CRABP1 Signalosomes in Non-Canonical Actions of Retinoic Acid—Maintaining Health and Preventing Thyroid Dysfunction in Aging
Abstract
1. Introduction
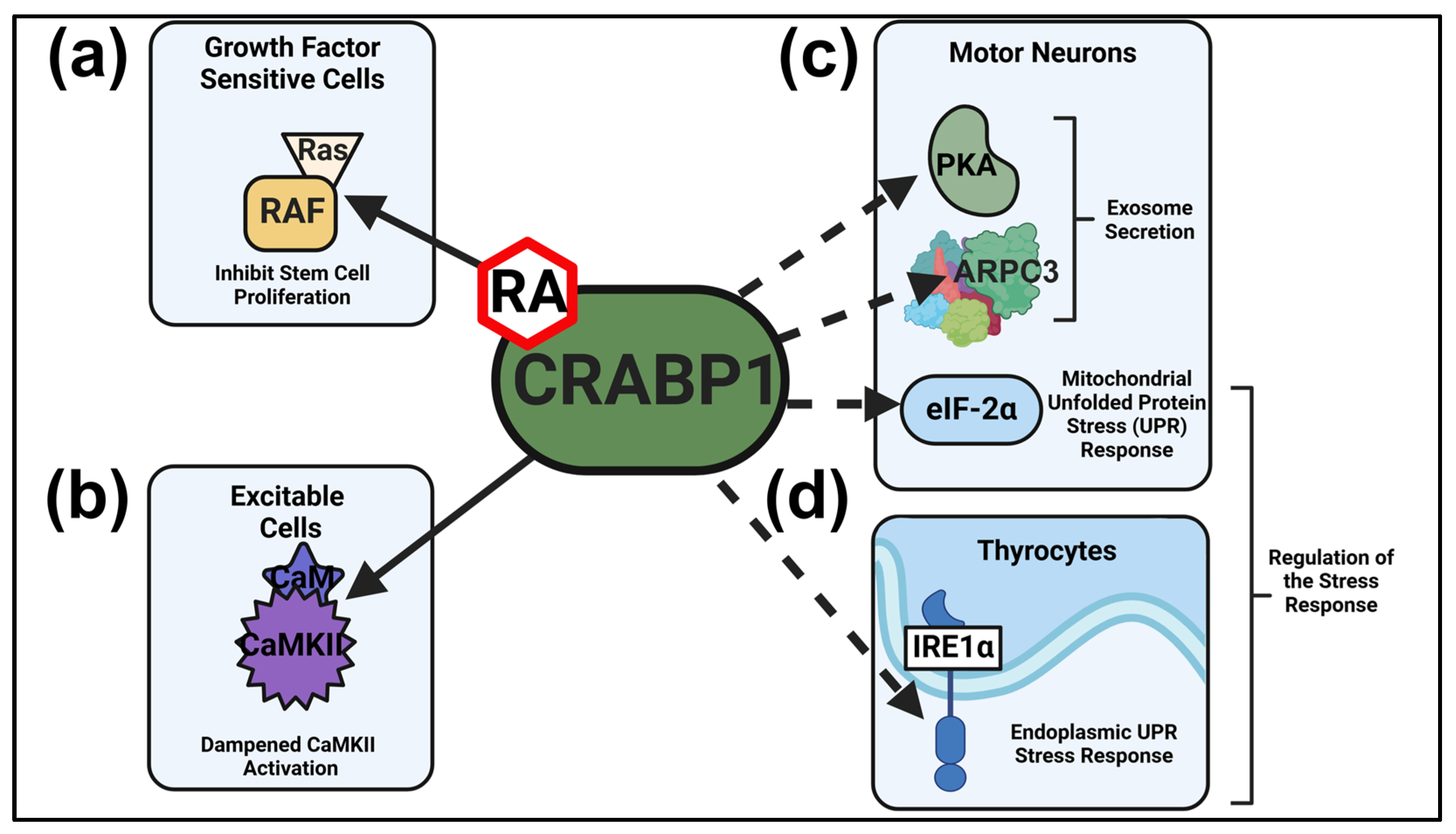
2. CRABP1 Signalosomes
2.1. CRABP1-RAF Signalosome (MAPK Signaling) in Dampening Stem Cell Proliferation
2.2. CRABP1-CaMKII Signalosome in Dampening Overexcitation-Induced Cellular Toxicity
2.3. CRABP1-PKA and CRABP1-Arp2/3 Signalosomes in Modulating Exosome Secretion
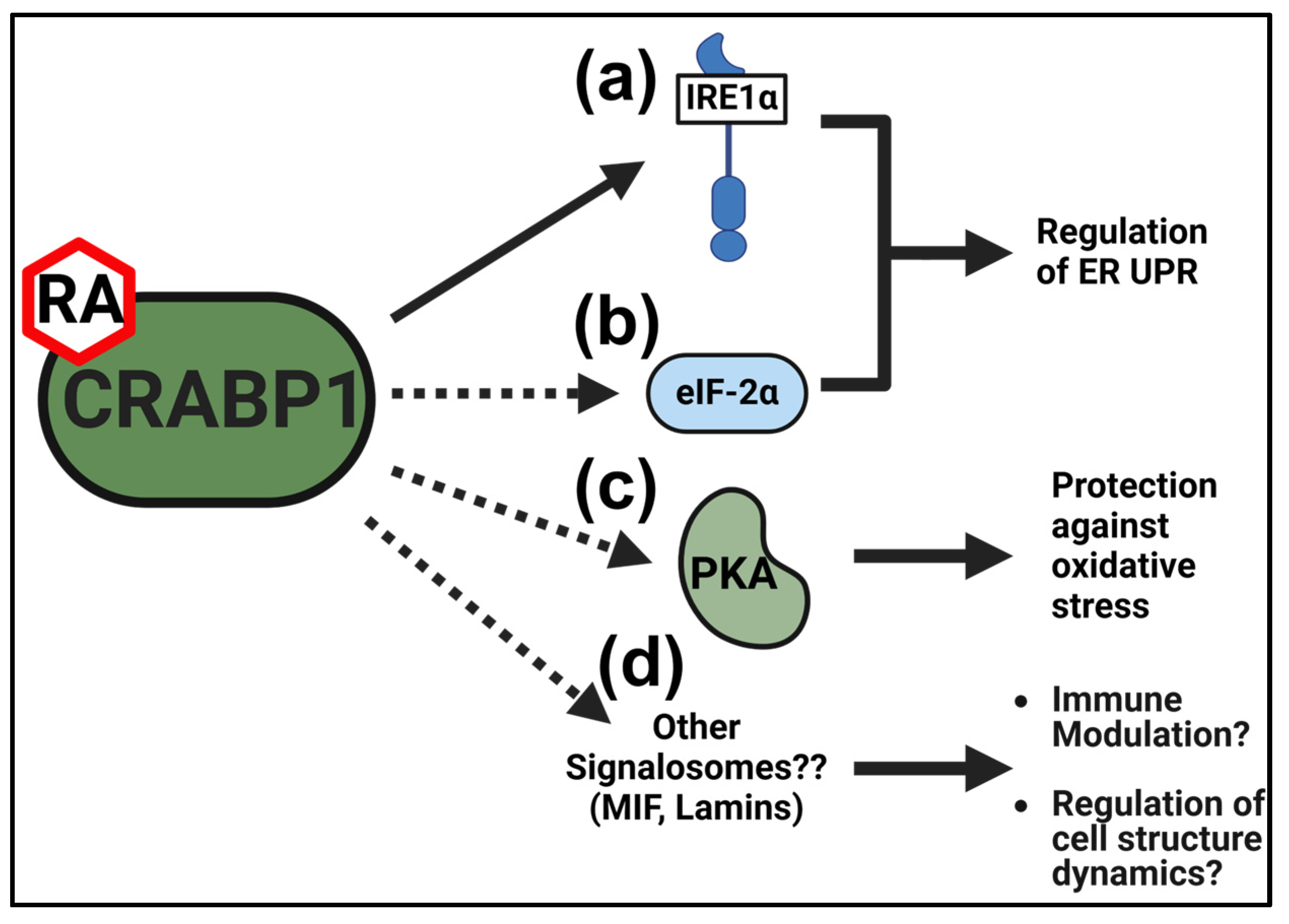
2.4. CRABP1-eIF2α and CRABP1 IRE1α Signalosomes in the Cellular Stress Response
3. Regulation of the CRABP1 Gene
3.1. Epigenetic Regulation of the Mouse Crabp1 Gene
3.2. Age-Dependent Changes in the Expression of CRABP1 in Human and Mouse Thyroid Glands
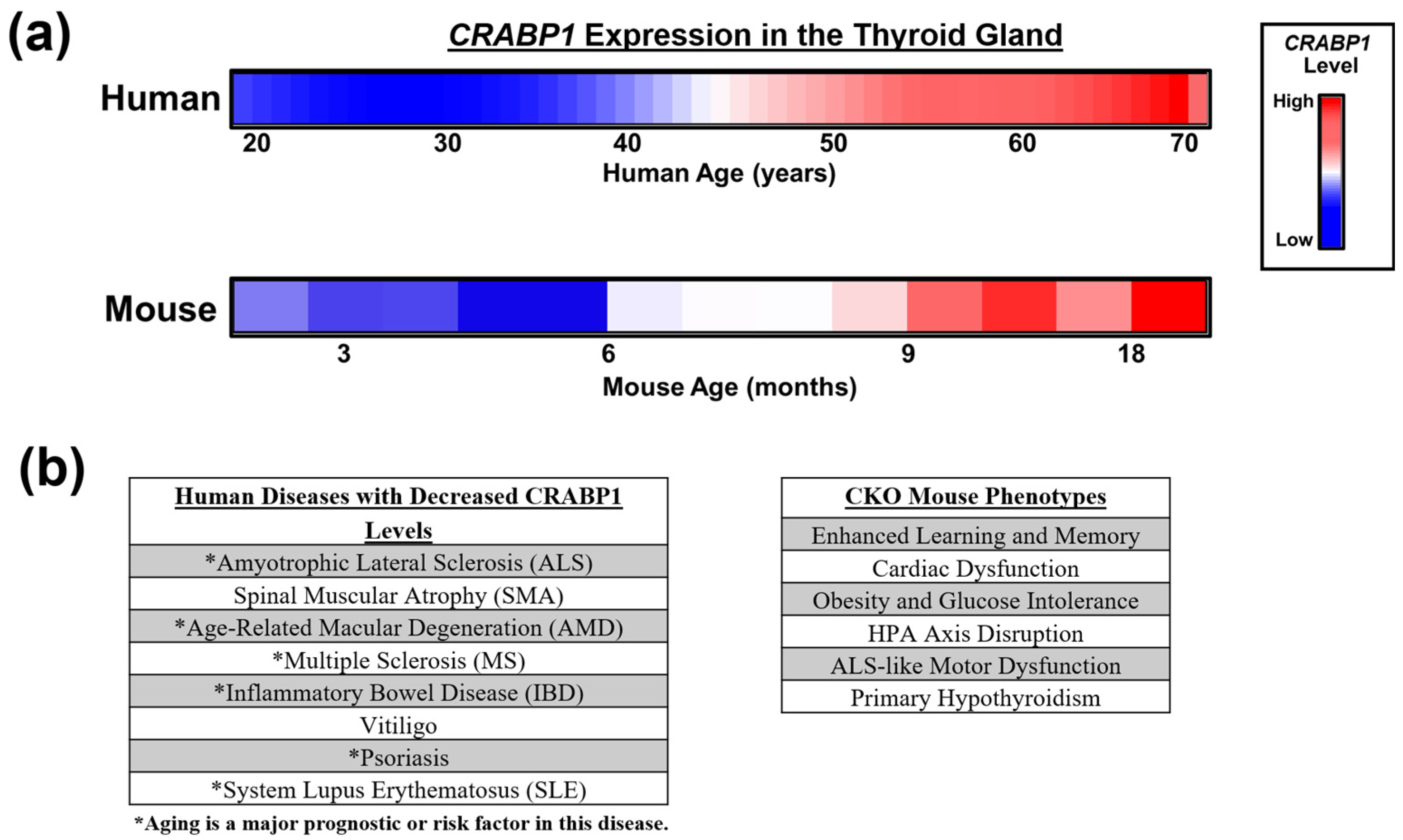
4. Clinical Relevance of CRABP1 in Thyroid Gland Health and Dysfunction
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015, 16, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, B.; Poon, M.M.; Nam, C.I.; Aoto, J.; Ting, P.; Chen, L. Retinoic acid regulates RARalpha-mediated control of translation in dendritic RNA granules during homeostatic synaptic plasticity. Proc. Natl. Acad. Sci. USA 2008, 105, 16015–16020. [Google Scholar] [CrossRef]
- Ochoa, W.F.; Torrecillas, A.; Fita, I.; Verdaguer, N.; Corbalán-García, S.; Gomez-Fernandez, J.C. Retinoic acid binds to the C2-domain of protein kinase C(alpha). Biochemistry 2003, 42, 8774–8779. [Google Scholar] [CrossRef] [PubMed]
- Persaud, S.D.; Lin, Y.-W.; Wu, C.-Y.; Kagechika, H.; Wei, L.-N. Cellular retinoic acid binding protein I mediates rapid non-canonical activation of ERK1/2 by all-trans retinoic acid. Cell. Signal. 2013, 25, 19–25. [Google Scholar] [CrossRef]
- Nagpal, I.; Wei, L.N. All-trans retinoic acid as a versatile cytosolic signal modulator mediated by CRABP1. Int. J. Mol. Sci. 2019, 20, 3610. [Google Scholar] [CrossRef]
- Nhieu, J.; Lin, Y.L.; Wei, L.N. CRABP1 in Non-Canonical Activities of Retinoic Acid in Health and Diseases. Nutrients 2022, 14, 1528. [Google Scholar] [CrossRef]
- Lin, Y.W.; Park, S.W.; Lin, Y.L.; Burton, F.H.; Wei, L.N. Cellular retinoic acid binding protein 1 protects mice from high-fat diet-induced obesity by decreasing adipocyte hypertrophy. Int. J. Obes. 2020, 44, 466–474. [Google Scholar] [CrossRef]
- Wei, C.W.; Nhieu, J.; Lin, Y.L.; Wei, L.N. Modulation of adipose inflammation by cellular retinoic acid-binding protein 1. Int. J. Obes. 2022, 46, 1759–1769. [Google Scholar] [CrossRef]
- Lin, Y.L.; Persaud, S.D.; Nhieu, J.; Wei, L.N. Cellular Retinoic Acid-Binding Protein 1 Modulates Stem Cell Proliferation to Affect Learning and Memory in Male Mice. Endocrinology 2017, 158, 3004–3014. [Google Scholar] [CrossRef]
