Neuroendocrinological Aspects of a Tailored Hormonal Contraception
Abstract
1. Hormonal Contraceptives and Neuroendocrinology
2. Neuroendocrine Effects of the Estrogenic Components in Hormonal Contraception
2.1. Ethinylestradiol (EE)
2.2. Estradiol (E2)
2.3. Estetrol (E4)
3. Progestin Components and Neuroendocrine Effects
4. Implications of Hormonal Contraception for Mood, Sexuality, and Behaviour
4.1. PMS and PMDD
4.2. Depression and Anxiety
4.3. Sexuality and Arousal
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Regidor, P.-A. Clinical relevance in present day hormonal contraception. Horm. Mol. Biol. Clin. Investig. 2018, 37, 20180030. [Google Scholar] [CrossRef]
- Bahamondes, L.; Valeria Bahamondes, M.; Shulman, L.P. Non-contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Hum. Reprod. Update 2015, 21, 640–651. [Google Scholar] [CrossRef]
- Rivera, R.; Yacobson, I.; Grimes, D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 1999, 181 Pt 1, 1263–1269. [Google Scholar] [CrossRef]
- Cooper, D.B.; Patel, P. Oral Contraceptive Pills. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK430882/ (accessed on 28 January 2025).
- Burkman, R.T. The transdermal contraceptive system. Am. J. Obstet. Gynecol. 2004, 190, S49–S53. [Google Scholar] [CrossRef]
- Hilz, E.N. Methods and considerations for the use of hormonal contraceptives in rat models of neurobehavior. Front. Neuroendocrinol. 2022, 66, 101011. [Google Scholar] [CrossRef] [PubMed]
- Skovlund, C.W.; Mørch, L.S.; Kessing, L.V.; Lidegaard, Ø. Association of Hormonal Contraception with Depression. JAMA Psychiatry 2016, 73, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Tronson, N.C.; Schuh, K.M. Hormonal contraceptives, stress, and the brain: The critical need for animal models. Front. Neuroendocrinol. 2022, 67, 101035. [Google Scholar] [CrossRef]
- Taxier, L.R.; Gross, K.S.; Frick, K.M. Oestradiol as a neuromodulator of learning and memory. Nat. Rev. Neurosci. 2020, 21, 535–550. [Google Scholar] [CrossRef]
- Moenter, S.M.; Starrett, J.R. Estradiol action in the female hypothalamo-pituitary-gonadal axis. J. Neuroendocrinol. 2024, 36, e13390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cui, D.; Wang, B.; Han, Y.-H.; Balimane, P.; Yang, Z.; Sinz, M.; Rodrigues, A.D. Pharmacokinetic drug interactions involving 17alpha-ethinylestradiol: A new look at an old drug. Clin. Pharmacokinet. 2007, 46, 133–157. [Google Scholar] [CrossRef]
- Odlind, V.; Milsom, I.; Persson, I.; Victor, A. Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills? Acta Obstet. Gynecol. Scand. 2002, 81, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Picazo, O.; Becerril-Montes, A.; Huidobro-Perez, D.; Garcia-Segura, L.M. Neuroprotective Actions of the Synthetic Estrogen 17α-Ethynylestradiol in the Hippocampus. Cell. Mol. Neurobiol. 2010, 30, 675–682. [Google Scholar] [CrossRef]
- Vega-Rivera, N.M.; López-Rubalcava, C.; Estrada-Camarena, E. The antidepressant-like effect of ethynyl estradiol is mediated by both serotonergic and noradrenergic systems in the forced swimming test. Neuroscience 2013, 250, 102–111. [Google Scholar] [CrossRef]
