 22.2 (2.3) | 0.0011 * |
| hs-CRP (mg/L) | 0.3 (0.0) | 0.3 (0.0) | 0.4 (0.1) | 0.1172 | 0.25(0.0) | 0.5 (0.1) | 0.0167 * |
| PAI-1 (ng/mL) | 23.1 (1.4) | 20.9 (1.6) | 25.9 (2.2) | 0.0487 * | 20.6 (1.2) | 29.2 (3.0) | 0.0078 * |
| MiRNA | Overall (n = 187) | Non-IR (n = 104) | IR (n = 83) | p-Value | Normal Weight (n = 121) | Overweight (n = 58) | p-Value |
|---|---|---|---|---|---|---|---|
| miR-15a | 1.4 (0.1) | 1.3 (1.1) | 1.6 (0.2) | 0.5372 | 1.3 (0.1) | 1.7(0.2) | 0.5715 |
| miR-16a | 1.4 (0.1) | 1.3 (0.1) | 1.6 (0.2) | 0.7936 | 1.3 (0.1) | 1.7 (0.2) | 0.7055 |
| miR-21 | 1.5 (0.1) | 1.3 (0.1) | 1.6 (0.2) | 0.3834 | 1.3 (0.1) | 1.8 (0.3) | 0.4245 |
| miR-28 | 1.7 (0.2) | 1.5 (0.2) | 1.9 (0.2) | 0.4035 | 1.5 (0.2) | 2.2 (0.3) | 0.3019 |
| miR-30a | 1.2 (0.0) | 1.1 (0.0) | 1.3 (0.1) | 0.3832 | 1.1 (0.0) | 1.3 (0.2) | 0.9969 |
| miR-30d | 1.4 (0.1) | 1.3 (0.1) | 1.6 (0.2) | 0.3079 | 1.3 (0.1) | 1.7 (0.3) | 0.2822 |
| miR-126 | 1.4 (0.0) | 1.3 (0.1) | 1.4 (0.1) | 0.581 | 1;2 (0.1) | 1.6 (0.2) | 0.4035 |
| miR-130b | 1.5 (0.1) | 1.4 (0.1) | 1.7 (0.2) | 0.7106 | 1.4 (0.2) | 1.8 (0.3) | 0.7203 |
| miR-140 | 2.0 (0.2) | 1.9 (0.4) | 2.0 (0.3) | 0.9306 | 1.8 (0.3) | 2.5 (0.5) | 0.4432 |
| miR-146a | 1.7 (0.1) | 1.5 (0.2) | 2.0 (0.3) | 0.364 | 1.5 (0.2) | 2.2 (0.3) | 0.3497 |
| miR-150 | 1.7 (0.2) | 1.6 (0.3) | 2.0 (0.3) | 0.5489 | 1.5 (0.3) | 2.3 (0.5) | 0.3553 |
| miR-222 | 1.7 (0.2) | 1.5 (0.2) | 1.9 (0.2) | 0.4341 | 1.6 (0.2) | 2.0 (0.3) | 0.6846 |
| miR-223 | 1.7 (0.1) | 1.5 (0.2) | 1.8 (0.2) | 0.5288 | 1.5 (0.1) | 2.1 (0.3) | 0.321 |
| miR-363 | 1.5 (0.1) | 1.4 (0.2) | 1.7 (0.2) | 0.618 | 1.4 (0.1) | 1.8 (0.3) | 0.7681 |
| miR-375 | 1.3 (0.0) | 1.1 (0.0) | 1.4 (0.2) | 0.1655 | 1;3 (0.1) | 1.1 (0.2) | 0.3131 |
| miR-376a | 2.0 (0.2) | 2.1(0.2) | 1.9 (0.3) | 0.6015 | 2.0 (0.2) | 2.1 (0.3) | 0.9821 |
| miR-486 | 2.3 (0.1) | 1.2 (0.1) | 1.4 (0.2) | 0.6631 | 1.2 (0.1) | 1.5 (0.2) | 0.8331 |
| miR-532 | 1.6 (0.1) | 1.5 (0.2) | 1.8 (0.2) | 0.7235 | 1.5 (0.2) | 2.0 (0.3) | 0.5688 |
| miR-let7c | 1.4 (0.1) | 1.3 (0.1) | 1.7 (0.2) | 0.1826 | 1.3 (0.1) | 1.8 (0.3) | 0.3395 |
| Variables | miR-122 (n = 187) | miR-139-3p (n = 157) | ||
|---|---|---|---|---|
| Coef. | p-Value | Coef. | p-Value | |
| BMI (kg/m2) | 0.05 | 0.421 | 0.09 | 0.122 |
| Waist circumference (cm) | 0.039 | 0.467 | 0.07 | 0.251 |
| SBP (mmHg) | 0.06 | 0.272 | 0.12 | 0.038 * |
| DBP (mmHg) | 0.07 | 0.202 | 0.14 | 0.023 * |