- Park, S.W.; Persaud, S.D.; Ogokeh, S.; Meyers, T.A.; Townsend, D.W.; Wei, L.N. CRABP1 protects the heart from isoproterenol-induced acute and chronic remodeling. J. Endocrinol. 2018, 236, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Nhieu, J.; Lin, Y.W.; Wei, L.N. All-trans retinoic acid attenuates isoproterenol-induced cardiac dysfunction through Crabp1 to dampen CaMKII activation. Eur. J. Pharmacol. 2019, 858, 172485. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Lin, Y.W.; Nhieu, J.; Zhang, X.; Wei, L.N. Sonic hedgehog-gli1 signaling and cellular retinoic acid binding protein 1 gene regulation in motor neuron differentiation and diseases. Int. J. Mol. Sci. 2020, 21, 4125. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Nhieu, J.; Liu, P.-Y.; Le, G.; Lee, D.J.; Wei, C.-W.; Lin, Y.-W.; Oh, S.-H.; Lowe, D.; Wei, L.N. CRABP1-CaMKII-Agrn regulates the maintenance of neuromuscular junction in spinal motor neuron. Cell Death Differ. 2022, 29, 1744–1756. [Google Scholar] [CrossRef]
- Najjar, F.; Nhieu, J.; Wei, C.-W.; Milbauer, L.; Burmeister, L.; Seelig, D.; Wei, L.-N. Deleting Cellular Retinoic-Acid-Binding Protein-1 (Crabp1) Gene Causes Adult-Onset Primary Hypothyroidism in Mice. Endocrines 2023, 4, 138–150. [Google Scholar] [CrossRef]
- Lin, Y.W.; Nhieu, J.; Wei, C.W.; Lin, Y.L.; Kagechika, H.; Wei, L.N. Regulation of exosome secretion by cellular retinoic acid binding protein 1 contributes to systemic anti-inflammation. Cell Commun. Signal. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Nhieu, J.; Wei, C.W.; Ludwig, M.; Drake, J.M.; Wei, L.N. CRABP1-complexes in exosome secretion. Cell Commun. Signal. 2024, 22, 1–17. [Google Scholar] [CrossRef]
- Ong, D.E.; Chytil, F. Cellular retinoic acid-binding protein from rat testis. Purification and characterization. J. Biol. Chem. 1978, 253, 4551–4554. [Google Scholar] [CrossRef]
- Fiorella, P.D.; Giguère, V.; Napoli, J.L. Expression of cellular retinoic acid-binding protein (type II) in Escherichia coli: Characterization and comparison to cellular retinoic acid-binding protein (type I). J. Biol. Chem. 1993, 268, 21545–21552. [Google Scholar] [CrossRef]
- Norris, A.W.; Cheng, L.; Giguère, V.; Rosenberger, M.; Li, E. Measurement of subnanomolar retinoic acid binding affinities for cellular retinoic acid binding proteins by fluorometric titration. Biochim. Biophys. Acta 1994, 1209, 10–18. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yan, H. Structure-function relationships of cellular retinoic acid-binding proteins: Quantitative analysis of the ligand binding properties of the wild-type proteins and site-directed mutants. J. Biol. Chem. 1997, 272, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Nhieu, J.; Miller, M.C.; Lerdall, T.A.; Mayo, K.H.; Wei, L.N. Molecular basis for cellular retinoic acid-binding protein 1 in modulating CaMKII activation. Front. Mol. Biosci. 2023, 10, 1268843. [Google Scholar] [CrossRef] [PubMed]
- Wook Park, S.; Nhieu, J.; Persaud, S.D.; Miller, M.C.; Xia, Y.; Lin, Y.W.; Lin, Y.L.; Kagechika, H.; Mayo, K.H.; Wei, L.N. A new regulatory mechanism for Raf kinase activation, retinoic acid-bound Crabp1. Sci. Rep. 2019, 9, 10929. [Google Scholar] [CrossRef]
- Wei, L.-N.; Lee, C.-H. Demethylation in the 5′-flanking region of mouse cellular retinoic acid binding protein-I gene is associated with its high level of expression in mouse embryos and facilitates its induction by retinoic acid in P19 embryonal carcinoma cells. Dev. Dyn. 1994, 201, 1–10. [Google Scholar] [CrossRef]