- Larsen, S.V.; Köhler-Forsberg, K.; Dam, V.H.; Poulsen, A.S.; Svarer, C.; Jensen, P.S.; Knudsen, G.M.; Fisher, P.M.; Ozenne, B.; Frokjaer, V.G. Oral contraceptives and the serotonin 4 receptor: A molecular brain imaging study in healthy women. Acta Psychiatr. Scand. 2020, 142, 294–306. [Google Scholar] [CrossRef]
- Mennenga, S.E.; Gerson, J.E.; Koebele, S.V.; Kingston, M.L.; Tsang, C.W.S.; Engler-Chiurazzi, E.B.; Baxter, L.C.; Bimonte-Nelson, H.A. Understanding the cognitive impact of the contraceptive estrogen Ethinyl Estradiol: Tonic and cyclic administration impairs memory, and performance correlates with basal forebrain cholinergic system integrity. Psychoneuroendocrinology 2015, 54, 1–13. [Google Scholar] [CrossRef]
- Simone, J.; Bogue, E.A.; Bhatti, D.L.; Day, L.E.; Farr, N.A.; Grossman, A.M.; Holmes, P.V. Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain-derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology 2015, 62, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Rybaczyk, L.A.; Bashaw, M.J.; Pathak, D.R.; Moody, S.M.; Gilders, R.M.; Holzschu, D.L. An overlooked connection: Serotonergic mediation of estrogen-related physiology and pathology. BMC Womens Health 2005, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; Hasegawa, H. Chapter 12—Tryptophan hydroxylase and serotonin synthesis regulation. In Handbook of Behavioral Neuroscience; Müller, C.P., Cunningham, K.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 239–256. Available online: https://www.sciencedirect.com/science/article/pii/B9780444641250000128 (accessed on 28 January 2025).
- Hampson, E.; Morley, E.E.; Evans, K.L.; Fleury, C. Effects of oral contraceptives on spatial cognition depend on pharmacological properties and phase of the contraceptive cycle. Front. Endocrinol. 2022, 13, 888510. Available online: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2022.888510/full (accessed on 20 July 2025). [CrossRef]
- Bendis, P.C.; Zimmerman, S.; Onisiforou, A.; Zanos, P.; Georgiou, P. The impact of estradiol on serotonin, glutamate, and dopamine systems. Front. Neurosci. 2024, 18, 1348551. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Winer, S.A.; Foidart, J.-M.; Archer, D.F. Comparison of estrogenic components used for hormonal contraception. Contraception 2024, 130, 110310. [Google Scholar] [CrossRef]
- Goodman, R.L.; Lehman, M.N.; Smith, J.T.; Coolen, L.M.; de Oliveira, C.V.R.; Jafarzadehshirazi, M.R.; Pereira, A.; Iqbal, J.; Caraty, A.; Ciofi, P.; et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007, 148, 5752–5760. [Google Scholar] [CrossRef]
- Menown, S.J.; Tello, J.A. Neurokinin 3 Receptor Antagonists Compared with Serotonin Norepinephrine Reuptake Inhibitors for Non-Hormonal Treatment of Menopausal Hot Flushes: A Systematic Qualitative Review. Adv. Ther. 2021, 38, 5025–5045. [Google Scholar] [CrossRef]
- Kuo, L.E.; Kitlinska, J.B.; Tilan, J.U.; Li, L.; Baker, S.B.; Johnson, M.D.; Lee, E.W.; Burnett, M.S.; Fricke, S.T.; Kvetnansky, R.; et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007, 13, 803–811. [Google Scholar] [CrossRef]
