Euglycemic Hyperinsulinemia Lowers Blood Pressure and Impedes Microvascular Perfusion More Effectively in Persons with Cardio-Metabolic Disease
Abstract
1. Introduction
2. Methods
2.1. Study Participants
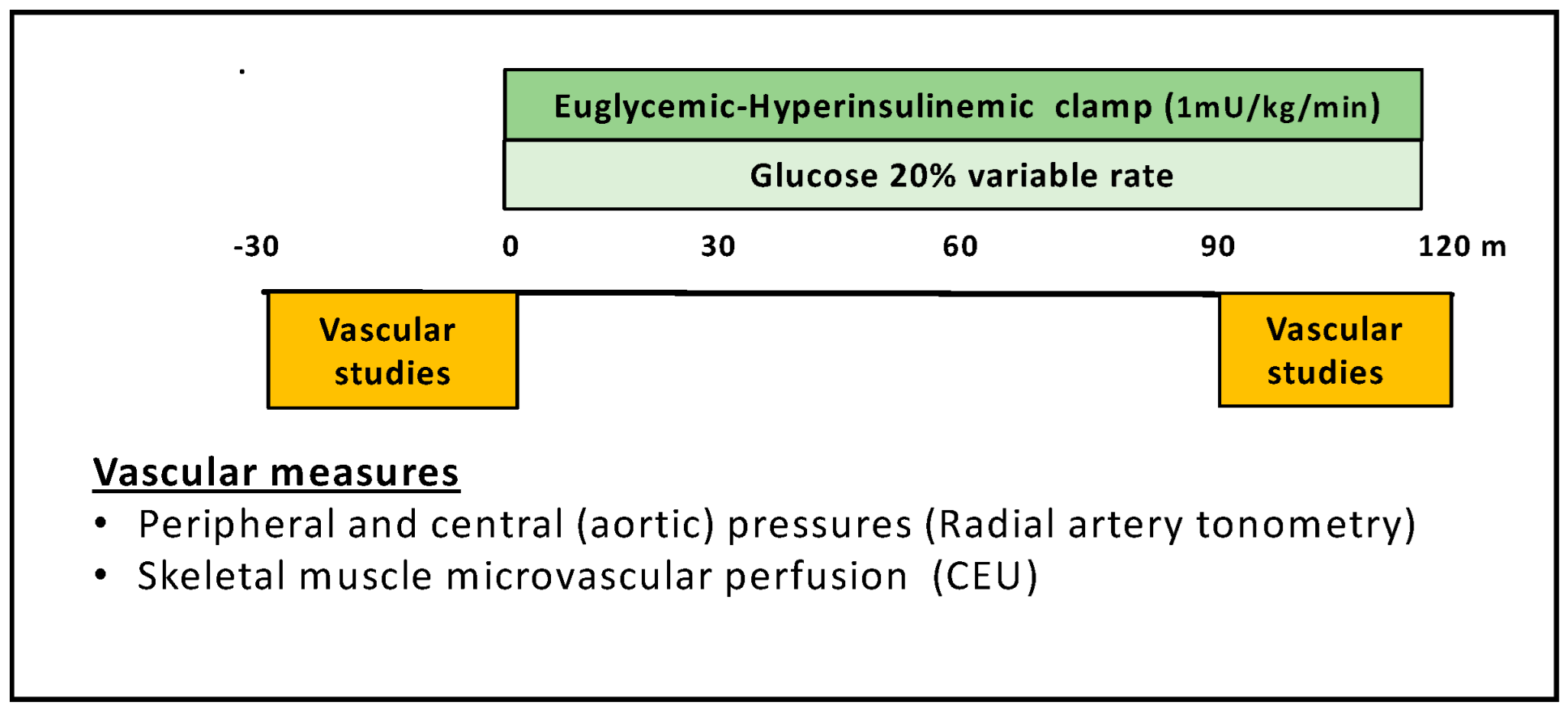
2.2. Experimental Protocol
2.3. Determination of Microvascular Perfusion by CEU
2.4. Recording of Peripheral and Central Blood Pressure
2.5. Euglycemic Hyperinsulinemic Clamp
2.6. Biochemical Analyses
2.7. Statistics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zeng, G.; Nystrom, F.H.; Ravichandran, L.V.; Cong, L.-N.; Kirby, M.; Mostowski, H.; Quon, M.J. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 2000, 101, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Quon, M.J. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J. Clin. Investig. 1996, 98, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Lin, Y.-W.; Clemont, A.; Feener, E.P.; Hein, K.D.; Igarashi, M.; Yamauchi, T.; White, M.F.; King, G.L. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J. Clin. Investig. 1999, 104, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.A.; Hoffman, R.P.; Balon, T.W.; Sinkey, C.A.; Mark, A.L. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J. Clin. Investig. 1991, 87, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.A.; Balon, T.W.; Hoffman, R.P.; Sinkey, C.A.; Mark, A.L. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension 1992, 19, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Limberg, J.K.; Smith, J.A.; Soares, R.N.; Harper, J.L.; Houghton, K.N.; Jacob, D.W.; Mozer, M.T.; Grunewald, Z.I.; Johnson, B.D.; Curry, T.B.; et al. Sympathetically mediated increases in cardiac output, not restraint of peripheral vasodilation, contribute to blood pressure maintenance during hyperinsulinemia. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H162–H170. [Google Scholar] [CrossRef] [PubMed]
- McMillan, N.J.; Soares, R.N.; Harper, J.L.; Shariffi, B.; Moreno-Cabanas, A.; Curry, T.B.; Manrique-Acevedo, C.; Padilla, J.; Limberg, J.K. Increased Muscle Sympathetic Nerve Activity with Acute Hyperinsulinemia: Role of Insulin-stimulated Peripheral Vasodilation and the Response of the Arterial Baroreflex. FASEB J. 2022, 36, R4106. [Google Scholar] [CrossRef]
- Heise, T.; Magnusson, K.; Heinemann, L.; Sawicki, P.T. Insulin resistance and the effect of insulin on blood pressure in essential hypertension. Hypertension 1998, 32, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Tamminen, M.; Westerbacka, J.; Vehkavaara, S.; Yki-Järvinen, H. Insulin-induced decreases in aortic wave reflection and central systolic pressure are impaired in type 2 diabetes. Diabetes Care 2002, 25, 2314–2319. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.A.; Hartline, L.M.; Nguyen, T.; Aylor, K.; Horton, W.B.; Liu, Z.; Barrett, E.J. Empagliflozin improves vascular insulin sensitivity and muscle perfusion in persons with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E258–E267. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Dawson, D.; Clark, A.; Lindner, J.; Rattigan, S.; Clark, M.; Barrett, E.J. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 2002, 51, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Coggins, M.; Lindner, J.; Rattigan, S.; Jahn, L.; Fasy, E.; Kaul, S.; Barrett, E. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 2001, 50, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, E.M.; Jahn, L.A.; Barrett, E.J. Early microvascular recruitment modulates subsequent insulin-mediated skeletal muscle glucose metabolism during lipid infusion. Diabetes Care 2013, 36, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Barrett, E.; Lindner, J.; Clark, M.; Rattigan, S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E123–E129. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Remash, D.; Russell, R.D.; Greenaway, T.; Rattigan, S.; Squibb, K.A.; Jones, G.; Premilovac, D.; Richards, S.M.; Keske, M.A. Impairments in adipose tissue microcirculation in type 2 diabetes mellitus assessed by real-time contrast-enhanced ultrasound. Circ. Cardiovasc. Imaging 2018, 11, e007074. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1250–E1255. [Google Scholar] [CrossRef] [PubMed]
- Clerk, L.H.; Vincent, M.A.; Jahn, L.A.; Liu, Z.; Lindner, J.R.; Barrett, E.J. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006, 55, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.A.; Hartline, L.; Rao, N.; Logan, B.; Kim, J.J.; Aylor, K.; Gan, L.-M.; Westergren, H.U.; Barrett, E.J. Insulin enhances endothelial function throughout the arterial tree in healthy but not metabolic syndrome subjects. J. Clin. Endocrinol. Metab. 2016, 101, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.A.; Logan, B.; Love, K.M.; Horton, W.B.; Eichner, N.Z.; Hartline, L.M.; Weltman, A.L.; Barrett, E.J. Nitric oxide-dependent micro-and macrovascular dysfunction occurs early in adolescents with type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2022, 322, E101–E108. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Simonsen, L.; Bulow, J.; Christensen, N.J. Measurement of forearm oxygen consumption: Role of heating the contralateral hand. Am. J. Physiol. 1988, 255, E572–E578. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.W.; Young, J.B.; Minaker, K.L.; Stevens, A.L.; Pallotta, J.; Landsberg, L. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes 1981, 30, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.D. Hemodynamic actions of insulin. Am. J. Physiol. 1994, 267, E187–E202. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.O.; Brechtel, G.; Johnson, A.; Fineberg, N.; Baron, A.D. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J. Clin. Investig. 1994, 94, 1172–1179. [Google Scholar] [PubMed]
- Scherrer, U.; Randin, D.; Vollenweider, P.; Vollenweider, L.; Nicod, P. Nitric oxide release accounts for insulin’s vascular effects in humans. J. Clin. Investig. 1994, 94, 2511–2515. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Lacy, P.S.; CAFE and the ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) Investigators. Impact of heart rate on central aortic pressures and hemodynamics: Analysis from the CAFE (Conduit Artery Function Evaluation) study: CAFE-Heart Rate. J. Am. Coll. Cardiol. 2009, 54, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Wang, H.; Upchurch, C.T.; Liu, Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E252–E263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Jahn, L.A.; Fowler, D.E.; Barrett, E.J. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J. Clin. Endocrinol. Metab. 2009, 94, 3543–3549. [Google Scholar] [CrossRef] [PubMed]