- Matallanas, D.; Birtwistle, M.; Romano, D.; Zebisch, A.; Rauch, J.; von Kriegsheim, A.; Kolch, W. Raf family kinases: Old dogs have learned new tricks. Genes Cancer 2011, 2, 232–260. [Google Scholar] [CrossRef]
- Guidez, F.; Parks, S.; Wong, H.; Jovanovic, J.V.; Mays, A.; Gilkes, A.F.; Mills, K.I.; Guillemin, M.-C.; Hobbs, R.M.; Pandolfi, P.P.; et al. RARalpha-PLZF overcomes PLZF-mediated repression of CRABPI, contributing to retinoid resistance in t(11;17) acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 2007, 104, 18694–18699. [Google Scholar] [CrossRef]
- Pfoertner, S.; Goelden, U.; Hansen, W.; Toepfer, T.; Geffers, R.; Ukena, S.N.; von Knobloch, R.; Hofmann, R.; Buer, J.; Schrader, A.J. Cellular retinoic acid binding protein I: Expression and functional influence in renal cell carcinoma. Tumour Biol. 2005, 26, 313–323. [Google Scholar] [CrossRef]
- Tanaka, K.; Imoto, I.; Inoue, J.; Kozaki, K.; Tsuda, H.; Shimada, Y.; Aiko, S.; Yoshizumi, Y.; Iwai, T.; Kawano, T.; et al. Frequent methylation-associated silencing of a candidate tumor-suppressor, CRABP1, in esophageal squamous-cell carcinoma. Oncogene 2007, 26, 6456–6468. [Google Scholar] [CrossRef]
- Miyake, T.; Ueda, Y.; Matsuzaki, S.; Miyatake, T.; Yoshino, K.; Fujita, M.; Nomura, T.; Enomoto, T.; Kimura, T. CRABP1-reduced expression is associated with poorer prognosis in serous and clear cell ovarian adenocarcinoma. J. Cancer Res. Clin. Oncol. 2011, 137, 715–722. [Google Scholar] [CrossRef]
- Wu, Q.; A Lothe, R.; Ahlquist, T.; Silins, I.; Tropé, C.G.; Micci, F.; Nesland, J.M.; Suo, Z.; E Lind, G. DNA methylation profiling of ovarian carcinomas and their in vitro models identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targets. Mol. Cancer 2007, 6, 45. [Google Scholar] [CrossRef]
- Hawthorn, L.; Stein, L.; Varma, R.; Wiseman, S.; Loree, T.; Tan, D.F. TIMP1 and SERPIN-A overexpression and TFF3 and CRABP1 underexpression as biomarkers for papillary thyroid carcinoma. Head Neck 2004, 26, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Celestino, R.; Nome, T.; Pestana, A.; Hoff, A.M.; Gonçalves, A.P.; Pereira, L.; Cavadas, B.; Eloy, C.; Bjøro, T.; Sobrinho-Simões, M.; et al. CRABP1, C1QL1 and LCN2 are biomarkers of differentiated thyroid carcinoma, and predict extrathyroidal extension. BMC Cancer 2018, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; de la Chapelle, A.; Pellegata, N.S. Hypermethylation, but not LOH, is associated with the low expression of MT1G and CRABP1 in papillary thyroid carcinoma. Int. J. Cancer 2003, 104, 735–744. [Google Scholar] [CrossRef]
- Lind, G.E.; Kleivi, K.; Meling, G.I.; Teixeira, M.R.; Thiis-Evensen, E.; Rognum, T.O.; Lothe, R.A. ADAMTS1, CRABP1, and NR3C1 identified as epigenetically deregulated genes in colorectal tumorigenesis. Cell. Oncol. 2006, 28, 259–272. [Google Scholar] [CrossRef]
- Erickson, J.R. Mechanisms of CaMKII activation in the heart. Front. Pharmacol. 2014, 5, 59. [Google Scholar] [CrossRef]
- Lisman, J.; Schulman, H.; Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002, 3, 175–190. [Google Scholar] [CrossRef]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Yao, Y.; Li, F.; Zhang, M.; Jin, L.; Xie, P.; Liu, D.; Zhang, J.; Hu, X.; Lv, F.; Shang, H.; et al. Targeting CaMKII-δ9 Ameliorates Cardiac Ischemia/Reperfusion Injury by Inhibiting Myocardial Inflammation. Circ. Res. 2022, 130, 887–903. [Google Scholar] [CrossRef]