- Santollo, J.; Eckel, L.A. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav. Brain Res. 2008, 191, 173–177. [Google Scholar] [CrossRef][Green Version]
- Lapchak, P.A. Effect of estradiol treatment on beta-endorphin content and release in the female rat hypothalamus. Brain Res. 1991, 554, 198–202. [Google Scholar] [CrossRef]
- Foy, M.R.; Xu, J.; Xie, X.; Brinton, R.D.; Thompson, R.F.; Berger, T.W. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J. Neurophysiol. 1999, 81, 925–929. [Google Scholar] [CrossRef]
- Holinka, C.F.; Diczfalusy, E.; Coelingh Bennink, H.J.T. Estetrol: A unique steroid in human pregnancy. J. Steroid Biochem. Mol. Biol. 2008, 110, 138–143. [Google Scholar] [CrossRef]
- Battipaglia, C.; Genazzani, A.D.; Nappi, R.E.; La Marca, A. Insights on estetrol, the native estrogen: From contraception to hormone replacement therapy. Minerva Obstet. Gynecol. 2024, 76, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Santoro, A.N.; Casarosa, E.; Giannini, A.; Genazzani, A.; Russo, M.; Russo, N.; Petignat, P.; Genazzani, A.R. Effect of estetrol administration on brain and serum allopregnanolone in intact and ovariectomized rats. J. Steroid Biochem. Mol. Biol. 2014, 143, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Diviccaro, S.; Cioffi, L.; Falvo, E.; Giatti, S.; Melcangi, R.C. Allopregnanolone: An overview on its synthesis and effects. J. Neuroendocrinol. 2022, 34, e12996. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Drakopoulos, P.; Casarosa, E.; Freschi, L.; Petignat, P.; Yaron, M.; Genazzani, A.R. Effect of estetrol on Beta-Endorphin level in female rats. Steroids 2015, 95, 104–110. [Google Scholar] [CrossRef]
- Tskitishvili, E.; Nisolle, M.; Munaut, C.; Pequeux, C.; Gerard, C.; Noel, A.; Foidart, J.-M. Estetrol attenuates neonatal hypoxic-ischemic brain injury. Exp. Neurol. 2014, 261, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Tskitishvili, E.; Pequeux, C.; Munaut, C.; Viellevoye, R.; Nisolle, M.; Noël, A.; Foidart, J.-M. Estrogen receptors and estetrol-dependent neuroprotective actions: A pilot study. J. Endocrinol. 2017, 232, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Tskitishvili, E.; Pequeux, C.; Munaut, C.; Viellevoye, R.; Nisolle, M.; Noël, A.; Foidart, J.-M. Use of estetrol with other steroids for attenuation of neonatal hypoxic-ischemic brain injury: To combine or not to combine? Oncotarget 2016, 7, 33722–33743. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone receptors: Form and function in brain. Front. Neuroendocrinol. 2008, 29, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Ducharme, S.; Karama, S. Effects of Sex Steroids in the Human Brain. Mol. Neurobiol. 2017, 54, 7507–7519. [Google Scholar] [CrossRef]
- Sitruk-Ware, R. New progestagens for contraceptive use. Hum. Reprod. Update 2006, 12, 169–178. [Google Scholar] [CrossRef]