| N (M/F) | Age (Years) | BMI (kg/m2) | |
|---|---|---|---|
| Metabolic Syndrome | 22 (11/11) | 46 ± 2 | 36 ± 1 |
| T2DM | 22 (9/13) | 52 ± 1 | 32 ± 1 |
| T1DM | 25 (16/9) | 28 ± 2 | 26 ± 1 |
| Obesity | 21 (5/16) | 32 ± 3 | 33 ± 2 |
| Control | 35 (17/18) | 23 ± 1 | 22 ± 1 |
| Fraction Affected | p-Value (Chi-Squared) | GIR (mg/min/kg) | p-Value (ANOVA) | |
|---|---|---|---|---|
| Metabolic Syndrome | 16/22 | <0.01 | 2.5 ± 0.2 | <0.001 |
| T2DM | 15/22 | <0.01 | 2.5 ± 0.3 | <0.001 |
| T1DM | 12/25 | <0.01 | 5.2 ± 0.4 | NS |
| Obesity | 14/21 | <0.01 | 3.6 ± 0.4 | <0.001 |
| Control | 4/35 | ----- | 6.2 ± 0.4 | ------ |
| Brachial Systolic Blood Pressure (mmHg) | Aortic Systolic Blood Pressure (mmHg) | Heart Rate (BPM) | ||||
|---|---|---|---|---|---|---|
| Baseline | 120 min | Baseline | 120 min | Baseline | 120 min | |
| Metabolic Syndrome | 130 ± 2 | 122 ± 2 * | 118 ± 2 | 108 ± 3 ** | 72 ± 3 | 75 ± 3 |
| T2DM | 128 ± 3 | 121 ± 3 * | 119 ± 3 | 112 ± 3 ** | 67 ± 2 | 70 ± 2 # |
| T1DM | 123 ± 3 | 118 ± 3 # | 108 ± 2 | 102 ± 2 ** | 70 ± 3 | 71 ± 2 |
| Obesity | 122 ± 3 | 116 ± 3 * | 108 ± 3 | 99 ± 3 ** | 61 ± 2 | 65 ± 2 * |
| Control | 109 ± 3 | 109 ± 2 | 95 ± 2 | 92 ± 2 ** | 55 ± 1 | 59 ± 1 * |
| Systolic Blood Pressure (mmHg) | Diastolic Blood Pressure (mmHg) | Heart Rate (BPM) | |||
|---|---|---|---|---|---|
| Brachial | Aortic | Brachial | Aortic | ||
| Metabolic Syndrome | −7.2 ± 2 * | −9.1 ± 1.9 * | −4.7 ± 1.7 | −4.7 ± 1.6 | 3.8 ± 1.5 |
| T2DM | −6.2 ± 2.1 # | −7.0 ± 1.3 # | −3.8 ± 2.1 | −4.0 ± 2.0 | 2.1 ± 1.3 |
| T1DM | −4.1 ± 1.7 | −5.6 ± 1.6 | −3.3 ± 1.4 | −3.0 ± 3.1 | 1.0 ± 2.4 |
| Obesity | −6.6 ± 1.8 # | −8.4 ± 1.8 * | −3.6 ± 1.2 | −2.8 ± 1.2 | 4.7 ± 0.9 |
| Control | −0.4 ± 1.9 | −3.1 ± 1.1 | −1.9 ± 1.0 | −2.0 ± 1.0 | 4.1 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Jahn, L.A.; Barrett, E.J. Euglycemic Hyperinsulinemia Lowers Blood Pressure and Impedes Microvascular Perfusion More Effectively in Persons with Cardio-Metabolic Disease. Endocrines 2025, 6, 36. https://doi.org/10.3390/endocrines6030036
Liu Z, Jahn LA, Barrett EJ. Euglycemic Hyperinsulinemia Lowers Blood Pressure and Impedes Microvascular Perfusion More Effectively in Persons with Cardio-Metabolic Disease. Endocrines. 2025; 6(3):36. https://doi.org/10.3390/endocrines6030036
Chicago/Turabian StyleLiu, Zhenqi, Linda A. Jahn, and Eugene J. Barrett. 2025. "Euglycemic Hyperinsulinemia Lowers Blood Pressure and Impedes Microvascular Perfusion More Effectively in Persons with Cardio-Metabolic Disease" Endocrines 6, no. 3: 36. https://doi.org/10.3390/endocrines6030036
APA StyleLiu, Z., Jahn, L. A., & Barrett, E. J. (2025). Euglycemic Hyperinsulinemia Lowers Blood Pressure and Impedes Microvascular Perfusion More Effectively in Persons with Cardio-Metabolic Disease. Endocrines, 6(3), 36. https://doi.org/10.3390/endocrines6030036