- Luczak, E.D.; Anderson, M.E. CaMKII oxidative activation and the pathogenesis of cardiac disease. J. Mol. Cell. Cardiol. 2014, 73, 112–116. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Yamamoto, M.; Kobayashi, Y.; Yoshihara, T.; Liang, Y.; Terao, S.; Takeuchi, H.; Ishigaki, S.; Katsuno, M.; Adachi, H.; et al. Gene expression profile of spinal motor neurons in sporadic amyotrophic lateral sclerosis. Ann. Neurol. 2005, 57, 236–251. [Google Scholar] [CrossRef] [PubMed]
- De Decker, M.; Zelina, P.; Moens, T.G.; Beckers, J.; Contardo, M.; Dittlau, K.S.; Van Schoor, E.; Ronisz, A.; Eggermont, K.; Moisse, M.; et al. C21ORF2 mutations point towards primary cilia dysfunction in amyotrophic lateral sclerosis. Brain 2024, 139, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Giese, K.P.; Fedorov, N.B.; Filipkowski, R.K.; Silva, A.J. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 1998, 279, 870–873. [Google Scholar] [CrossRef]
- Islam, A.; Jones, H.; Hiroi, T.; Lam, J.; Zhang, J.; Moss, J.; Vaughan, M.; Levine, S.J. cAMP-dependent protein kinase A (PKA) signaling induces TNFR1 exosome-like vesicle release via anchoring of PKA regulatory subunit RIIβ to BIG2. J. Biol. Chem. 2008, 283, 25364–25371. [Google Scholar] [CrossRef]
- Sinha, S.; Hoshino, D.; Hong, N.H.; Kirkbride, K.C.; Grega-Larson, N.E.; Seiki, M.; Tyska, M.J.; Weaver, A.M. Cortactin promotes exosome secretion by controlling branched actin dynamics. J. Cell Biol. 2016, 214, 197–213. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Hinnebusch, A.G.; Ivanov, I.P.; Sonenberg, N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 2016, 352, 1413–1416. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef]
- Anderson, N.S.; Haynes, C.M. Folding the Mitochondrial UPR into the Integrated Stress Response. Trends Cell Biol. 2020, 30, 428–439. [Google Scholar] [CrossRef]
- Wei, C.-W.; Lerdall, T.; Najjar, F.; Wei, L.-N. Depleting Cellular Retinoic Acid Binding Protein 1 Impairs UPRmt. J. Cell. Signal. 2023, 4, 151–162. [Google Scholar] [CrossRef]
- Yadav, A.; Matson, K.J.E.; Li, L.; Hua, I.; Petrescu, J.; Kang, K.; Alkaslasi, M.R.; Lee, D.I.; Hasan, S.; Galuta, A.; et al. A cellular taxonomy of the adult human spinal cord. Neuron 2023, 111, 328–344.e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Brandizzi, F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013, 23, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Shore, G.C.; Papa, F.R.; Oakes, S.A. Signaling cell death from the endoplasmic reticulum stress response. Curr. Opin. Cell Biol. 2011, 23, 143–149. [Google Scholar] [CrossRef]
- Hetz, C.; Mollereau, B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 2014, 15, 233–249. [Google Scholar] [CrossRef]
- English, A.M.; Green, K.M.; Moon, S.L. A (dis)integrated stress response: Genetic diseases of eIF2α regulators. Wiley Interdiscip. Rev. RNA 2022, 13, e1689. [Google Scholar] [CrossRef]