- Schindler, A.E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J.R.; Schweppe, K.W.; Thijssen, J.H.H. Classification and pharmacology of progestins. Maturitas 2008, 61, 171–180. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Fidecicchi, T.; Arduini, D.; Giannini, A.; Simoncini, T. Hormonal and natural contraceptives: A review on efficacy and risks of different methods for an informed choice. Gynecol. Endocrinol. 2023, 39, 2247093. [Google Scholar] [CrossRef]
- Pluchino, N.; Cubeddu, A.; Giannini, A.; Merlini, S.; Cela, V.; Angioni, S.; Genazzani, A.R. Progestogens and brain: An update. Maturitas 2009, 62, 349–355. [Google Scholar] [CrossRef]
- Mitchell, V.E.; Welling, L.L.M. Not All Progestins are Created Equally: Considering Unique Progestins Individually in Psychobehavioral Research. Adapt. Hum. Behav. Physiol. 2020, 6, 381–412. [Google Scholar] [CrossRef]
- Porcu, P.; Mostallino, M.C.; Sogliano, C.; Santoru, F.; Berretti, R.; Concas, A. Long-term administration with levonorgestrel decreases allopregnanolone levels and alters GABAA receptor subunit expression and anxiety-like behavior. Pharmacol. Biochem. Behav. 2012, 102, 366–372. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Pluchino, N.; Begliuomini, S.; Pieri, M.; Centofanti, M.; Freschi, L.; Casarosa, E.; Luisi, M. Drospirenone increases central and peripheral β-endorphin in ovariectomized female rats. Menopause 2007, 14, 63. [Google Scholar] [CrossRef]
- Pluchino, N.; Lenzi, E.; Merlini, S.; Giannini, A.; Cubeddu, A.; Casarosa, E.; Begliuomini, S.; Luisi, M.; Cela, V.; Genazzani, A.R. Selective effect of chlormadinone acetate on brain allopregnanolone and opioids content. Contraception 2009, 80, 53–62. [Google Scholar] [CrossRef]
- Lenzi, E.; Pluchino, N.; Begliuomini, S.; Ninni, F.; Freschi, L.; Centofanti, M.; Casarosa, E.; Luisi, S.; Valentino, V.; Luisi, M.; et al. Effects of nomegestrol acetate administration on central and peripheral beta-endorphin and allopregnanolone in ovx rats. J. Steroid Biochem. Mol. Biol. 2008, 110, 67–75. [Google Scholar] [CrossRef]
- Pluchino, N.; Luisi, M.; Lenzi, E.; Centofanti, M.; Begliuomini, S.; Freschi, L.; Ninni, F.; Genazzani, A.R. Progesterone and progestins: Effects on brain, allopregnanolone and β-endorphin. J. Steroid Biochem. Mol. Biol. 2006, 102, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yonkers, K.A.; O’Brien, P.M.S.; Eriksson, E. Premenstrual syndrome. Lancet 2008, 371, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Epperson, C.N.; Steiner, M.; Hartlage, S.A.; Eriksson, E.; Schmidt, P.J.; Jones, I.; Yonkers, K.A. Premenstrual dysphoric disorder: Evidence for a new category for DSM-5. Am. J. Psychiatry 2012, 169, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, K.M.; Dimmock, P.W.; Ismail, K.M.K.; Jones, P.W.; O’Brien, P.M.S. The effectiveness of GnRHa with and without “add-back” therapy in treating premenstrual syndrome: A meta analysis. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 585–593. [Google Scholar] [CrossRef]
- Gingnell, M.; Morell, A.; Bannbers, E.; Wikström, J.; Sundström Poromaa, I. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm. Behav. 2012, 62, 400–406. [Google Scholar] [CrossRef]