| Total cholesterol (mg/dL) | 0.04 | 0.477 | 0.07 | 0.239 |
| HDL-c (mg/dL) | 0.03 | 0.596 | −0.07 | 0.217 |
| LDL-c (mg/dL) | −0.02 | 0.734 | 0.09 | 0.098 |
| VLDL-c (mg/dL) | 0.15 | 0.002 ** | 0.05 | 0.391 |
| Non-HDL cholesterol (mg/dL) | 0.02 | 0.666 | 0.09 | 0.066 |
| Triacylglycerol (mg/dL) | 0.15 | 0.002 ** | 0.05 | 0.416 |
| Fasting blood glucose (mg/dL) | −0.03 | 0.635 | 0.007 | 0.911 |
| Fasting insulin (µUI/mL) | 0.09 | 0.102 | 0.107 | 0.093 |
| HOMA-IR | 0.09 | 0.128 | 0.109 | 0.091 |
| hs-CRP (mg/L) | −0.00 | 0.988 | 0.110 | 0.076 |
| PAI-1 (ng/mL) | 0.04 | 0.453 | 0.027 | 0.630 |
| Leptin (ng/mL) | −0.00 | 0.976 | 0.050 | 0.373 |
| Adiponectin (ng/mL) | 0.06 | 0.316 | −0.12 | 0.028 * |
| TNF-α (pg/mL) | 0.09 | 0.092 | 0.01 | 0.839 |
| MCP-1 (ng/mL) | 0.04 | 0.438 | 0.04 | 0.554 |
| ICAM (ng/mL) | −0.00 | 0.992 | 0.09 | 0.091 |
| VCAM (ng/mL) | 0.07 | 0.186 | −0.04 | 0.458 |
| IL-6 (pg/mL) | 0.12 | 0.01 * | 0.06 | 0.269 |
| IL-10 (pg/mL) | 0.17 | 0.002 ** | 0.02 | 0.769 |
| IL-1β (pg/mL) | 0.14 | 0.008 ** | −0.02 | 0.764 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payolla, T.B.; Brandão-Lima, P.N.; de Carvalho, G.B.; Sarti, F.M.; Fisberg, R.M.; Rogero, M.M. Circulating miR-122 and miR-139-3p: Association with Lipid, Inflammatory, and Glycemic Profile in Adolescents with Insulin-Resistant and Overweight. Endocrines 2025, 6, 51. https://doi.org/10.3390/endocrines6040051
Payolla TB, Brandão-Lima PN, de Carvalho GB, Sarti FM, Fisberg RM, Rogero MM. Circulating miR-122 and miR-139-3p: Association with Lipid, Inflammatory, and Glycemic Profile in Adolescents with Insulin-Resistant and Overweight. Endocrines. 2025; 6(4):51. https://doi.org/10.3390/endocrines6040051
Chicago/Turabian StylePayolla, Tanyara Baliani, Paula Nascimento Brandão-Lima, Gabrielli Barbosa de Carvalho, Flávia Mori Sarti, Regina Mara Fisberg, and Marcelo Macedo Rogero. 2025. "Circulating miR-122 and miR-139-3p: Association with Lipid, Inflammatory, and Glycemic Profile in Adolescents with Insulin-Resistant and Overweight" Endocrines 6, no. 4: 51. https://doi.org/10.3390/endocrines6040051
APA StylePayolla, T. B., Brandão-Lima, P. N., de Carvalho, G. B., Sarti, F. M., Fisberg, R. M., & Rogero, M. M. (2025). Circulating miR-122 and miR-139-3p: Association with Lipid, Inflammatory, and Glycemic Profile in Adolescents with Insulin-Resistant and Overweight. Endocrines, 6(4), 51. https://doi.org/10.3390/endocrines6040051