- Junjappa, R.P.; Patil, P.; Bhattarai, K.R.; Kim, H.R.; Chae, H.J. IRE1α implications in endoplasmic reticulum stress-mediated development and pathogenesis of autoimmune diseases. Front. Immunol. 2018, 9, 362725. [Google Scholar] [CrossRef]
- Scheper, W.; Hoozemans, J.J.M. The unfolded protein response in neurodegenerative diseases: A neuropathological perspective. Acta Neuropathol. 2015, 130, 315. [Google Scholar] [CrossRef]
- Huang, S.; Xing, Y.; Liu, Y. Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease. J. Biol. Chem. 2019, 294, 18726–18741. [Google Scholar] [CrossRef]
- Wei, L.-N. Non-canonical activity of retinoic acid in epigenetic control of embryonic stem cell. Transcription 2013, 4, 158–161. [Google Scholar] [CrossRef]
- Wei, L.N.; Chen, G.J.; Chu, Y.S.; Tsao, J.L.; Nguyen-Huu, M.C. A 3 kb sequence from the mouse cellular retinoic-acid-binding protein gene upstream region mediates spatial and temporal LacZ expression in transgenic mouse embryos. Development 1991, 112, 847–854. [Google Scholar] [CrossRef]
- Wei, L.N. Chromatin remodeling and epigenetic regulation of the CrabpI gene in adipocyte differentiation. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 206–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, L.N. Cellular retinoic acid binding proteins: Genomic and non-genomic functions and their regulation. In Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2016; Volume 81, pp. 163–178. [Google Scholar] [CrossRef]
- Sung, W.P.; Li, G.; Lin, Y.P.; Barrero, M.J.; Ge, K.; Roeder, R.G.; Wei, L.N. Thyroid hormone-induced juxtaposition of regulatory elements/factors and chromatin remodeling of Crabp1 dependent on MED1/TRAP220. Mol. Cell 2005, 19, 643–653. [Google Scholar] [CrossRef]
- Park, S.W.; Huang, W.H.; Persaud, S.D.; Wei, L.N. RIP140 in thyroid hormone-repression and chromatin remodeling of Crabp1 gene during adipocyte differentiation. Nucleic Acids Res. 2009, 37, 7085–7094. [Google Scholar] [CrossRef]
- Bi, J.; Hu, X.; Zhou, F.C.; Wei, L.N. Upregulation of cellular retinoic acid-binding protein I expression by ethanol. Dev. Growth Differ. 2001, 43, 553–561. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Wegmüller, R.; Zeder, C.; Chaouki, N.; Torresani, T. The effects of vitamin A deficiency and vitamin A supplementation on thyroid function in goitrous children. J. Clin. Endocrinol. Metab. 2004, 89, 5441–5447. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Jooste, P.L.; Mabapa, N.S.; Schoeman, S.; Biebinger, R.; Mushaphi, L.F.; Mbhenyane, X. Vitamin A supplementation in iodine-deficient African children decreases thyrotropin stimulation of the thyroid and reduces the goiter rate. Am. J. Clin. Nutr. 2007, 86, 1040–1044. [Google Scholar] [CrossRef]
- Ingre, C.; Roos, P.M.; Piehl, F.; Kamel, F.; Fang, F. Risk factors for amyotrophic lateral sclerosis. Clin. Epidemiol. 2015, 7, 181. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Graves, J.S.; Krysko, K.M.; Hua, L.H.; Absinta, M.; Franklin, R.J.M.; Segal, B.M. Ageing and multiple sclerosis. Lancet Neurol. 2023, 22, 66–77. [Google Scholar] [CrossRef]