- Tiranini, L.; Nappi, R.E. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Fac. Rev. 2022, 11, 11. [Google Scholar] [CrossRef]
- Pearlstein, T.B.; Bachmann, G.A.; Zacur, H.A.; Yonkers, K.A. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception 2005, 72, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.M.; Kaptein, A.A.; Helmerhorst, F.M. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst. Rev. 2012, 2, CD006586. [Google Scholar] [CrossRef] [PubMed]
- Robertson, E.; Thew, C.; Thomas, N.; Karimi, L.; Kulkarni, J. Pilot Data on the Feasibility and Clinical Outcomes of a Nomegestrol Acetate Oral Contraceptive Pill in Women with Premenstrual Dysphoric Disorder. Front. Endocrinol. 2021, 12, 704488. Available online: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2021.704488/full (accessed on 2 February 2025). [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Tiller, J.W.G. Depression and anxiety. Med. J. Aust. 2013, 199, S28–S31. [Google Scholar] [CrossRef]
- Buggio, L.; Barbara, G.; Facchin, F.; Ghezzi, L.; Dridi, D.; Vercellini, P. The influence of hormonal contraception on depression and female sexuality: A narrative review of the literature. Gynecol. Endocrinol. 2022, 38, 193–201. [Google Scholar] [CrossRef]
- Duke, J.M.; Sibbritt, D.W.; Young, A.F. Is there an association between the use of oral contraception and depressive symptoms in young Australian women? Contraception 2007, 75, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Keyes, K.M.; Cheslack-Postava, K.; Westhoff, C.; Heim, C.M.; Haloossim, M.; Walsh, K.; Koenen, K. Association of hormonal contraceptive use with reduced levels of depressive symptoms: A national study of sexually active women in the United States. Am. J. Epidemiol. 2013, 178, 1378–1388. [Google Scholar] [CrossRef]
- Stenhammar, E.; Wikman, P.; Gemzell Danielsson, K.; Kopp-Kallner, H.; Sundström Poromaa, I. Levonorgestrel intrauterine device and depression: A Swedish register-based cohort study. Int. J. Psychophysiol. 2023, 193, 112230. [Google Scholar] [CrossRef] [PubMed]
- Anderl, C.; Li, G.; Chen, F.S. Oral contraceptive use in adolescence predicts lasting vulnerability to depression in adulthood. J. Child. Psychol. Psychiatry 2020, 61, 148–156. [Google Scholar] [CrossRef]
- Burrows, L.J.; Basha, M.; Goldstein, A.T. The effects of hormonal contraceptives on female sexuality: A review. J. Sex. Med. 2012, 9, 2213–2223. [Google Scholar] [CrossRef]
- Simon, J.; Braunstein, G.; Nachtigall, L.; Utian, W.; Katz, M.; Miller, S.; Waldbaum, A.; Bouchard, C.; Derzko, C.; Buch, A.; et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J. Clin. Endocrinol. Metab. 2005, 90, 5226–5233. [Google Scholar] [CrossRef]
- Shepherd, J.E. Therapeutic options in female sexual dysfunction. J. Am. Pharm. Assoc. 2002, 42, 479–487; quiz 487–488. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, G.; Liu, J.; Li, Y.; Du, P. Is There an Association Between Contraception and Sexual Dysfunction in Women? A Systematic Review and Meta-analysis Based on Female Sexual Function Index. J. Sex. Med. 2020, 17, 1942–1955. [Google Scholar] [CrossRef]