- Faye, A.S.; Colombel, J.F. Aging and IBD: A New Challenge for Clinicians and Researchers. Inflamm. Bowel Dis. 2022, 28, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Kwon, I.H.; Youn, J.I. Clinical study of psoriasis occurring over the age of 60 years: Is elderly-onset psoriasis a distinct subtype? Int. J. Dermatol. 2012, 51, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, D. Elderly-onset systemic lupus erythematosus: Prevalence, clinical course and treatment. Drugs Aging 2007, 24, 701–715. [Google Scholar] [CrossRef]
- Schneider, A.L.; Martins-Silva, R.; Kaizeler, A.; Saraiva-Agostinho, N.; Barbosa-Morais, N.L. voyAGEr, a free web interface for the analysis of age-related gene expression alterations in human tissues. eLife 2024, 12, RP88623. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Toro, F.; Jialal, I. Physiology, Thyroid Stimulating Hormone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wolf, G. The regulation of the thyroid-stimulating hormone of the anterior pituitary gland by thyroid hormone and by 9-cis-retinoic acid. Nutr. Rev. 2002, 60, 374–377. [Google Scholar] [CrossRef]
- Rugge, J.B.; Bougatsos, C.; Chou, R. Screening and treatment of thyroid dysfunction: An evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2015, 162, 35–45. [Google Scholar] [CrossRef]
- Bianco, A.C. Emerging Therapies in Hypothyroidism. Annu Rev Med. 2024, 75, 307–319. [Google Scholar] [CrossRef]
- McAninch, E.A.; Bianco, A.C. New insights into the variable effectiveness of levothyroxine monotherapy for hypothyroidism. Lancet Diabetes Endocrinol. 2015, 3, 756–758. [Google Scholar] [CrossRef]
- Chaker, L.; Razvi, S.; Bensenor, I.M.; Azizi, F.; Pearce, E.N.; Peeters, R.P. Hypothyroidism. Nat. Rev. Dis. Primers 2022, 8, 1–17. [Google Scholar] [CrossRef]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Chiovato, L.; Magri, F.; Carlé, A. Hypothyroidism in Context: Where We’ve Been and Where We’re Going. Adv. Ther. 2019, 36, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Diab, N.; Daya, N.R.; Juraschek, S.P.; Martin, S.S.; McEvoy, J.W.; Schultheiß, U.T.; Köttgen, A.; Selvin, E. Prevalence and Risk Factors of Thyroid Dysfunction in Older Adults in the Community. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, N.; Brown, T.R.; Parish, R.F. Thyroid Dysfunction in Adults Over Age 55 Years: A Study in an Urban US Community. Arch. Intern. Med. 1990, 150, 785–787. [Google Scholar] [CrossRef]
- Zhang, X.; Kellogg, A.P.; Citterio, C.E.; Zhang, H.; Larkin, D.; Morishita, Y.; Targovnik, H.M.; Balbi, V.A.; Arvan, P. Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death. JCI Insight 2021, 6, e148496. [Google Scholar] [CrossRef]
- Zhang, X.; Young, C.; Liao, X.-H.; Refetoff, S.; Torres, M.; Tomer, Y.; Stefan-Lifshitz, M.; Zhang, H.; Larkin, D.; Fang, D.; et al. Perturbation of endoplasmic reticulum proteostasis triggers tissue injury in the thyroid gland. JCI Insight 2023, 8, e169937. [Google Scholar] [CrossRef]
- Kainov, Y.; Favorskaya, I.; Delektorskaya, V.; Chemeris, G.; Komelkov, A.; Zhuravskaya, A.; Trukhanova, L.; Zueva, E.; Tavitian, B.; Dyakova, N.; et al. CRABP1 provides high malignancy of transformed mesenchymal cells and contributes to the pathogenesis of mesenchymal and neuroendocrine tumors. Cell Cycle 2014, 13, 1530. [Google Scholar] [CrossRef]
- Tomás, G.; Tarabichi, M.; Gacquer, D.; Hébrant, A.; Dom, G.; Dumont, J.E.; Keutgen, X.; Fahey, T.J.; Maenhaut, C.; Detours, V. A general method to derive robust organ-specific gene expression-based differentiation indices: Application to thyroid cancer diagnostic. Oncogene 2012, 31, 4490–4498. [Google Scholar] [CrossRef]
- Beamer, W.G.; Maltais, L.J.; Debaets, M.H.; Eicher, E.M. Inherited congenital goiter in mice. Endocrinology 1987, 120, 838–840. [Google Scholar] [CrossRef]
- Takabayashi, S.; Umeki, K.; Yamamoto, E.; Suzuki, T.; Okayama, A.; Katoh, H. A novel hypothyroid dwarfism due to the missense mutation Arg479Cys of the thyroid peroxidase gene in the mouse. Mol. Endocrinol. 2006, 20, 2584–2590. [Google Scholar] [CrossRef]
- Ferrandino, G.; Kaspari, R.R.; Reyna-Neyra, A.; Boutagy, N.E.; Sinusas, A.J.; Carrasco, N. An extremely high dietary iodide supply forestalls severe hypothyroidism in Na+/I− symporter (NIS) knockout mice. Sci. Rep. 2017, 7, 5329. [Google Scholar] [CrossRef] [PubMed]
- Faustino, L.C.; Li, C.W.; Stefan-Lifshitz, M.; Kim, K.; Clarke, O.B.; Tomer, Y. A Novel Mouse Model of Autoimmune Thyroiditis Induced by Immunization with Adenovirus Containing Full-Length Thyroglobulin cDNA: Implications to Genetic Studies of Thyroid Autoimmunity. Thyroid 2020, 30, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Tsujio, M.; Yoshioka, K.; Satoh, M.; Watahiki, Y.; Mutoh, K. Skin morphology of thyroidectomized rats. Vet. Pathol. 2008, 45, 505–511. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Luan, H.; Roberts, R.L. Effect of methylimidazole-induced hypothyroidism in a model of low retinal neovascular incidence. Investig. Ophthalmol. Vis. Sci. 2004, 45, 919–921. [Google Scholar] [CrossRef]
- Chen, C.R.; Hamidi, S.; Braley-Mullen, H.; Nagayama, Y.; Bresee, C.; Aliesky, H.A.; Rapoport, B.; McLachlan, S.M. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology 2010, 151, 4583–4593. [Google Scholar] [CrossRef]
- Michael Gale, J.; Tan, S.-L.; Katze, M.G. Translational Control of Viral Gene Expression in Eukaryotes. Microbiol. Mol. Biol. Rev. 2000, 64, 239. [Google Scholar] [CrossRef]
- Ivanova, N.; Serzhanova, V.; Demina, N.; Guseva, D.; Skoblov, M. mRNA analysis revealed a novel pathogenic EIF2S3 variant causing MEHMO syndrome. Eur. J. Med. Genet. 2022, 65, 104421. [Google Scholar] [CrossRef]
- Ho, C.Y.; Lammerding, J. Lamins at a glance. J. Cell Sci. 2012, 125, 2087–2093. [Google Scholar] [CrossRef]
- Vantyghem, M.C.; Faivre-Defrance, F.; Marcelli-Tourvieille, S.; Fermon, C.; Evrard, A.; Bourdelle-Hego, M.F.; Vigouroux, C.; Defebvre, L.; Delemer, B.; Wemeau, J.L. Familial partial lipodystrophy due to the LMNA R482W mutation with multinodular goitre, extrapyramidal syndrome and primary hyperaldosteronism. Clin. Endocrinol. 2007, 67, 247–249. [Google Scholar] [CrossRef]
- Harris, J.; VanPatten, S.; Deen, N.S.; Al-Abed, Y.; Morand, E.F. Rediscovering MIF: New Tricks for an Old Cytokine. Trends Immunol. 2019, 40, 447–462. [Google Scholar] [CrossRef]