- King, S.R. Emerging roles for neurosteroids in sexual behavior and function. J. Androl. 2008, 29, 524–533. [Google Scholar] [CrossRef]
- Both, S.; Lew-Starowicz, M.; Luria, M.; Sartorius, G.; Maseroli, E.; Tripodi, F.; Lowenstein, L.; Nappi, R.E.; Corona, G.; Reisman, Y.; et al. Hormonal Contraception and Female Sexuality: Position Statements from the European Society of Sexual Medicine (ESSM). J. Sex. Med. 2019, 16, 1681–1695. [Google Scholar] [CrossRef]
- van Lunsen, R.H.W.; Zimmerman, Y.; Coelingh Bennink, H.J.T.; Termeer, H.M.M.; Appels, N.; Fauser, B.C.J.M.; Laan, E. Maintaining physiologic testosterone levels during combined oral contraceptives by adding dehydroepiandrosterone: II. Effects on sexual function. A phase II randomized, double-blind, placebo-controlled study. Contraception 2018, 98, 56–62. [Google Scholar] [CrossRef]
- Casey, E.; Reese, M.; Okafor, E.; Chun, D.; Gagnon, C.; Nigl, F.; Dhaher, Y.Y. Influence of Menstrual Cycle and Oral Contraceptive Phase on Spinal Excitability. PM R 2016, 8, 860–868. [Google Scholar] [CrossRef] [PubMed]
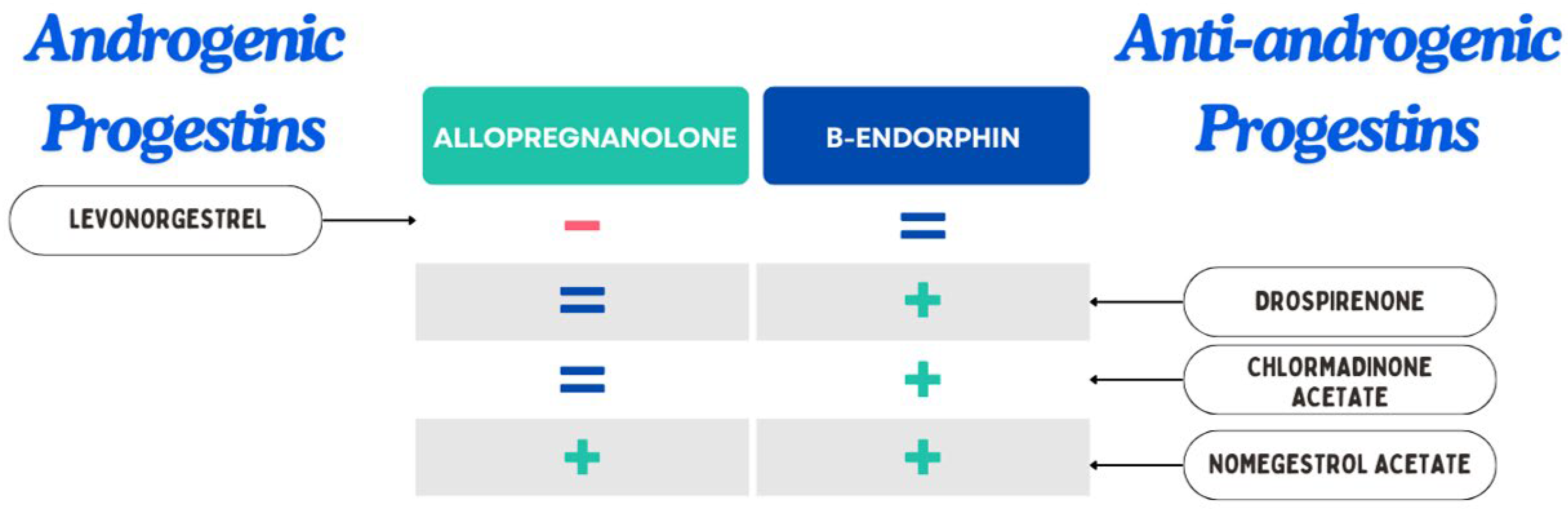
| Component | Class | Neuroendocrine Changes | Clinical Considerations |
|---|---|---|---|
| Ethinylestradiol (EE) | Estrogen | ↑ SHBG, modulates 5-HT1A, may ↓ serotonin tone | Probably better to avoid higher dosages in mood-sensitive users |
| Estradiol (E2) | Estrogen | ↑ β-END, ↑ serotonin and dopamine | Potential mood-stabilizing effect |
| Estetrol (E4) | Estrogen | ↑ allopregnanolone, ↑ β-END | May stabilize mood and improve anxiety |
| Levonorgestrel (LNG) | Androgenic Progestin | ↓ allopregnanolone, ↓ dopamine | May worsen anxiety and be associated with depressive symptoms |
| Drospirenone (DRSP) | Anti-Androgenic Progestin | ↑ β-END, preserves allopregnanolone levels | May show benefit on mood and PMDD |
| Nomegestrol Acetate | Anti-Androgenic Progestin | ↑ allopregnanolone, ↑ β-END | Favourable effect for mood-sensitive patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battipaglia, C.; Szeliga, A.; Setti, V.; Bala, G.; Chedraui, P.; Genazzani, A.D.; Meczekalski, B. Neuroendocrinological Aspects of a Tailored Hormonal Contraception. Endocrines 2025, 6, 37. https://doi.org/10.3390/endocrines6030037
Battipaglia C, Szeliga A, Setti V, Bala G, Chedraui P, Genazzani AD, Meczekalski B. Neuroendocrinological Aspects of a Tailored Hormonal Contraception. Endocrines. 2025; 6(3):37. https://doi.org/10.3390/endocrines6030037
Chicago/Turabian StyleBattipaglia, Christian, Anna Szeliga, Veronica Setti, Gregory Bala, Peter Chedraui, Alessandro D. Genazzani, and Blazej Meczekalski. 2025. "Neuroendocrinological Aspects of a Tailored Hormonal Contraception" Endocrines 6, no. 3: 37. https://doi.org/10.3390/endocrines6030037
APA StyleBattipaglia, C., Szeliga, A., Setti, V., Bala, G., Chedraui, P., Genazzani, A. D., & Meczekalski, B. (2025). Neuroendocrinological Aspects of a Tailored Hormonal Contraception. Endocrines, 6(3), 37. https://doi.org/10.3390/endocrines6030037