- Martínez-Hernández, R.; Sánchez de la Blanca, N.; Sacristán-Gómez, P.; Serrano-Somavilla, A.; Muñoz De Nova, J.L.; Sánchez Cabo, F.; Heyn, H.; Sampedro-Núñez, M.; Marazuela, M. Unraveling the molecular architecture of autoimmune thyroid diseases at spatial resolution. Nat. Commun. 2024, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.S.; Yang, J.; Wu, J.; Haste, N.M.; Radzio-Andzelm, E.; Anand, G. PKA: A portrait of protein kinase dynamics. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2004, 1697, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Morshed, S.A.; Ma, R.; Latif, R.; Davies, T.F. How one TSH receptor antibody induces thyrocyte proliferation while another induces apoptosis. J. Autoimmun. 2013, 47, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Persaud, S.D.; Park, S.W.; Ishigami-Yuasa, M.; Koyano-Nakagawa, N.; Kagechika, H.; Wei, L.N. All trans-retinoic acid analogs promote cancer cell apoptosis through non-genomic Crabp1 mediating ERK1/2 phosphorylation. Sci. Rep. 2016, 6, 22396. [Google Scholar] [CrossRef]
- Nhieu, J.; Milbauer, L.; Lerdall, T.; Najjar, F.; Wei, C.W.; Ishida, R.; Ma, Y.; Kagechika, H.; Wei, L.N. Targeting Cellular Retinoic Acid Binding Protein 1 with Retinoic Acid-like Compounds to Mitigate Motor Neuron Degeneration. Int. J. Mol. Sci. 2023, 24, 4980. [Google Scholar] [CrossRef]
| Gene Name | Description | Thyroid Gland Phenotype |
|---|---|---|
| EIF2S3 (eIF2γ) | GTP-binding component of the eIF2 complex (eIF2α, eIF2β, and eIF2γ) that binds the initiator tRNA required for translation initiation [98]. | Patients with MEHMO syndrome due to the EIF2S3 c.820C > G variant presented with hypothyroidism [99]. |
| LMNA | An intermediate filament that forms major structural components of the nuclear lamina [100]. | Patients with LMNA R482W/Q mutations presented with multi-nodal goiter [101] |
| MIF | A pleiotropic cytokine that regulates innate and adaptive immunity by activating monocytes/macrophages [102]. | MIF expression is increased in thyroid tissues of patients with auto-immune thyroid diseases, GD and HT [103]. |
| PRKACA | Catalytic subunit of PKA responsible for kinase activity [104]. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nhieu, J.; Najjar, F.; Wei, L.-N. CRABP1 Signalosomes in Non-Canonical Actions of Retinoic Acid—Maintaining Health and Preventing Thyroid Dysfunction in Aging. Endocrines 2025, 6, 26. https://doi.org/10.3390/endocrines6020026
Nhieu J, Najjar F, Wei L-N. CRABP1 Signalosomes in Non-Canonical Actions of Retinoic Acid—Maintaining Health and Preventing Thyroid Dysfunction in Aging. Endocrines. 2025; 6(2):26. https://doi.org/10.3390/endocrines6020026
Chicago/Turabian StyleNhieu, Jennifer, Fatimah Najjar, and Li-Na Wei. 2025. "CRABP1 Signalosomes in Non-Canonical Actions of Retinoic Acid—Maintaining Health and Preventing Thyroid Dysfunction in Aging" Endocrines 6, no. 2: 26. https://doi.org/10.3390/endocrines6020026
APA StyleNhieu, J., Najjar, F., & Wei, L.-N. (2025). CRABP1 Signalosomes in Non-Canonical Actions of Retinoic Acid—Maintaining Health and Preventing Thyroid Dysfunction in Aging. Endocrines, 6(2), 26. https://doi.org/10.3390/endocrines6020026






